Key Drivers Influencing the Presence and Absence of Micropterus salmoides and Their Effect on Native Fish Communities and Biotic Integrity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
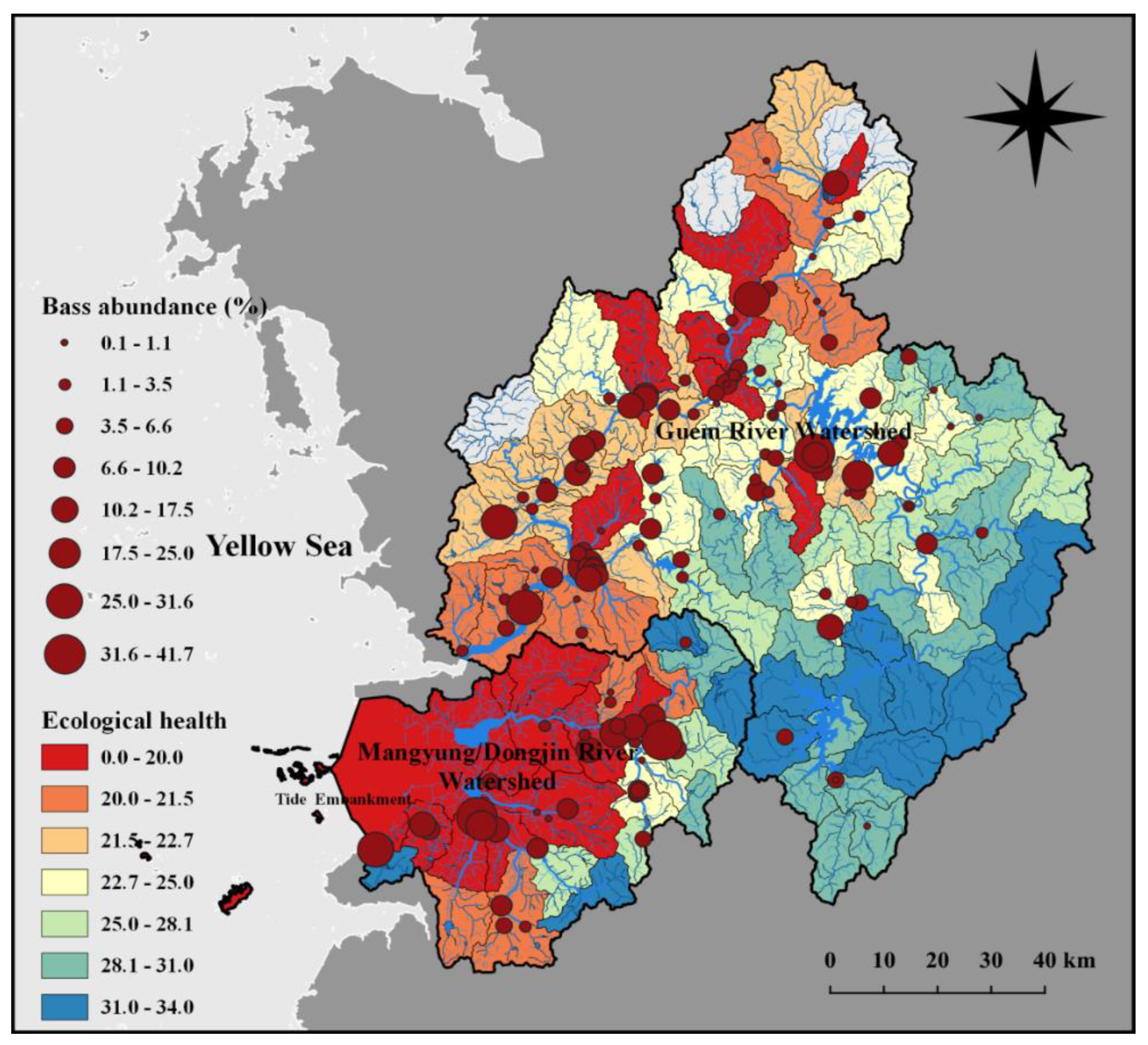
2.2. Geographical Distribution of Fish Sampling Sites
2.3. Fish Sampling
2.4. Chemical Water Quality Analysis
2.5. Physical Habitat Evaluation
2.6. Ecological Health Assessment
2.7. Statistical Analyses
3. Results
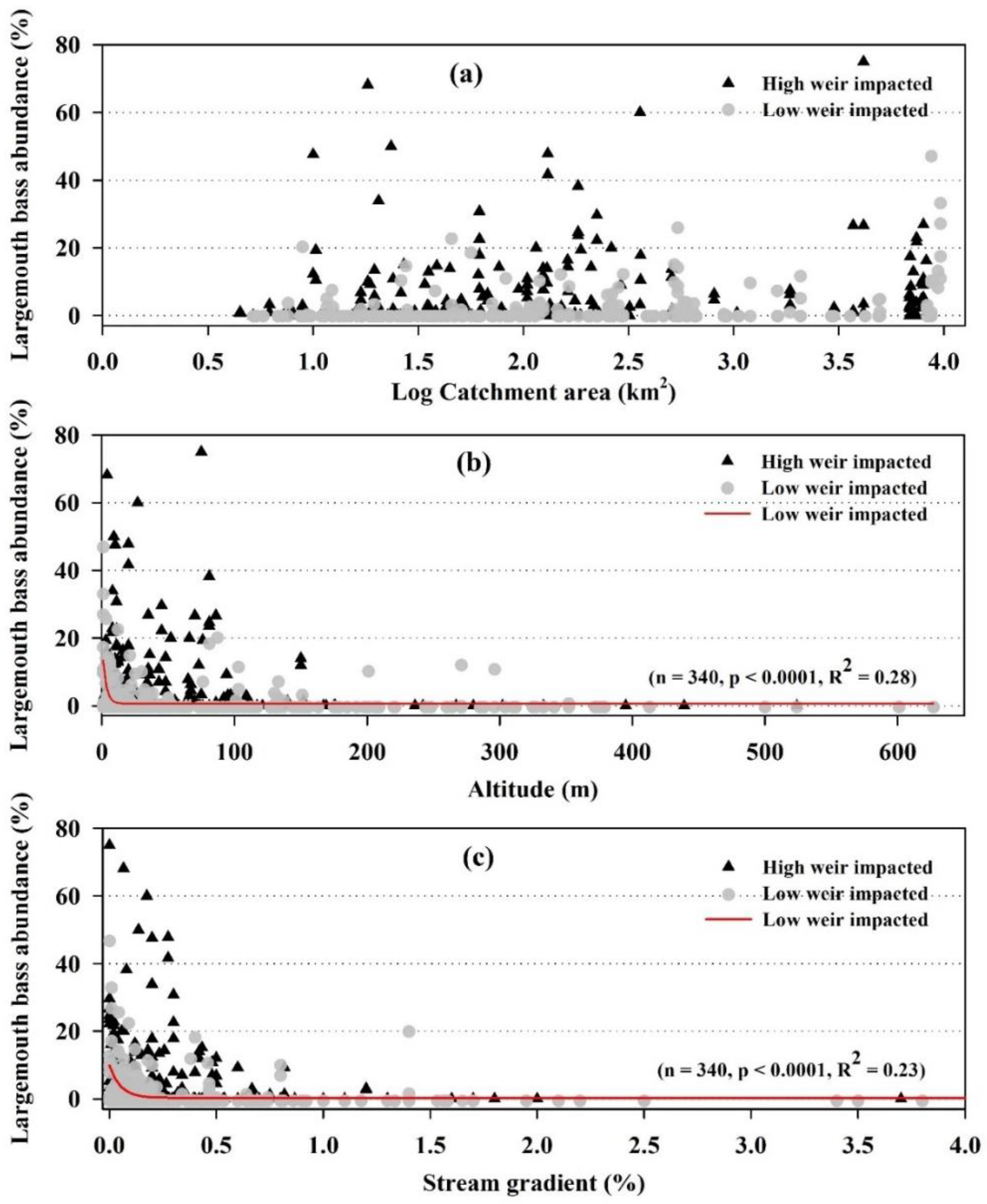
3.1. Geomorphological Characteristic and Largemouth Bass
3.2. Hydro-Chemical Factors and Largemouth Bass Distribution
3.3. Habitat Quality and Largemouth Bass
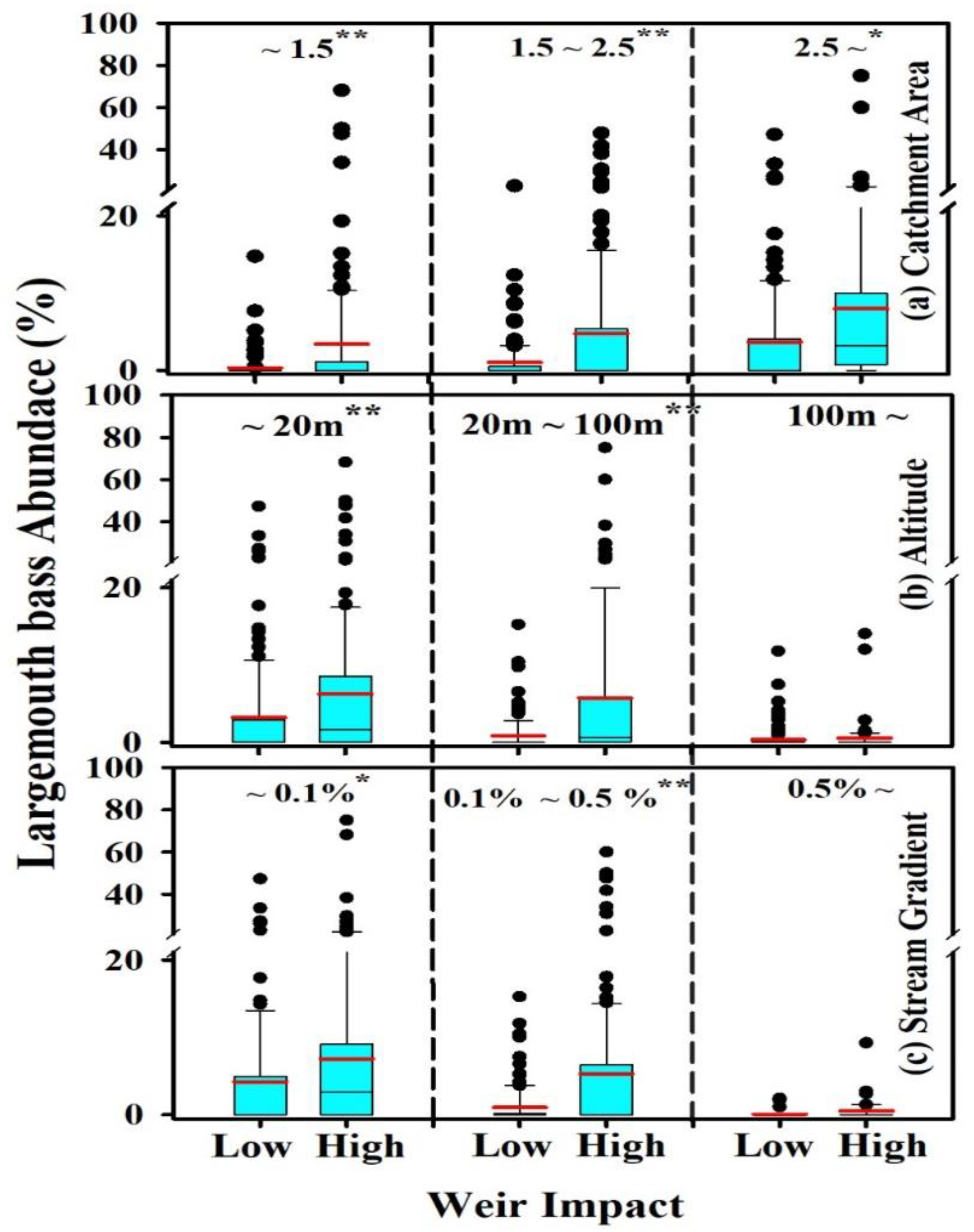
3.4. Relationship between Weir Impacts and Largemouth Bass
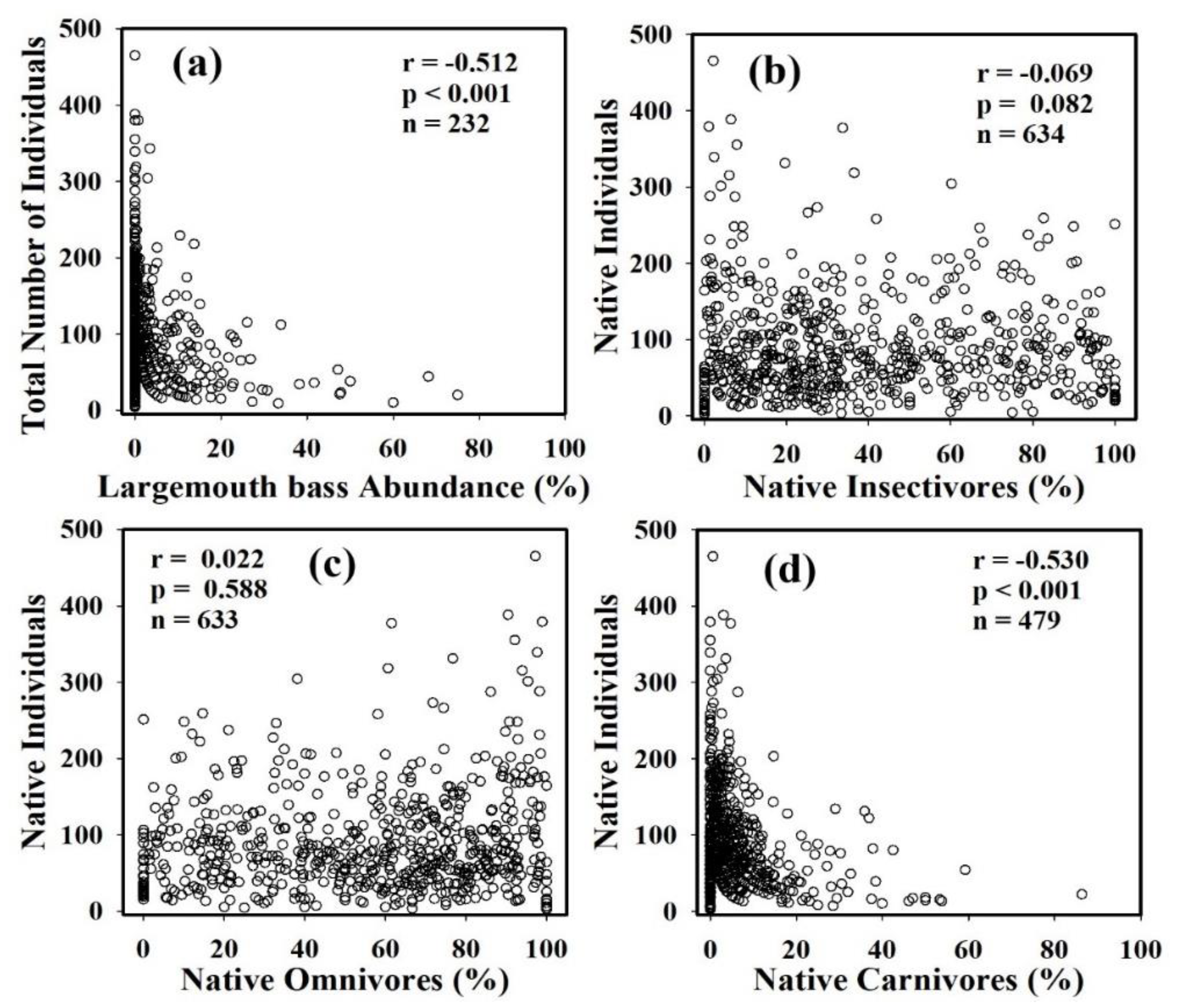
3.5. Occurrence and Impact of Largemouth Bass on Native Fish Communities
3.6. Native vs. Native’ and Largemouth Bass Abundance and Constancy
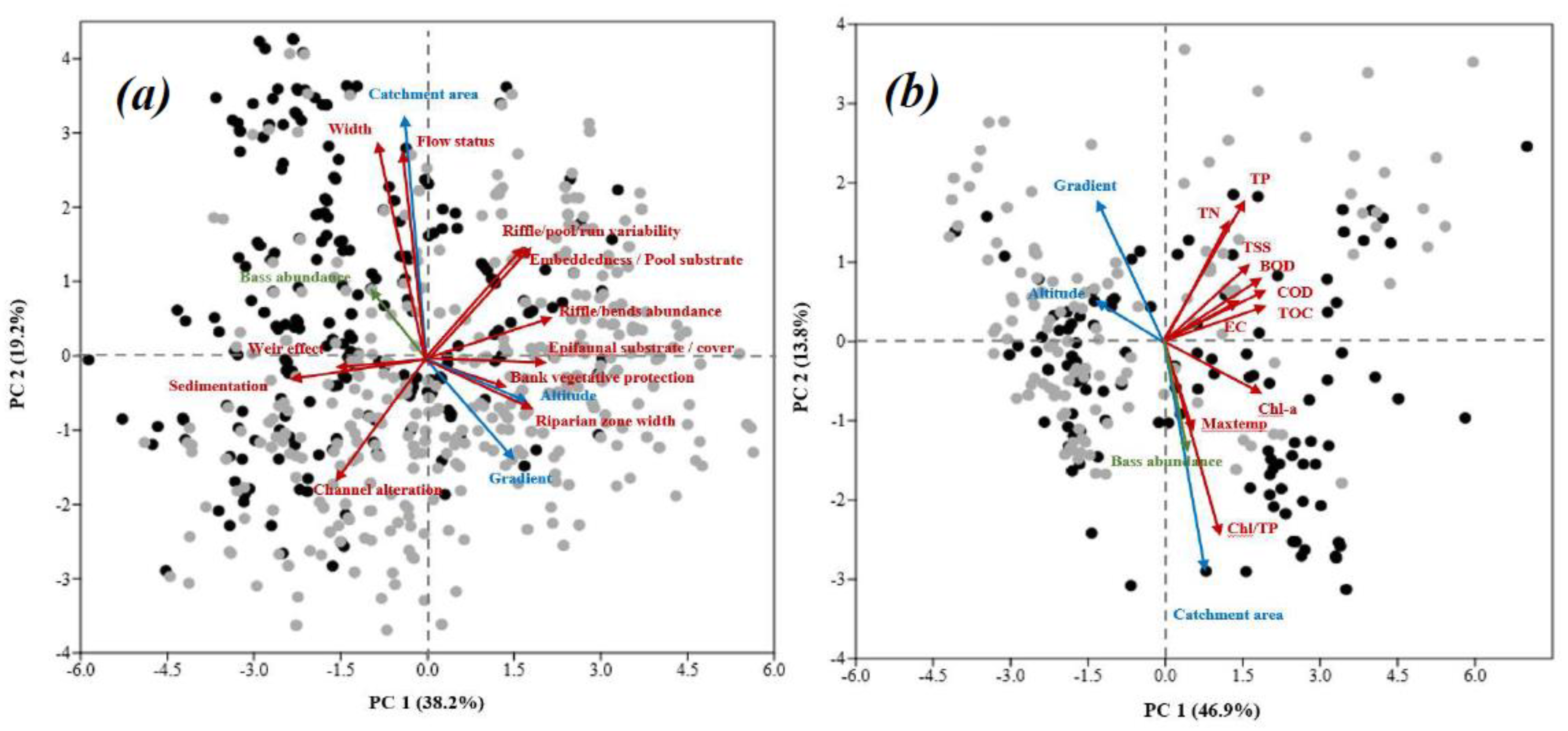
3.7. Linking Largemouth Bass, Geomorphology, Habitat, and Water Quality
3.8. Biotic Integrity and Largemouth Bass Abundance
4. Discussion
4.1. Largemouth Bass in South Korea
4.2. Water Chemistry and Physical Habitat Quality
4.3. Influence of Weir
4.4. Geomorphology, Catchment Size and Largemouth Bass Distribution
4.5. Largemouth Bass and Native Fish Communities
4.6. River Health and Exotic Fish Species
5. Conclusions and Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitule, J.R.S.; Freire, C.A.; Simberloff, D. Introduction of non-native freshwater fish can certainly be bad. Fish Fish. 2009, 10, 98–108. [Google Scholar] [CrossRef]
- Kim, J.-J.; Atique, U.; An, K.-G. Long-Term Ecological Health Assessment of a Restored Urban Stream Based on Chemical Water Quality, Physical Habitat Conditions and Biological Integrity. Water 2019, 11, 114. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Atique, U.; An, K.-G. Relative Abundance and Invasion Dynamics of Alien Fish Species Linked to Chemical Conditions, Ecosystem Health, Native Fish Assesmblage, and Stream Order. Water 2021, 13, 158. [Google Scholar] [CrossRef]
- Essl, F.; Lenzner, B.; Bacher, S.; Bailey, S.; Capinha, C.; Daehler, C.; Dullinger, S.; Genovesi, P.; Hui, C.; Hulme, P.E.; et al. Drivers of future alien species impacts: An expert-based assessment. Glob. Chang. Biol. 2020, 26, 4880–4893. [Google Scholar] [CrossRef]
- Atique, U.; Byungjin, L.; Johee, Y.; An, K.-G. Biological Health Assessments of Lotic Waters by Biotic Integrity Indices and their Relations to Water Chemistry. Water 2019, 11, 436. [Google Scholar] [CrossRef] [Green Version]
- Early, R.; Bradley, B.A.; Dukes, J.S.; Lawler, J.J.; Olden, J.D.; Blumenthal, D.M.; Gonzalez, P.; Grosholz, E.D.; Ibañez, I.; Miller, L.P.; et al. Global threats from invasive alien species in the twenty-first century and national response capacities. Nat. Commun. 2016, 7, 1–9. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Wei, H.; Li, S.; Piria, M.; Al-Faisal, A.J.; Almeida, D.; Atique, U.; Al-Wazzan, Z.; Bakiu, R.; et al. Speaking their language—Development of a multilingual decision-support tool for communicating invasive species risks to decision makers and stakeholders. Environ. Model. Softw. 2021, 135, 104900. [Google Scholar] [CrossRef]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefani, F.; Schiavon, A.; Tirozzi, P.; Gomarasca, S.; Marziali, L. Functional response of fish communities in a multistressed freshwater world. Sci. Total Environ. 2020, 740, 139902. [Google Scholar] [CrossRef]
- Preston, D.L.; Hedman, H.D.; Johnson, P.T.J. Nutrient availability and invasive fish jointly drive community dynamics in an experimental aquatic system. Ecosphere 2018, 9, e02153. [Google Scholar] [CrossRef]
- Flood, P.J.; Duran, A.; Barton, M.; Mercado-Molina, A.E.; Trexler, J.C. Invasion impacts on functions and services of aquatic ecosystems. Hydrobiologia 2020, 847, 1571–1586. [Google Scholar] [CrossRef]
- Kennard, M.J.; Arthington, A.H.; Pusey, B.J.; Harch, B.D. Are alien fish a reliable indicator of river health? Freshw. Biol. 2005, 50, 174–193. [Google Scholar] [CrossRef] [Green Version]
- Roy, H.E.; Bacher, S.; Essl, F.; Adriaens, T.; Aldridge, D.C.; Bishop, J.D.D.; Blackburn, T.M.; Branquart, E.; Brodie, J.; Carboneras, C.; et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2019, 25, 1032–1048. [Google Scholar] [CrossRef] [Green Version]
- Haque, M.A.; Paul, S.; Jewel, M.A.S.; Atique, U.; Ahmed, Z.; Paul, A.K.; Iqbal, S.; Mahboob, S. Seasonal analysis of food items and feeding habits of endangered riverine catfish Rita rita (Hamilton, 1822). Braz. J. Biol. 2021, 82, 1–11. [Google Scholar] [CrossRef]
- Kim, J.Y.; Atique, U.; Mamun, M.; An, K.-G. Long-Term Interannual and Seasonal Links between the Nutrient Regime, Sestonic Chlorophyll and Dominant Bluegreen Algae under the Varying Intensity of Monsoon Precipitation in a Drinking Water Reservoir. Int. J. Environ. Res. Public Health 2021, 18, 2871. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000. [Google Scholar]
- Hermoso, V.; Blanco-Garrido, F.; Prenda, J. Spatial distribution of exotic fish species in the Guadiana river basin, with two new records. Limnetica 2008, 27, 189–194. [Google Scholar]
- Xiong, W.; Sui, X.; Liang, S.-H.; Chen, Y. Non-native freshwater fish species in China. Rev. Fish Biol. Fish. 2015, 25, 651–687. [Google Scholar] [CrossRef]
- Maezono, Y.; Kobayashi, R.; Kusahara, M.; Miyashita, T. Direct and indirect effects of EXOTIC Bass and Bluegill on exotic and native organisms in farm ponds. Ecol. Appl. 2005, 15, 638–650. [Google Scholar] [CrossRef]
- Wasserman, R.J.; Strydom, N.A.; Weyl, O.L.F. Diet of largemouth bass, Micropterus salmoides (Centrarchidae), an invasive alien in the lower reaches of an Eastern Cape River, South Africa. Afr. Zool. 2011, 46, 378–386. [Google Scholar] [CrossRef]
- Atique, U.; Kwon, S.; An, K.G. Linking weir imprints with riverine water chemistry, microhabitat alterations, fish assemblages, chlorophyll-nutrient dynamics, and ecological health assessments. Ecol. Indic. 2020, 117, 106652. [Google Scholar] [CrossRef]
- Cucherousset, J.; Olden, J.D. Ecological Impacts of Nonnative Freshwater Fishes. Fisheries 2011, 36, 215–230. [Google Scholar] [CrossRef]
- Pereira, F.W.; Vitule, J.R.S. The largemouth bass Micropterus salmoides (Lacepède, 1802): Impacts of a powerful freshwater fish predator outside of its native range. Rev. Fish Biol. Fish. 2019, 29, 639–652. [Google Scholar] [CrossRef]
- Tsunoda, H.; Mitsuo, Y.; Ohira, M.; Doi, M.; Senga, Y. Change of fish fauna in ponds after eradication of invasive piscivorous largemouth bass, Micropterus salmoides, in north-eastern Japan. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 710–716. [Google Scholar] [CrossRef]
- Goudswaard, P.C.; Witte, F.; Katunzi, E.F.B. The tilapiine fish stock of Lake Victoria before and after the Nile perch upsurge. J. Fish Biol. 2002, 60, 838–856. [Google Scholar] [CrossRef]
- Witte, F.; Msuku, B.S.; Wanink, J.H.; Seehausen, O.; Katunzi, E.F.B.; Goudswaard, P.C.; Goldschmidt, T. Recovery of cichlid species in Lake Victoria: An examination of factors leading to differential extinction. Rev. Fish Biol. Fish. 2000, 10, 233–241. [Google Scholar] [CrossRef]
- Power, M.E.; Matthews, W.J.; Stewart, A.J. Grazing Minnows, Piscivorous Bass, and Stream Algae: Dynamics of a Strong Interaction. Ecology 1985, 66, 1448–1456. [Google Scholar] [CrossRef]
- Parker, I.M.; Simberloff, D.; Lonsdale, W.M.; Goodell, K.; Wonham, M.; Kareiva, P.M.; Williamson, M.H.; Von Holle, B.; Moyle, P.B.; Byers, J.E.; et al. Impact: Toward a Framework for Understanding the Ecological Effects of Invaders. Biol. Invasions 1999, 1, 3–19. [Google Scholar] [CrossRef]
- Dick, J.T.A.; Alexander, M.E.; Jeschke, J.M.; Ricciardi, A.; Macisaac, H.J.; Robinson, T.B.; Kumschick, S.; Weyl, O.L.F.; Dunn, A.M.; Hatcher, M.J.; et al. Advancing impact prediction and hypothesis testing in invasion ecology using a comparative functional response approach. Biol. Invasions 2014, 16, 735–753. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.E.; Dick, J.T.A.; Weyl, O.L.F.; Robinson, T.B.; Richardson, D.M. Existing and emerging high impact invasive species are characterized by higher functional responses than natives. Biol. Lett. 2014, 10, 20130946. [Google Scholar] [CrossRef]
- Almeida, D.; Almodóvar, A.; Nicola, G.G.; Elvira, B.; Grossman, G.D. Trophic plasticity of invasive juvenile largemouth bass Micropterus salmoides in Iberian streams. Fish. Res. 2012, 113, 153–158. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, S.-H.; Kim, H.-T.; Kim, J.-G.; Park, J.-Y.; Kim, H.-S. Study on feeding habits of Micropterus salmoides in habitat types from Korea. Korean J. Ichthyol. 2019, 31, 39–53. [Google Scholar] [CrossRef]
- Egnew, N.; Renukdas, N.; Ramena, Y.; Yadav, A.K.; Kelly, A.M.; Lochmann, R.T.; Sinha, A.K. Physiological insights into largemouth bass (Micropterus salmoides) survival during long-term exposure to high environmental ammonia. Aquat. Toxicol. 2019, 207, 72–82. [Google Scholar] [CrossRef]
- Stuber, R.J.; Gebhart, G.; Maughan, O.E. Habitat Suitability Index Models: Largemouth Bass; FWS/OBS-82/10.16; U.S. Department of the Interior: Washington, DC, USA, 1982; p. 32.
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Atique, U.; An, K.-G. Stream health evaluation using a combined approach of multi-metric chemical pollution and biological integrity models. Water 2018, 10, 661. [Google Scholar] [CrossRef] [Green Version]
- Atique, U.; An, K.-G. Reservoir Water Quality Assessment Based on Chemical Parameters and the Chlorophyll Dynamics in Relation to Nutrient Regime. Pol. J. Environ. Stud. 2019, 28, 1043–1061. [Google Scholar] [CrossRef]
- Buffington, J.M.; Montgomery, D.R.; Greenberg, H.M. Basin-scale availability of salmonid spawning gravel as influenced by channel type and hydraulic roughness in mountain catchments. Can. J. Fish. Aquat. Sci. 2004, 61, 2085–2096. [Google Scholar] [CrossRef]
- Montgomery, D.R.; Buffington, J.M. Channel-reach morphology in mountain drainage basins. GSA Bull. 1997, 109, 596–611. [Google Scholar] [CrossRef]
- McGarvey, D.J. Quantifying ichthyofaunal zonation and species richness along a 2800-km reach of the Rio Chama and Rio Grande (USA). Ecol. Freshw. Fish 2011, 20, 231–242. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Ward, G.M. Scale dependence in the species-discharge relationship for fishes of the southeastern U.S.A. Freshw. Biol. 2008, 53, 2206–2219. [Google Scholar] [CrossRef]
- Rahel, F.J.; Hubert, W.A. Fish assemblages and habitat gradients in a Rocky Mountain–Great Plains stream: Biotic zonation and additive patterns of community change. Trans. Am. Fish. Soc. 1991, 120, 319–332. [Google Scholar] [CrossRef]
- Massong, T.M.; Montgomery, D.R. Influence of sediment supply, lithology, and wood debris on the distribution of bedrock and alluvial channels. GSA Bull. 2000, 112, 591–599. [Google Scholar] [CrossRef]
- Nagel, D.; Buffington, J.M.; Isaak, D. Estimating stream gradient using NHD stream lines and DEM data. In Proceedings of the ESRI International User Conference, San Diego, CA, USA, 12–16 July 2010. [Google Scholar]
- Nagel, D.; Buffington, J.; Isaak, D. Comparison of Methods for Estimating Stream Channel Gradient Using GIS; USDA Forest Service, Rocky Mountain Research Station Boise Aquatic Sciences Lab: Boise, ID, USA, 2006.
- Ohio Environmental Protection Agency. Biological criteria for the protection of aquatic life. In Standardized Biological Field Sampling and Laboratory Methods for Assessing Fish and Macroinvertebrate Communities; Ohio Environmental Protection Agency: Columbus, OH, USA, 1989; Volume III. [Google Scholar]
- Barbour, M.T.; Gerritsen, J.; Snyder, B.D.; Stribling, J.B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish; US Environmental Protection Agency, Office of Water: Washington, DC, USA, 1999.
- Plafkin, J.; Barbour, M.; Porter, K.; Gross, S.; Hughes, R. Rapid Assessment Protocols for Use in Streams and Rivers: Binthic Macroinvertebrats and Fish; EPA/444/4-89-001; Office of Water Regulations and Standards, US EPA: Washington, DC, USA, 1989.
- Kim, J.-H.; An, K.-G. A diagnosis of ecological health using a physical habitat assessment and multi-metric fish model in Daejeon Stream. Korean J. Ecol. Environ. 2005, 38, 361–371. [Google Scholar]
- Karr, J.R. Assessment of biotic integrity using fish communities. Fisheries 1981, 6, 21–27. [Google Scholar] [CrossRef]
- Choi, J.W.; An, K.G. Hydrodynamic fish modeling for potential expansion evaluations of exotic species (largemouth bass) on waterway tunnel of Andong-Imha reservoir. J. Ecol. Environ. 2016, 40, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Crooks, J.A.; Chang, A.L.; Ruiz, G.M. Aquatic pollution increases the relative success of invasive species. Biol. Invasions 2011, 13, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Atique, U.; An, K.-G. Landscape heterogeneity impacts water chemistry, nutrient regime, organic matter and chlorophyll dynamics in agricultural reservoirs. Ecol. Indic. 2020, 110, 105813. [Google Scholar] [CrossRef]
- Bae, D.-Y.; Atique, U.; Yoon, J.; Lim, B.; An, K.-G. Ecological Risk Assessment of Urban Streams Using Fish Biomarkers of DNA Damages and Physiological Responses. Pol. J. Environ. Stud. 2020, 29, 1–10. [Google Scholar] [CrossRef]
- Moon, W.-K.; Atique, U.; An, K.-G. Ecological risk assessments and eco-toxicity analyses using chemical, biological, physiological responses, DNA damages and gene-level biomarkers in Zebrafish (Danio rerio) in an urban stream. Chemosphere 2020, 239, 124754. [Google Scholar] [CrossRef]
- Marchetti, M.P.; Light, T.; Moyle, P.B.; Viers, J.H. Fish invasions in california watersheds: Testing hypotheses using landscape patterns. Ecol. Appl. 2004, 14, 1507–1525. [Google Scholar] [CrossRef]
- Johnson, P.T.; Olden, J.D.; Vander Zanden, M.J. Dam invaders: Impoundments facilitate biological invasions into freshwaters. Front. Ecol Environ. 2008, 6, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.; Pander, J.; Geist, J. The effects of weirs on structural stream habitat and biological communities. J. Appl. Ecol. 2011, 48, 1450–1461. [Google Scholar] [CrossRef]
- Gido, K.B.; Propst, D.L.; Olden, J.D.; Bestgen, K.R. Multidecadal responses of native and introduced fishes to natural and altered flow regimes in the American Southwest. Can. J. Fish. Aquat. Sci. 2013, 70, 554–564. [Google Scholar] [CrossRef]
- Bae, M.J.; Murphy, C.A.; García-Berthou, E. Temperature and hydrologic alteration predict the spread of invasive Largemouth Bass (Micropterus salmoides). Sci. Total Environ. 2018, 639, 58–66. [Google Scholar] [CrossRef]
- Jo, H.; Jeppesen, E.; Ventura, M.; Buchaca, T.; Gim, J.S.; Yoon, J.D.; Kim, D.H.; Joo, G.J. Responses of fish assemblage structure to large-scale weir construction in riverine ecosystems. Sci. Total Environ. 2019, 657, 1334–1342. [Google Scholar] [CrossRef] [Green Version]
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the world’s free-flowing rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Januchowski-Hartley, S.R.; Mantel, S.; Celi, J.; Hermoso, V.; White, J.C.; Blankenship, S.; Olden, J.D. Small instream infrastructure: Comparative methods and evidence of environmental and ecological responses. Ecol. Solut. Evid. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Olden, J.D. Challenges and opportunities for fish conservation in dam-impacted waters. In Conservation of Freshwater Fishes; Closs, G.P., Krkosek, M., Olden, J.D., Eds.; Cambridge University Press: Cambridge, UK, 2016; pp. 107–148. [Google Scholar]
- HaRa, J.; Atique, U.; An, K.-G. Multiyear Links between Water Chemistry, Algal Chlorophyll, Drought-Flood Regime, and Nutrient Enrichment in a Morphologically Complex Reservoir. Int. J. Environ. Res. Public Health 2020, 17, 3139. [Google Scholar] [CrossRef] [PubMed]
- Oele, D.L.; Gaeta, J.W.; Rypel, A.L.; McIntyre, P.B. Growth and recruitment dynamics of young-of-year northern pike: Implications for habitat conservation and management. Ecol. Freshw. Fish 2019, 28, 285–301. [Google Scholar] [CrossRef]
- Sax, D.F. Latitudinal gradients and geographic ranges of exotic species: Implications for biogeography. J. Biogeogr. 2001, 28, 139–150. [Google Scholar] [CrossRef]
- Boon, P.J. The catchment approach as the scientific basis of river basin management. River Syst. 2005, 16, 29–58. [Google Scholar] [CrossRef]
- Van Wilgen, B.W.; Nel, J.L.; Rouget, M. Invasive alien plants and South African rivers: A proposed approach to the prioritization of control operations. Freshw. Biol. 2007, 52, 711–723. [Google Scholar] [CrossRef]
- Forsyth, G.G.; Le Maitre, D.C.; O’farrell, P.J.; Van Wilgen, B.W. The prioritisation of invasive alien plant control projects using a multi-criteria decision model informed by stakeholder input and spatial data. J. Environ. Manag. 2012, 103, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Magliozzi, C.; Tsiamis, K.; Vigiak, O.; Deriu, I.; Gervasini, E.; Cardoso, A.C. Assessing invasive alien species in European catchments: Distribution and impacts. Sci. Total Environ. 2020, 732, 138677. [Google Scholar] [CrossRef]
- Weyl, P.S.R.; de Moor, F.C.; Hill, M.P.; Weyl, O.L.F. The effect of largemouth bass Micropterus salmoides on aquatic macroinvertebrate communities in the Wit River, Eastern Cape, South Africa. Afr. J. Aquat. Sci. 2010, 35, 273–281. [Google Scholar] [CrossRef]
- Gilinsky, E. The role of fish predation and spatial heterogeneity in determining benthic community structure. Ecology 1984, 65, 455–468. [Google Scholar] [CrossRef]
- Flecker, A.S.; Townsend, C.R. Community-Wide Consequences of Trout Introduction in New Zealand Streams. Ecol. Appl. 1994, 4, 798–807. [Google Scholar] [CrossRef]
- Chick, J.H.; Gibson-Reinemer, D.K.; Soeken-Gittinger, L.; Casper, A.F. Invasive silver carp is empirically linked to declines of native sport fish in the Upper Mississippi River System. Biol. Invasions 2020, 22, 723–734. [Google Scholar] [CrossRef] [Green Version]
- Haubrock, P.J.; Pilotto, F.; Innocenti, G.; Cianfanelli, S.; Haase, P. Two centuries for an almost complete community turnover from native to non-native species in a riverine ecosystem. Glob. Chang. Biol. 2020, 27, 606–623. [Google Scholar] [CrossRef]
- Simon, K.S.; Townsend, C.R. Impacts of freshwater invaders at different levels of ecological organisation, with emphasis on salmonids and ecosystem consequences. Freshw. Biol. 2003, 48, 982–994. [Google Scholar] [CrossRef] [Green Version]
- Maezono, Y.; Miyashita, T. Community-level impacts induced by introduced largemouth bass and bluegill in farm ponds in Japan. Biol. Conserv. 2002, 109, 111–121. [Google Scholar] [CrossRef]
- Beatty, S.J.; Morgan, D.L. Introduced freshwater fishes in a global endemic hotspot and implications of habitat and climatic change. BioInvasions Rec. 2013, 2, 1–9. [Google Scholar] [CrossRef]
- Bezerra, L.A.V.; Angelini, R.; Vitule, J.R.S.; Coll, M.; Sa´nchez-Botero, J.I. Food web changes associated with drought and invasive species in a tropical semiarid reservoir. Hydrobiologia 2018, 817, 475–489. [Google Scholar] [CrossRef]
- Rolls, R.J.; Hayden, B.; Kahilainen, K.K. Conceptualising the interactive effects of cli- mate change and biological invasions on subarctic freshwater fish. Ecol. Evol. 2017, 7, 4109–4128. [Google Scholar] [CrossRef] [PubMed]
- Khanom, D.A.; Nesa, A.; Jewel, M.A.S.; Haque, M.A.; Paul, A.K.; Iqbal, S.; Atique, U.; Alam, L. Muscular Tissue Bioaccumulation and Health Risk Assessment of Heavy Metals in Two Edible Fish Species (Gudusia chapra and Eutropiichthys vacha) in Padma River, Bangladesh. Punjab Univ. J. Zool. 2020, 35, 81–89. [Google Scholar] [CrossRef]
- Haque, M.A.; Jewel, M.A.S.; Akhi, M.M.; Atique, U.; Paul, A.K.; Iqbal, S.; Islam, M.S.; Das, S.K.; Alam, M.M. Seasonal dynamics of phytoplankton community and functional groups in a tropical river. Environ. Monit. Assess. 2021, 193, 1–16. [Google Scholar] [CrossRef]
- Atique, U.; Iqbal, S.; Khan, N.; Qazi, B.; Javeed, A.; Anjum, K.M.; Haider, M.S.; Khan, T.A.; Mahmood, S.; Sherzada, S. Multivariate Assessment of Water Chemistry and Metals in a River Impacted by Tanning Industry. Fresenius Environ. Bull. 2020, 29, 3013–3025. [Google Scholar]
- Colin, N.; Villéger, S.; Wilkes, M.; de Sostoa, A.; Maceda-Veiga, A. Functional diversity measures revealed impacts of non-native species and habitat degradation on species-poor freshwater fish assemblages. Sci. Total Environ. 2018, 625, 861–871. [Google Scholar] [CrossRef]
- Alexander, M.E.; Kaiser, H.; Weyl, O.L.F.; Dick, J.T.A. Habitat simplification increases the impact of a freshwater invasive fish. Environ. Biol. Fishes 2015, 98, 477–486. [Google Scholar] [CrossRef] [Green Version]



| Variables | Abbr. & Unit | Largemouth Bass | Significance (t-Test) | SpearMan’s Coefficient | ||
|---|---|---|---|---|---|---|
| Overall (SD) | Bass Present (SD) | Bass Absent (SD) | ||||
| Geomorphological stream characteristics | ||||||
| Stream order | - | 3.1 (1.3) | 3.7 (1.3) | 2.7 (1.1) | ** | 0.332 ** |
| Catchment area (km2) | - | 604.9 (1754.0) | 1135.8 (2448.2) | 173.2 (554.9) | ** | 0.321 ** |
| Altitude (m) | - | 89.3 (113.1) | 43.6 (57.8) | 126.5 (132.1) | ** | −0.351 ** |
| Stream gradient (%) | Gradient | 0.53 (0.79) | 0.21 (0.26) | 0.79 (0.96) | ** | −0.462 ** |
| Stream width (m) | Width | 130.7 (175.4) | 207.2 (233.2) | 68.9 (57.7) | ** | 0.392 ** |
| Physicochemical water quality | ||||||
| pH | - | 7.9 (0.4) | 7.9 (0.4) | 7.9 (0.4) | NS | NS |
| Dissolved oxygen | DO (mg/L) | 10.0 (1.1) | 10.0 (1.1) | 10.0 (1.1) | NS | NS |
| Biological oxygen demand | BOD (mg/L) | 2.2 (1.8) | 2.6 (2.0) | 1.8 (1.6) | ** | 0.250 ** |
| Chemical oxygen demand | COD (mg/L) | 5.8 (3.2) | 6.5 (3.4) | 5.3 (2.9) | ** | 0.210 ** |
| Total suspended solids | TSS (mg/L) | 9.3 (10.0) | 10.6 (9.6) | 8.1 (10.3) | * | 0.242 ** |
| Total nitrogen | TN (mg/L) | 2.5 (1.1) | 2.6 (1.1) | 2.4 (1.1) | NS | 0.158** |
| Total phosphorus | TP (µg/L) | 71.1 (59.9) | 77.0 (47.2) | 66.1 (68.5) | NS | 0.233 ** |
| Total organic carbon | TOC (mg/L) | 3.6 (2.0) | 4.1 (2.0) | 3.2 (1.9) | ** | 0.232 ** |
| Electrical conductivity | EC (mS/cm) | 283.8 (132.8) | 306.3 (116.1) | 264.9 (143.1) | * | 0.225 ** |
| Chlorophyll-a | Chl-a (µg/L) | 22.0 (26.4) | 30.3 (30.4) | 14.2 (19.2) | ** | 0.291 ** |
| Chlorophyll-a: TP | CHL/TP | 0.32 (0.27) | 0.42 (0.31) | 0.25 (0.21) | ** | 0.295 ** |
| Flow (m3/s) | - | 16.1 (22.8) | 19.4 (25.2) | 14.0 (21.1) | ** | 0.308 ** |
| Max. summer temperature | Max temp (°C) | 28.5 (3.1) | 29.1 (2.7) | 27.9 (3.3) | ** | 0.199 ** |
| QHEI Metrics | Abbr. | Largemouth Bass | Significance (t-Test) | Spearman’s Coefficient | ||
|---|---|---|---|---|---|---|
| Overall (SD) | Present (SD) | Absent (SD) | ||||
| Epifaunal substrate/available cover (0–20) | M1 | 12.8 (5.5) | 10.2 (4.7) | 14.2 (5.4) | ** | −0.349 ** |
| Embeddedness (0–20) | M2-1 | 7.1 (5.8) | 9.3 (4.9) | 6.7 (5.9) | ** | 0.194 ** |
| Pool substrate characterization (0–20) | M2-2 | 13.7 (4.5) | 12.9 (4.0) | 14.5 (4.9) | ** | −0.202 ** |
| Velocity/depth combinations (0–20) | M3-1 | 11.8 (4.7) | 10.8 (4.2) | 12.2 (4.8) | ** | −0.124 * |
| Pool variability (0–20) | M3-2 | 13.9 (3.5) | 12.8 (3.0) | 15.2 (3.6) | ** | −0.351 ** |
| Sediment deposition (0–20) | M4 | 11.0 (6.3) | 14.0 (4.3) | 9.4 (6.6) | ** | 0.327 ** |
| Channel flow status (0–20) | M5 | 15.4 (4.1) | 17.0 (3.4) | 14.5 (4.1) | ** | 0.330 ** |
| Channel alteration (0–20) | M6 | 8.6 (4.1) | 8.9 (4.1) | 8.4 (4.1) | NS | NS |
| Frequency of riffles (0–20) | M7-1 | 10.4 (5.9) | 8.0 (5.1) | 11.1 (6.0) | ** | −0.231 ** |
| Channel sinuosity (0–20) | M7-2 | 8.3 (4.5) | 7.3 (3.7) | 9.6 (5.2) | ** | −0.219 ** |
| Bank stability (0–20) | M8 | 19.3 (2.1) | 19.6 (1.4) | 19.2 (2.4) | NS | NS |
| Bank vegetative protection (0–20) | M9 | 14.2 (4.6) | 13.1 (4.7) | 14.8 (4.5) | ** | −0.198 ** |
| Riparian vegetative zone width (0–20) | M10 | 10.9 (5.5) | 8.7 (5.2) | 12.0 (5.3) | ** | −0.302 ** |
| Weir effect (0–20) | M11 | 7.3 (7.2) | 10.4 (7.3) | 5.7 (6.6) | ** | 0.319 ** |
| Scientific Name | Tro. G. | Tol. G. | Ori | GR Basin | MDR Basin | TNI | RA (%) | Presence Sites (n = 303) | Const. (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fish ind. | RA (%) | Fish ind. | RA (%) | ||||||||
| Zacco platypus | O | IS | N | 12,784 | 28.0 | 3593 | 21.8 | 16,377 | 26.4 | 252 | 83.2 |
| Zacco koreanus | I | SS | N | 6590 | 14.4 | 1884 | 11.5 | 8474 | 13.6 | 137 | 45.2 |
| Carassius auratus | O | TS | N | 2721 | 6.0 | 1755 | 10.7 | 4476 | 7.2 | 183 | 60.4 |
| Acheilognathus lanceolatus | O | IS | N | 2214 | 4.8 | 148 | 0.9 | 2362 | 3.8 | 147 | 48.5 |
| Rhinogobius brunneus | I | IS | N | 1802 | 3.9 | 427 | 2.6 | 2229 | 3.6 | 199 | 65.7 |
| Pungtungia herzi | I | IS | N | 1779 | 3.9 | 385 | 2.3 | 2164 | 3.5 | 152 | 50.2 |
| Pseudogobio esocinus | I | IS | N | 1706 | 3.7 | 330 | 2.0 | 2036 | 3.3 | 184 | 60.7 |
| Pseudorasbora parva | O | TS | N | 954 | 2.1 | 572 | 3.5 | 1526 | 2.5 | 117 | 38.6 |
| Hemiculter eigenmanni | O | TS | N | 610 | 1.3 | 873 | 5.3 | 1483 | 2.4 | 83 | 27.4 |
| Rhynchocypris oxycephalus | I | SS | N | 1046 | 2.3 | 292 | 1.8 | 1338 | 2.2 | 72 | 23.8 |
| Micropterus salmoides | C | TS | E | 801 | 1.8 | 493 | 3.0 | 1294 | 2.1 | 138 | 45.5 |
| Squalidus japonicus coreanus | O | TS | N | 793 | 1.7 | 490 | 3.0 | 1283 | 2.1 | 66 | 21.8 |
| Oryzias sinensis | O | TS | N | 786 | 1.7 | 397 | 2.4 | 1183 | 1.9 | 58 | 19.1 |
| Lepomis macrochirus | I | TS | E | 774 | 1.7 | 312 | 1.9 | 1086 | 1.7 | 70 | 23.1 |
| Iksookimia koreensis | I | IS | N | 707 | 1.5 | 323 | 2.0 | 1030 | 1.7 | 110 | 36.3 |
| Hemibarbus labeo | I | TS | N | 729 | 1.6 | 202 | 1.2 | 931 | 1.5 | 92 | 30.4 |
| Odontobutis interrupta | C | IS | N | 736 | 1.6 | 176 | 1.1 | 912 | 1.5 | 171 | 56.4 |
| Acheilognathus koreensis | O | IS | N | 739 | 1.6 | 6 | 0.0 | 745 | 1.2 | 46 | 15.2 |
| Coreoleuciscus splendidus | I | SS | N | 665 | 1.5 | 28 | 0.2 | 693 | 1.1 | 56 | 18.5 |
| Zacco temminckii | I | SS | N | 23 | 0.1 | 665 | 4.0 | 688 | 1.1 | 10 | 3.3 |
| Opsarichthys uncirostris amurensis | C | TS | N | 486 | 1.1 | 122 | 0.7 | 608 | 1.0 | 90 | 29.7 |
| Squalidus gracilis majimae | I | IS | N | 272 | 0.6 | 279 | 1.7 | 551 | 0.9 | 55 | 18.2 |
| Rhodeus notatus | O | IS | N | 369 | 0.8 | 166 | 1.0 | 535 | 0.9 | 53 | 17.5 |
| Misgurnus anguillicaudatus | O | TS | N | 367 | 0.8 | 163 | 1.0 | 530 | 0.9 | 116 | 38.3 |
| Squaliobarbus curriculus | O | IS | N | 456 | 1.0 | 27 | 0.2 | 483 | 0.8 | 44 | 14.5 |
| Hemibarbus longirostris | I | IS | N | 421 | 0.9 | 42 | 0.3 | 463 | 0.7 | 95 | 31.4 |
| Acanthorhodeus macropterus | O | IS | N | 361 | 0.8 | 85 | 0.5 | 446 | 0.7 | 43 | 14.2 |
| Tridentiger brevispinis | I | IS | N | 389 | 0.9 | 48 | 0.3 | 437 | 0.7 | 60 | 19.8 |
| Acanthorhodeus gracilis | O | IS | N | 218 | 0.5 | 213 | 1.3 | 431 | 0.7 | 54 | 17.8 |
| Microphysogobio yaluensis | O | IS | N | 392 | 0.9 | 23 | 0.1 | 415 | 0.7 | 74 | 24.4 |
| Rhodeus uyekii | O | IS | N | 289 | 0.6 | 115 | 0.7 | 404 | 0.7 | 53 | 17.5 |
| Pseudopungtungia nigra | I | SS | N | 332 | 0.7 | 332 | 0.5 | 25 | 8.3 | ||
| Odontobutis platycephala | C | SS | N | 228 | 0.5 | 95 | 0.6 | 323 | 0.5 | 85 | 28.1 |
| Total number of species | 68 | 63 | 74 | ||||||||
| Total number of individuals | 45,677 | 16,444 | 62,121 | ||||||||
| Native vs. Invasive | Number of Sites | Constancy Value | Mean Dominance at Sites of Occurrence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ~0.1% n = 80 | 0.1~0.5% n = 128 | 0.5%~ n = 95 | Total Sites n = 303 | ~0.1% | 0.1~0.5% | 0.5%~ | Total Sites | ~0.1% | 0.1~0.5% | 0.5%~ | Total Sites | |
| Invasive carnivore species | ||||||||||||
| Micropterus salmoides | 62 | 64 | 12 | 138 | 77.5 | 50.0 | 12.6 | 45.5 | 9.92 | 8.62 | 5.61 | 9.1 |
| Native carnivore species | ||||||||||||
| Odontobutis interrupta | 39 | 84 | 48 | 171 | 48.8 | 65.6 | 50.5 | 56.4 | 4.97 | 5.52 | 7.26 | 5.9 |
| Opsarichthys uncirostris | 42 | 46 | 1 | 89 | 52.5 | 35.9 | 1.1 | 29.4 | 7.94 | 4.22 | 1.85 | 6.3 |
| Odontobutis platycephala | 4 | 41 | 40 | 85 | 5.0 | 32.0 | 42.1 | 28.1 | 1.37 | 2.79 | 3.84 | 3.2 |
| Erythroculter erythropterus | 31 | 5 | 0 | 36 | 38.8 | 3.9 | 0.0 | 11.9 | 8.64 | 2.86 | NA | 7.8 |
| Pseudobagrus fulvidraco | 29 | 35 | 6 | 70 | 36.3 | 27.3 | 6.3 | 23.1 | 2.97 | 2.52 | 2.79 | 2.8 |
| Coreoperca herzi | 3 | 27 | 20 | 50 | 3.8 | 21.1 | 21.1 | 16.5 | 3.25 | 3.10 | 2.85 | 3.0 |
| Silurus asotus | 12 | 19 | 7 | 38 | 15.0 | 14.8 | 7.4 | 12.5 | 2.12 | 2.44 | 2.90 | 2.4 |
| Channa argus | 9 | 7 | 3 | 19 | 11.3 | 5.5 | 3.2 | 6.3 | 3.81 | 2.57 | 0.85 | 2.9 |
| Siniperca scherzeri | 2 | 12 | 1 | 15 | 2.5 | 9.4 | 1.1 | 5.0 | 0.67 | 1.50 | 3.33 | 1.5 |
| Monopterus albus | 5 | 9 | 3 | 17 | 6.3 | 7.0 | 3.2 | 5.6 | 1.90 | 1.26 | 1.58 | 1.5 |
| Anguilla japonicai | 1 | 0 | 0 | 1 | 1.3 | 0.0 | 0.0 | 0.3 | 0.85 | NA | NA | 0.9 |
| Category | Metrics | Scoring Criteria | Bass Present | Bass Absent | df | Significance Level (t-Test) | ||
|---|---|---|---|---|---|---|---|---|
| 5 | 3 | 1 | ||||||
| Species richness and composition | M1. Total number of native fish species | Metric score vary with stream order | 3.2 (1.5) | 3.9 (1.3) | 661 | ** | ||
| M2. Number of riffle benthic dwelling species | Metric score vary with stream order | 1.5 (1.1) | 1.6 (1.2) | 510.3 | NS | |||
| M3. Number of sensitive species | Metric score vary with stream order | 1.1 (0.4) | 1.9 (1.3) | 548.6 | ** | |||
| M4. Proportion of individual as tolerant species | <5% | 5~20% | >20% | 3.6 (1.5) | 4.3 (1.4) | 423.2 | ** | |
| Trophic composition | M5. Proportion of individuals as tolerant species | <20% | 20~45% | >45% | 2.2 (1.3) | 3.0 1.6) | 580.9 | ** |
| M6. Proportion of individuals as native insectivore species | >45% | 20~45% | <20% | 2.5 (1.5) | 3.4 (1.7) | 521.2 | ** | |
| Fish abundance and condition | M7. Total number of native individuals | Metric score vary with stream order | 2.6 (1.7) | 3.4 (1.6) | 661 | ** | ||
| M8. Proportion of individuals as abnormalities | 0 | 0~1 | >1 | 4.6 (1.0) | 4.8 (0.9) | 406.4 | NS | |
| Total scores | 21.4 (4.9) | 26.3 (6.2) | 572.9 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-J.; Atique, U.; An, K.-G. Key Drivers Influencing the Presence and Absence of Micropterus salmoides and Their Effect on Native Fish Communities and Biotic Integrity. Water 2021, 13, 3430. https://doi.org/10.3390/w13233430
Kim J-J, Atique U, An K-G. Key Drivers Influencing the Presence and Absence of Micropterus salmoides and Their Effect on Native Fish Communities and Biotic Integrity. Water. 2021; 13(23):3430. https://doi.org/10.3390/w13233430
Chicago/Turabian StyleKim, Jung-Jae, Usman Atique, and Kwang-Guk An. 2021. "Key Drivers Influencing the Presence and Absence of Micropterus salmoides and Their Effect on Native Fish Communities and Biotic Integrity" Water 13, no. 23: 3430. https://doi.org/10.3390/w13233430







