Calcium Carbonate Scale Inhibition with Ultrasonication and a Commercial Antiscalant
Abstract
:1. Introduction
2. Experiments
2.1. Reagents and Instruments
2.2. Saturated Calcium Carbonate Solution
2.3. Application of Ultrasonic Radiation and Study of Effect of Ultrasonic Amplitude
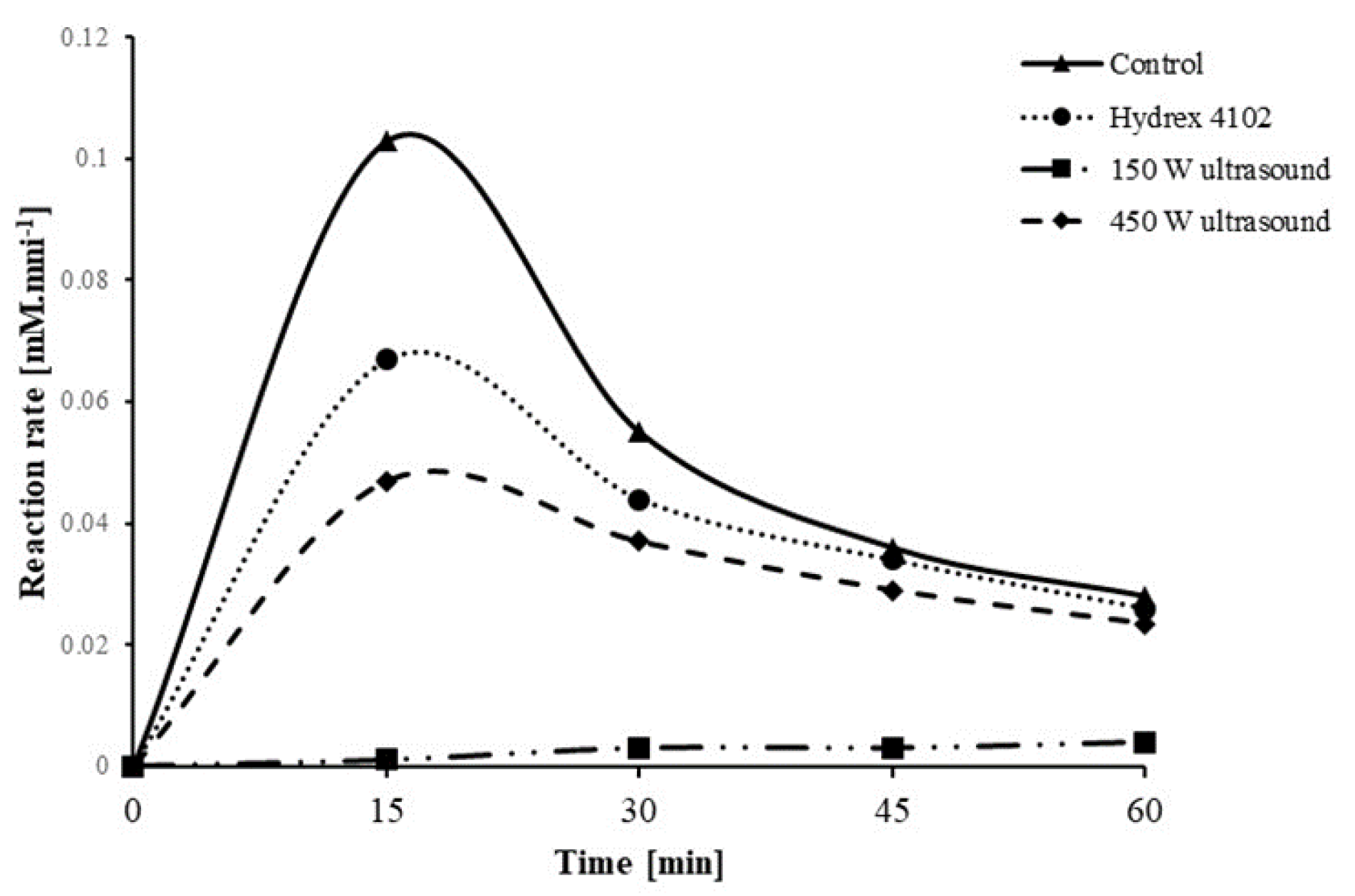
3. Results and Discussions
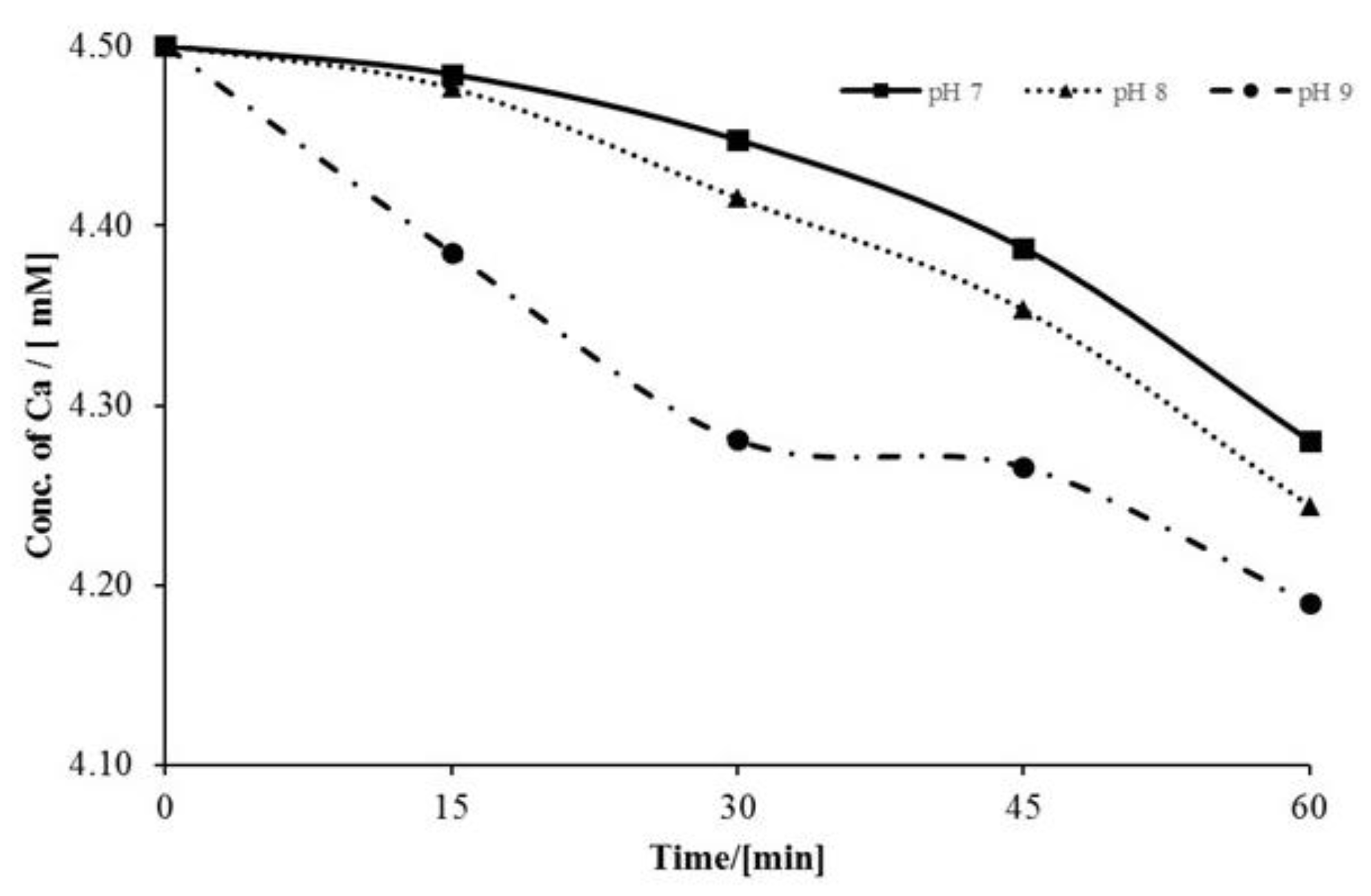
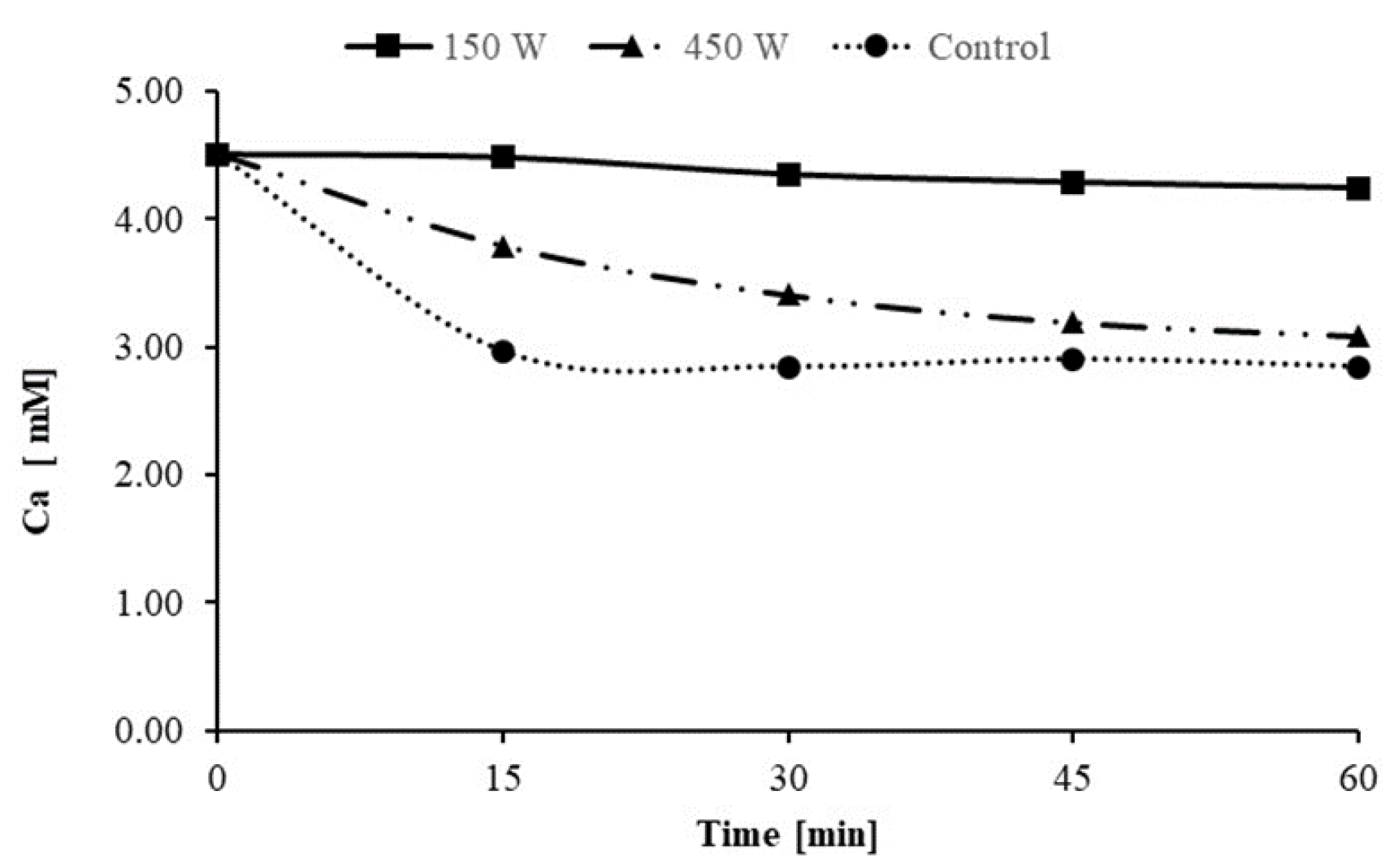
3.1. Factors Affecting Scale Inhibition
3.2. Characterization of Scale Deposits
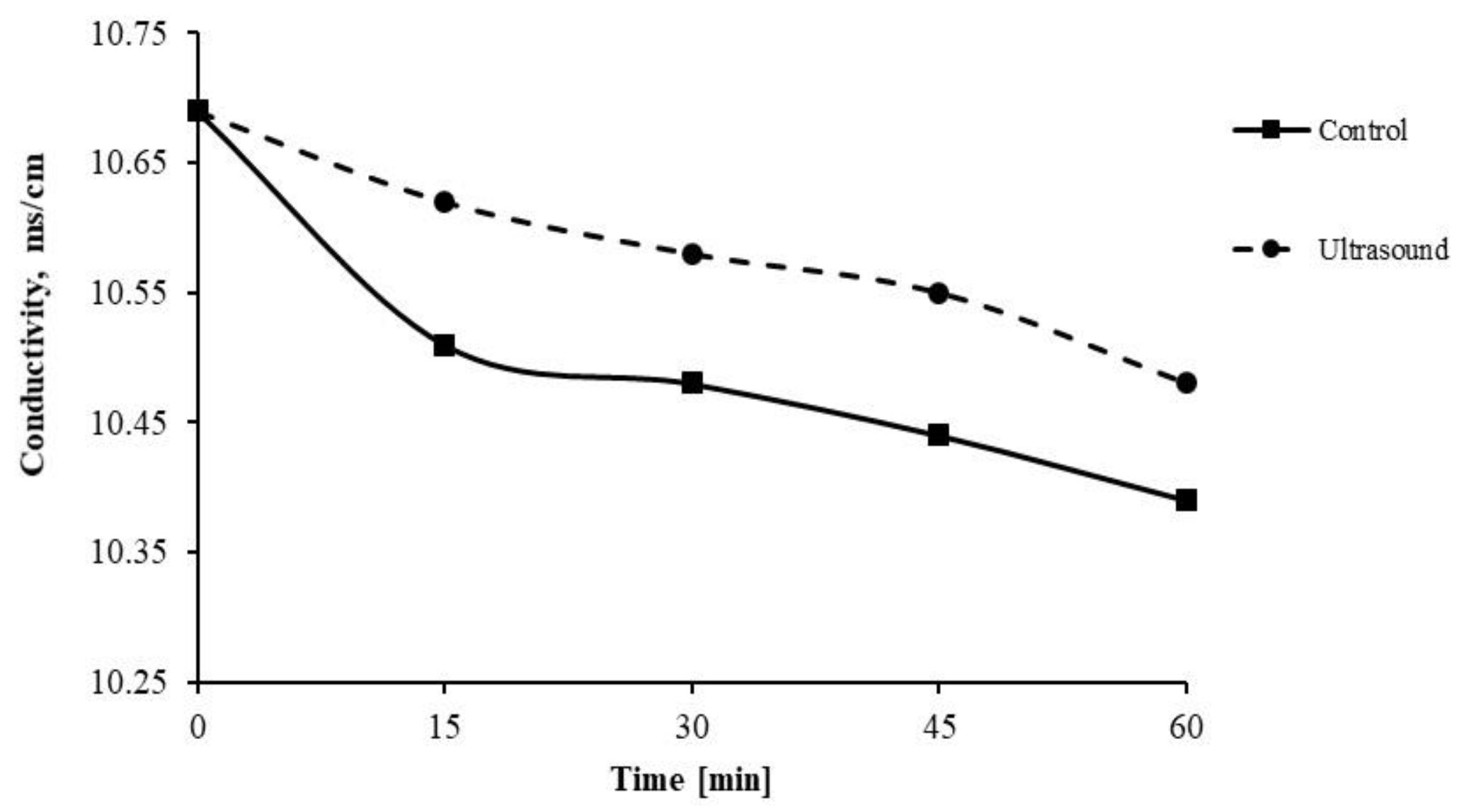
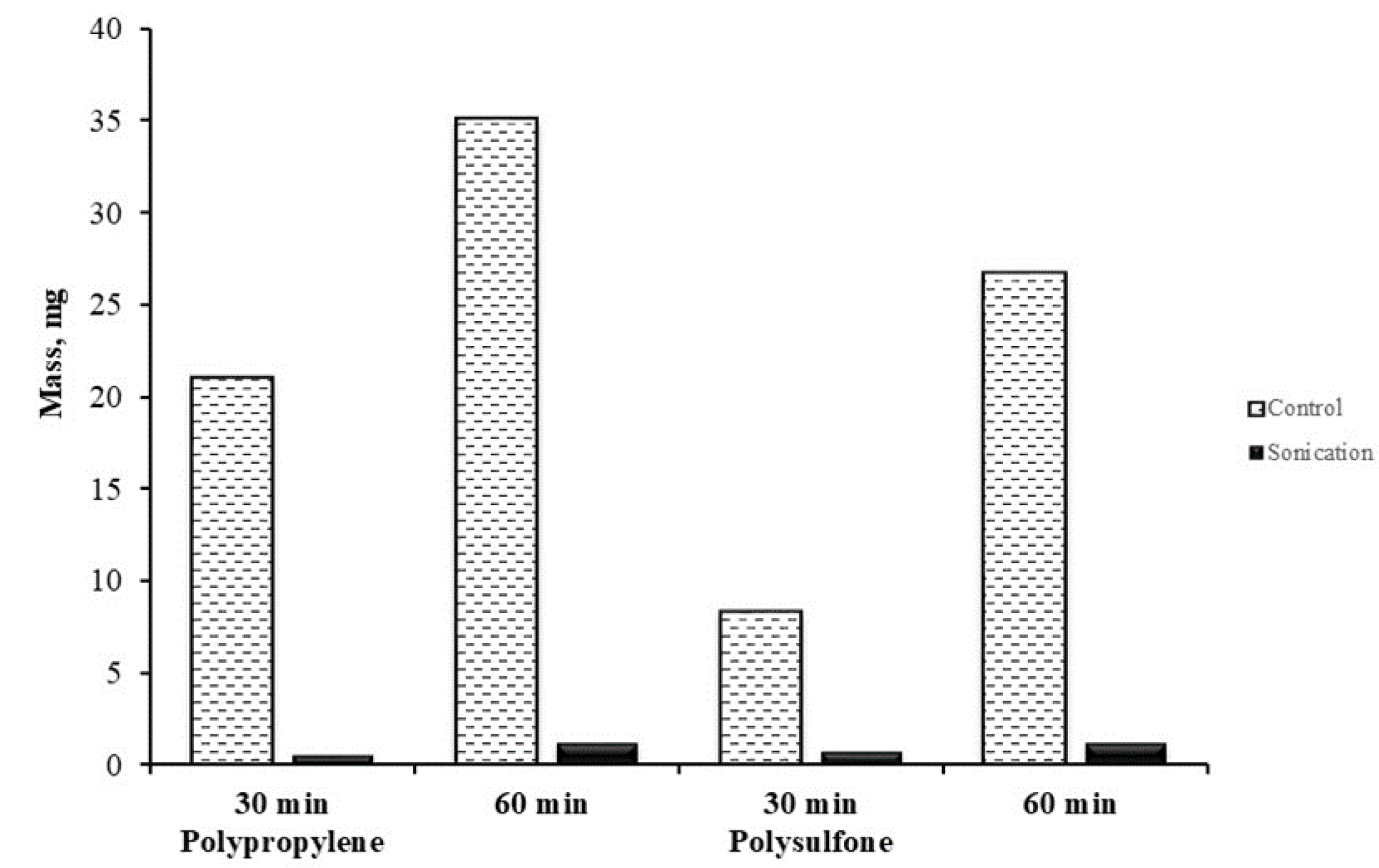
3.2.1. Membrane Scale Deposit Measurements
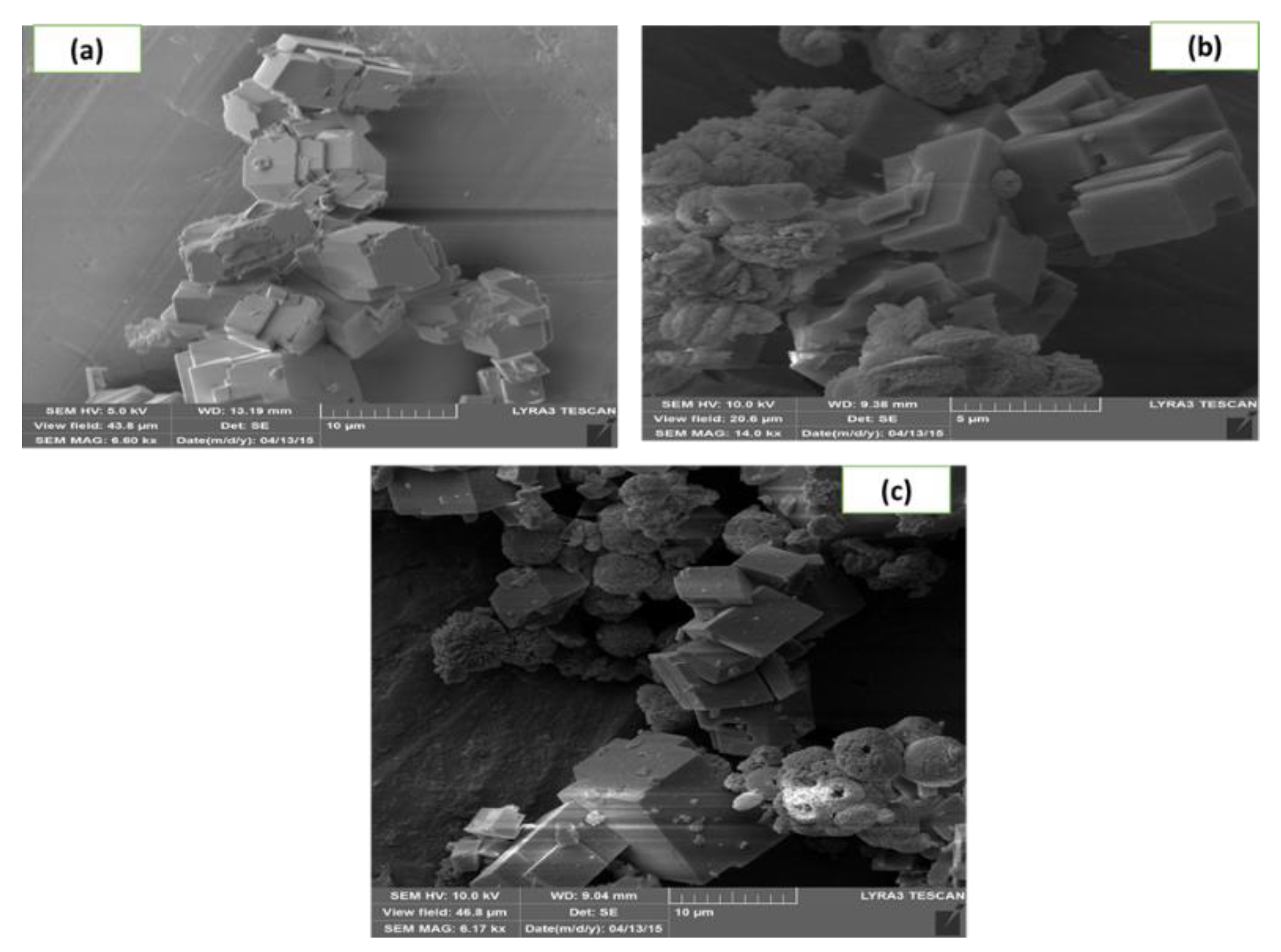
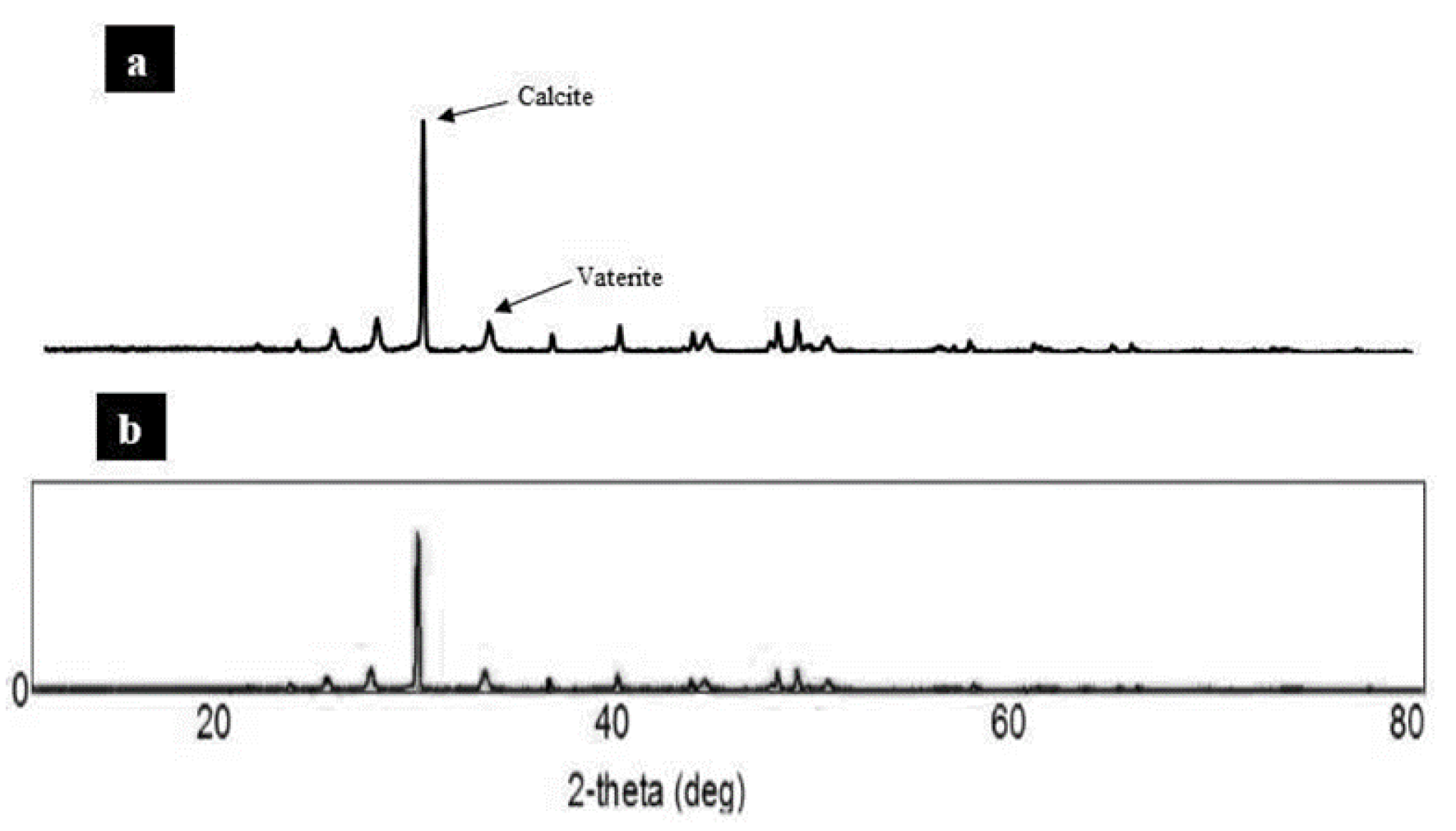
3.2.2. SEM Micrograph and XRD Analysis
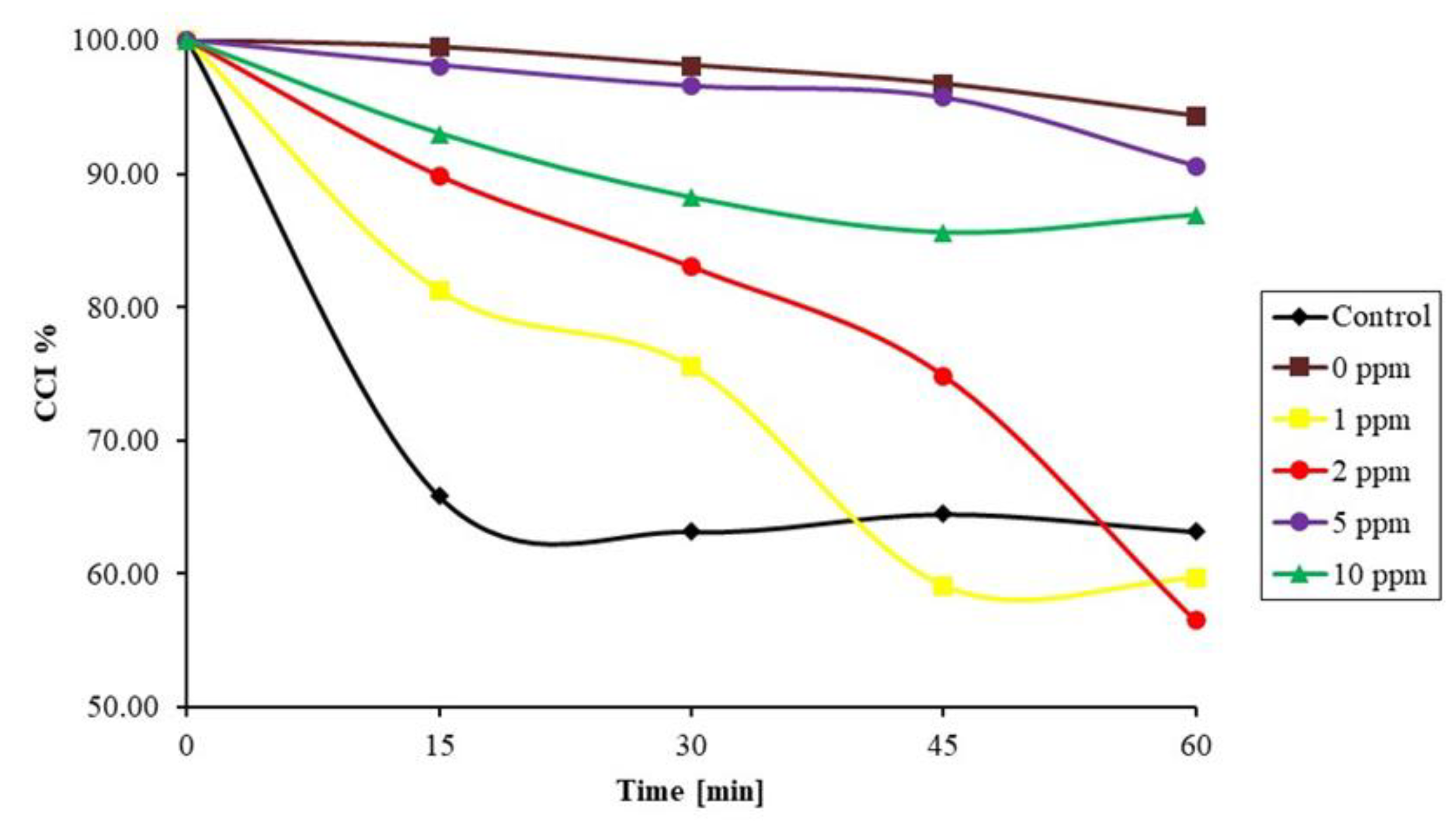
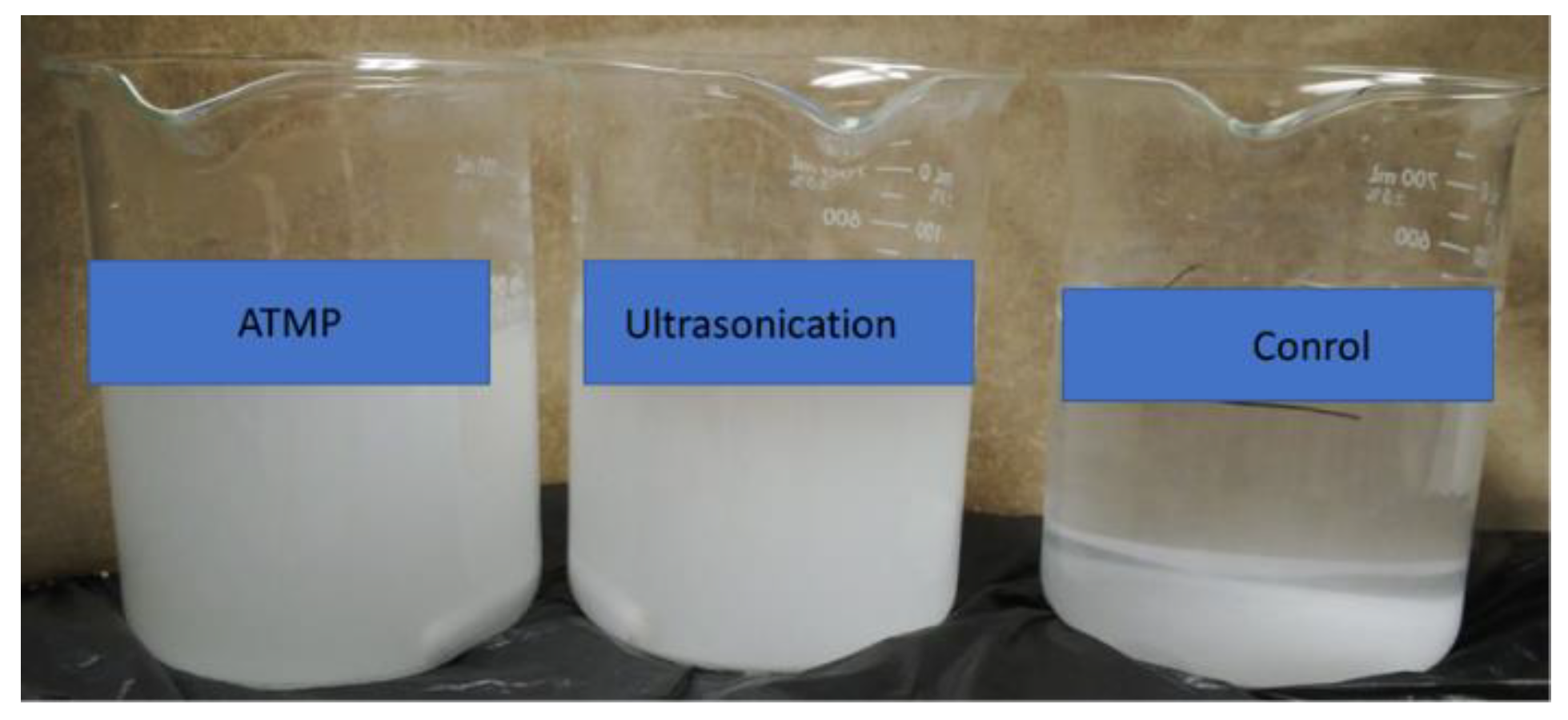
3.3. Comparison of Ultrasound with Commercial Antiscalants
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zarga, Y.; Boubaker, H.B.; Ghaffour, N.; Elfil, H. Study of calcium carbonate and sulfate co-precipitation. Chem. Eng. Sci. 2013, 96, 33–41. [Google Scholar] [CrossRef]
- Younes, A.A.; El-Maghrabi, H.H.; Ali, H.R. Novel polyacrylamide-based solid scale inhibitor. J. Hazard. Mater. 2017, 334, 1–9. [Google Scholar] [CrossRef]
- Antony, A.; Low, J.H.; Gray, S.; Childress, A.E.; Le-Clech, P.; Leslie, G. Scale formation and control in high-pressure membrane water treatment systems: A review. J. Memb. Sci. 2011, 383, 1–16. [Google Scholar] [CrossRef]
- Chen, T.; Neville, A.; Yuan, M. Calcium carbonate scale formation—assessing the initial stages of precipitation and deposition. J. Pet. Sci. Eng. 2005, 46, 185–194. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, H.; Starikovskiy, A.; Fridman, A.; Cho, Y.I. Application of pulsed spark discharge for calcium carbonate precipitation in hard water. Water Res. 2010, 44, 3659–3668. [Google Scholar] [CrossRef]
- Demadis, K.D.; Mavredaki, E.; Stathoulopoulou, A.; Neofotistou, E.; Mantzaridis, C. Industrial water systems: Problems, challenges, and solutions for the process industries. Desalination 2007, 213, 38–46. [Google Scholar] [CrossRef]
- Shen, Z.; Shi, J.; Zhang, S.; Fan, J.; Li, J. Effect of optimized three-component antiscalant mixture on calcium carbonate scale deposition. Water Sci. Technol. 2017, 75, 255–262. [Google Scholar] [CrossRef]
- Al Nasser, W.N.; Al-Salhi, F.H.; Hounslow, M.J.; Salman, A.D. Inline monitoring the effect of chemical inhibitor on the calcium carbonate precipitation and agglomeration. Chem. Eng. Res. Des. 2011, 89, 500–511. [Google Scholar] [CrossRef]
- Siva, T.; Muralidharan, S.; Sathiyanarayanan, S.; Manikandan, E.; Jayachandran, M. Enhanced Polymer Induced Precipitation of Polymorphous in Calcium Carbonate: Calcite Aragonite Vaterite Phases. J. Inorg. Organomet. Polym. Mater. 2017, 27, 770–778. [Google Scholar] [CrossRef]
- Al-Hamzah, A.A.; East, C.P.; Doherty, W.O.S.; Fellows, C.M. Inhibition of homogenous formation of calcium carbonate by poly (acrylic acid). The effect of molar mass and end-group functionality. Desalination 2014, 338, 93–105. [Google Scholar] [CrossRef] [Green Version]
- de Leeuw, N.H.; Parker, S.C. Surface Structure and Morphology of Calcium Carbonate Polymorphs Calcite, Aragonite, and Vaterite: An Atomistic Approach. J. Phys. Chem. B. 1998, 102, 2914–2922. [Google Scholar] [CrossRef]
- Gerdts, C.J.; Tereshko, V.; Yadav, M.K.; Dementieva, I.; Collart, F.; Joachimiak, A.; Stevens, R.C.; Kuhn, P.; Kossiakoff, A.; Ismagilov, R.F. Time-Controlled Microfluidic Seeding in nL-Volume Droplets To Separate Nucleation and Growth Stages of Protein Crystallization. Angew. Chem. 2006, 118, 8336–8340. [Google Scholar] [CrossRef]
- Lindfors, L.; Forssén, S.; Westergren, J.; Olsson, U. Nucleation and crystal growth in supersaturated solutions of a model drug. J. Colloid Interface Sci. 2008, 325, 404–413. [Google Scholar] [CrossRef]
- Szcześ, A. Influence of SDS on particle size and adhesion of precipitating calcium carbonate. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 320, 98–103. [Google Scholar] [CrossRef]
- Wu, Z.; Davidson, J.H.; Francis, L.F. Effect of water chemistry on calcium carbonate deposition on metal and polymer surfaces. J. Colloid Interface Sci. 2010, 343, 176–187. [Google Scholar] [CrossRef]
- Venkatesan, A.; Wankat, P.C. Simulation of ion exchange water softening pretreatment for reverse osmosis desalination of brackish water. Desalination 2011, 271, 122–131. [Google Scholar] [CrossRef]
- Liu, D.; Dong, W.; Li, F.; Hui, F.; Lédion, J. Comparative performance of polyepoxysuccinic acid and polyaspartic acid on scaling inhibition by static and rapid controlled precipitation methods. Desalination 2012, 304, 1–10. [Google Scholar] [CrossRef]
- Alabi, A.; Chiesa, M.; Garlisi, C.; Palmisano, G. Advances in anti-scale magnetic water treatment. Environ. Sci. Water Res. Technol. 2015, 1, 408–425. [Google Scholar] [CrossRef]
- Ou, H.-H.; Hsieh, L.-H.C. A synergistic effect of sodium gluconate and 2-phosphonobutane-1,2,4-tricarboxylic acid on the inhibition of CaCO3 scaling formation. Powder Technol. 2016, 302, 160–167. [Google Scholar] [CrossRef]
- Tran, H.V.; Tran, L.D.; Vu, H.D.; Thai, H. Facile surface modification of nanoprecipitated calcium carbonate by adsorption of sodium stearate in aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 366, 95–103. [Google Scholar] [CrossRef]
- Tijing, L.D.; Lee, D.-H.; Kim, D.-W.; Cho, Y.I.; Kim, C.S. Effect of high-frequency electric fields on calcium carbonate scaling. Desalination 2011, 279, 47–53. [Google Scholar] [CrossRef]
- Lipus, L.C.; Ačko, B.; Hamler, A. Electromagnets for high-flow water processing. Chem. Eng. Process. Process Intensif. 2011, 50, 952–958. [Google Scholar] [CrossRef]
- MacAdam, J.; Parsons, S.A. Calcium carbonate scale formation and control. Rev. Environ. Sci. Bio./Technol. 2004, 3, 159–169. [Google Scholar] [CrossRef]
- Liu, G.; Xue, M.; Liu, Q.; Yang, H.; Yang, J.; Zhou, Y. Maleic anhydride–allylpolyethoxy carboxylate copolymer as an effective and environmentally benign inhibitor for calcium carbonate in industrial cooling systems. RSC Adv. 2017, 7, 24723–24729. [Google Scholar] [CrossRef] [Green Version]
- Shen, Z.; Li, J.; Xu, K.; Ding, L.; Ren, H. The effect of synthesized hydrolyzed polymaleic anhydride (HPMA) on the crystal of calcium carbonate. Desalination 2012, 284, 238–244. [Google Scholar] [CrossRef]
- Bai, S.; Naren, G.; Nakano, M.; Okaue, Y.; Yokoyama, T. Effect of polysilicic acid on the precipitation of calcium carbonate. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 445, 54–58. [Google Scholar] [CrossRef]
- Sousa, M.F.B.; Bertran, C.A. New methodology based on static light scattering measurements for evaluation of inhibitors for in bulk CaCO3 crystallization. J. Colloid Interface Sci. 2014, 420, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, K.; Raj, K.S.; Bhuvaneswari, S.; Subramanian, V.K. A novel phenomenon of effect of metal on calcium carbonate scale, morphology, polymorphism and its deposition. Mater. Res. Innov. 2017, 21, 294–303. [Google Scholar] [CrossRef]
- Kim, S.; Wei, C.; Kiang, S. Crystallization Process Development of an Active Pharmaceutical Ingredient and Particle Engineering via the Use of Ultrasonics and Temperature Cycling. Org. Process Res. Dev. 2003, 7, 997–1001. [Google Scholar] [CrossRef]
- Su, M.; Han, J.; Li, Y.; Chen, J.; Zhao, Y.; Chadwick, K. Ultrasonic Crystallization of Calcium Carbonate in the presence of Seawater Ions. Desalination 2015, 369, 85–90. [Google Scholar] [CrossRef]
- Hu, A.; Zheng, J.; Qiu, T. Industrial experiments for the Application of ultrasound on scale control in the Chinese sugar industry. Ultrason. Sonochem. 2006, 13, 329–333. [Google Scholar] [CrossRef]
- Dalas, E. The effect of ultrasonic field on calcium carbonate scale formation. J. Cryst. Growth 2001, 222, 287–292. [Google Scholar] [CrossRef]
- Nishida, I. Precipitation of calcium carbonate by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 423–428. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.; Jipa, I.; Dobre, T.; Dobre, L. Ultrasound influence upon calcium carbonate precipitation on bacterial cellulose membranes. Ultrason. Sonochem. 2012, 19, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Kojima, Y.; Yamaguchi, K.; Nishimiya, N. Effect of amplitude and frequency of ultrasonic irradiation on morphological characteristics control of calcium carbonate. Ultrason. Sonochem. 2010, 17, 617–620. [Google Scholar] [CrossRef]
- Revelle, R. Physico-Chemical Factors Affecting the Solubility of Calcium Carbonate in Sea Water. SEPM J. Sediment. Res. 1934, 4, 103–110. [Google Scholar] [CrossRef]
- Shivkumara, C.; Singh, P.; Gupta, A.; Hegde, M.S. Synthesis of vaterite CaCO3 by direct precipitation using glycine and l-alanine as directing agents. Mater. Res. Bull. 2006, 41, 1455–1460. [Google Scholar] [CrossRef]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Crystallization and transformation of vaterite at controlled pH. J. Cryst. Growth 2006, 289, 269–274. [Google Scholar] [CrossRef]

| pH | [CO32−]/mM |
|---|---|
| 7 | 4.44 × 10−9 |
| 8 | 4.44 × 10−8 |
| 9 | 4.44 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basheer, C.; Shaikh, A.A.; Al-Mutairi, E.M.; Noor El Deen, M.; Qureshi, K.K. Calcium Carbonate Scale Inhibition with Ultrasonication and a Commercial Antiscalant. Water 2021, 13, 3428. https://doi.org/10.3390/w13233428
Basheer C, Shaikh AA, Al-Mutairi EM, Noor El Deen M, Qureshi KK. Calcium Carbonate Scale Inhibition with Ultrasonication and a Commercial Antiscalant. Water. 2021; 13(23):3428. https://doi.org/10.3390/w13233428
Chicago/Turabian StyleBasheer, Chanbasha, Amjad A. Shaikh, Eid M. Al-Mutairi, Mokhtar Noor El Deen, and Khurram Karim Qureshi. 2021. "Calcium Carbonate Scale Inhibition with Ultrasonication and a Commercial Antiscalant" Water 13, no. 23: 3428. https://doi.org/10.3390/w13233428






