An Experimental Study of Fluoride Removal from Wastewater by Mn-Ti Modified Zeolite
Abstract
:1. Introduction
2. Material Preparation and Experimental Methodology
2.1. Preparation of the Mn-Ti Modified Zeolite
2.1.1. Nano-Manganese Oxide Synthesis
2.1.2. Mn-Ti Modified Zeolite Preparation
2.2. Adsorbent Characterization
2.3. Experimental Methodology
2.3.1. Static Experiments
2.3.2. Dynamic Experiments Using an Adsorption Column
3. Results and Discussion
3.1. Material Characterization and Crystallinity
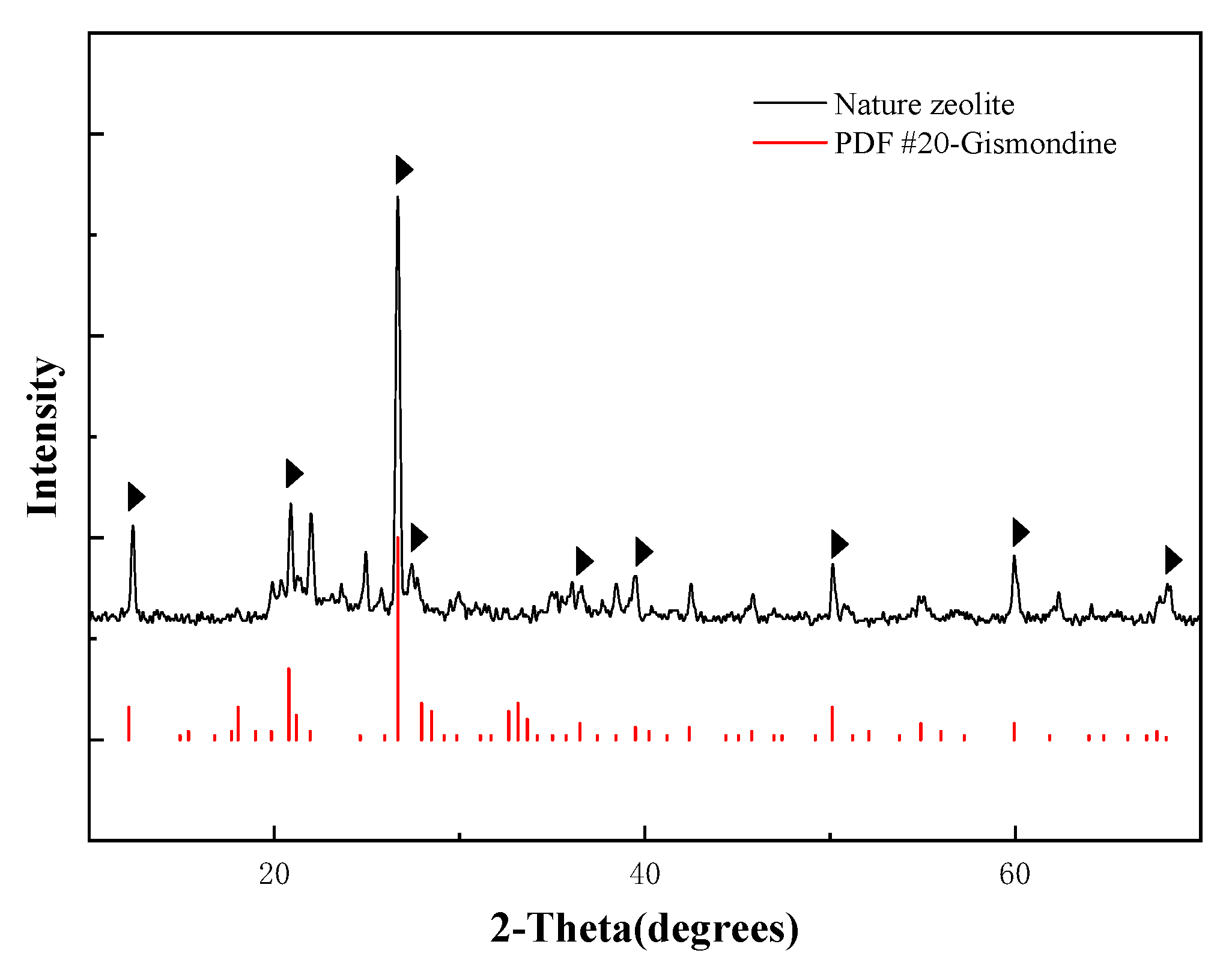
3.1.1. The Natural Zeolite Composition
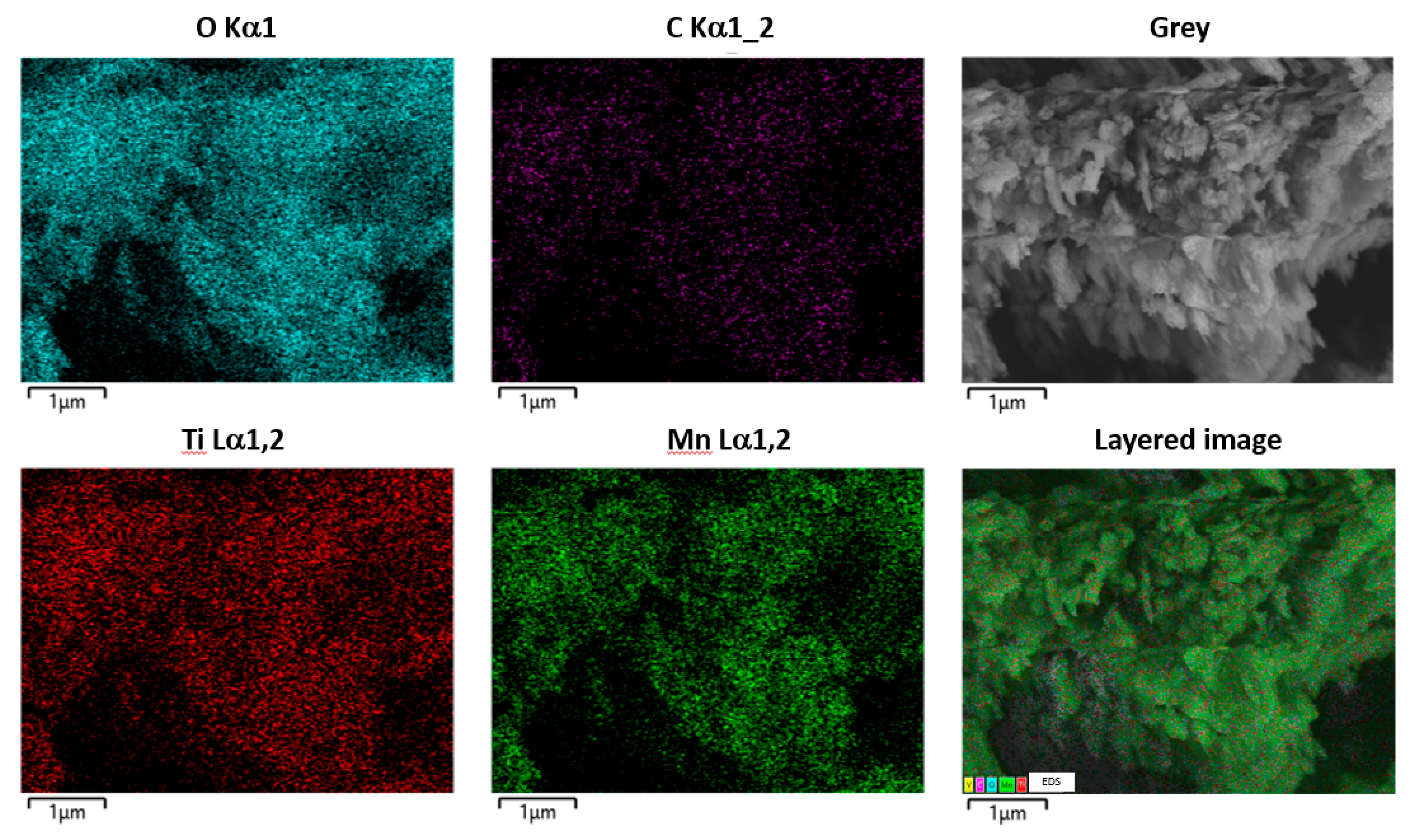
3.1.2. SEM and EDS Analyses of Physical and Chemical Properties
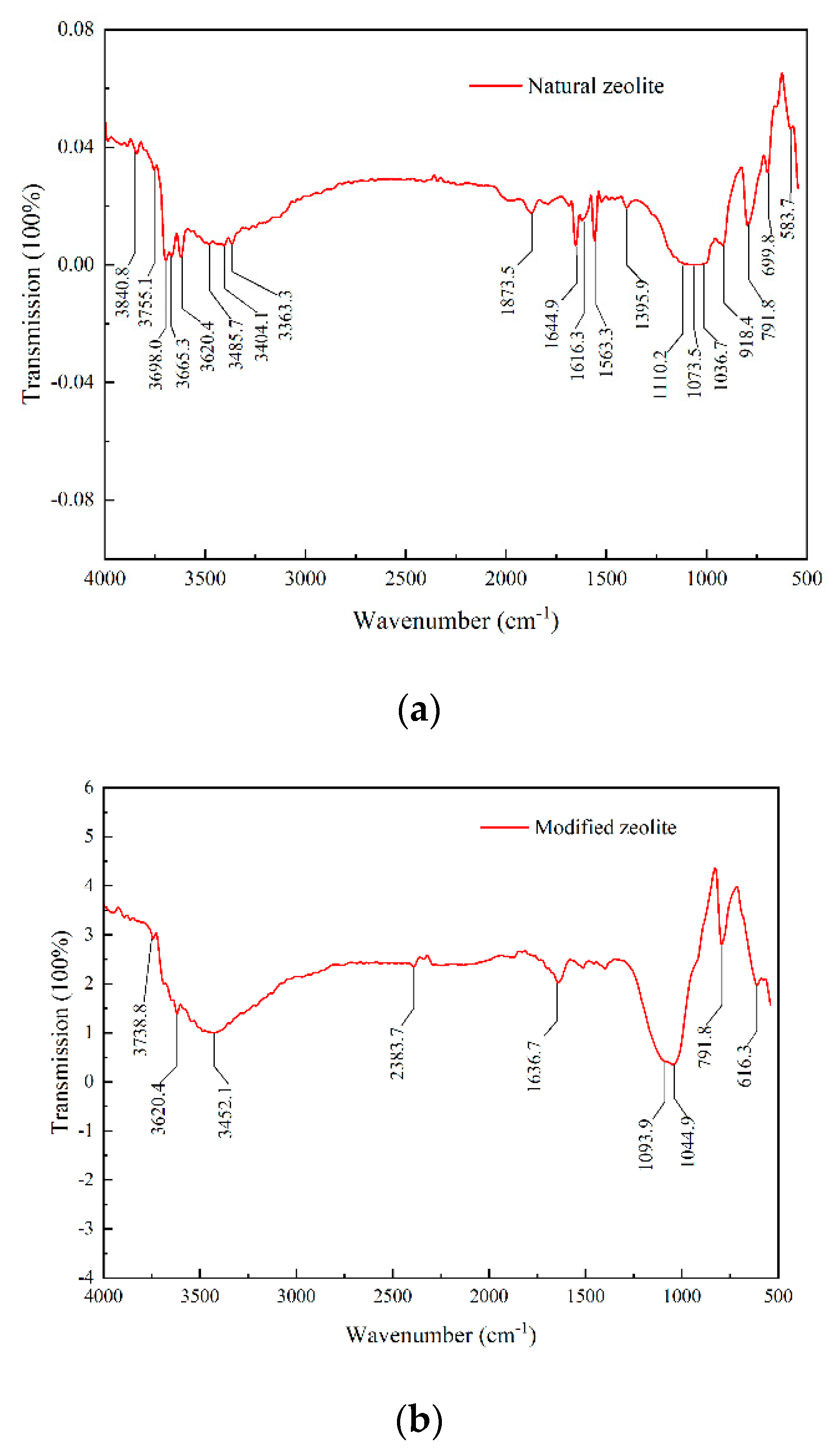
3.1.3. FTIR Analysis of Composition
3.2. Static Adsorption
3.2.1. Adsorbent Dosage
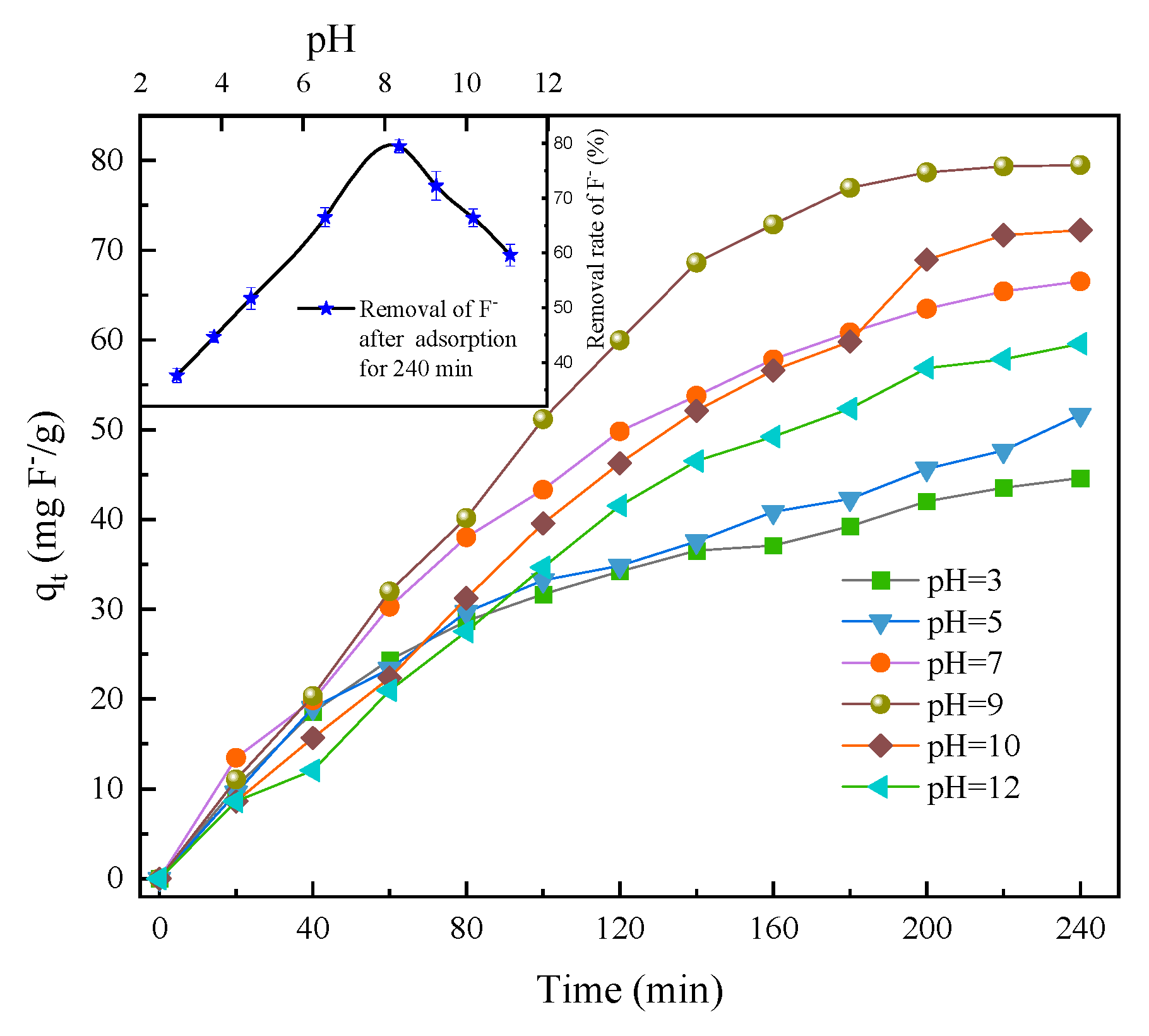
3.2.2. PH
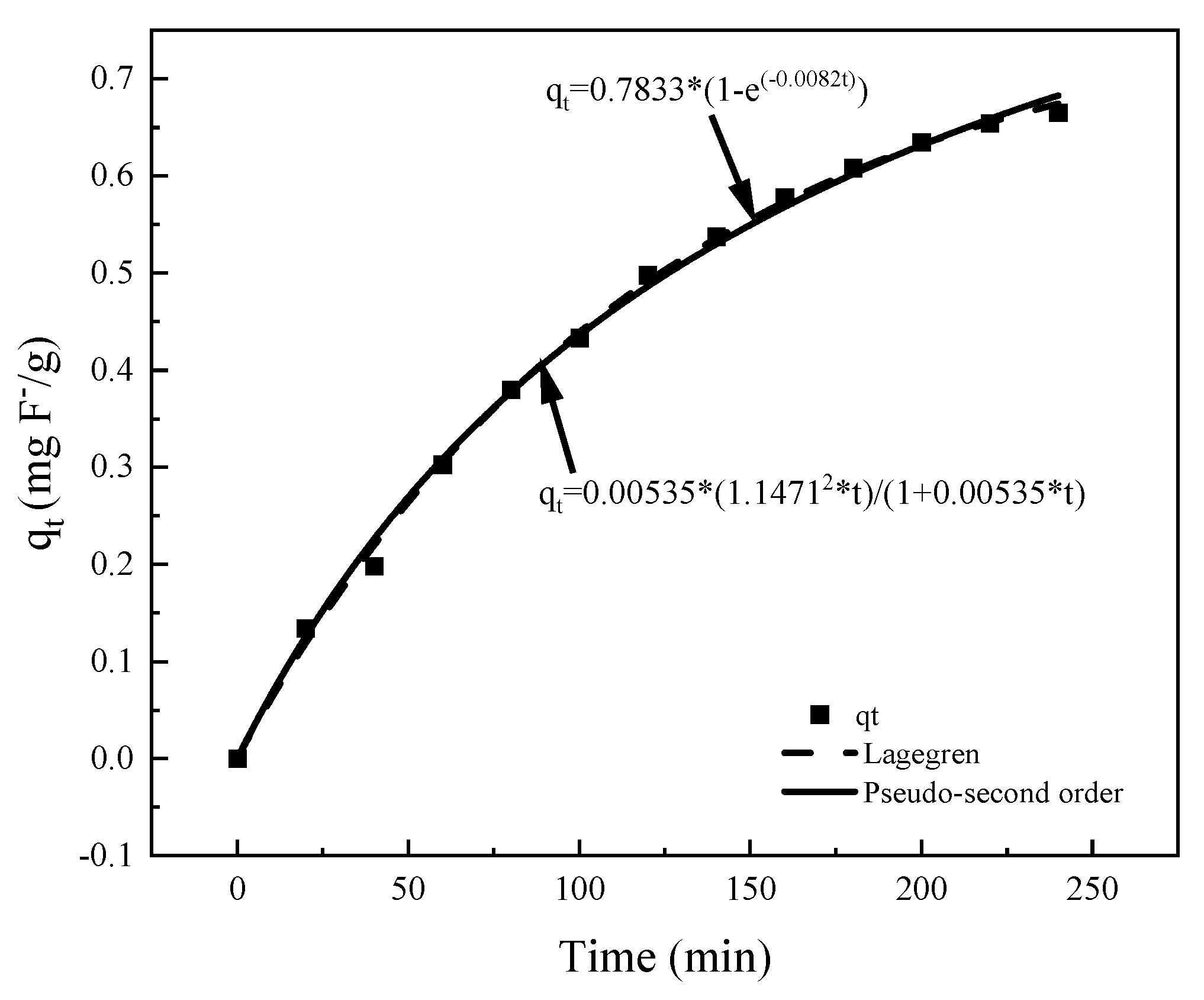
3.2.3. Adsorption Kinetics
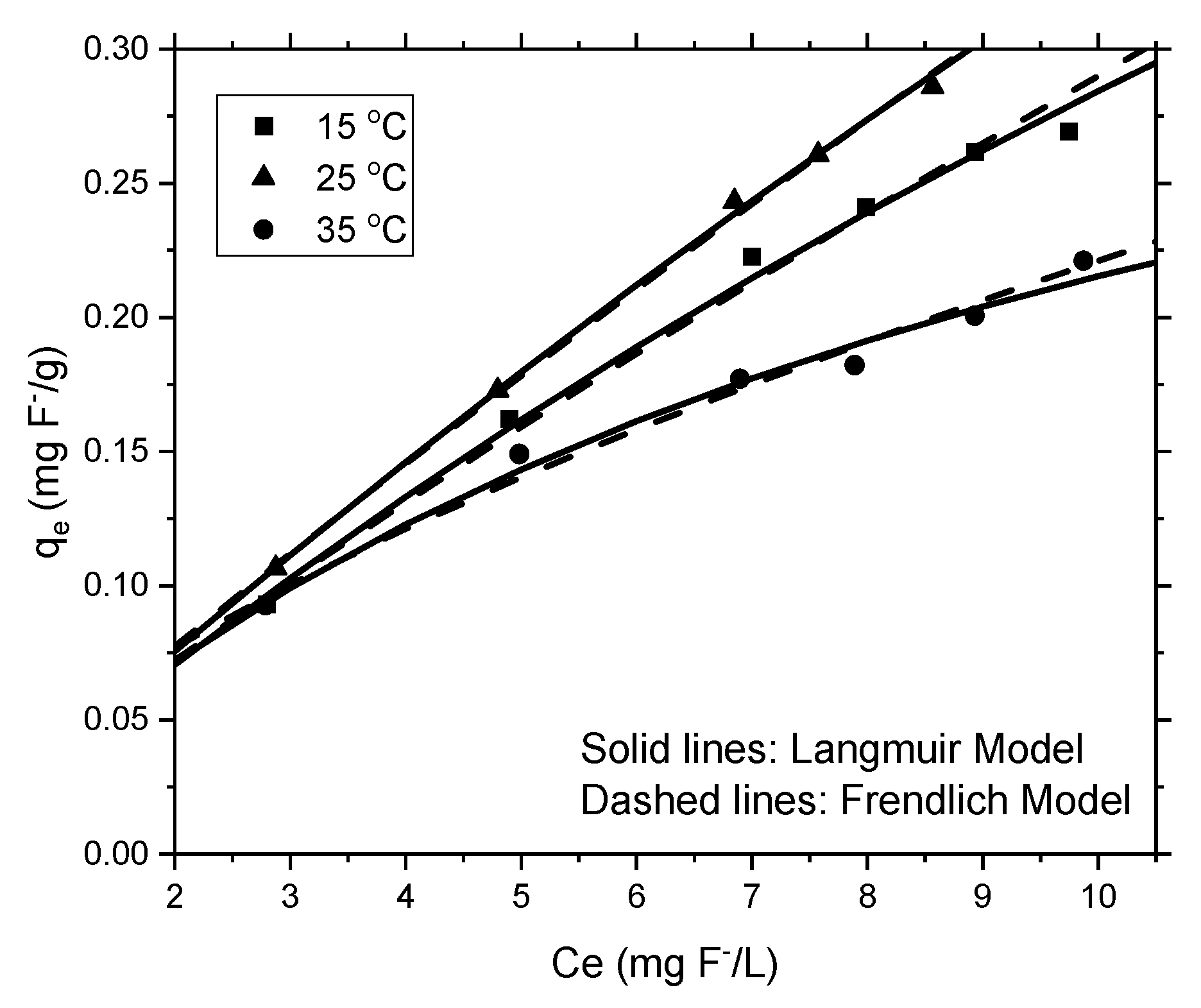
3.2.4. Adsorption Isotherms
3.2.5. Competitive Ions
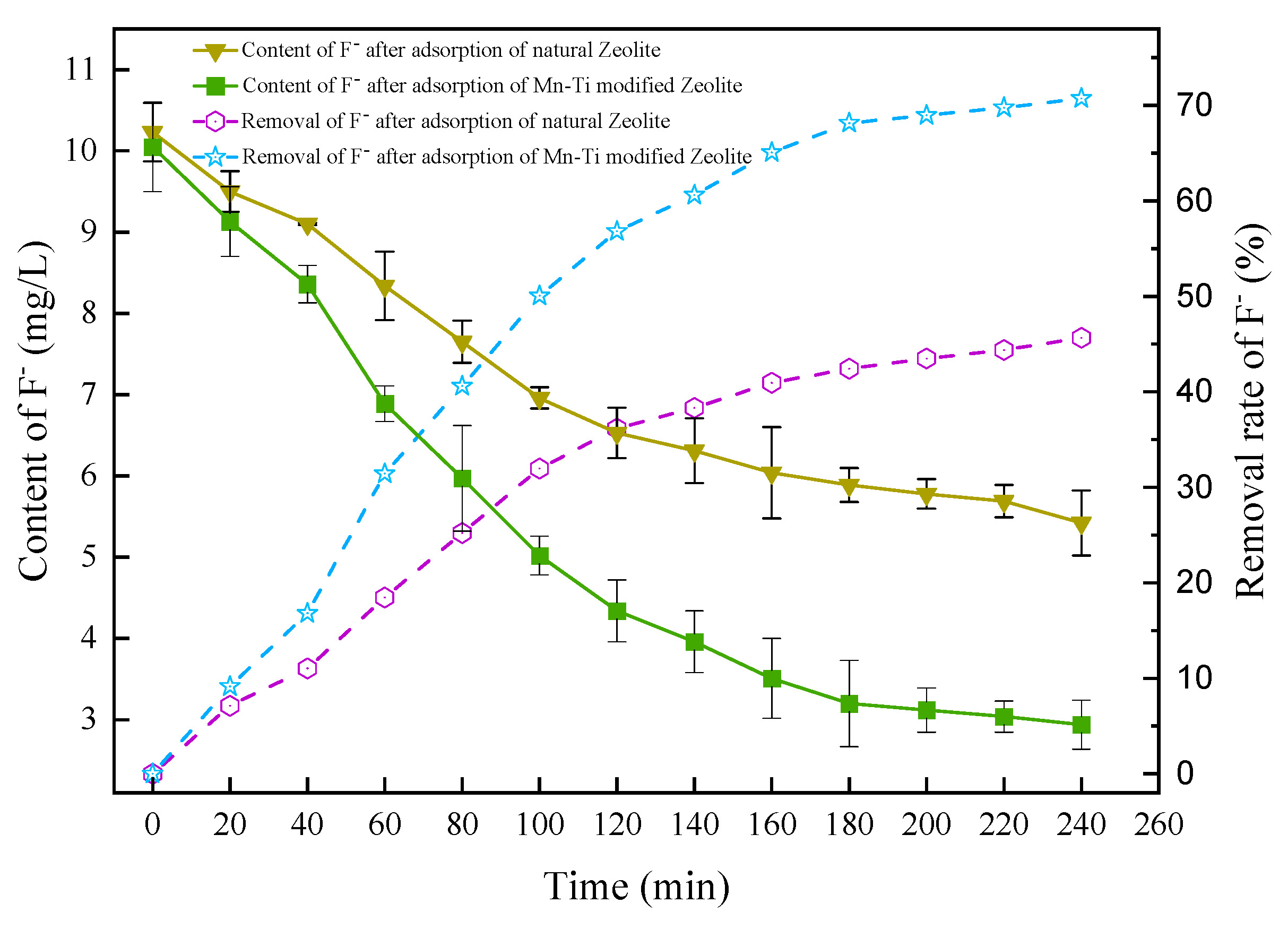
3.2.6. Comparison between Natural and Mn-Ti Modified Zeolites
3.2.7. Comparison with Studies in the Literature
3.3. Dynamic Adsorption
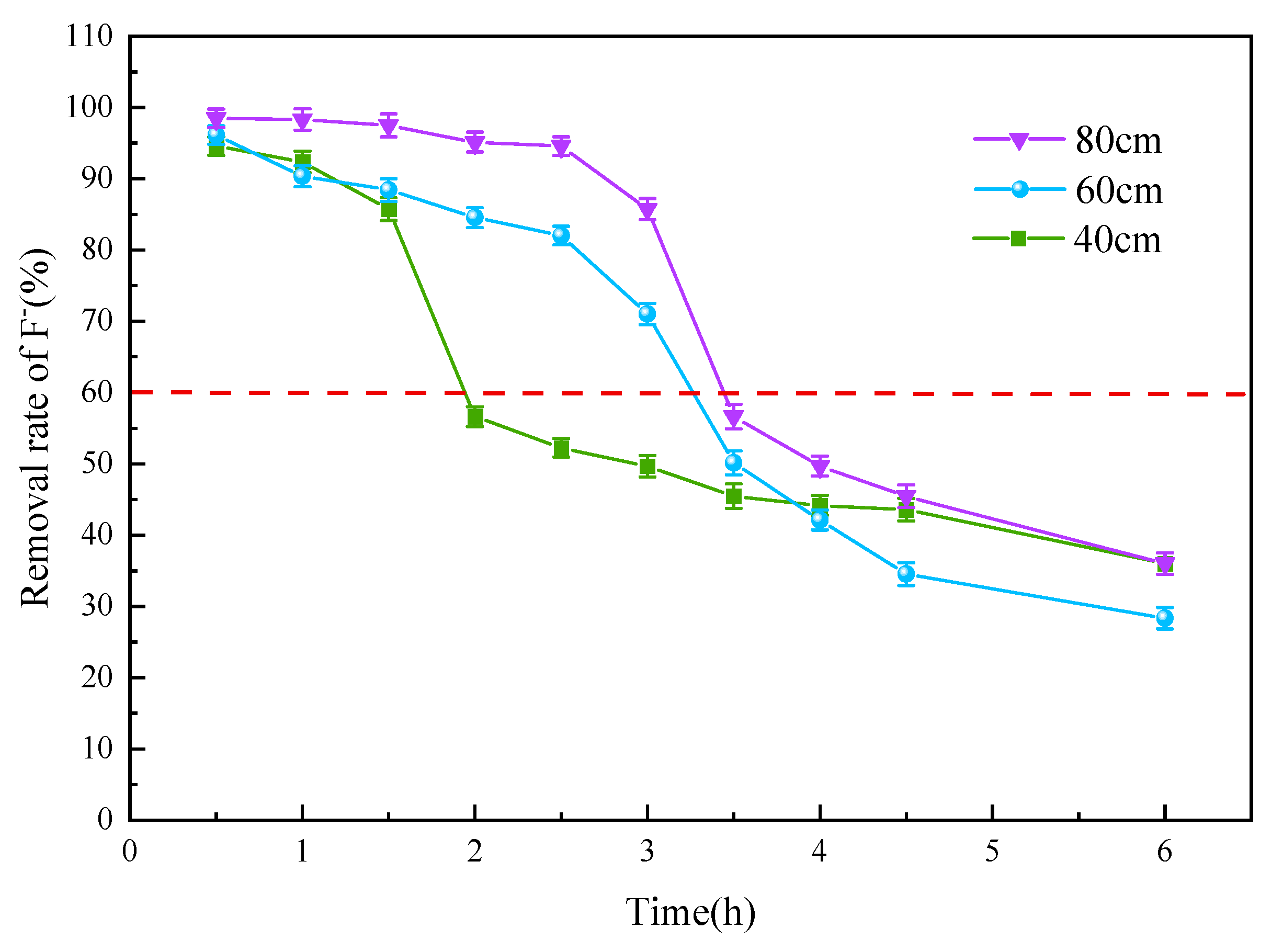
3.3.1. Adsorbent Thickness
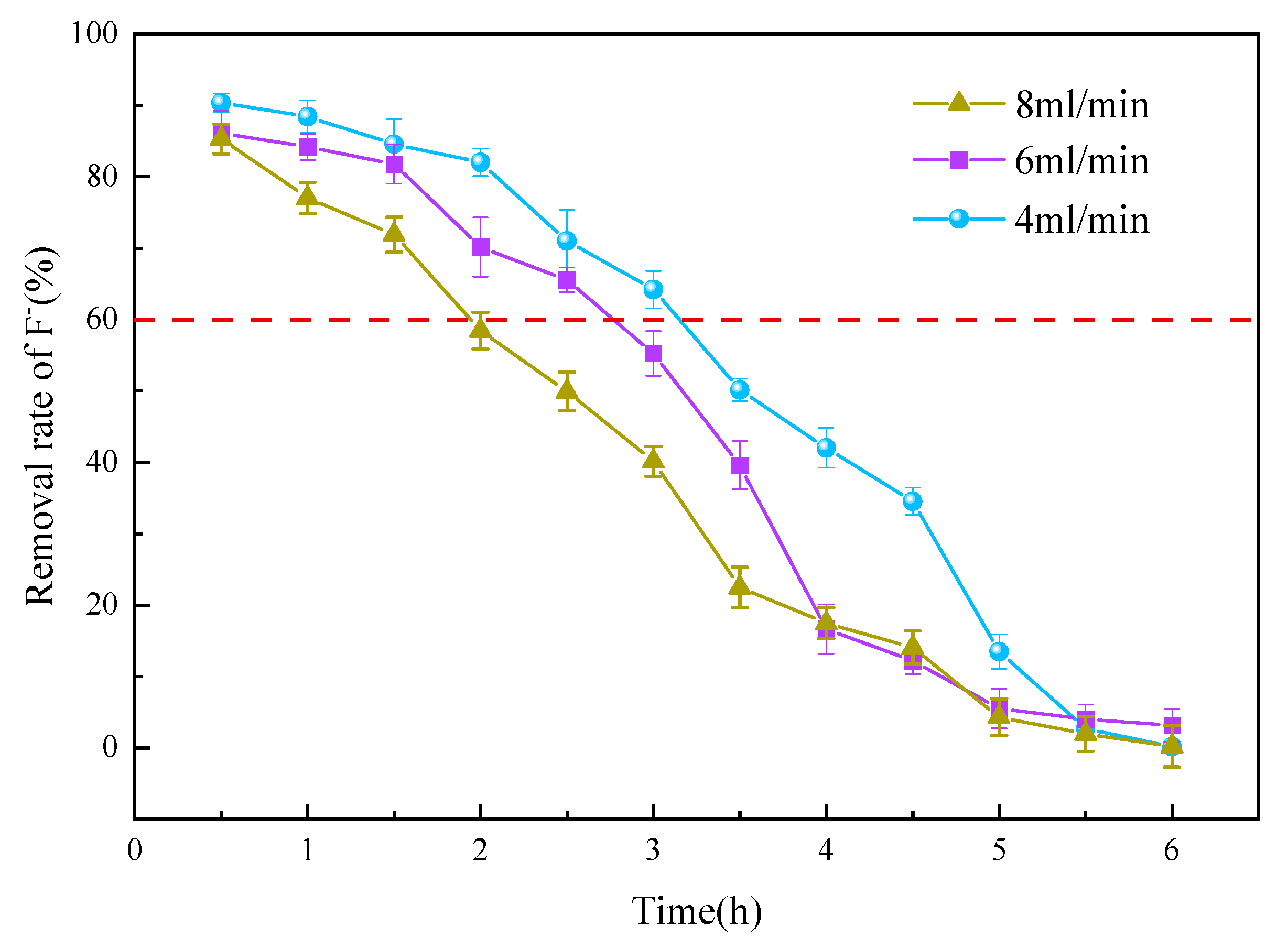
3.3.2. Flow Velocity
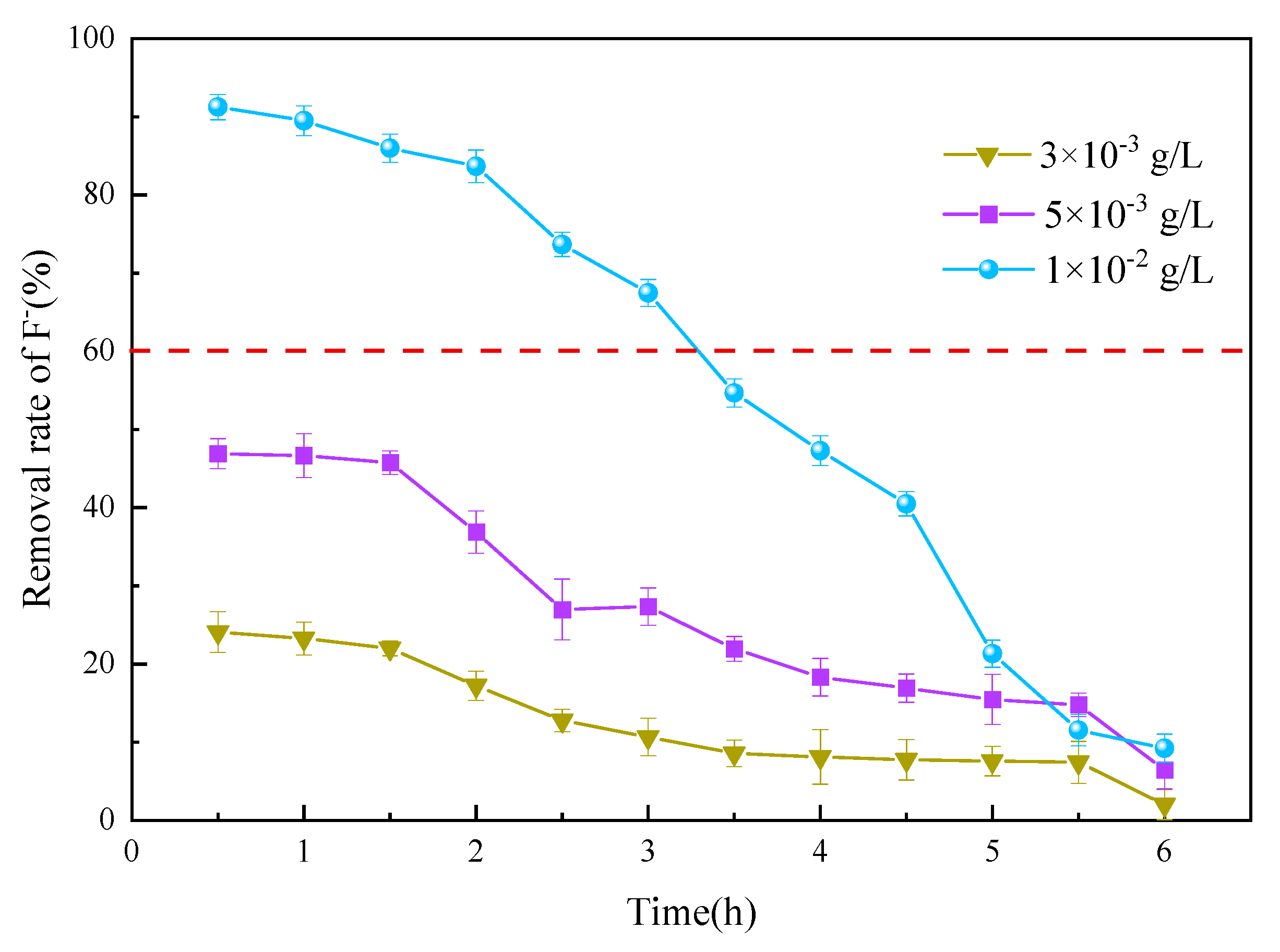
3.3.3. Initial Fluoride Concentration
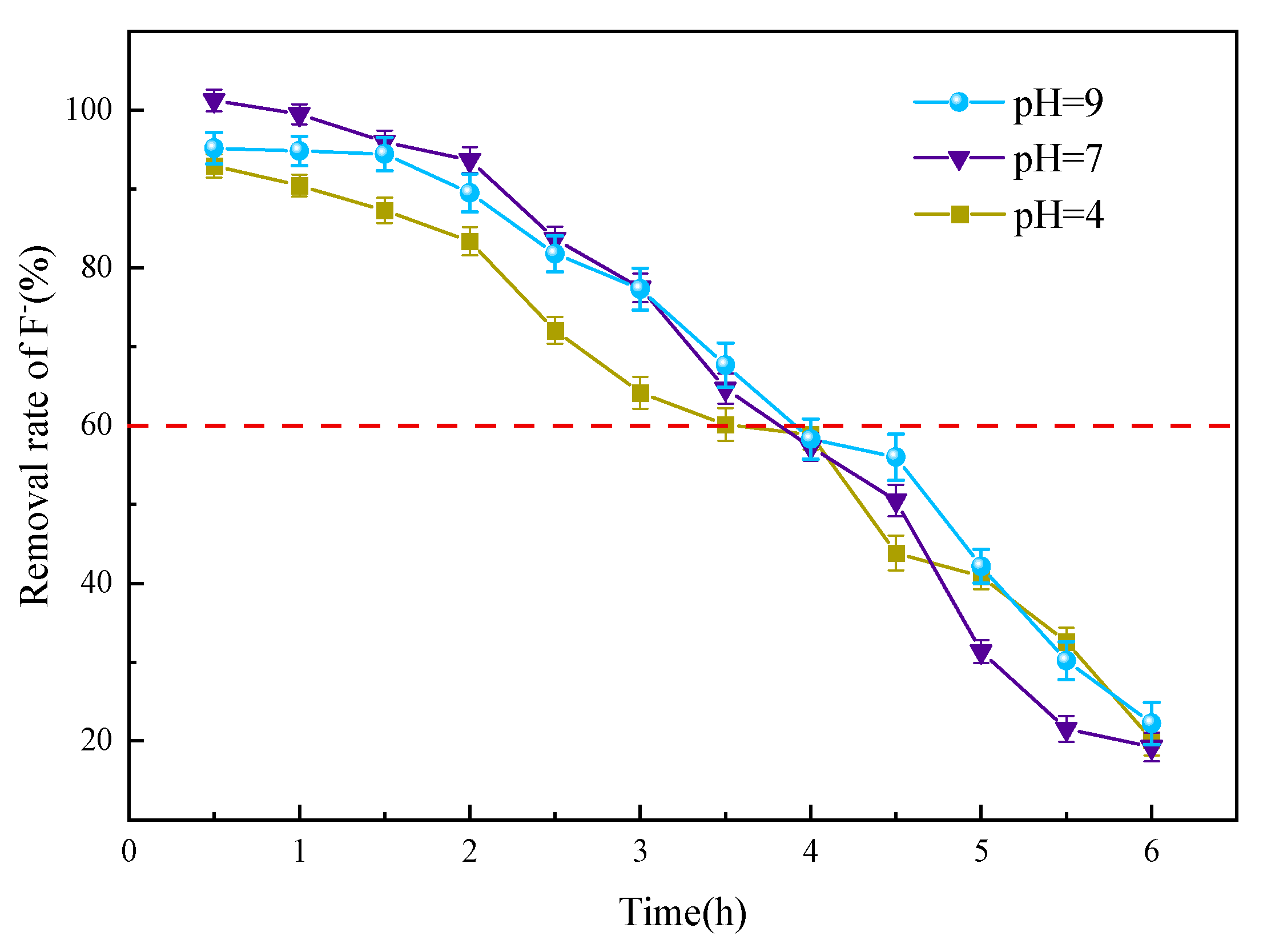
3.3.4. pH
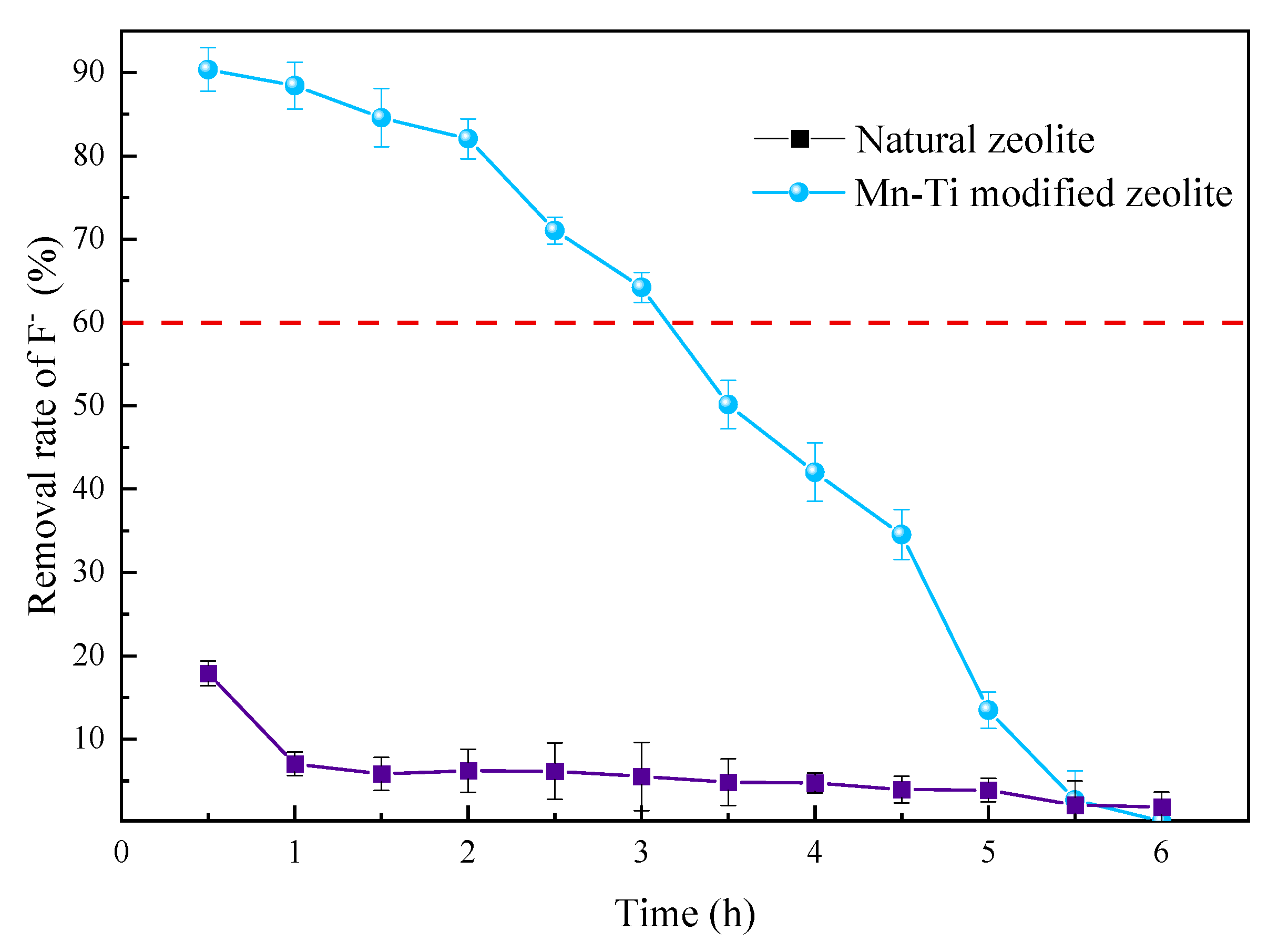
3.3.5. Comparison between Natural and Mn-Ti Modified Zeolites
3.4. Adsorption Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edition, F. Guidelines for drinking water quality. WHO Chron. 2011, 38, 104–108. [Google Scholar]
- Handa, B.K. Geochemistry and genesis of fluoride-containing ground waters in India. Ground Water 1975, 13, 275–281. [Google Scholar] [CrossRef]
- Fuhong, R.; Shuqin, J. Distribution and formation of high-fluorine groundwater in China. Environ. Earth Sci. 1988, 12, 3–10. [Google Scholar] [CrossRef]
- Malago, J.; Makoba, E.; Muzuka, A.N.N. fluoride levels in surface and groundwater in Africa: A review. Am. J. Water Sci. Eng. 2017, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Armienta, M.A.; Segovia, N. Arsenic and fluoride in the groundwater of Mexico. Environ. Geochem. Health 2008, 30, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Drondina, R.; Romanov, A.; Matveevich, V.; Motspan, V.; Shafranskij, V. Electrochemical method to remove fluorine from waters. The study of its mechanism. J. Fluor. Chem. 1989, 45, 58. [Google Scholar] [CrossRef]
- Singh, R.K.; Multari, N.; Nau-Hix, C.; Woodard, S.; Nickelsen, M.; Thagard, S.M.; Holsen, T.M. Removal of poly- and per-fluorinated compounds from ion exchange regenerant still bottom samples in a plasma reactor. Environ. Sci. Technol. 2020, 54, 13973–13980. [Google Scholar] [CrossRef]
- Turner, B.D.; Binning, P.; Stipp, S.L.S. Fluoride removal by calcite: Evidence for fluorite precipitation and surface adsorption. Environ. Sci. Technol. 2005, 39, 9561–9568. [Google Scholar] [CrossRef] [PubMed]
- Amor, Z.; Bariou, B.; Mameri, N.; Taky, M.; Nicolas, S.; Elmidaoui, A. Fluoride removal from brackish water by electrodialysis. Desalination 2001, 133, 215–223. [Google Scholar] [CrossRef]
- Zeni, M.; Riveros, R.; Melo, K.; Primieri, R.; Lorenzini, S. Study on fluoride reduction in artesian well—water from electrodialysis process. Desalination 2005, 185, 241–244. [Google Scholar] [CrossRef]
- Keri, R.S.; Hosamani, K.M.; Reddy, H.R.S.; Nataraj, S.K.; Aminabhavi, T. Application of the electrodialytic pilot plant for fluoride removal. J. Water Chem. Technol. 2011, 33, 293–300. [Google Scholar] [CrossRef]
- Sehn, P. Fluoride removal with extra low energy reverse osmosis membranes: Three years of large scale field experience in Finland. Desalination 2008, 223, 73–84. [Google Scholar] [CrossRef]
- Schneiter, R.; Middlebrooks, E. Arsenic and fluoride removal from groundwater by reverse osmosis. Environ. Int. 1983, 9, 289–291. [Google Scholar] [CrossRef]
- Min, B.R.; Gill, A.L.; Gill, W.N. A note on fluoride removal by reverse osmosis. Desalination 1984, 49, 89–93. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A. Removal of fluoride and uranium by nanofiltration and reverse osmosis: A review. Chemosphere 2014, 117, 679–691. [Google Scholar] [CrossRef]
- Velazquez-Peña, G.C.; Olguín-Gutiérrez, M.T.; Solache-Ríos, M.J.; Fall, C. Significance of FeZr-modified natural zeolite networks on fluoride removal. J. Fluor. Chem. 2017, 202, 41–53. [Google Scholar] [CrossRef]
- Tabi, R.N.; Agyemang, F.O.; Mensah-Darkwa, K.; Arthur, E.K.; Gikunoo, E.; Momade, F. Zeolite synthesis and its application in water defluorination. Mater. Chem. Phys. 2021, 261, 124229. [Google Scholar] [CrossRef]
- Gao, Y.; Li, M.; Ru, Y.; Fu, J. Fluoride removal from water by using micron zirconia/zeolite molecular sieve: Characterization and mechanism. Groundw. Sustain. Dev. 2021, 13, 100567. [Google Scholar] [CrossRef]
- Vinati, A.; Mahanty, B.; Behera, S. Clay and clay minerals for fluoride removal from water: A state-of-the-art review. Appl. Clay Sci. 2015, 114, 340–348. [Google Scholar] [CrossRef]
- Karthikeyan, G.; Pius, A.; Alagumuthu, G. Fluoride adsorption studies of montmorillonite clay. Indian J. Chem. Technol. 2005, 12, 263–272. [Google Scholar]
- Nabbou, N.; Belhachemi, M.; Boumelik, M.; Merzougui, T.; Lahcene, D.; Harek, Y.; Zorpas, A.A.; Jeguirim, M. Removal of fluoride from groundwater using natural clay (kaolinite): Optimization of adsorption conditions. C. R. Chim. 2019, 22, 105–112. [Google Scholar] [CrossRef]
- Liang, S.; Xue, Y.; Gao, B.; Yang, K. Removal of fluoride from aqueous solution by TiO2-based composites. J. Taiwan Inst. Chem. Eng. 2017, 74, 205–210. [Google Scholar] [CrossRef]
- Fukahori, S.; Ito, M.; Fujiwara, T. Removal mechanism of sulfamethazine and its intermediates from water by a rotating advanced oxidation contactor equipped with TiO2–high-silica zeolite composite sheets. Environ. Sci. Pollut. Res. 2018, 25, 29017–29025. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Amal, R. TiO2-coated natural zeolite: Rapid humic acid adsorption and effective photocatalytic regeneration. Chem. Eng. Sci. 2014, 105, 46–52. [Google Scholar] [CrossRef]
- Con, T.H.; Thao, P.; Dai, T.X.; Loan, D.K. Application of nano dimensional MnO2 for high effective sorption of arsenic and fluoride in drinking water. Environ. Sci. 2013, 1, 69–77. [Google Scholar] [CrossRef] [Green Version]
- Mudzielwana, R.; Gitari, M.W. Removal of fluoride from groundwater using MnO2 bentonite-smectite rich clay soils composite. Groundw. Sustain. Dev. 2021, 14, 100623. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Akinyemi, S.A.; Msagati, T.A.M. Synthesis and physicochemical characterization of MnO2 coated Na-bentonite for groundwater defluoridation: Adsorption modelling and mechanistic aspect. Appl. Surf. Sci. 2017, 422, 745–753. [Google Scholar] [CrossRef]
- Bahiraei, A.; Behin, J. Sonochemical immobilization of MnO2 nanoparticles on NaP-zeolite for enhanced Hg (II) adsorption from water. J. Environ. Chem. Eng. 2020, 8, 103790. [Google Scholar] [CrossRef]
- Zeng, Y.; Xue, Y.; Liang, S.; Zhang, J. Removal of fluoride from aqueous solution by TiO2 and TiO2–SiO2 nanocomposite. Chem. Speciat. Bioavailab. 2016, 29, 25–32. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.A.; Cozens, F.L.; Schepp, N.P. Photogeneration and migration of electrons and holes in zeolite NaY. J. Phys. Chem. B 2001, 105, 12746–12758. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, R.; Wei, S.; Wang, J.; Liu, Y.; Li, M.; Liu, R. Selective removal of H2S from biogas using a regenerable hybrid TiO2/zeolite composite. Fuel 2015, 157, 183–190. [Google Scholar] [CrossRef]
- Hashimoto, K.; Wasada, K.; Osaki, M.; Shono, E.; Adachi, K.; Toukai, N.; Kominami, H.; Kera, Y. Photocatalytic oxidation of nitrogen oxide over titania–zeolite composite catalyst to remove nitrogen oxides in the atmosphere. Appl. Catal. B Environ. 2001, 30, 429–436. [Google Scholar] [CrossRef]
- Camacho, L.M.; Parra, R.R.; Deng, S. Arsenic removal from groundwater by MnO2-modified natural clinoptilolite zeolite: Effects of pH and initial feed concentration. J. Hazard. Mater. 2011, 189, 286–293. [Google Scholar] [CrossRef]
- Zou, W.; Han, R.; Chen, Z.; Shi, J.; Liu. Characterization and properties of manganese oxide coated zeolite as adsorbent for removal of copper(II) and lead(II) ions from solution. J. Chem. Eng. Data 2006, 51, 534–541. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Li, H.; Li, Y.; Shi, J. Copper(II) and lead(II) removal from aqueous solution in fixed-bed columns by manganese oxide coated zeolite. J. Hazard. Mater. 2006, 137, 934–942. [Google Scholar] [CrossRef]
- Lyu, C.; Yang, X.; Zhang, S.; Zhang, Q.; Su, X. Preparation and performance of manganese-oxide-coated zeolite for the removal of manganese-contamination in groundwater. Environ. Technol. 2019, 40, 878–887. [Google Scholar] [CrossRef]
- Zhu, B.L.; Lv, L.; Wang, X.J. Preparation and magnetic properties of α-MnO2 nanoparticles. In Key Engineering Materials; Trans Tech Publications Ltd.: Zürich, Switzerland, 2012; Volume 492, pp. 264–267. [Google Scholar]
- Huang, X.; Lv, D.; Yue, H.; Attia, A.; Yang, Y. Controllable synthesis of α-and β-MnO2: Cationic effect on hydrothermal crystallization. Nanotechnology 2008, 19, 225606. [Google Scholar] [CrossRef] [Green Version]
- Hashemzadeh, F.; Kashani-Motlagh, M. Hydrothermal synthesis and characterisation of MnO2 nanostructures. Int. J. Nanomanuf. 2010, 5, 260–267. [Google Scholar] [CrossRef]
- Fischer, K. The crystal structure determination of the zeolite gismondite. CaAl2Si2O8· 4H2O. Am. Mineral. J. Earth Planet. Mater. 1963, 48, 664–672. [Google Scholar]
- Akgül, M. Enhancement of the anionic dye adsorption capacity of clinoptilolite by Fe3+-grafting. J. Hazard. Mater. 2014, 267, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Góra-Marek, K.; Brylewska, K.; Tarach, K.A.; Rutkowska, M.; Jabłońska, M.; Choi, M.; Chmielarz, L. IR studies of Fe modified ZSM-5 zeolites of diverse mesopore topologies in the terms of their catalytic performance in NH3-SCR and NH3-SCO processes. Appl. Catal. B Environ. 2015, 179, 589–598. [Google Scholar] [CrossRef]
- Breck, D.W.; Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry, and Use; John Wiley & Sons: Hoboken, NJ, USA, 1973. [Google Scholar]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and infrared spectra of carbonates of calcite structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Asgari, G.; Roshani, B.; Ghanizadeh, G. The investigation of kinetic and isotherm of fluoride adsorption onto functionalize pumice stone. J. Hazard. Mater. 2012, 217–218, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Bia, G.; De Pauli, C.P.; Borgnino, L. The role of Fe(III) modified montmorillonite on fluoride mobility: Adsorption experiments and competition with phosphate. J. Environ. Manag. 2012, 100, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aksakal, O.; Ucun, H. Equilibrium, kinetic and thermodynamic studies of the biosorption of textile dye (Reactive Red 195) onto Pinus sylvestris L. J. Hazard. Mater. 2010, 181, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Wang, L. Comparison between linear and non-linear forms of pseudo-first-order and pseudo-second-order adsorption kinetic models for the removal of methylene blue by activated carbon. Front. Environ. Sci. Eng. China 2009, 3, 320–324. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon: Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J. Hazard. Mater. 2007, 147, 381–394. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Chen, J.P. Adsorption of fluoride by Fe–Mg–La triple-metal composite: Adsorbent preparation, illustration of performance and study of mechanisms. Chem. Eng. J. 2015, 262, 839–846. [Google Scholar] [CrossRef]
- Samatya, S.; Yüksel, Ü.; Yüksel, M.; Kabay, N. Removal of fluoride from water by metal ions (Al3+, La3+ and ZrO2+) loaded natural zeolite. Sep. Sci. Technol. 2007, 42, 2033–2047. [Google Scholar] [CrossRef]
- Becerril, J.J.; Solache-Ríos, M.; García-Sosa, I. Fluoride removal from aqueous solutions by boehmite. Water Air Soil Pollut. 2011, 223, 1073–1078. [Google Scholar] [CrossRef]
- Gao, S.; Sun, R.; Wei, Z.; Zhao, H.; Li, H.; Hu, F. Size-dependent defluoridation properties of synthetic hydroxyapatite. J. Fluor. Chem. 2009, 130, 550–556. [Google Scholar] [CrossRef]
- Fan, X.; Parker, D.J.; Smith, M.D. Adsorption kinetics of fluoride on low cost materials. Water Res. 2003, 37, 4929–4937. [Google Scholar] [CrossRef]
- Sujana, M.; Anand, S. Ferric hydroxide: Preparation, characterisation and fluoride removal studies from water. Desalin. Water Treat. 2013, 52, 6453–6463. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Q.; Weng, X.; Mang, C.; Si, L.; Guan, Z.; Cheng, L. Fluoride ion adsorption from wastewater using magnesium(II), aluminum(III) and titanium(IV) modified natural zeolite: Kinetics, thermodynamics, and mechanistic aspects of adsorption. J. Water Reuse Desalin. 2017, 8, 479–489. [Google Scholar] [CrossRef]
- Raichur, A.; Basu, M.J. Adsorption of fluoride onto mixed rare earth oxides. Sep. Purif. Technol. 2001, 24, 121–127. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Processes; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Balistrieri, L.S.; Murray, J.W. The surface chemistry of goethite (alpha FeOOH) in major ion seawater. Am. J. Sci. 1981, 281, 788–806. [Google Scholar] [CrossRef]
- Tor, A. Removal of fluoride from an aqueous solution by using montmorillonite. Desalination 2006, 201, 267–276. [Google Scholar] [CrossRef]
- Sarkar, M.; Banerjee, A.; Pramanick, P.P. Kinetics and mechanism of fluoride removal using laterite. Ind. Eng. Chem. Res. 2006, 45, 5920–5927. [Google Scholar] [CrossRef]





| Name | Height CPS | FWHM eV | Area (P) CPS.eV | Area (N) TPP-2M | Atomic % |
|---|---|---|---|---|---|
| Si2p | 3119.57 | 1.85 | 6653.8 | 0.09 | 30.59 |
| C1s | 4037.57 | 1.68 | 9192.49 | 0.12 | 39.92 |
| Ti2p | 8175.82 | 1.49 | 28,072.26 | 0.06 | 19.94 |
| Mn2p | 3694.47 | 3.67 | 23,789.49 | 0.03 | 9.54 |
| Lagergren | Pseudo-Second Order | ||||
|---|---|---|---|---|---|
| qe (mg/g) | KL (1/min) | R2 | qe (mg/g) | k (g/mg·min) | R2 |
| 0.7833 | 0.008220 | 0.9984 | 1.1467 | 0.005350 | 0.9972 |
| Langmuir Model | Frendlich Model | |||||
|---|---|---|---|---|---|---|
| Qmax (mg/g) | KL (L/mg) | R2 | KF (L/mg) | 1/n | R2 | |
| 15 °C | 1.1726 | 0.0320 | 0.813 | 0.03956 | 0.8653 | 0.989 |
| 25 °C | 2.1752 | 0.0180 | 0.913 | 0.04114 | 0.9111 | 0.998 |
| 35 °C | 0.4327 | 0.0995 | 0.950 | 0.04890 | 0.6553 | 0.981 |
| Adsorbents | Experimental Conditions | qe (mg/g) | |||
|---|---|---|---|---|---|
| pH | Time (h) | Temperature (°C) | Initial F− Concentration (mg/L) | ||
| Zeolite-Zr [51] | 7 | 24 | 30 | 2.5 | 4.427 |
| Zeolite-La [51] | 7 | 24 | 30 | 2.5 | 1.691 |
| Zeolite-Al [51] | 7 | 24 | 30 | 2.5 | 1.71 |
| Boehmite [52] | 6.8 | 24 | 25 | 10 | 2.057 |
| Synthetic hydroxyapatite [53] | 2 | 2 | 25 | 5 | 0.489 |
| Fe3+þactivated quartz [54] | 6 | 1.6 | 20 | 3 × 10−5 | 1.16 |
| Mn−Ti modified zeolite | 7 | 4 | 25 | 10 | 2.175 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, B.; Sun, G.; Quan, B.; Tang, J.; Zhang, C.; Jia, C.; Tang, Y.; Wang, X.; Zhao, M.; Wang, W.; et al. An Experimental Study of Fluoride Removal from Wastewater by Mn-Ti Modified Zeolite. Water 2021, 13, 3343. https://doi.org/10.3390/w13233343
Yang B, Sun G, Quan B, Tang J, Zhang C, Jia C, Tang Y, Wang X, Zhao M, Wang W, et al. An Experimental Study of Fluoride Removal from Wastewater by Mn-Ti Modified Zeolite. Water. 2021; 13(23):3343. https://doi.org/10.3390/w13233343
Chicago/Turabian StyleYang, Bo, Guirong Sun, Bingxu Quan, Jiawei Tang, Chunhui Zhang, Chaomin Jia, Yuanhui Tang, Xinling Wang, Mengmeng Zhao, Wenqian Wang, and et al. 2021. "An Experimental Study of Fluoride Removal from Wastewater by Mn-Ti Modified Zeolite" Water 13, no. 23: 3343. https://doi.org/10.3390/w13233343






