Sustainable Treatment of Food Industry Wastewater Using Membrane Technology: A Short Review
Abstract
:1. Introduction
2. Characteristics of Food Industry Wastewater
3. Membrane Technologies for Food Wastewater Treatment
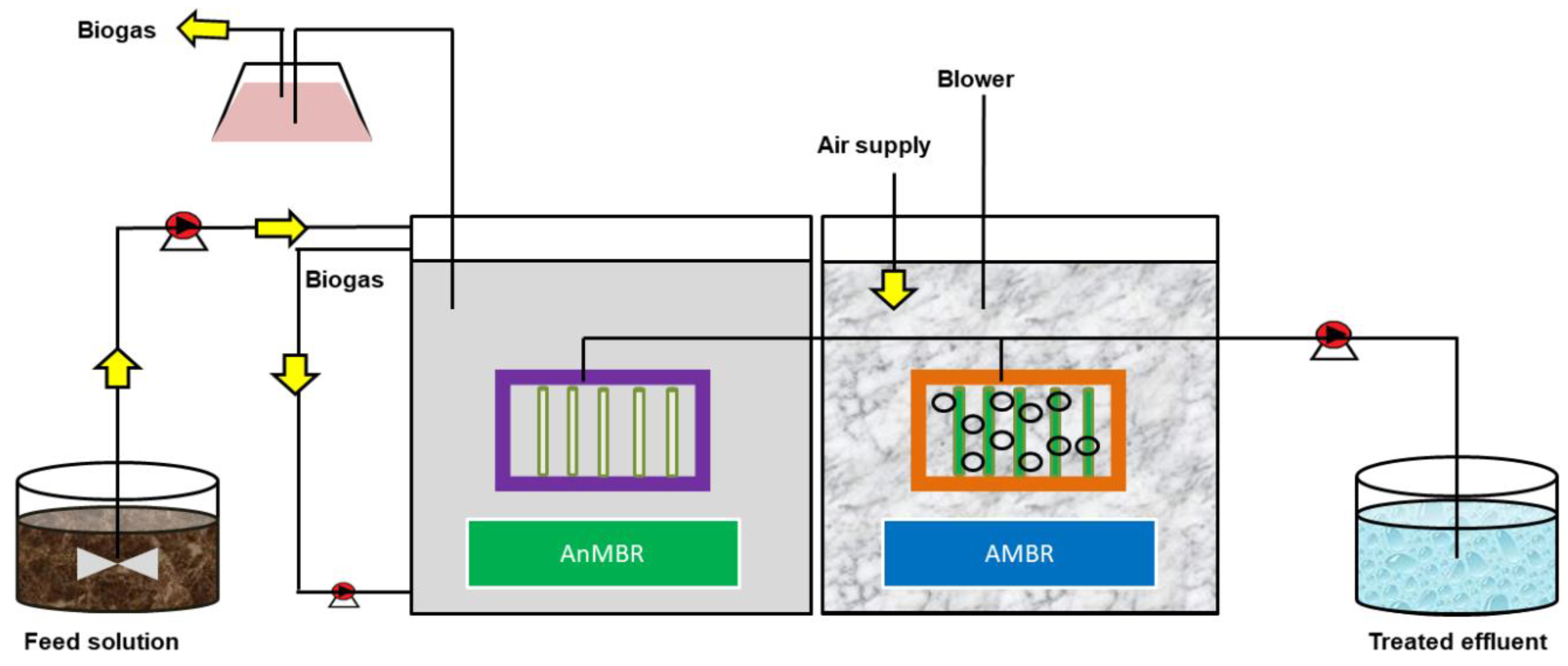
3.1. Membrane Bioreactor
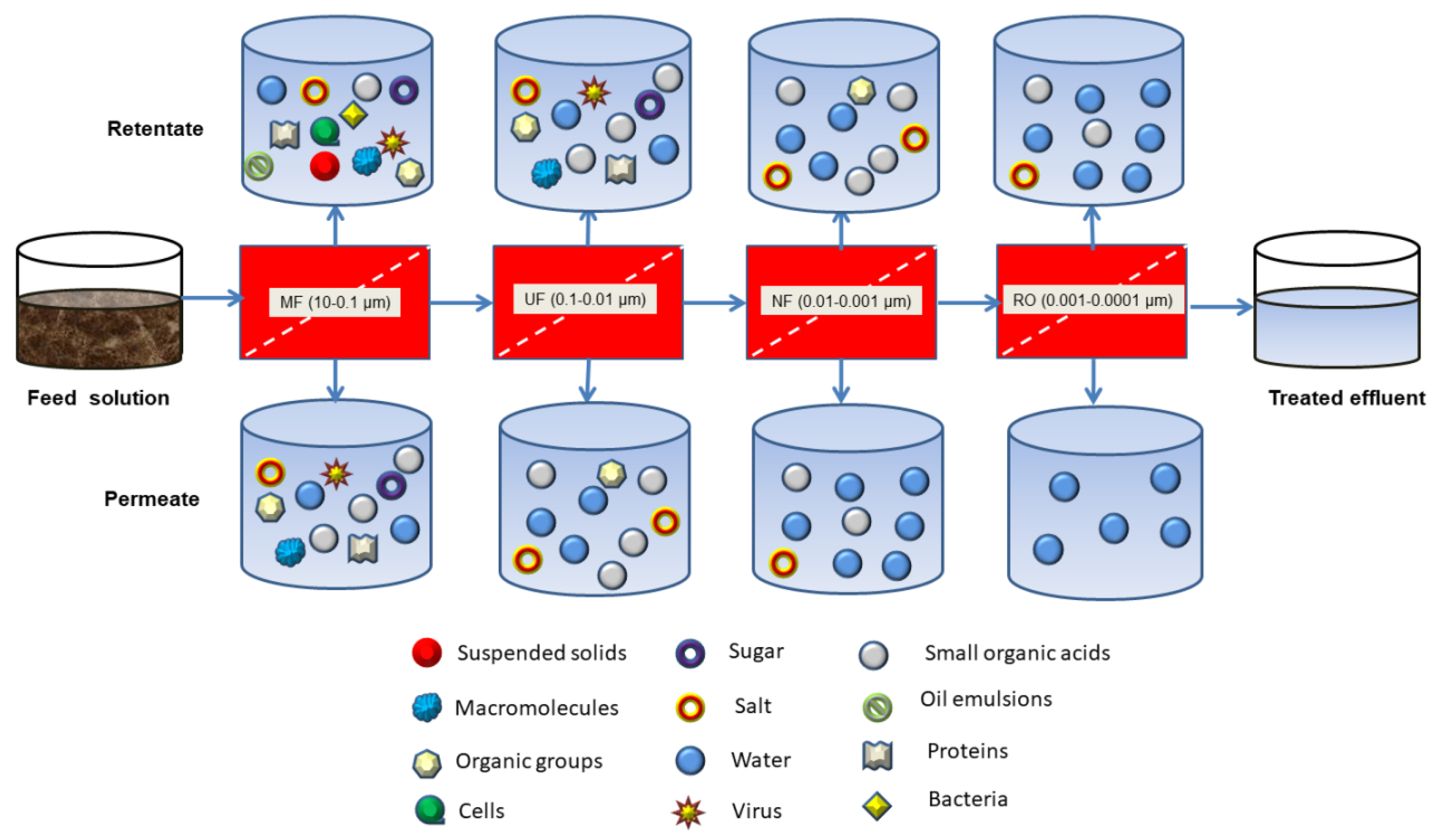
3.2. Pressure-Driven Membrane Filtration
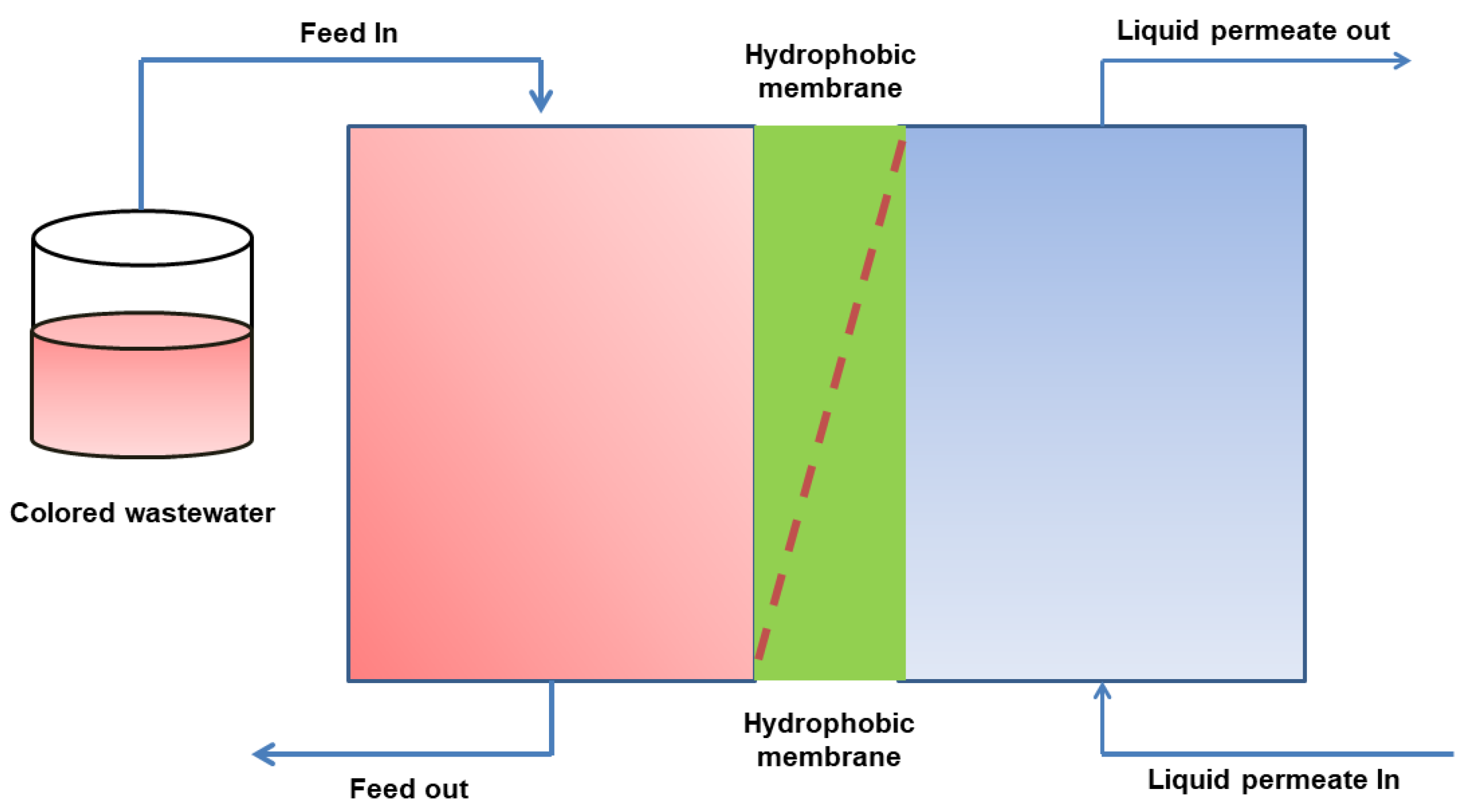
3.3. Membrane Distillation
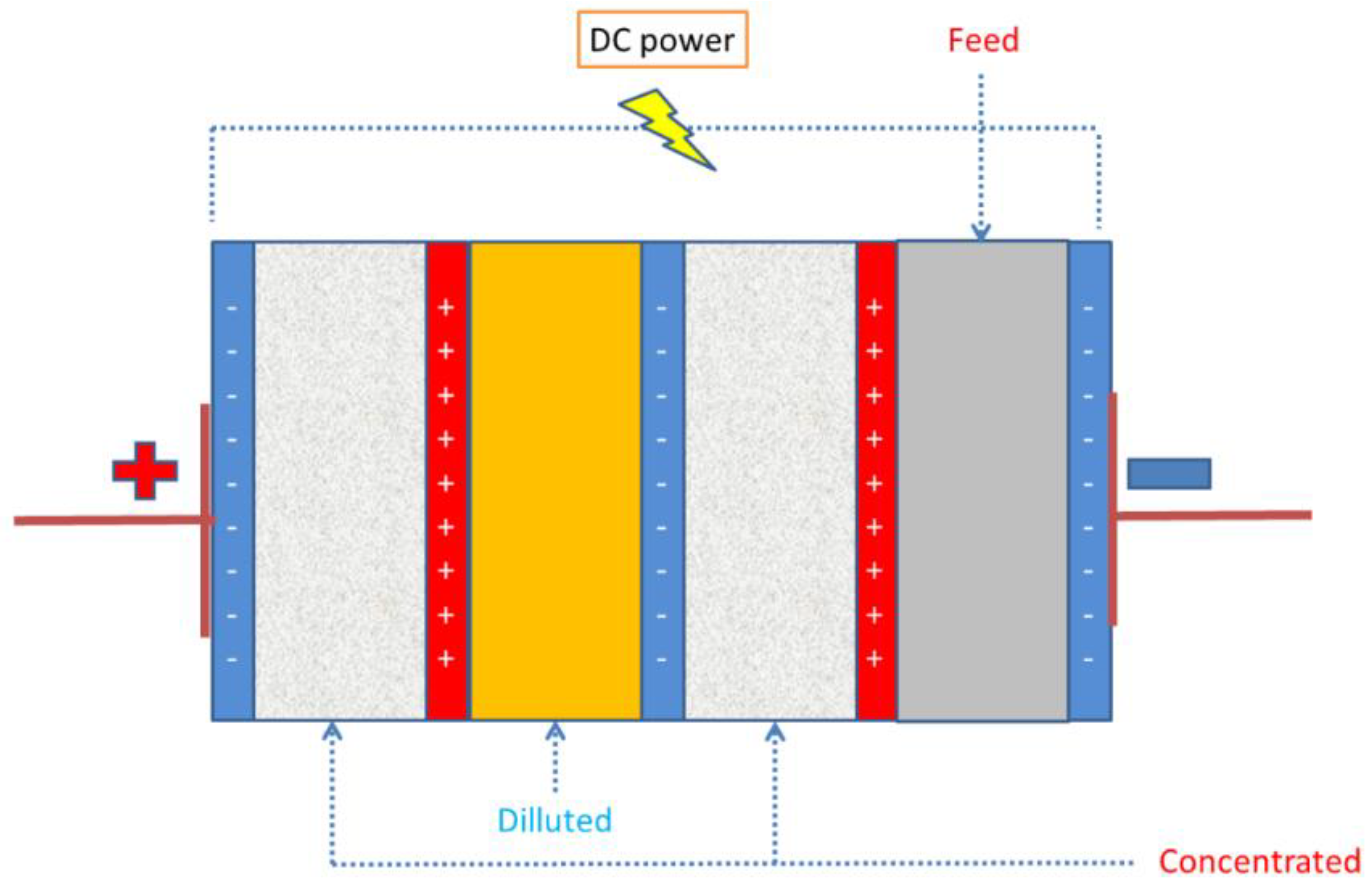
3.4. Electrodialysis
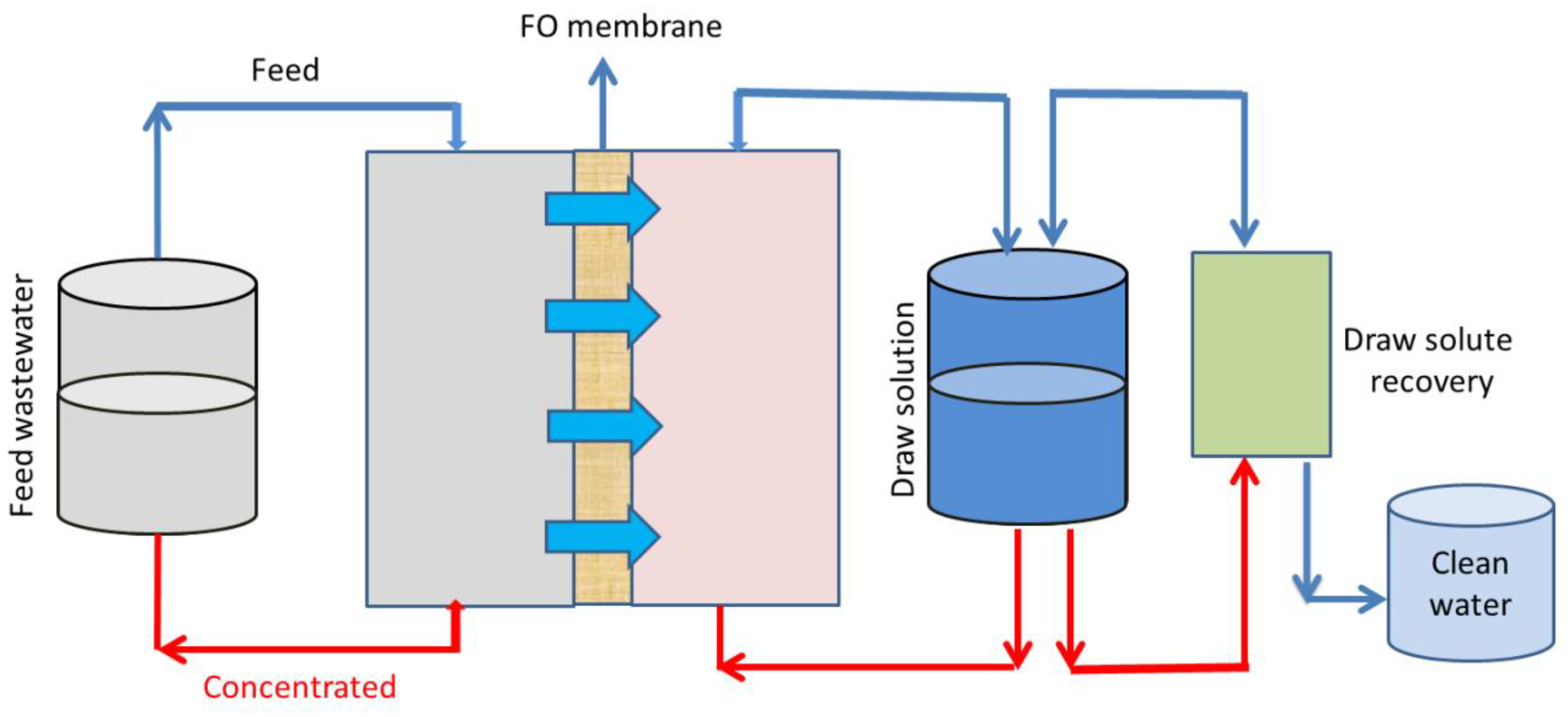
3.5. Forward Osmosis
3.6. Electrospun Nanofiber Membranes
4. Membrane Fouling
5. Challenges and Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awange, J. Lake Victoria Monitored from Space; Springer: Cham, Swizterland; Gewerbestr, Germany, 2021. [Google Scholar]
- Tapera, M. Towards greener preservation of edible oils: A mini-review. Asian J. Appl. Chem. Res. 2019, 4, 1–8. [Google Scholar] [CrossRef]
- Pervez, M.N.; Wei, Y.; Sun, P.; Qu, G.; Naddeo, V.; Zhao, Y. α-FeOOH quantum dots impregnated graphene oxide hybrids enhanced arsenic adsorption: The mediation role of environmental organic ligands. Sci. Total Environ. 2021, 781, 146726. [Google Scholar] [CrossRef]
- Pervez, M.N.; Fu, D.; Wang, X.; Bao, Q.; Yu, T.; Naddeo, V.; Tian, H.; Cao, C.; Zhao, Y. A bifunctional α-FeOOH@GCA nanocomposite for enhanced adsorption of arsenic and photo Fenton-like catalytic conversion of As(III). Environ. Technol. Innov. 2021, 22, 101437. [Google Scholar] [CrossRef]
- Naddeo, V.; Korshin, G. Water, energy and waste: The great European deal for the environment. Sci. Total. Environ. 2021, 764, 142911. [Google Scholar] [CrossRef]
- Pervez, M.N.; He, W.; Zarra, T.; Naddeo, V.; Zhao, Y. New Sustainable Approach for the Production of Fe3O4/Graphene Oxide-Activated Persulfate System for Dye Removal in Real Wastewater. Water 2020, 12, 733. [Google Scholar] [CrossRef] [Green Version]
- Jing, J.; Pervez, M.N.; Sun, P.; Cao, C.; Li, B.; Naddeo, V.; Jin, W.; Zhao, Y. Highly efficient removal of bisphenol A by a novel Co-doped LaFeO3 perovskite/PMS system in salinity water. Sci. Total Environ. 2021, 801, 149490. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, V. One planet, one health, one future: The environmental perspective. Water Environ. Res. 2021, 93, 1472–1475. [Google Scholar] [CrossRef] [PubMed]
- Naddeo, V.; Liu, H. Editorial Perspectives: 2019 novel coronavirus (SARS-CoV-2): What is its fate in urban water cycle and how can the water research community respond? Environ. Sci.-Water Res. Technol. 2020, 6, 1939. [Google Scholar] [CrossRef]
- Walsh, B.P.; Cusack, D.O.; O’Sullivan, D.T.J. An industrial water management value system framework development. Sustain. Prod. Consum. 2016, 5, 82–93. [Google Scholar] [CrossRef]
- Flörke, M.; Kynast, E.; Bärlund, I.; Eisner, S.; Wimmer, F.; Alcamo, J. Domestic and industrial water uses of the past 60 years as a mirror of socio-economic development: A global simulation study. Glob. Environ. Chang. 2013, 23, 144–156. [Google Scholar] [CrossRef]
- Compton, M.; Willis, S.; Rezaie, B.; Humes, K. Food processing industry energy and water consumption in the Pacific northwest. Innov. Food Sci. Emerg. Technol. 2018, 47, 371–383. [Google Scholar] [CrossRef]
- Jing, J.; Cao, C.; Ma, S.; Li, Z.; Qu, G.; Xie, B.; Jin, W.; Zhao, Y. Enhanced defect oxygen of LaFeO3/GO hybrids in promoting persulfate activation for selective and efficient elimination of bisphenol A in food wastewater. Chem. Eng. J. 2021, 407, 126890. [Google Scholar] [CrossRef]
- Sakcharoen, T.; Ratanatamskul, C.; Chandrachai, A. Factors affecting technology selection, techno-economic and environmental sustainability assessment of a novel zero-waste system for food waste and wastewater management. J. Clean. Prod. 2021, 314, 128103. [Google Scholar] [CrossRef]
- Barbera, M.; Gurnari, G. Wastewater Treatment and Reuse in the Food Industry; Springer: Cham, Swizterland; Gewerbestr, Germany, 2018. [Google Scholar]
- Sellaoui, L.; Dhaouadi, F.; Li, Z.; Cadaval, T.R., Jr.; Igansi, A.V.; Pinto, L.A.; Dotto, G.L.; Bonilla-Petriciolet, A.; Pinto, D.; Chen, Z. Implementation of a multilayer statistical physics model to interpret the adsorption of food dyes on a chitosan film. J. Environ. Chem. Eng. 2021, 9, 105516. [Google Scholar] [CrossRef]
- Leifeld, V.; Dos Santos, T.P.M.; Zelinski, D.W.; Igarashi-Mafra, L. Ferrous ions reused as catalysts in Fenton-like reactions for remediation of agro-food industrial wastewater. J. Environ. Manag. 2018, 222, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Pervez, M.N.; Telegin, F.Y.; Cai, Y.; Xia, D.; Zarra, T.; Naddeo, V. Efficient Degradation of Mordant Blue 9 Using the Fenton-Activated Persulfate System. Water 2019, 11, 2532. [Google Scholar] [CrossRef] [Green Version]
- Norton, T.; Misiewicz, P. Ozone for water treatment and its potential for process water reuse in the food industry. In Ozone in Food Processing; O’Donnell, C., Tiwari, B.K., Cullen, P.J., Rice, R.G., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 177–199. [Google Scholar]
- Li, S.; Zhao, S.; Yan, S.; Qiu, Y.; Song, C.; Li, Y.; Kitamura, Y. Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production—A review. Chin. J. Chem. Eng. 2019, 27, 2845–2856. [Google Scholar] [CrossRef]
- Sehar, S. Wastewater treatment of food industries through constructed wetland: A review. Int. J. Environ. Sci. Technol. 2020, 17, 4667–4668. [Google Scholar] [CrossRef]
- De Nardi, I.R.; Del Nery, V.; Amorim, A.K.B.; dos Santos, N.G.; Chimenes, F. Performances of SBR, chemical–DAF and UV disinfection for poultry slaughterhouse wastewater reclamation. Desalination 2011, 269, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Patange, A.; Boehm, D.; Giltrap, M.; Lu, P.; Cullen, P.J.; Bourke, P. Assessment of the disinfection capacity and eco-toxicological impact of atmospheric cold plasma for treatment of food industry effluents. Sci. Total Environ. 2018, 631–632, 298–307. [Google Scholar] [CrossRef]
- Cecconet, D.; Molognoni, D.; Callegari, A.; Capodaglio, A.G. Agro-food industry wastewater treatment with microbial fuel cells: Energetic recovery issues. Int. J. Hydrogen Energy 2018, 43, 500–511. [Google Scholar] [CrossRef]
- Valta, K.; Kosanovic, T.; Malamis, D.; Moustakas, K.; Loizidou, M. Overview of water usage and wastewater management in the food and beverage industry. Desalination Water Treat. 2015, 53, 3335–3347. [Google Scholar] [CrossRef]
- Asgharnejad, H.; Khorshidi Nazloo, E.; Madani Larijani, M.; Hajinajaf, N.; Rashidi, H. Comprehensive review of water management and wastewater treatment in food processing industries in the framework of water-food-environment nexus. Compr. Rev. Food Sci. Food Saf. 2021, 45, 19642–19663. [Google Scholar] [CrossRef]
- Morshed, M.N.; Pervez, M.N.; Behary, N.; Bouazizi, N.; Guan, J.; Nierstrasz, V.A. Statistical modeling and optimization of heterogeneous Fenton-like removal of organic pollutant using fibrous catalysts: A full factorial design. Sci. Rep. 2020, 10, 16133. [Google Scholar] [CrossRef]
- Millanar-Marfa, J.M.J.; Borea, L.; Castrogiovanni, F.; Hasan, S.W.; Choo, K.-H.; Korshin, G.V.; de Luna, M.D.G.; Ballesteros, F.C., Jr.; Belgiorno, V.; Naddeo, V. Self-forming Dynamic Membranes for Wastewater Treatment. Sep. Purif. Rev. 2021, 1–17. [Google Scholar] [CrossRef]
- Pervez, M.N.; Balakrishnan, M.; Hasan, S.W.; Choo, K.-H.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment. NPJ Clean Water 2020, 3, 43. [Google Scholar] [CrossRef]
- Ng, L.Y.; Chua, H.S.; Ng, C.Y. Incorporation of graphene oxide-based nanocomposite in the polymeric membrane for water and wastewater treatment: A review on recent development. J. Environ. Chem. Eng. 2021, 9, 105994. [Google Scholar] [CrossRef]
- Ng, C.Y.; Khoo, L.H.; Ng, L.Y.; Ong, C.B.; Mahmoudi, E.; Rohani, R.; Mohammad, A.W. Novel polyethersulfone-cellulose composite thin film using sustainable empty fruit bunches from Elaeis guineensis for methylene blue removal. Polym. Test. 2020, 86, 106494. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Wei, C.; Wang, P.; Gao, P.; Zhou, L.; Wen, G. One step co-sintering of silicon carbide ceramic membrane with the aid of boron carbide. J. Eur. Ceram. Soc. 2021, 41, 1181–1188. [Google Scholar] [CrossRef]
- Molodkina, L.M.; Kolosova, D.D.; Leonova, E.I.; Kudoyarov, M.F.; Patrova, M.Y.; Vedmetskii, Y.V. Track membranes in post-treatment of domestic wastewater. Pet. Chem. 2012, 52, 487–493. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Fan, X.; Chen, S.; Yu, H.; Quan, X. A controlled wet-spinning and dip-coating process for preparation of high-permeable TiO2 hollow fiber membranes. Water Sci. Technol. 2015, 73, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Pervez, M.N.; Stylios, G.K. An Experimental Approach to the Synthesis and Optimisation of a ‘Green’ Nanofibre. Nanomaterials 2018, 8, 383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervez, M.N.; Stylios, G.K. Investigating the Synthesis and Characterization of a Novel “Green” H2O2-Assisted, Water-Soluble Chitosan/Polyvinyl Alcohol Nanofiber for Environmental End Uses. Nanomaterials 2018, 8, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pervez, M.N.; Stylios, G.K.; Liang, Y.; Ouyang, F.; Cai, Y. Low-temperature synthesis of novel polyvinylalcohol (PVA) nanofibrous membranes for catalytic dye degradation. J. Clean. Prod. 2020, 262, 121301. [Google Scholar] [CrossRef]
- Bjorge, D.; Daels, N.; De Vrieze, S.; Dejans, P.; Van Camp, T.; Audenaert, W.; Hogie, J.; Westbroek, P.; De Clerck, K.; Van Hulle, S.W.H. Performance assessment of electrospun nanofibers for filter applications. Desalination 2009, 249, 942–948. [Google Scholar] [CrossRef]
- Lotfikatouli, S.; Hadi, P.; Yang, M.; Walker, H.W.; Hsiao, B.S.; Gobler, C.; Reichel, M.; Mao, X. Enhanced anti-fouling performance in Membrane Bioreactors using a novel cellulose nanofiber-coated membrane. Sep. Purif. Technol. 2021, 275, 119145. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Li, B.; Hsiao, B.S.; Chu, B. Electrospun nanofibrous membranes for high flux microfiltration. J. Membr. Sci. 2012, 392–393, 167–174. [Google Scholar] [CrossRef]
- Dobosz, K.M.; Kuo-Leblanc, C.A.; Martin, T.J.; Schiffman, J.D. Ultrafiltration membranes enhanced with electrospun nanofibers exhibit improved flux and fouling resistance. Ind. Eng. Chem. Res. 2017, 56, 5724–5733. [Google Scholar] [CrossRef] [PubMed]
- Shen, K.; Cheng, C.; Zhang, T.; Wang, X. High performance polyamide composite nanofiltration membranes via reverse interfacial polymerization with the synergistic interaction of gelatin interlayer and trimesoyl chloride. J. Membr. Sci. 2019, 588, 117192. [Google Scholar] [CrossRef]
- Zhou, T.; Li, J.; Guo, X.; Yao, Y.; Zhu, P.; Xiang, R. Freestanding PTFE electrospun tubular membrane for reverse osmosis brine concentration by vacuum membrane distillation. Desalin. Water Treat 2019, 165, 63–72. [Google Scholar] [CrossRef]
- Zheng, G.; Jiang, J.; Wang, X.; Li, W.; Liu, J.; Fu, G.; Lin, L. Nanofiber membranes by multi-jet electrospinning arranged as arc-array with sheath gas for electrodialysis applications. Mater. Des. 2020, 189, 108504. [Google Scholar] [CrossRef]
- Deka, B.J.; Lee, E.-J.; Guo, J.; Kharraz, J.; An, A.K. Electrospun Nanofiber Membranes Incorporating PDMS-Aerogel Superhydrophobic Coating with Enhanced Flux and Improved Antiwettability in Membrane Distillation. Environ. Sci. Technol. 2019, 53, 4948–4958. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Heath, D.E.; Kentish, S.E. Improved carbon dioxide stripping by membrane contactors using hydrophobic electrospun poly(vinylidene fluoride-co-hexafluoro propylene) (PVDF-HFP) membranes. Chem. Eng. J. 2022, 428, 131247. [Google Scholar] [CrossRef]
- Obaid, M.; Abdelkareem, M.A.; Kook, S.; Kim, H.-Y.; Hilal, N.; Ghaffour, N.; Kim, I.S. Breakthroughs in the fabrication of electrospun-nanofiber-supported thin film composite/nanocomposite membranes for the forward osmosis process: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1727–1795. [Google Scholar] [CrossRef]
- Aderibigbe, D.O.; Giwa, A.-R.A.; Bello, I.A. Characterization and treatment of wastewater from food processing industry: A review. IMAM J. Appl. Sci. 2017, 2, 27. [Google Scholar]
- Qiu, Y.; Zu, Y.; Song, C.; Xie, M.; Qi, Y.; Kansha, Y.; Kitamura, Y. Soybean processing wastewater purification via Chlorella L166 and L38 with potential value-added ingredients production. Bioresour. Technol. Rep. 2019, 7, 100195. [Google Scholar] [CrossRef]
- Karadag, D.; Köroğlu, O.E.; Ozkaya, B.; Cakmakci, M. A review on anaerobic biofilm reactors for the treatment of dairy industry wastewater. Process Biochem. 2015, 50, 262–271. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Torregrosa, J.; García, J.; Dominguez, J.R.; Tierno, J.C. Degradation of olive mill wastewater by the combination of Fenton’s reagent and ozonation processes with an aerobic biological treatment. Water Sci. Technol. 2001, 44, 103–108. [Google Scholar] [CrossRef]
- Chatzipaschali, A.A.; Stamatis, A.G. Biotechnological utilization with a focus on anaerobic treatment of cheese whey: Current status and prospects. Energies 2012, 5, 3492–3525. [Google Scholar] [CrossRef] [Green Version]
- Sroka, E.; Kamiński, W.; Bohdziewicz, J. Biological treatment of meat industry wastewater. Desalination 2004, 162, 85–91. [Google Scholar] [CrossRef]
- Arantes, M.K.; Alves, H.J.; Sequinel, R.; da Silva, E.A. Treatment of brewery wastewater and its use for biological production of methane and hydrogen. Int. J. Hydrogen Energy 2017, 42, 26243–26256. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Borghetti, I.A.; Cartas, L.C.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Biorefinery integration of microalgae production into cassava processing industry: Potential and perspectives. Bioresour. Technol. 2018, 247, 1165–1172. [Google Scholar] [CrossRef]
- Barrera-Díaz, C.; Roa-Morales, G.; Ávila-Córdoba, L.; Pavón-Silva, T.; Bilyeu, B. Electrochemical Treatment Applied to Food-Processing Industrial Wastewater. Ind. Eng. Chem. Res. 2006, 45, 34–38. [Google Scholar] [CrossRef]
- Chiacchierini, E.; Restuccia, D.; Vinci, G. Bioremediation of Food Industry Effluents: Recent Applications of Free and Immobilised Polyphenoloxidases. Food Sci. Technol. Int. 2004, 10, 373–382. [Google Scholar] [CrossRef]
- Qasim, W.; Mane, A.V. Characterization and treatment of selected food industrial effluents by coagulation and adsorption techniques. Water Resour. Ind. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Benamar, A.; Mahjoubi, F.Z.; Barka, N.; Kzaiber, F.; Boutoial, K.; Ali, G.A.M.; Oussama, A. Olive mill wastewater treatment using infiltration percolation in column followed by aerobic biological treatment. SN Appl. Sci. 2020, 2, 655. [Google Scholar] [CrossRef] [Green Version]
- Değermenci, N.; Cengiz, İ.; Yildiz, E.; Nuhoglu, A. Performance investigation of a jet loop membrane bioreactor for the treatment of an actual olive mill wastewater. J. Environ. Manag. 2016, 184, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Bustillo-Lecompte, C.; Mehrvar, M.; Quiñones-Bolaños, E. Slaughterhouse wastewater characterization and treatment: An economic and public health necessity of the meat processing industry in Ontario, Canada. J. Geosci. Environ. Prot. 2016, 4, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Puchlik, M.; Struk-Sokołowska, J. Comparison of the Composition of Wastewater from Fruit and Vegetables as well as Dairy Industry. In Proceedings of the E3S Web of Conferences, 9th Conference on Interdisciplinary Problems in Environmental Protection and Engineering EKO-DOK 2017, Boguszow Gorce, Bialystok, Poland; EDP Sciences: Les Ulis, France, 2017; p. 77. [Google Scholar]
- Ahmad, N.N.R.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current advances in membrane technologies for saline wastewater treatment: A comprehensive review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Nayyar, D.; Nawaz, T.; Noore, S.; Singh, A.P. Food Processing Wastewater Treatment: Current Practices and Future Challenges. In Pollution Control Technologies; Springer: Berlin/Heidelberg, Germany, 2021; pp. 177–208. [Google Scholar]
- Melin, T.; Jefferson, B.; Bixio, D.; Thoeye, C.; De Wilde, W.; de Koning, J.; van der Graaf, J.; Wintgens, T. Membrane bioreactor technology for wastewater treatment and reuse. Desalination 2006, 187, 271–282. [Google Scholar] [CrossRef]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Goswami, L.; Vinoth Kumar, R.; Borah, S.N.; Arul Manikandan, N.; Pakshirajan, K.; Pugazhenthi, G. Membrane bioreactor and integrated membrane bioreactor systems for micropollutant removal from wastewater: A review. J. Water Process Eng. 2018, 26, 314–328. [Google Scholar] [CrossRef]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Xu, P.; Li, C.; Zhang, B. High-concentration food wastewater treatment by an anaerobic membrane bioreactor. Water Res. 2005, 39, 4110–4118. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, X.; Yuan, Q. Nitrogen and carbon removals from food processing wastewater by an anoxic/aerobic membrane bioreactor. Process Biochem. 2005, 40, 1733–1739. [Google Scholar] [CrossRef]
- Galib, M.; Elbeshbishy, E.; Reid, R.; Hussain, A.; Lee, H.-S. Energy-positive food wastewater treatment using an anaerobic membrane bioreactor (AnMBR). J. Environ. Manag. 2016, 182, 477–485. [Google Scholar] [CrossRef]
- Katayon, S.; Megat Mohd Noor, M.J.; Ahmad, J.; Abdul Ghani, L.A.; Nagaoka, H.; Aya, H. Effects of mixed liquor suspended solid concentrations on membrane bioreactor efficiency for treatment of food industry wastewater. Desalination 2004, 167, 153–158. [Google Scholar] [CrossRef]
- Acharya, C.; Nakhla, G.; Bassi, A.; Kurian, R. Treatment of High-Strength Pet Food Wastewater Using Two-Stage Membrane Bioreactors. Water Environ. Res. 2006, 78, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Acharya, C.; Nakhla, G.; Bassi, A. Operational Optimization and Mass Balances in a Two-Stage MBR Treating High Strength Pet Food Wastewater. J. Environ. Eng. 2006, 132, 810–817. [Google Scholar] [CrossRef]
- Mahat, S.B.; Omar, R.; Che Man, H.; Mohamad Idris, A.I.; Mustapa Kamal, S.M.; Idris, A.; Shreeshivadasan, C.; Jamali, N.S.; Abdullah, L.C. Performance of dynamic anaerobic membrane bioreactor (DAnMBR) with phase separation in treating high strength food processing wastewater. J. Environ. Chem. Eng. 2021, 9, 105245. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22, 46–56. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Martinez-Ferez, A. A focus on pressure-driven membrane technology in olive mill wastewater reclamation: State of the art. Water Sci. Technol. 2012, 66, 2505–2516. [Google Scholar] [CrossRef] [PubMed]
- Muro, C.; Riera, F.; del Carmen Díaz, M. Membrane separation process in wastewater treatment of food industry. In Food Industrial Processes—Methods and Equipment; InTech: Rijeka, Croatia, 2012; pp. 253–280. [Google Scholar]
- Hart, M.R.; Huxsoll, C.C.; Tsai, I.-S.; Ng, K.C. Preliminary Studies of Microfiltration for Food Processing Water Reuse1. J. Food Prot. 1988, 51, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.V.; Goswami, L.; Pakshirajan, K.; Pugazhenthi, G. Dairy wastewater treatment using a novel low cost tubular ceramic membrane and membrane fouling mechanism using pore blocking models. J. Water Process Eng. 2016, 13, 168–175. [Google Scholar] [CrossRef]
- Hua, F.L.; Tsang, Y.F.; Wang, Y.J.; Chan, S.Y.; Chua, H.; Sin, S.N. Performance study of ceramic microfiltration membrane for oily wastewater treatment. Chem. Eng. J. 2007, 128, 169–175. [Google Scholar] [CrossRef]
- Zielińska, M.; Galik, M. Use of Ceramic Membranes in a Membrane Filtration Supported by Coagulation for the Treatment of Dairy Wastewater. Water Air Soil Pollut. 2017, 228, 173. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.F.; Jiang, Y.; Field, R.W. Fundamentals of pressure-driven membrane separation processes. In Membrane Technology; Cui, Z.F., Muralidhara, H.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1–18. [Google Scholar]
- Zulaikha, S.; Lau, W.J.; Ismail, A.F.; Jaafar, J. Treatment of restaurant wastewater using ultrafiltration and nanofiltration membranes. J. Water Process Eng. 2014, 2, 58–62. [Google Scholar] [CrossRef]
- Ochando-Pulido, J.M.; Rodriguez-Vives, S.; Hodaifa, G.; Martinez-Ferez, A. Impacts of operating conditions on reverse osmosis performance of pretreated olive mill wastewater. Water Res. 2012, 46, 4621–4632. [Google Scholar] [CrossRef] [PubMed]
- Vourch, M.; Balannec, B.; Chaufer, B.; Dorange, G. Treatment of dairy industry wastewater by reverse osmosis for water reuse. Desalination 2008, 219, 190–202. [Google Scholar] [CrossRef]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]
- Giacobbo, A.; Meneguzzi, A.; Bernardes, A.M.; de Pinho, M.N. Pressure-driven membrane processes for the recovery of antioxidant compounds from winery effluents. J. Clean. Prod. 2017, 155, 172–178. [Google Scholar] [CrossRef]
- Alkhudhiri, A.; Darwish, N.; Hilal, N. Membrane distillation: A comprehensive review. Desalination 2012, 287, 2–18. [Google Scholar] [CrossRef]
- Alsebaeai, M.K.; Ahmad, A.L. Membrane distillation: Progress in the improvement of dedicated membranes for enhanced hydrophobicity and desalination performance. J. Ind. Eng. Chem. 2020, 86, 13–34. [Google Scholar] [CrossRef]
- Anvari, A.; Azimi Yancheshme, A.; Kekre, K.M.; Ronen, A. State-of-the-art methods for overcoming temperature polarization in membrane distillation process: A review. J. Membr. Sci. 2020, 616, 118413. [Google Scholar] [CrossRef]
- Drioli, E.; Ali, A.; Macedonio, F. Membrane distillation: Recent developments and perspectives. Desalination 2015, 356, 56–84. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Hafidi, A.; García-Payo, M.C.; Khayet, M. Treatment of olive mill wastewater by membrane distillation using polytetrafluoroethylene membranes. Sep. Purif. Technol. 2012, 98, 55–61. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Hafidi, A.; Khayet, M.; García-Payo, M.C. Integrated direct contact membrane distillation for olive mill wastewater treatment. Desalination 2013, 323, 31–38. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Khayet, M.; Kiai, H.; Hafidi, A.; García-Payo, M.C. Treatment of crude olive mill wastewaters by osmotic distillation and osmotic membrane distillation. Sep. Purif. Technol. 2013, 104, 327–332. [Google Scholar] [CrossRef]
- Garcia-Castello, E.; Cassano, A.; Criscuoli, A.; Conidi, C.; Drioli, E. Recovery and concentration of polyphenols from olive mill wastewaters by integrated membrane system. Water Res. 2010, 44, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Luiz, A.; McClure, D.D.; Lim, K.; Leslie, G.; Coster, H.G.L.; Barton, G.W.; Kavanagh, J.M. Potential upgrading of bio-refinery streams by electrodialysis. Desalination 2017, 415, 20–28. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T. Electrodialysis with bipolar membranes for sustainable development. Environ. Sci. Technol. 2006, 40, 5233–5243. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Valero, D.; García-García, V.; Expósito, E.; Aldaz, A.; Montiel, V. Application of electrodialysis for the treatment of almond industry wastewater. J. Membr. Sci. 2015, 476, 580–589. [Google Scholar] [CrossRef] [Green Version]
- Hestekin, J.; Ho, T.; Potts, T. Electrodialysis in the food industry. In Membrane Technology: Volume 3: Membranes for Food Applications, Volume 3; Wiley: Hoboken, NJ, USA, 2010; Volume 3, pp. 75–104. [Google Scholar]
- Chung, T.-S.; Li, X.; Ong, R.C.; Ge, Q.; Wang, H.; Han, G. Emerging forward osmosis (FO) technologies and challenges ahead for clean water and clean energy applications. Curr. Opin. Chem. Eng. 2012, 1, 246–257. [Google Scholar] [CrossRef]
- Francis, L.; Ogunbiyi, O.; Saththasivam, J.; Lawler, J.; Liu, Z. A comprehensive review of forward osmosis and niche applications. Environ. Sci. Water Res. Technol. 2020, 6, 1986–2015. [Google Scholar] [CrossRef]
- Zamani, F.; Chew, J.W.; Akhondi, E.; Krantz, W.B.; Fane, A.G. Unsteady-state shear strategies to enhance mass-transfer for the implementation of ultrapermeable membranes in reverse osmosis: A review. Desalination 2015, 356, 328–348. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Curcio, E.; Poerio, T.; Mazzei, R.; Di Profio, G.; Drioli, E.; Giorno, L. Treatment of Olive Mill Wastewater by Forward Osmosis. Sep. Purif. Technol. 2015, 147, 292–302. [Google Scholar] [CrossRef]
- Haupt, A.; Lerch, A. Forward osmosis treatment of effluents from dairy and automobile industry—Results from short-term experiments to show general applicability. Water Sci. Technol. 2018, 78, 467–475. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Petrinic, I.; Hélix-Nielsen, C.; Basu, S.; Balakrishnan, M. Influence of Forward Osmosis (FO) membrane properties on dewatering of molasses distillery wastewater. J. Water Process Eng. 2019, 32, 100921. [Google Scholar] [CrossRef]
- Talukder, M.E.; Pervez, M.N.; Jianming, W.; Gao, Z.; Stylios, G.K.; Hassan, M.M.; Song, H.; Naddeo, V. Chitosan-functionalized sodium alginate-based electrospun nanofiber membrane for As (III) removal from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 106693. [Google Scholar] [CrossRef]
- Talukder, M.E.; Hasan, K.M.F.; Wang, J.; Yao, J.; Li, C.; Song, H. Novel fibrin functionalized multilayered electrospun nanofiber membrane for burn wound treatment. J. Mater. Sci. 2021, 56, 12814–12834. [Google Scholar] [CrossRef]
- Chen, K.; Nikam, S.P.; Zander, Z.K.; Hsu, Y.-H.; Dreger, N.Z.; Cakmak, M.; Becker, M.L. Continuous Fabrication of Antimicrobial Nanofiber Mats Using Post-Electrospinning Functionalization for Roll-to-Roll Scale-Up. ACS Appl. Polym. Mater. 2020, 2, 304–316. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Hossain, M.Y.; Zhu, W.; Pervez, M.N.; Yang, X.; Sarker, S.; Hassan, M.M.; Hoque, M.I.U.; Naddeo, V.; Cai, Y. Adsorption, kinetics, and thermodynamic studies of cacao husk extracts in waterless sustainable dyeing of cotton fabric. Cellulose 2021, 28, 2521–2536. [Google Scholar] [CrossRef]
- Wang, Q.; Ju, J.; Tan, Y.; Hao, L.; Ma, Y.; Wu, Y.; Zhang, H.; Xia, Y.; Sui, K. Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr. Polym. 2019, 205, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Wang, C. Synthesis of β-Cyclodextrin-Based Electrospun Nanofiber Membranes for Highly Efficient Adsorption and Separation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 26649–26657. [Google Scholar] [CrossRef]
- Thamer, B.M.; El-Hamshary, H.; Al-Deyab, S.S.; El-Newehy, M.H. Functionalized electrospun carbon nanofibers for removal of cationic dye. Arab. J. Chem. 2019, 12, 747–759. [Google Scholar] [CrossRef]
- Bai, L.; Jia, L.; Yan, Z.; Liu, Z.; Liu, Y. Plasma-etched electrospun nanofiber membrane as adsorbent for dye removal. Chem. Eng. Res. Des. 2018, 132, 445–451. [Google Scholar] [CrossRef]
- Shalan, A.E.; Afifi, M.; El-Desoky, M.M.; Ahmed, M.K. Electrospun nanofibrous membranes of cellulose acetate containing hydroxyapatite co-doped with Ag/Fe: Morphological features, antibacterial activity and degradation of methylene blue in aqueous solution. New J. Chem. 2021, 45, 9212–9220. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.-S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef] [PubMed]
- Moradi, E.; Ebrahimzadeh, H.; Mehrani, Z.; Asgharinezhad, A.A. The efficient removal of methylene blue from water samples using three-dimensional poly (vinyl alcohol)/starch nanofiber membrane as a green nanosorbent. Environ. Sci. Pollut. Res. 2019, 26, 35071–35081. [Google Scholar] [CrossRef]
- Ibupoto, A.S.; Qureshi, U.A.; Ahmed, F.; Khatri, Z.; Khatri, M.; Maqsood, M.; Brohi, R.Z.; Kim, I.S. Reusable carbon nanofibers for efficient removal of methylene blue from aqueous solution. Chem. Eng. Res. Des. 2018, 136, 744–752. [Google Scholar] [CrossRef]
- Dogan, Y.E.; Satilmis, B.; Uyar, T. Crosslinked PolyCyclodextrin/PolyBenzoxazine electrospun microfibers for selective removal of methylene blue from an aqueous system. Eur. Polym. J. 2019, 119, 311–321. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Jiang, Z.; Wang, C. Water-insoluble sericin/β-cyclodextrin/PVA composite electrospun nanofibers as effective adsorbents towards methylene blue. Colloids Surf. B Biointerfaces 2015, 136, 375–382. [Google Scholar] [CrossRef]
- Mohammad, N.; Atassi, Y. Adsorption of methylene blue onto electrospun nanofibrous membranes of polylactic acid and polyacrylonitrile coated with chloride doped polyaniline. Sci. Rep. 2020, 10, 13412. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Binagag, F.F.; Haider, A.; Mahmood, A.; Shah, N.; Al-Masry, W.A.; Khan, S.U.-D.; Ramay, S.M. Adsorption kinetic and isotherm of methylene blue, safranin T and rhodamine B onto electrospun ethylenediamine-grafted-polyacrylonitrile nanofibers membrane. Desalination Water Treat. 2015, 55, 1609–1619. [Google Scholar] [CrossRef]
- Haider, S.; Binagag, F.F.; Haider, A.; Al-Masry, W.A. Electrospun oxime-grafted-polyacrylonitrile nanofiber membrane and its application to the adsorption of dyes. J. Polym. Res. 2014, 21, 371. [Google Scholar] [CrossRef]
- Ali, A.S.M.; El-Aassar, M.R.; Hashem, F.S.; Moussa, N.A. Surface Modified of Cellulose Acetate Electrospun Nanofibers by Polyaniline/β-cyclodextrin Composite for Removal of Cationic Dye from Aqueous Medium. Fibers Polym. 2019, 20, 2057–2069. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.-H.; Li, J. A mini-review on membrane fouling. Bioresour. Technol. 2012, 122, 27–34. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Membr. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Shirazi, S.; Lin, C.-J.; Chen, D. Inorganic fouling of pressure-driven membrane processes—A critical review. Desalination 2010, 250, 236–248. [Google Scholar] [CrossRef]
- Subrahmanya, T.M.; Arshad, A.B.; Lin, P.T.; Widakdo, J.; Makari, H.K.; Austria, H.F.; Hu, C.C.; Lai, J.Y.; Hung, W.S. A review of recent progress in polymeric electrospun nanofiber membranes in addressing safe water global issues. RSC Adv. 2021, 11, 9638–9663. [Google Scholar]
- Bouchareb, R.; Bilici, Z.; Dizge, N. Potato Processing Wastewater Treatment Using a Combined Process of Chemical Coagulation and Membrane Filtration. CLEAN-Soil Air Water 2021, 49, 2100017. [Google Scholar] [CrossRef]
- Fane, A.G. Membranes and the water cycle: Challenges and opportunities. Appl. Water Sci. 2011, 1, 3–9. [Google Scholar] [CrossRef] [Green Version]
| Common Parameters | Standard Volume (mg/L) |
|---|---|
| Total suspended solids | 50 |
| Total nitrogen | 10 |
| Total Phosphorus | 2 |
| Biochemical oxygen demand | 50 |
| Chemical oxygen demand | 250 |
| Oil and grease | 10 |
| pH | 5.5–9.0 |
| Sources | Main Components of Wastewater | Characteristics | Ref |
|---|---|---|---|
| Dairy | Proteins, detergents, lactose, and lipids | BOD = 442 mg/L COD = 8960 mg/L TDS = 253.6 mg/L pH = 7.10 | [58] |
| Olive mill | Phenols, pectin, sugars, fats, oil, salts and carbohydrates | BOD = 4426 mg/L COD = 55,730–156,000 mg/L Total phenol = 2439–8300 mg/L pH = 5.6 | [59,60] |
| Slaughterhouse | Nitrogen, sodium, potassium, calcium and fats | BOD = 1209 mg/L COD = 4221 mg/L Total nitrogen = 427 mg/L pH = 6.95 | [61] |
| Fruits | Carbohydrates, minerals, nitrogen phosphorus and salts | BOD = 860 mg/L COD = 919 mg/L Total nitrogen = 40 mg/L pH = 5.5–7.2 | [62] |
| Seafood | Sodium chlorides, phosphorus, nitrogen, salts, fats and grease | BOD = 3250 mg/L COD = 13,180 mg/L Salts = 2–5% (w/v) pH = 5–7 | [63] |
| Type of Membrane Filtration | Source | Characteristics | Performance | Ref |
|---|---|---|---|---|
| MF | Dairy wastewater | BOD = 890 ± 92 mg/L COD = 3536 ± 328 mg/L Turbidity = 623 ± 140 NTU TSS = 1860 ± 220 mg/L pH = 7.3 ± 0.3 | COD Removal (%) = 89 ± 2 Color Removal (%) = 93 ± 5 Turbidity Removal (%) = 98 ± 4 | [83] |
| UF | Dairy wastewater | BOD = 890 ± 92 mg/L COD = 3536 ± 328 mg/L Turbidity = 623 ± 140 NTU TSS = 1860 ± 220 mg/L pH = 7.3 ± 0.3 | COD Removal (%) = 95 ± 1 Color Removal (%) = 97 ± 6 Turbidity Removal (%) = 99 ± 5 | [83] |
| NF | Restaurant wastewater | BOD = 816.17–1097.25 mg/L COD = 10,356.67–16,443.33 mg/L Turbidity = 402.67–1208 NTU TSS = 1860 ± 220 mg/L pH = 4.49–6.15 | COD Removal (%) = 99.4 BOD Removal (%) = 86.8 Turbidity Removal (%) = 99.9 | [85] |
| RO | Olive wastewater | Suspended matter = 14–16 mg/L COD = 120.5–226.6 mg/L | COD Removal (%) = 99.8 | [86] |
| Membrane Materials | Membrane Characteristics | Operational Conditions | Adsorption Capacity, qe, max (mg/g) | Ref. |
|---|---|---|---|---|
| Sodium alginate (SA), poly (ethylene oxide) (PEO) | Fiber diameter = 150 nm, surface area = 13.97 m2/g | Initial MB conc. = 200 to 1500 mg/L, V = 50 mL, pH = 6, adsorbent weight = 20 mg | 2230 | [114] |
| β-cyclodextrin, poly(acrylic acid) (PAA) | Fiber diameter = 20.56 nm, surface area = 34.88 m2/g | Initial MB conc. = 40 mg/L, V = 80 mL, pH = 9, adsorbent weight = 6 mg | 826.45 | [115] |
| Plasma etched poly(l-lactic acid) (PLLA) | Fiber diameter = N/A, surface area = 22.84 m2/g | Initial MB conc. = 4 mg/L, V = 3 mL, pH = N/A, adsorbent weight = 10 mg | 8.73 | [117] |
| Poly(vinyl alcohol) (PVA)/starch | Fiber diameter = 350–450, surface area = 45.61 m2/g | Initial MB conc. = 250 mg/L, V = 60 mL, pH = 8.5, adsorbent weight = 5 mg | 400 | [120] |
| Polyacrylonitrile (PAN) | Fiber diameter = 250–300, surface area = N/A | Initial MB conc. = 25 mg/L, V = 10 mL, pH = 10, adsorbent weight = 7 mg | 72.46 | [121] |
| Hydroxypropyl-β-cyclodextrin (HPβCD) and benzoxazine monomer (BA-a) | Fiber diameter = N/A, surface area = N/A | Initial MB conc. = 10–100 mg/L, V = 5 mL, pH = N/A, adsorbent weight = 5 mg | 46 | [122] |
| Sericin/-cyclodextrin/poly (vinyl alcohol) | Fiber diameter = N/A, surface area = N/A | Initial MB conc. = 20 mg/L, V = 80 mL, pH = 8, adsorbent weight = 14 mg | 187 | [123] |
| Poly-L-lactic acid (pLLA), polyaniline (PANI) | Fiber diameter = 518 nm, surface area = 7.0 ± 0.4 | Initial MB conc. = 250 mg/L, V = 10 mL, pH = 6, adsorbent weight = 10 mg | 239 | [124] |
| Polyacrylonitrile (PAN), polyaniline (PANI) | Fiber diameter = 418 nm, surface area = 10.0 ± 0.3 | Initial MB conc. = 250 mg/L, V = 10 mL, pH = 6, adsorbent weight = 10 mg | 398 | [124] |
| polyacrylonitrile (PAN) | Fiber diameter = 225 nm, surface area = N/A | Initial MB conc. = 400 mg/L, V = 10 mL, pH = N/A, adsorbent weight = 10 mg | 42 | [125] |
| ethylenediamine (EDA)-grafted polyacrylonitrile (PAN) | Fiber diameter = 230 nm, surface area = N/A | Initial MB conc. = 400 mg/L, V = 10 mL, pH = N/A, adsorbent weight = 10 mg | 94 | [125] |
| Oxime grafted polyacrylonitrile (OX-g-PAN) | Fiber diameter = 231 nm, surface area = N/A | Initial MB conc. = 400 mg/L, V = 10 mL, pH = 6, adsorbent weight = 10 mg | 102 | [126] |
| Cellulose acetate (CA) | Fiber diameter = 752 ± 311 nm, surface area = N/A | Initial MB conc. = 30 mg/L, V = 100 mL, pH = 8, adsorbent weight = 80 mg | 45 | [127] |
| Cellulose acetate (CA)/polyaniline/ β-cyclodextrin (PANI/β-CD) | Fiber diameter = 1085 ± 325 nm, surface area = N/A | Initial MB conc. = 30 mg/L, V = 100 mL, pH = 8, adsorbent weight = 80 mg | 49 | [127] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pervez, M.N.; Mishu, M.R.; Stylios, G.K.; Hasan, S.W.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. Sustainable Treatment of Food Industry Wastewater Using Membrane Technology: A Short Review. Water 2021, 13, 3450. https://doi.org/10.3390/w13233450
Pervez MN, Mishu MR, Stylios GK, Hasan SW, Zhao Y, Cai Y, Zarra T, Belgiorno V, Naddeo V. Sustainable Treatment of Food Industry Wastewater Using Membrane Technology: A Short Review. Water. 2021; 13(23):3450. https://doi.org/10.3390/w13233450
Chicago/Turabian StylePervez, Md. Nahid, Monira Rahman Mishu, George K. Stylios, Shadi W. Hasan, Yaping Zhao, Yingjie Cai, Tiziano Zarra, Vincenzo Belgiorno, and Vincenzo Naddeo. 2021. "Sustainable Treatment of Food Industry Wastewater Using Membrane Technology: A Short Review" Water 13, no. 23: 3450. https://doi.org/10.3390/w13233450









