1. Introduction
In 2018, there were 790.5 million pigs in the whole world, and global pork production was about 118.8 million tons [
1]. Swine wastewater has been one of the most widely distributed pollution sources globally, and the discharge of pig wastewater has increased gradually over recent years. Furthermore, China is one of the countries with the highest pork production, with pork accounting for 65% of all meat consumed in China [
2]. A previous study estimated that each pig farm in China produces approximately 1300 m
3 of pig wastewater each year [
3]. In South Korea, swine wastewater accounted for approximately 53% on average of all livestock excreta [
4].
Antibiotic pollution has become an increasingly serious concern in the past few decades, increasingly attracting attention. Antibiotics are also a non-negligible part of swine wastewater, and swine wastewater is known to be a major source of environmental antibiotic input. The livestock and poultry industries consume more than 84,000 tons of veterinary antibiotics every year in China, and about 58% of these are directly or indirectly discharged into the natural environment [
5]. It is estimated that from 2010 to 2030, global antibiotic use will increase by 67%. In Brazil, Russia, India, China, and South Africa, the rate of usage is expected to double [
6].
Swine wastewater contains various types of antibiotics, including tetracyclines, sulfonamides, macrolides, fluoroquinolones, and other veterinary antibiotics [
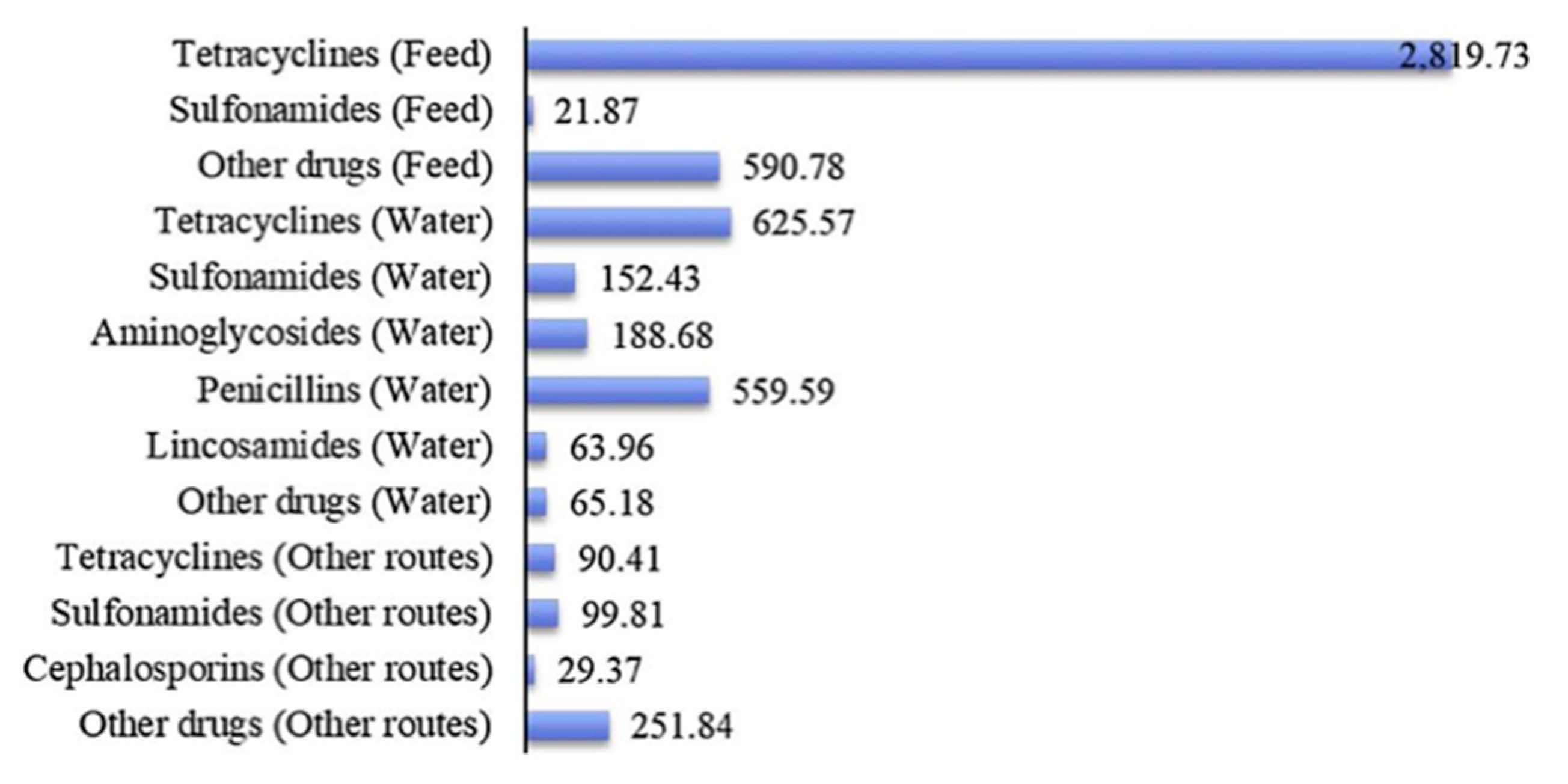
7]. Tetracyclines are the most common veterinary antibiotics in pig fodder, as well as the most threatening antibiotics to the natural environment, with concentrations as high as 685.60 µg/L being used [
8]. An American Food and Drug Administration report showed that 80% of antibiotics sold in the United States are used for livestock production. The ways that different types of antibiotics were used in livestock production in 2017 are shown in
Figure 1.
Antibiotics are difficult to degrade in the natural environment and can have a terrible impact on soil and water. Antibiotic administration has direct effects on the enteric and pathogenic bacteria in livestock animals, and environmental bacteria are also affected if antibiotic residues and metabolites are released into the environment [
9]. Currently, antibiotic-resistant bacteria with resistance to sulfonamides and tetracyclines are detected ubiquitously in Asia [
10].
This requires pig farms to treat swine wastewater to remove residual antibiotics before it is discharged; however, conventional treatment technology only shows good performance in the removal of general polluting factors, such as chemical oxygen demand (COD) and total nitrogen (TN). A study reporting on sewage treatment plants in the Jiulongjiang Basin found that the average removal efficiency of TCs in sewage treatment plants is mainly based on anaerobic–anoxic–oxic processes (A2/O), and adsorption biodegradation (A/B) was between −71.6% and 56.3% [
11]. Zhang et al.’s research found that the removal rate of TCs by composting was less than 63.7%, but that the composting cycle was 171 days [
12]. Glutathione S-transferase (GST) is a type of protein that can be used to specifically degrade refractory and impediment antibiotics; however, the degradation efficiency of this method for TCs was found to be only 30% [
13]. On account of this, there is an urgent need to find a swine wastewater treatment process that can efficiently degrade TCs.
Anaerobic digestion is a common way to degrade swine wastewater, as it has a high degradation efficiency, low energy consumption, and is easy to carry out. Anaerobic digestion has also been shown to have an excellent performance when it comes to the degradation of TCs. Researchers evaluated the impact of different concentrations of tetracycline on the performance of anaerobic treatment and found no major sustained impact on methane production, demonstrating that anaerobic digestion was stable and reliable in the presence of tetracycline [
14]. During the anaerobic digestion of manure from medicated calves, the chlortetracycline (CTC) concentration decreased approximately 75% during the 33-day digestion period [
15]. Wang et al.’s results showed that the removal rate of TCs in the supernatant could be up to 90%–100% during swine manure anaerobic digestion. However, their removal rates in the solid phase were only around 40% [
16]. The OTC removal efficiency in a different thermophilic composting study was found to be over 90% [
17].
Ma et al.’s study indicated that thermophilic anaerobic digesters more effectively reduced concentrations of TCs compared to mesophilic digesters [
18]. During anaerobic digestion, the number of antibiotic resistance genes decreased sharply with increasing temperature [
19]. However, the removal rate did not increase with increasing temperature. Despite this, some studies have shown that when the anaerobic digestion temperature exceeds 60 °C, the reduction of antibiotic resistance genes is not as high as when the temperature is below 55 °C [
20].
OTC is a crucial broad-spectrum antibiotic [
21] and can reliably inhibit a variety of bacteria at low cost [
22]. OTC has been used widely in pig farms to control diseases caused by various bacteria [
23]. However, there are few studies looking at how changes in OTC content affect the difficulty of removing pollutants from swine wastewater during anaerobic digestion.
The most common and effective method used to deal with swine wastewater and degrade antibiotics has been high-temperature digestion. As the content of antibiotics varies in different collections of swine wastewater, there are few studies aiming to examine the effect of antibiotic content on high-temperature swine wastewater digestion and how various components in the wastewater affect each other in the process. This study focused on the effect of OTC addition in high-temperature anaerobic digestion (55 °C) and the interactions between TCs, heavy metals, and the microbial community, aiming at providing a theoretical basis for the study of high-temperature anaerobic digestion of TCs.
2. Materials and Methods
2.1. Swine Wastewater and Granular Sludge
The swine wastewater used in this study was taken from a pig farm in Jiaxing City, Zhejiang Province, China. It was fresh pig manure. The advantage of fresh pig manure was that it could to avoid the degradation of antibiotics due to time and temperature. The solid content of pig manure was about 5%, and the basic indicators, such as pH, total organic carbon (TOC), total phosphorus (TP), and total nitrogen (TN), are shown in
Table 1. The basic indicators were tested after mixing, centrifuging, and diluting. TOC and TN were detected by a TOC analyzer, and TP was analyzed manually by the molybdate calibration method.
The inoculated sludge in this study was granular sludge obtained from the Shanghai Songjiang Tsingtao Brewery and was a black granular solid with many extracellular polymers. Solid and liquid reagents were stored at 4 °C, and ordinary sampling reagents were stored at −20 °C.
2.2. Experimental Design
High-temperature anaerobic digestion was carried out in a digestion reactor, with a water bath maintaining the temperature in the reactor at 55 °C. For the uniformity and completeness of the reaction process, each digestion reactor was equipped with a stirring device, which could constantly stir with 300 r/min. The reactor provided a closed anaerobic environment for the digestion process.
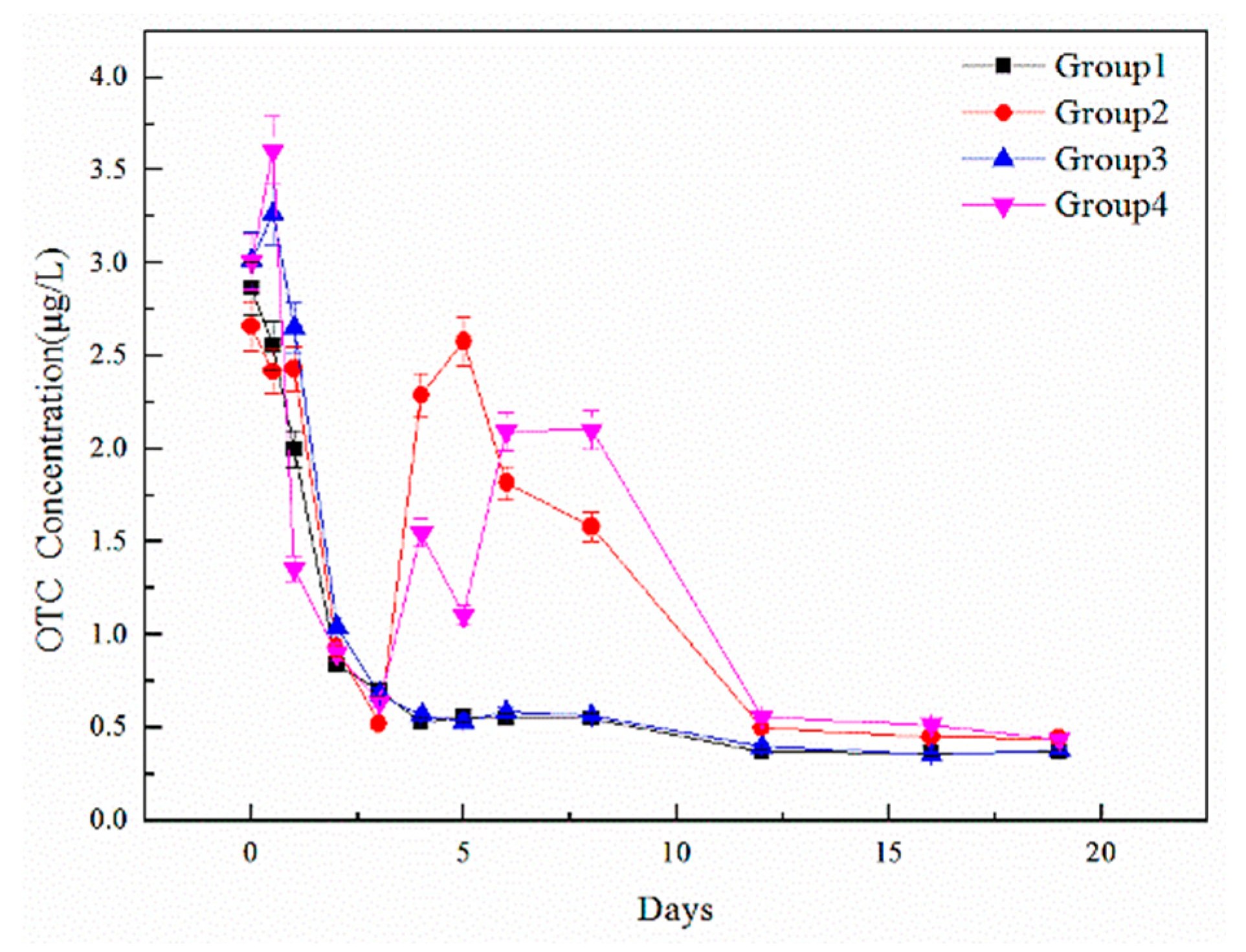
The main experimental process was carried out in four groups of anaerobic digestion reactors, each with a capacity of 1000 mL. Each reactor was supplemented with a total of 1000 mL of swine wastewater and 50 g of solidified granular sludge. Before digestion, all reactors were infused with nitrogen for 30 min to obtain an anaerobic environment. Reactors were then subjected to high-temperature anaerobic digestion at 55 °C. After the digestion was started, groups 2, 3 and 4 were spiked with 1 µg of OTC by different methods. More details are shown in
Table 2.
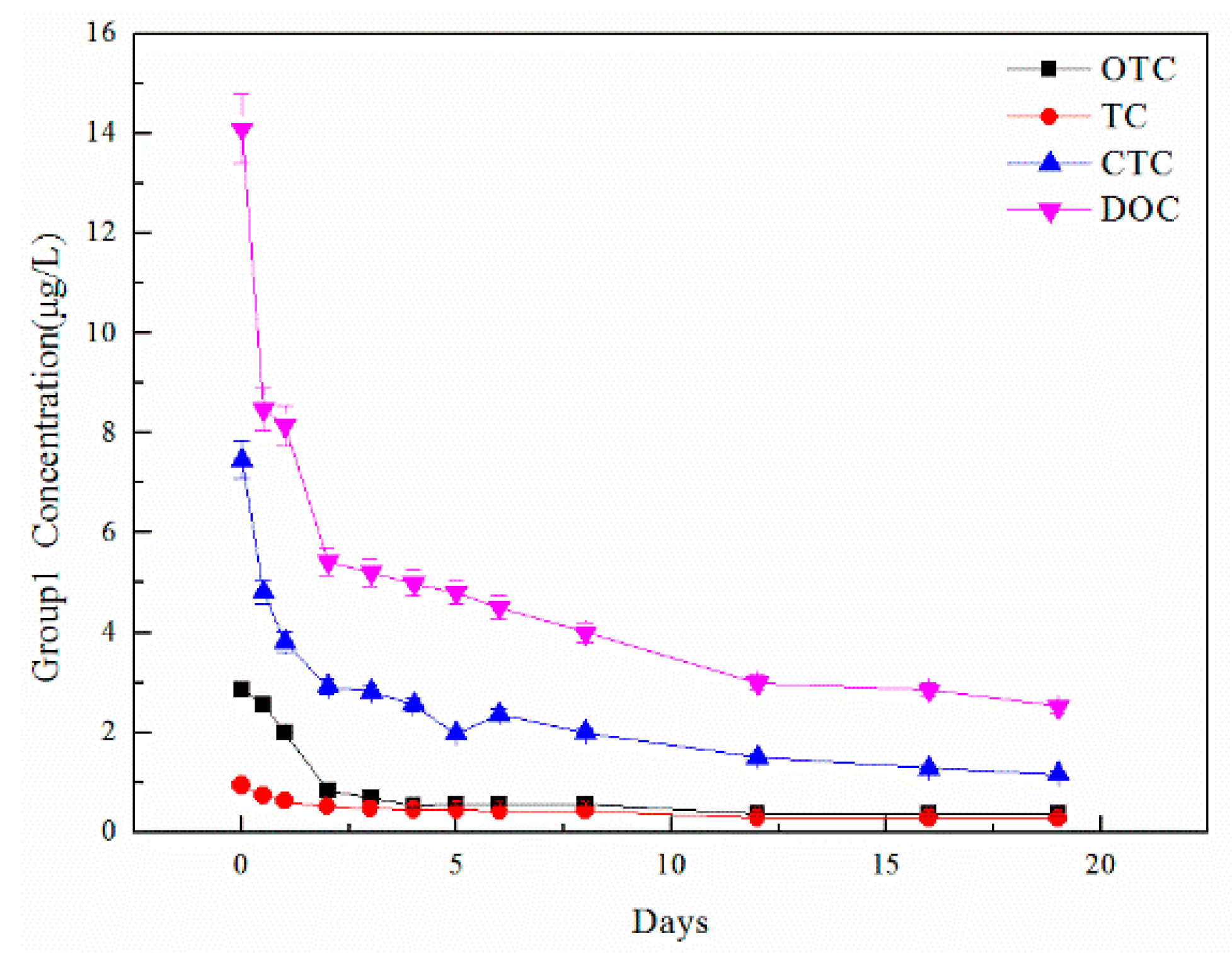
The high-temperature anaerobic digestion process took 40–60 days to optimize; however, the core anaerobic digestion process mainly occurred in the first 20 days. With the focus being on the process of high-temperature anaerobic degradation of TCs and the effects of OTC addition on this process, the main experimental study took place over the first 20 days. During the reaction process, 12 samples were taken to do further analysis at 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 16, and 19 days. A total of 5 mL of mixture was taken in each sampling by syringe, which aimed at keeping oxygen from entering the reactors. At the end of each sampling, reactors were refilled with nitrogen to ensure an anaerobic environment. The 5 mL mixture was used to detect basic indicators, TCs, microorganisms, and heavy metals.
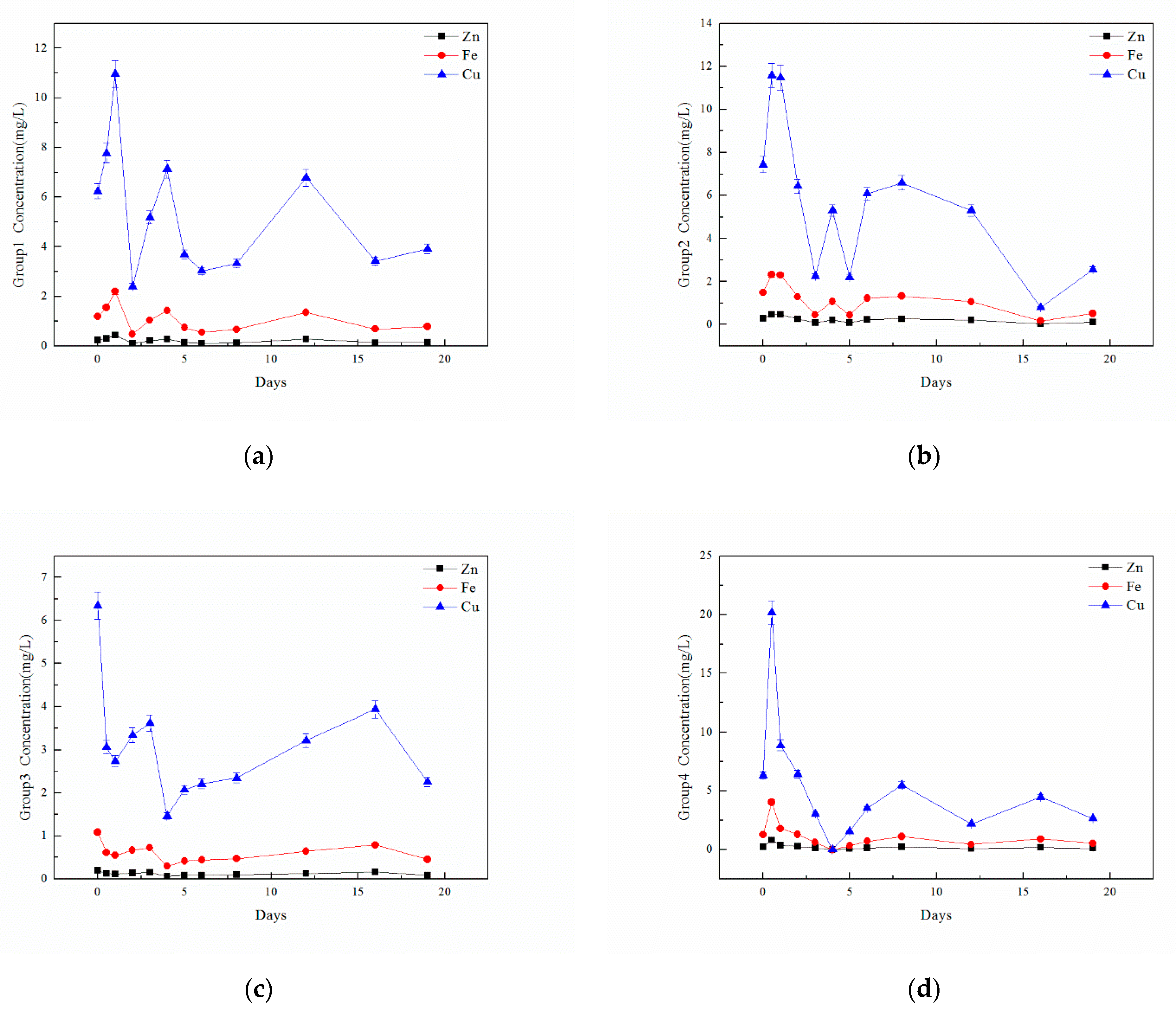
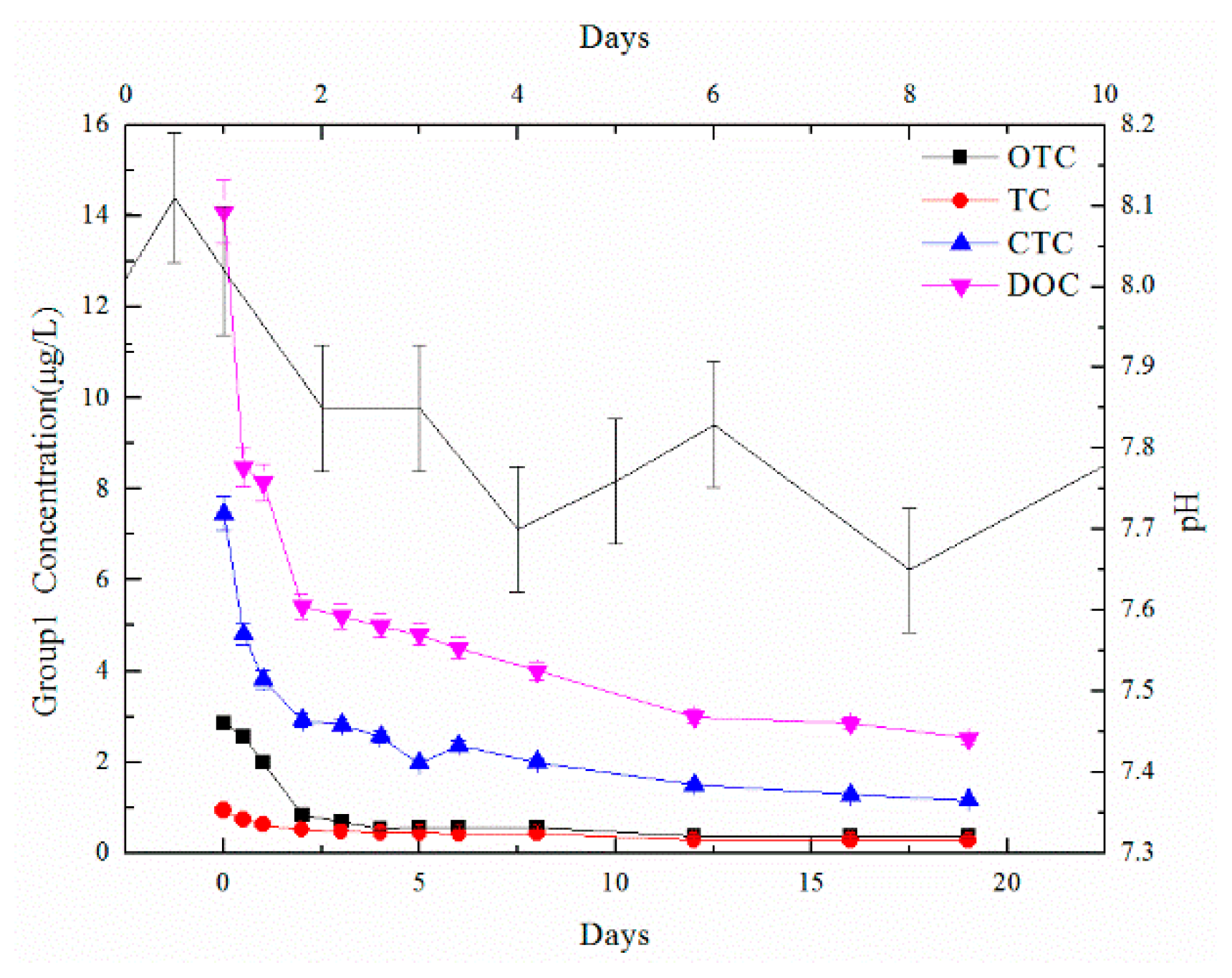
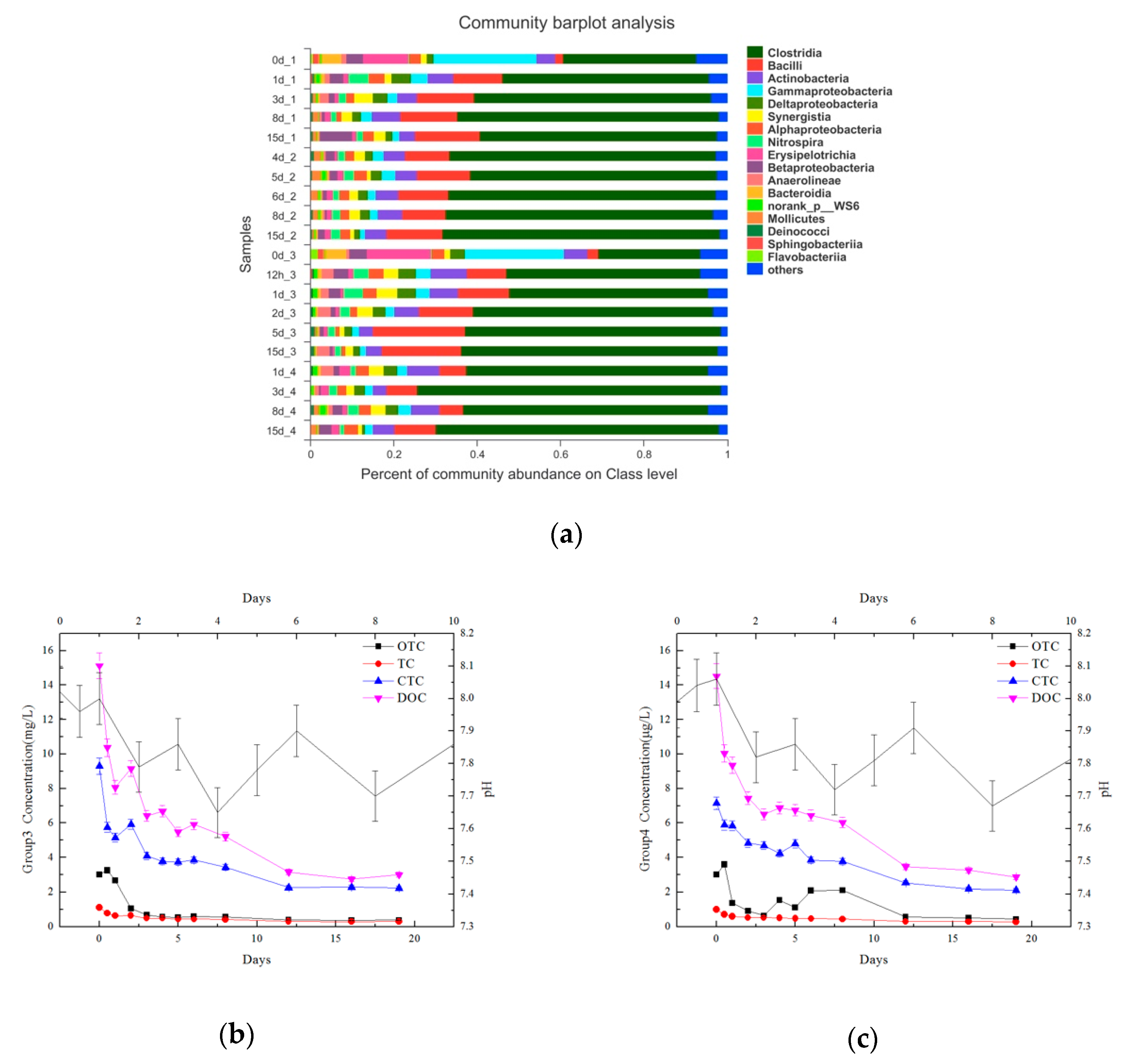
Water indicators showed the chemical changes and treatment effects during high-temperature anaerobic digestion, including TOC, TN, and TP, which were obtained using a TOC analyzer and chemical detection methods. Heavy metal content (Cu, Zn, and Fe) was detected by a heavy metal analyzer after microwave digestion with strong acid to display the migration and transformation of heavy metals during the digestion process. Microbial communities were detected by Majorbio to analyze community changes during the digestion process. After solid phase extraction, the concentrations of antibiotic contents, including tetracycline (TC), oxytetracycline (OTC), chlortetracycline (CTC), and doxycycline (DOC), were detected by liquid chromatography and mass spectrometry so that the degradation effect from high-temperature anaerobic digestion could be explored.
2.3. Analysis Instruments and Methods
2.3.1. Instruments
Ultra-performance liquid chromatography (UPLC) was used in the analysis, and the mass spectrometer employed was a Xevo TQ-S (Waters, Milford, MA, USA). The TOC and TN were detected by TOC-L CPH (Shimadzu, Kyoto, Japan). The heavy metals were determined using a ContrAA 300 (Jena, Germany). The water used in this study was treated with Milli-Q when needed for sample analysis.
2.3.2. Solid Phase Extraction
The four major tetracycline antibiotics in this study, including TC, OTC, CTC, and DOC, were purchased from the Aladdin Company. Methanol and formic acid (HPLC 99.9%) used in sample detection were purchased from J&K Scientific, and the solid phase extraction column used in the solid phase extraction process was purchased from Waters.
Antibiotic contents of swine wastewater were extracted by solid phase extraction. First, swine wastewater was centrifuged at 10,000 r/min for 10 min, and 5 mL supernatant was taken and diluted 10-fold with ultrapure water. For storage, 0.2 g of EDTA-Na2 was added before storage at 4 °C in darkness. The obtained samples were treated within 3 days. The water samples needed to be filtered through a 0.45 µm glass fiber filter membrane, and pH was adjusted to 2.5–4 using 6 mol/L hydrochloric acid. After adjustment, HLB solid-phase extraction cartridges were activated with a triple rinse of a 2 mL methanol, 2 mL water, and 2 mL hydrochloric acid solution with pH = 3. After passing the water sample through the solid-phase extraction instrument, the instrument was pumped to dry at 5 mL/min, and continued to be vacuum dried for 30 min. The small column was rinsed with 2 mL of 5% methanol, before slowly eluting with 6 mL of methanol solution, which was collected with a 10 mL centrifuge tube. Nitrogen was blown until the sample was near dryness, and the sample was topped up to 1 mL with methanol, filtered with a 0.22 µm organic filter, and transferred to a 2 mL brown sample bottle which was protected from light.
2.3.3. Liquid Chromatography Conditions
The UPLC used in this experiment was a quaternary pump system with a vacuum degasser, an autosampler, and a constant temperature column box. The chromatography column was a Waters ACQUITY UPLC BEH C18 with a particle size of 1.7 µm. During the detection process, the temperatures of the sample chamber of the liquid chromatograph and column were 25 °C and 30 °C, respectively. The sample flow rate was 0.3 mL/min, and the injection volume was 20 µL per needle. The experimental analysis using a binary solvent system included mobile phase A (0.1% formic acid in water) and phase B (methanol). The gradient concentration of the mobile phase changed within the first minute, using 90% A and 10% B. Between 1 and 1.5 min, the mobile phase was changed linearly to 10% A and 90% B, which was maintained from 1.5–4 min. After that, from 4–4.2 min, the gradient of the mobile phase was changed back to the initial mix and was maintained until the end of 5 min.
2.3.4. Mass Spectrometry Conditions
A three-stage quadrupole mass spectrometer was used for the mass spectrometry analysis, with an accuracy reaching one part per trillion (ppt) levels. The mass spectrometer was equipped with an ESI source and Masslynex software was used for instrument control, data acquisition, and evaluation. The gas flow was transferred from the LC column to the MS using the two strongest and most specific fragment ions to determine the multi-residue analysis. The entire process was analyzed in cation mode, with a scan time of 0.5 s, a scan width of 0.2, argon gas used as the collision gas, a flow rate of 0.15 mL/min, a spray voltage of 3.5 kV, a cone voltage of 75 V, a desolvation gas temperature of 400 °C, a desolvation gas flow rate of 800 L/h, and a cone gas flow rate of 150 L/h.
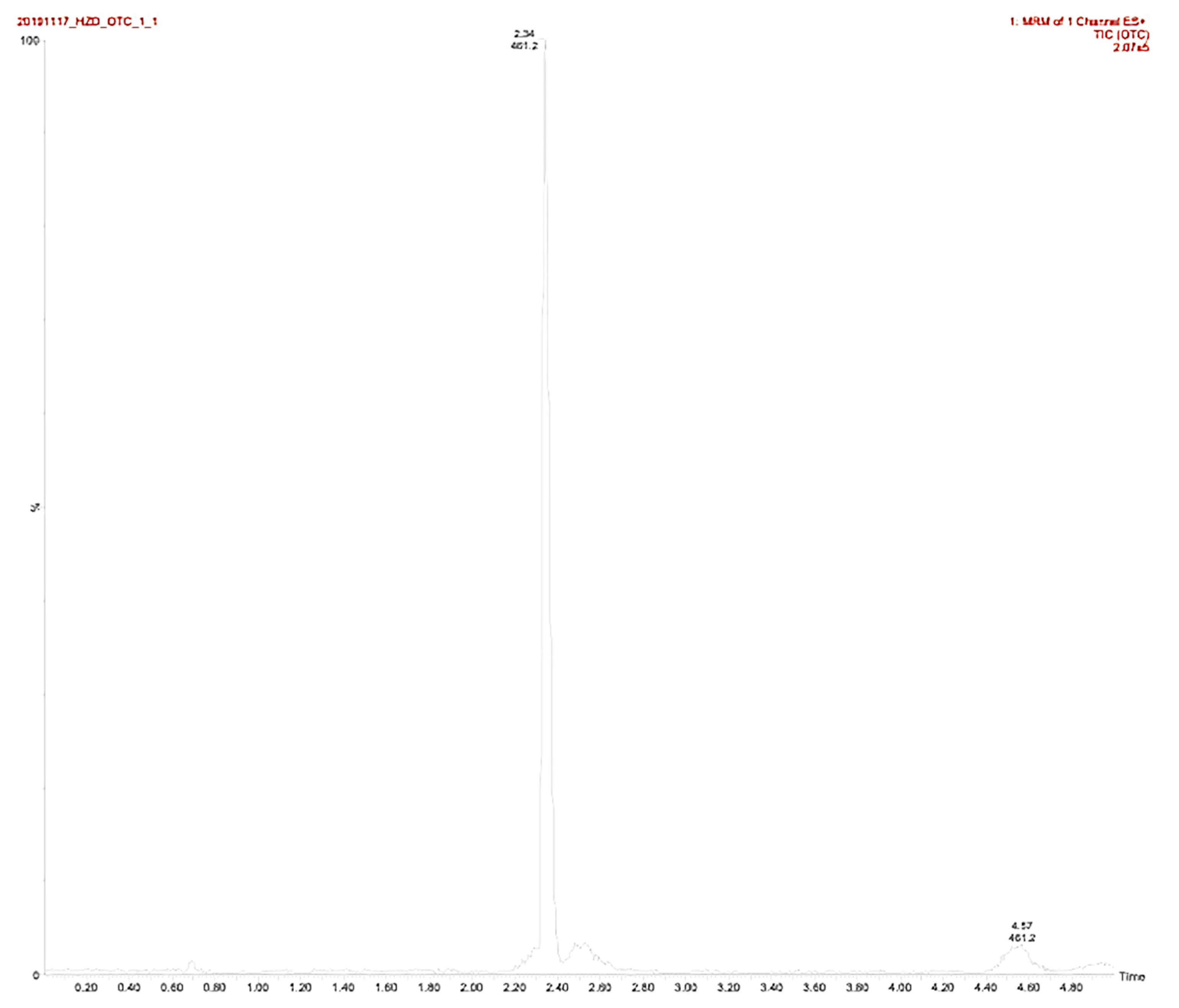
Table 3 and
Figure 2 show the analytic parameters and the oxytetracycline mass spectrogram, respectively.
2.3.5. Standard Curve
The sample concentrations were calculated by the external standard method based on the peak area of the product ions monitored. The chromatographically pure analyte was dissolved in methanol to prepare a 100 mg/L stock solution and stored in an amber vial at 4 °C in darkness. The standard curves of the four analytes were diluted to 0.1, 1.0, 2.0, 5.0, 10.0, 20.0, 50.0, and 100.0 µg/L in the initial concentration mobile phase mixture. The standard curve was used within 2 h each time. The R2 of the standard curve used in this experiment was above 0.9999.
2.3.6. Microbial Community Analysis
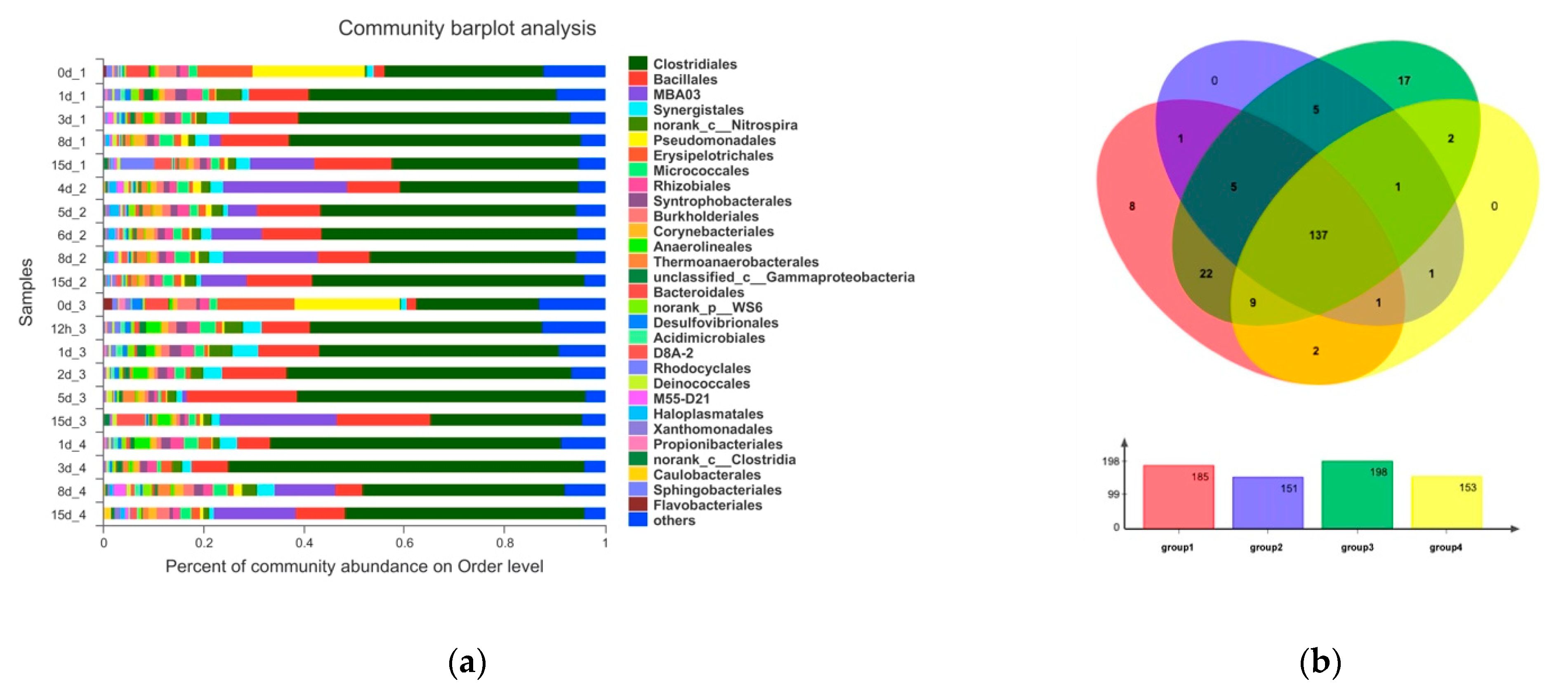
The solid matter in the wastewater was centrifuged at 10,000 rpm for 10 min to remove pore water and stored at −80 °C for further analysis. High-throughput sequencing was performed by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China) on the Illumina MiSeq platform. The study used 338F and 806R primers to focus on the V3 and V4 regions of 16S rRNA. The diversity of microbial community structure was analyzed on the free online Majorbio I-Sanger cloud platform (
www.i-sanger.com, accessed on 20 June 2020).