On the Degradation of Glyphosate by Photocatalysis Using TiO2/Biochar Composite Obtained from the Pyrolysis of Rice Husk
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. TiO2 Coated Biochar Preparation
2.3. Research Methods
2.3.1. Characterization Methods
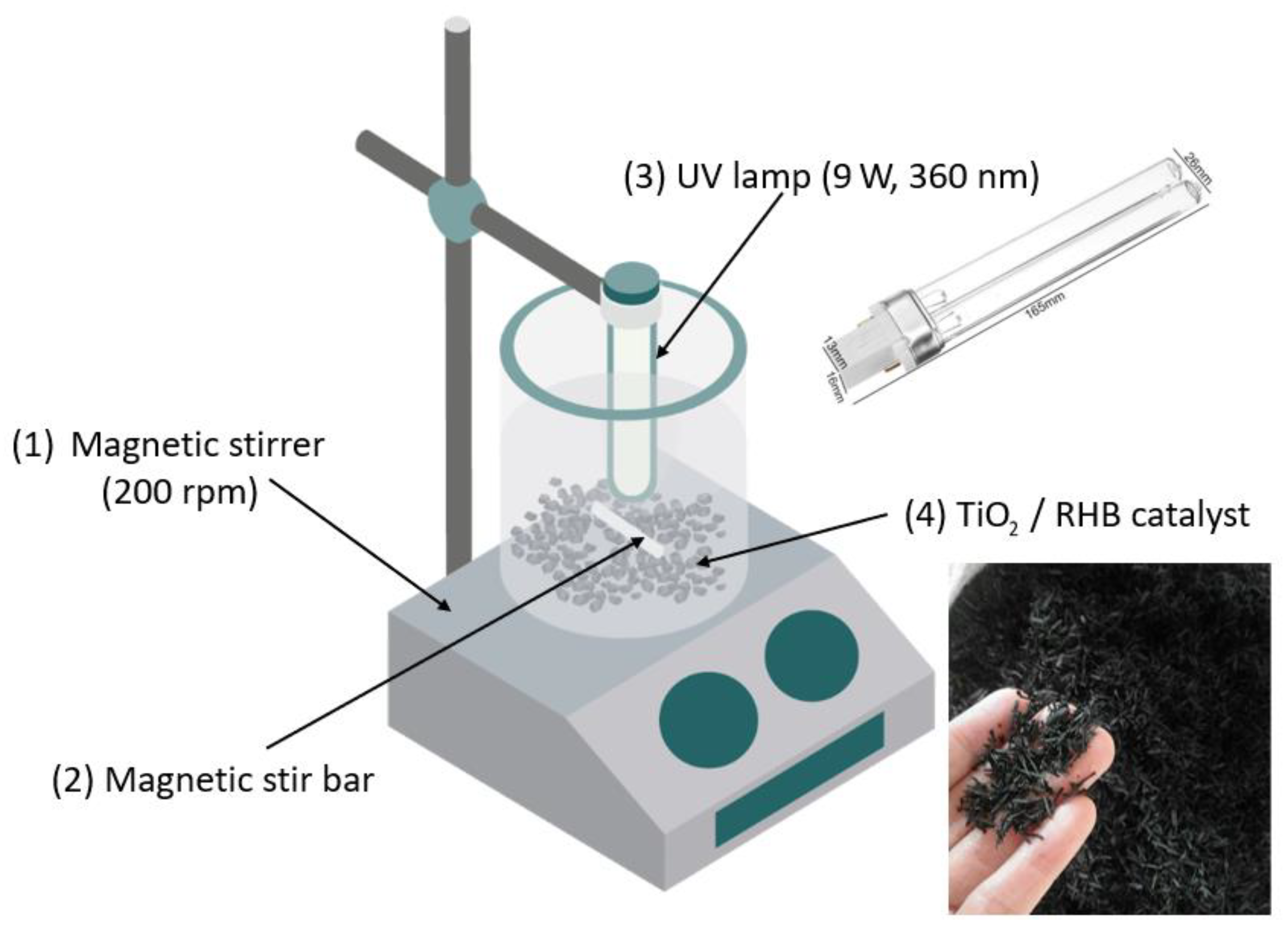
2.3.2. Experimental Setup
2.3.3. Evaluation of Photocatalysis Activity
3. Results
3.1. Characterization of TiO2/RHB Composites
3.1.1. Surface Morphology and Porous Structure of the Composite Catalysts
3.1.2. Elemental Compositions of TiO2/RHB Composites
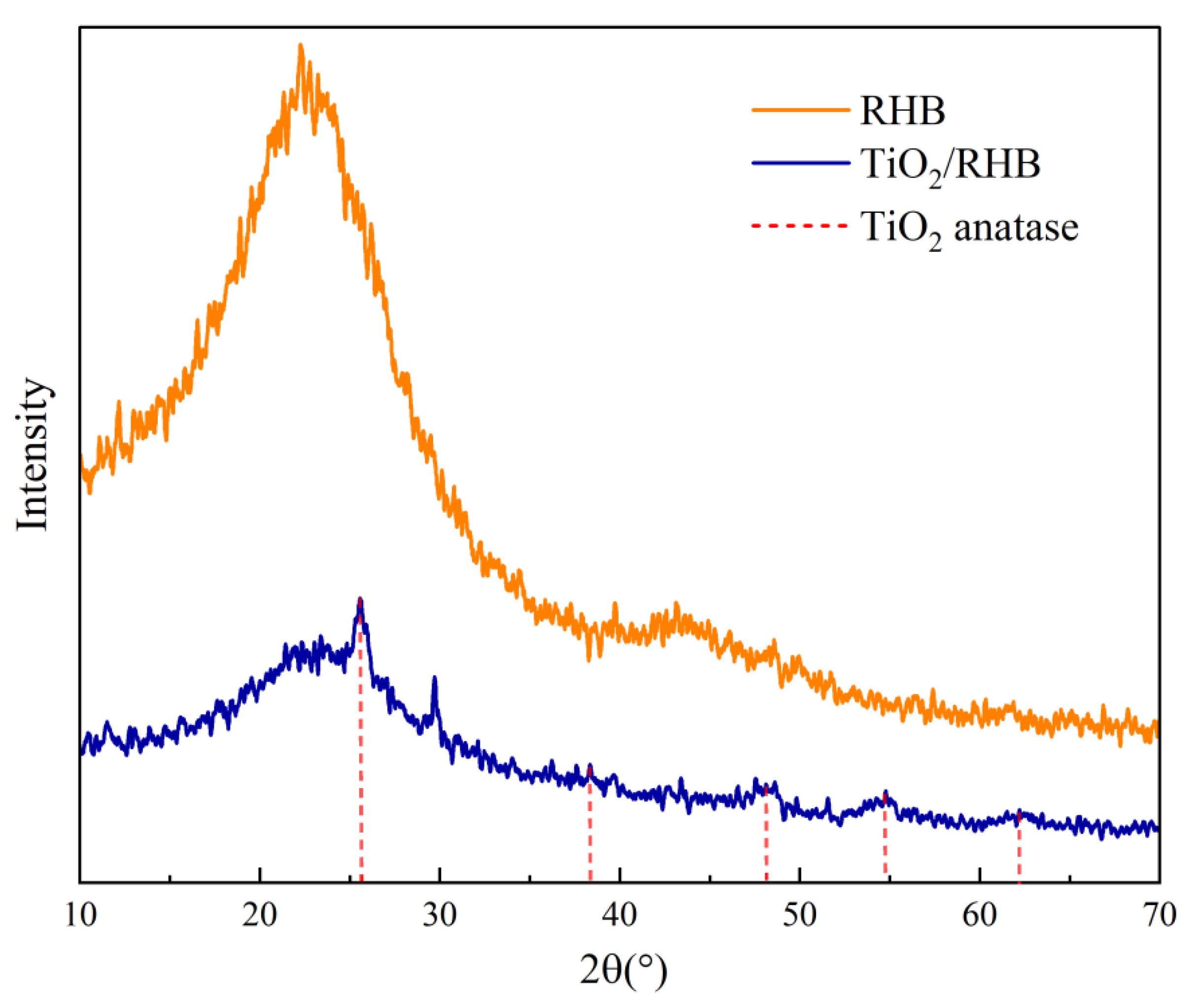
3.1.3. The Crystalline Structure of TiO2/RHB
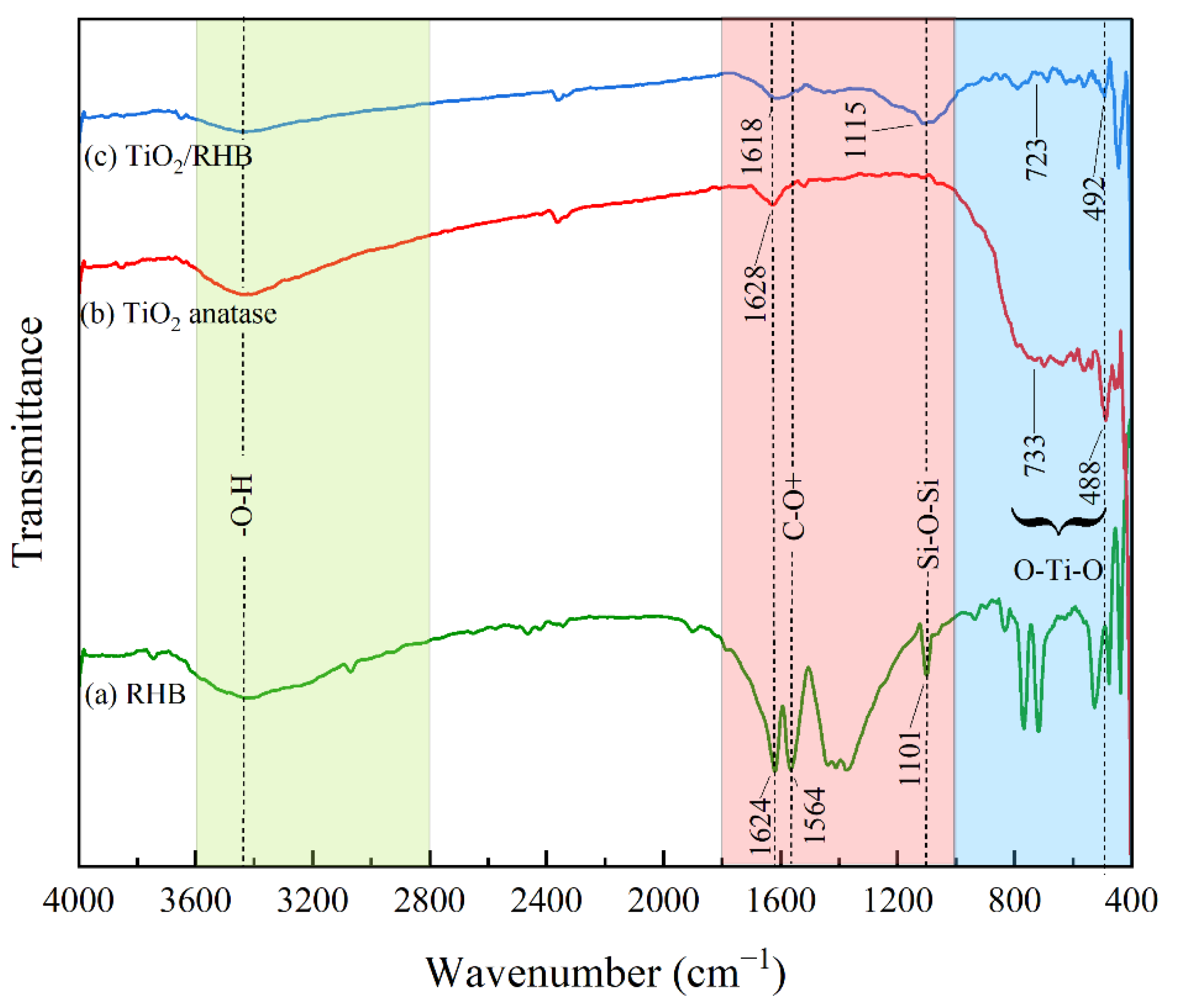
3.1.4. Functional Groups of TiO2/RHB Composites
3.2. Photodegradation of Glyphosate in the Presence of TiO2/RHB Catalysts
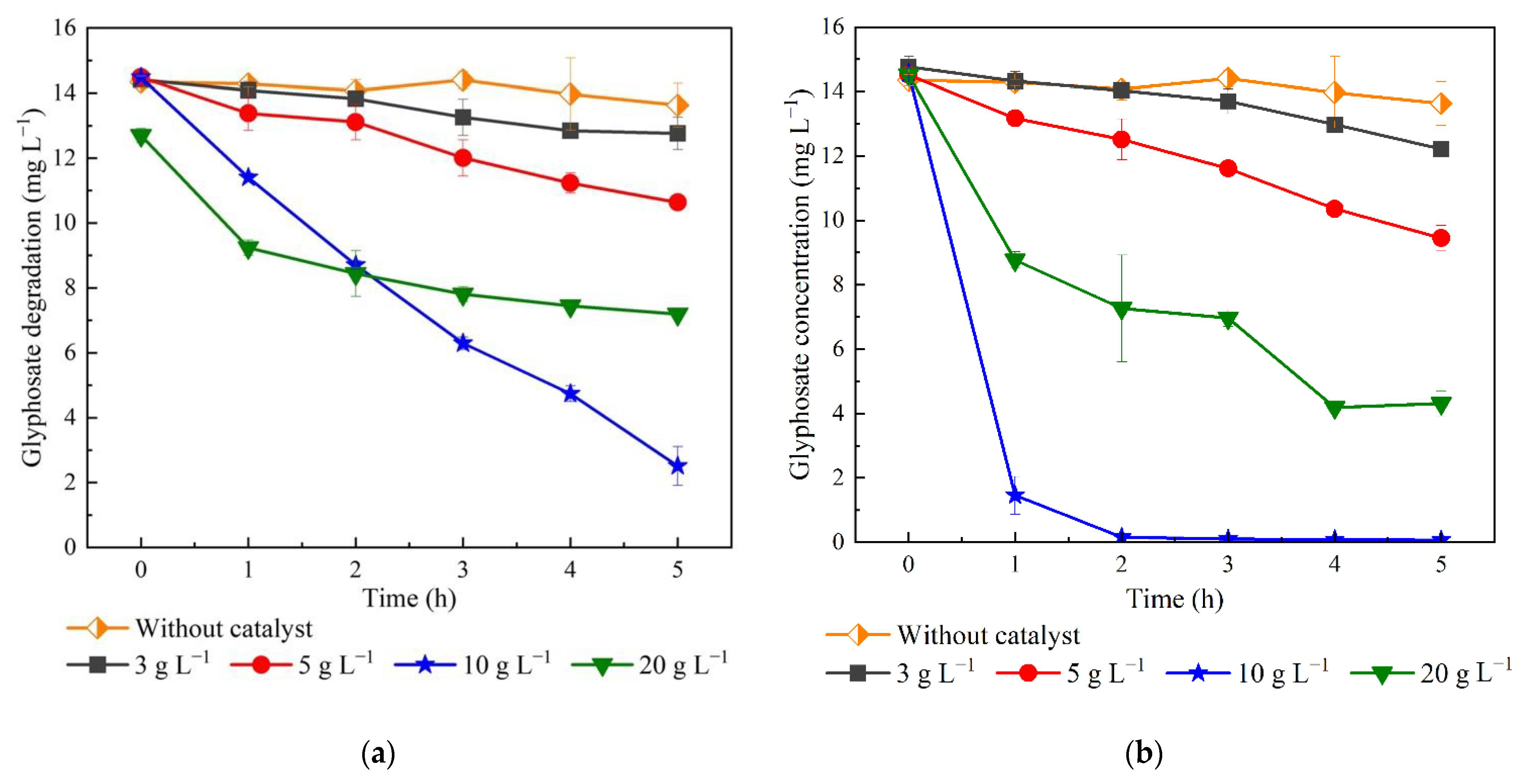
3.2.1. Effects of TiO2/RHB Catalyst Dosage
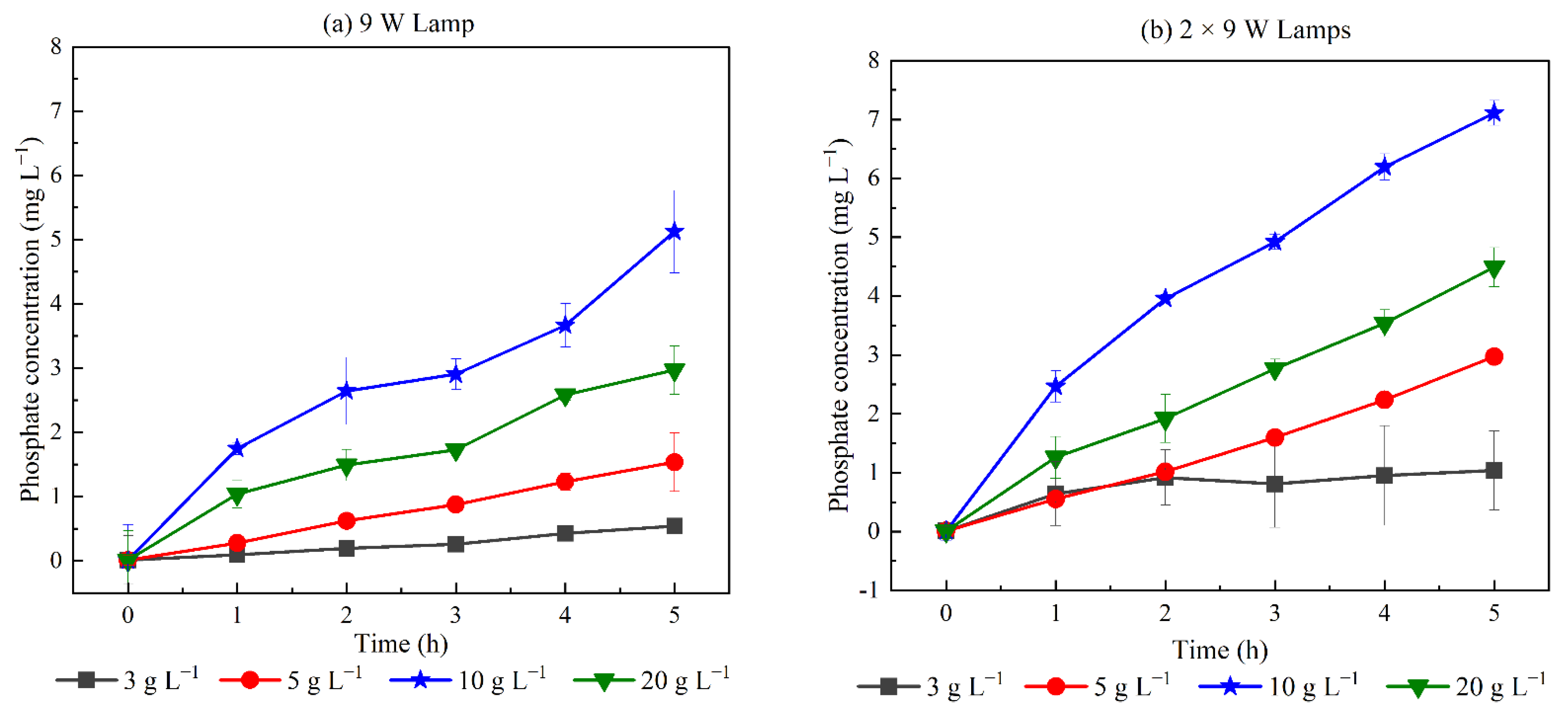
3.2.2. Effects of Irradiation Intensity
3.2.3. Mineralization Ability of Glyphosate
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

Appendix B
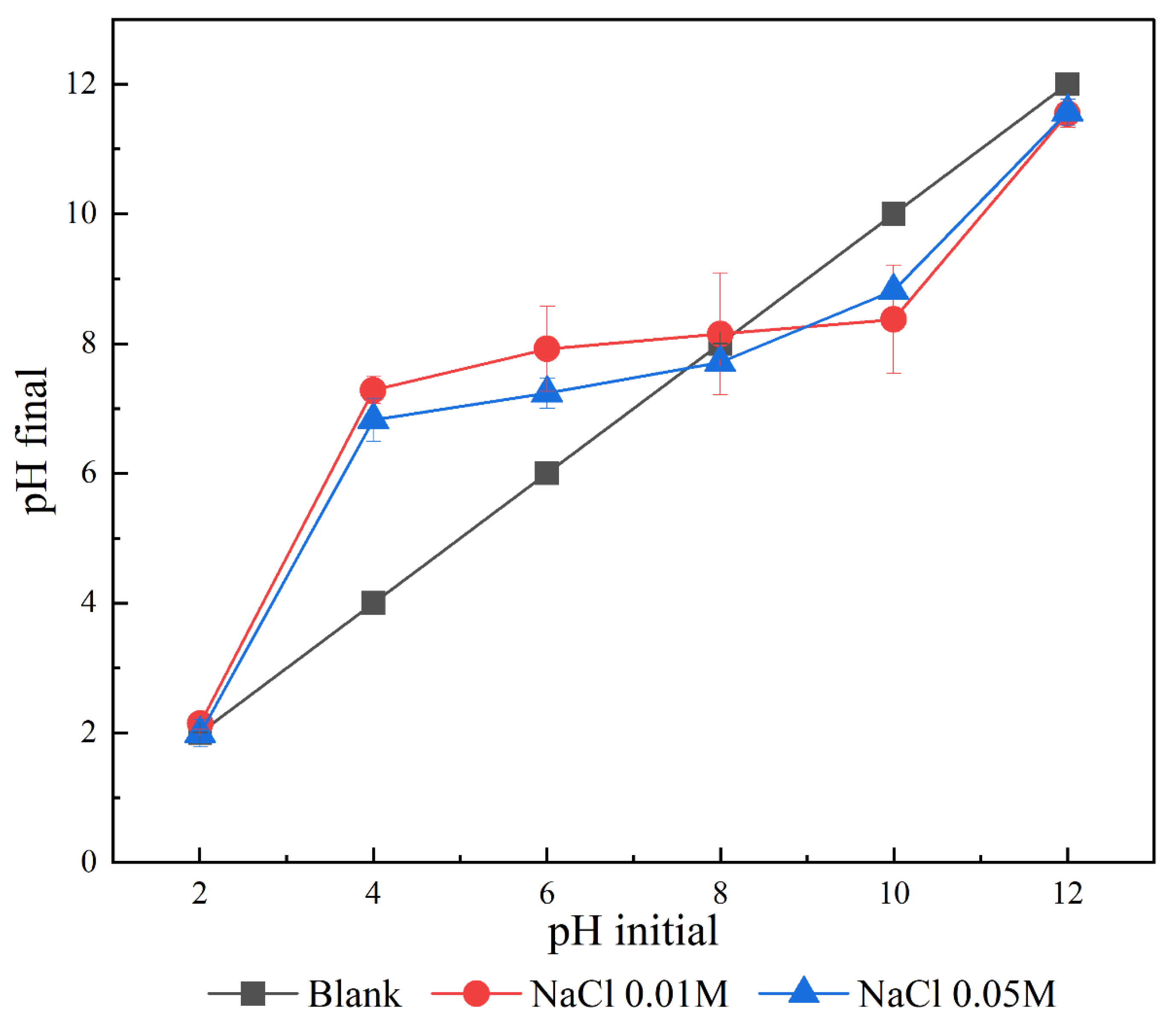
Appendix C
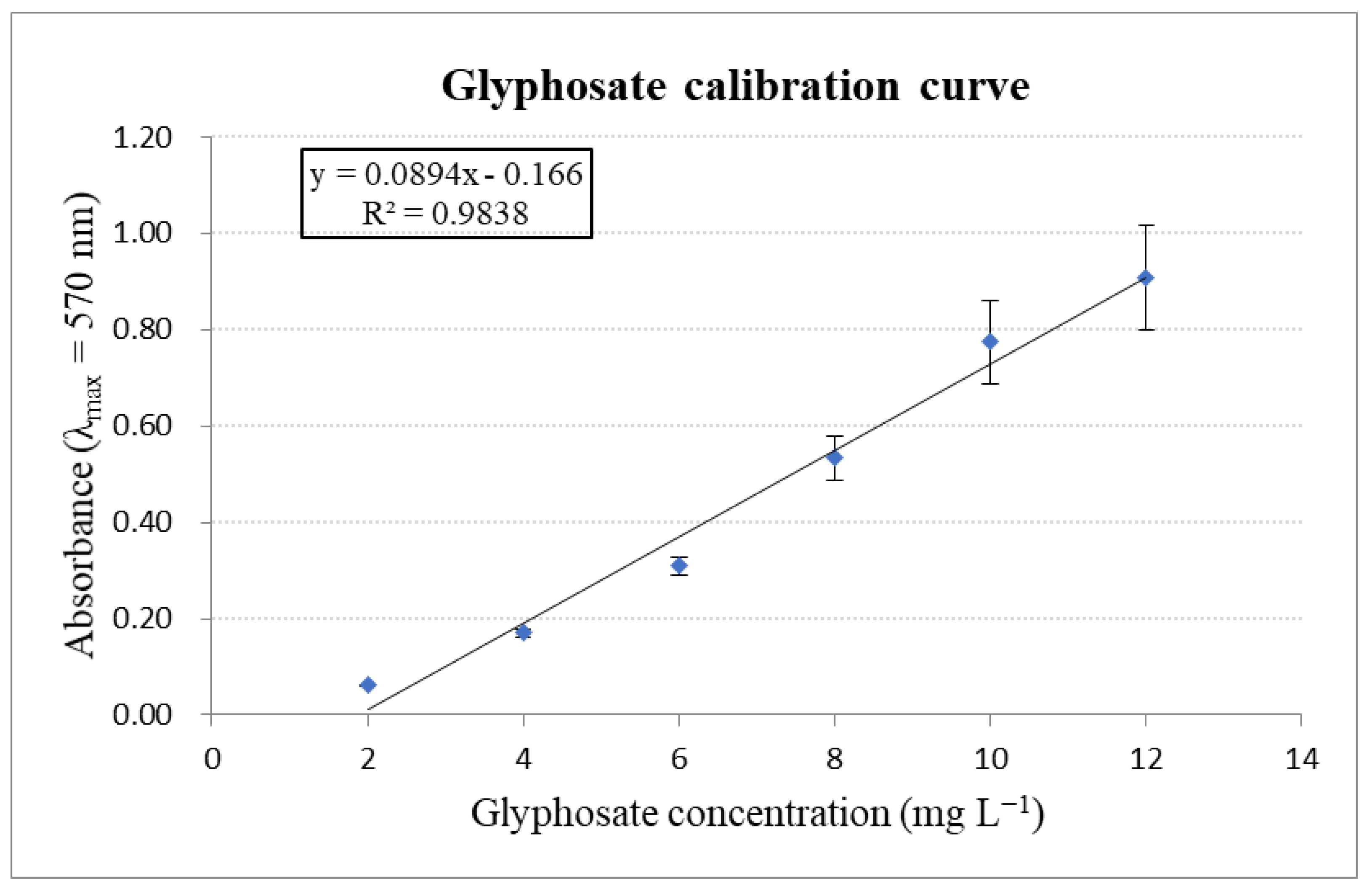
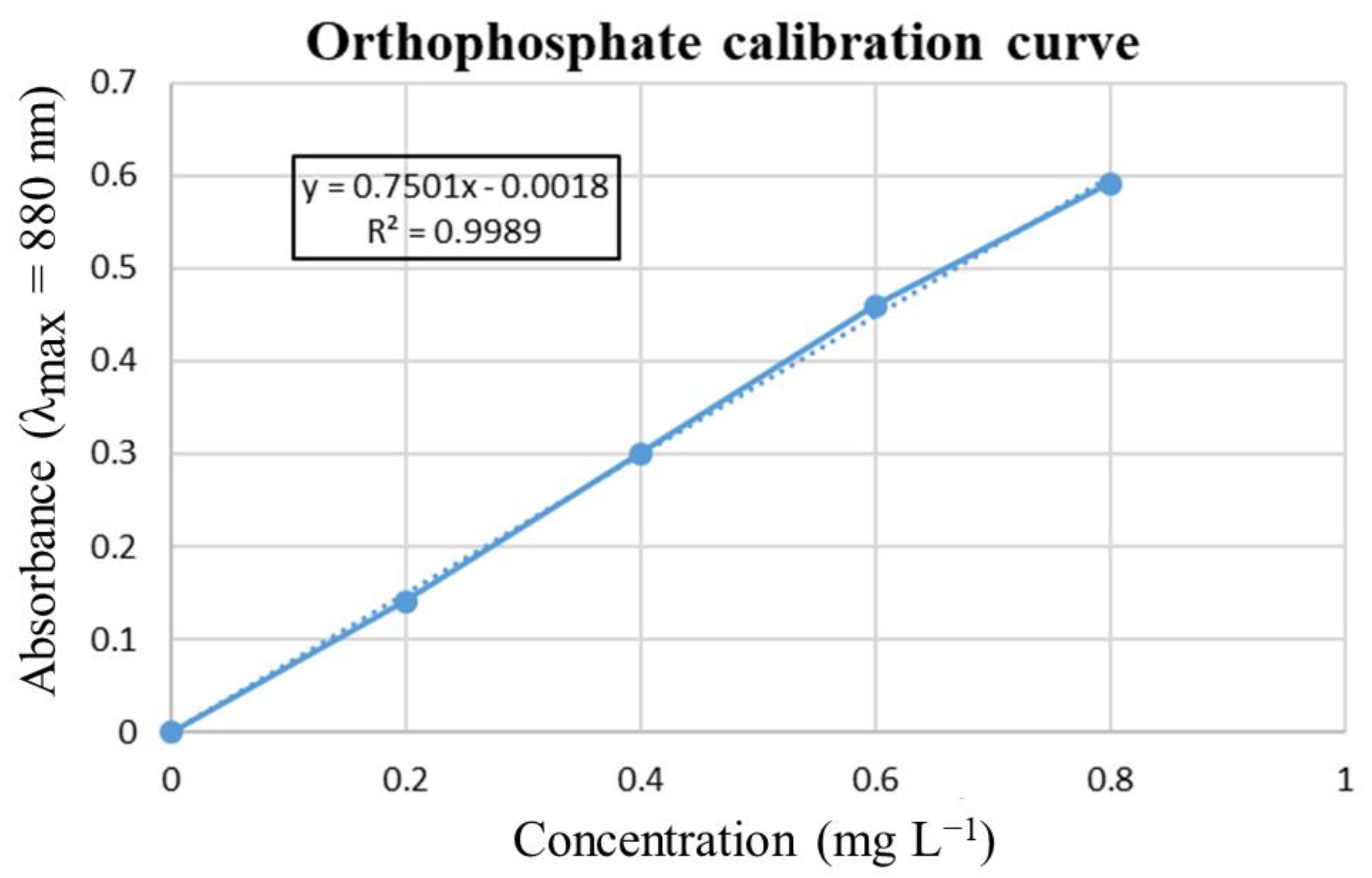
Appendix D. Calibration Curve of Orthophosphate
Appendix E. The Gradient Elution Profile and the Compounds Information
| Time (mn) | % A | % B |
|---|---|---|
| 0.01 2.00 3.00 7.00 7.01 | 100 100 50 50 100 | 0 0 50 50 0 |
| 10.00 | 100 | 0 |
| Compound | m/z | E (V) | RT (mn) |
|---|---|---|---|
| Glyphosate | 168 > 63 | −30 | 4.07 |
| AMPA | 110 > 60 | −26 | 2.87 |
Appendix F. SEM Images of Raw RHB


Appendix G. EDX Analyzed Position


References
- Baylis, A.D. Why glyphosate is a global herbicide: Strengths, weakness and prospects. Pest Manag. Sci. 2000, 56, 299–308. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Kolpin, D.W.; Scribner, E.A.; Kuivila, K.M.; Sandstrom, M.W. Glyphosate, Other Herbicides, and Transformation Products in Midwestern Streams, 20021. JAWRA J. Am. Water Resour. Assoc. 2005, 41, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Battaglin, W.A.; Rice, K.C.; Focazio, M.J.; Salmons, S.; Barry, R.X. The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005–2006. Environ. Monit. Assess. 2009, 155, 281–307. [Google Scholar] [CrossRef] [PubMed]
- Coupe, R.H.; Kalkhoff, S.J.; Capel, P.D.; Gregoire, C. Fate and transport of glyphosate and aminomethylphosphonic acid in surface waters of agricultural basins. Pest Manag. Sci. 2012, 68, 16–30. [Google Scholar] [CrossRef]
- Mahler, B.J.; Van Metre, P.C.; Burley, T.E.; Loftin, K.A.; Meyer, M.T.; Nowell, L.H. Similarities and differences in occurrence and temporal fluctuations in glyphosate and atrazine in small Midwestern streams (USA) during the 2013 growing season. Sci. Total. Environ. 2017, 579, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Liu, Y. Study on the photocatalytic degradation of glyphosate by TiO2 photocatalyst. Chemosphere 2007, 67, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-L.; Gao, Y.; Li, Y.; Li, X.; Zhang, H.; Han, X.X.; Zhao, B.; Su, L. Indirect glyphosate detection based on ninhydrin reaction and surface-enhanced Raman scattering spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 197, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, B.; Anvaripour, B.; Jorfi, S.; Jaafarzadeh, N. Enhanced Photocatalytic Degradation and Mineralization of Furfural Using UVC/TiO2/GAC Composite in Aqueous Solution. Int. J. Photoenergy 2016, 2016, 2782607. [Google Scholar] [CrossRef]
- Jonsson, J.; Camm, R.; Hall, T. Removal and degradation of glyphosate in water treatment: A review. J. Water Supply Res. Technol. 2013, 62, 395–408. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Jayawardhana, O.; Mayakaduwage, S.; Bandara, T.; Wickramasinghe, S.; Vithanage, M. Rice husk derived engineered biochar for glyphosate removal in aqueous media; engineered biochar for pesticides removal. In Proceedings of the International Conference Environmental Conservation, Clean Water, Air & Soil (CleanWAS), Beijing, China, 26–28 August 2016; Ashraf, M.A., Aqma, W.S., Eds.; IWA Publishing: London, UK, 2017; p. 164. [Google Scholar]
- Herath, G.A.D.; Poh, L.S.; Ng, W.J. Statistical optimization of glyphosate adsorption by biochar and activated carbon with response surface methodology. Chemosphere 2019, 227, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Montero, P.J.; Vega-Verduga, C.; Alulema-Pullupaxi, P.; Fernández, L.; Paz, J.L. Technologies Employed in the Treatment of Water Contaminated with Glyphosate: A Review. Molecules 2020, 25, 5550. [Google Scholar] [CrossRef] [PubMed]
- Lawrinenko, M.; Laird, D.A. Anion exchange capacity of biochar. Green Chem. 2015, 17, 4628–4636. [Google Scholar] [CrossRef] [Green Version]
- Speth, T.F. Glyphosate Removal from Drinking Water. J. Environ. Eng. 1993, 119, 1139–1157. [Google Scholar] [CrossRef]
- Lu, L.; Shan, R.; Shi, Y.; Wang, S.; Yuan, H. A novel TiO2/biochar composite catalysts for photocatalytic degradation of methyl orange. Chemosphere 2019, 222, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.-C.; Chen, J.-N.; Chang, K.-T. Effect of adsorbents coated with titanium dioxide on the photocatalytic degradation of propoxur. Chemosphere 1999, 38, 617–627. [Google Scholar] [CrossRef]
- Nghia, N.M.; Negishi, N.; Hue, N.T. Enhanced Adsorption and Photocatalytic Activities of Co-Doped TiO2 Immobilized on Silica for Paraquat. J. Electron. Mater. 2017, 47, 692–700. [Google Scholar] [CrossRef]
- Marien, C.B.D.; Marchal, C.; Koch, A.; Robert, D.; Drogui, P. Sol-gel synthesis of TiO2 nanoparticles: Effect of Pluronic P123 on particle’s morphology and photocatalytic degradation of paraquat. Environ. Sci. Pollut. Res. 2016, 24, 12582–12588. [Google Scholar] [CrossRef] [PubMed]
- Bhaskara, B.L.; Nagaraja, P. Direct Sensitive Spectrophotometric Determination of Glyphosate by Using Ninhydrin as a Chromogenic Reagent in Formulations and Environmental Water Samples. Helv. Chim. Acta 2006, 89, 2686–2693. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Severo, F.F.; Da Silva, L.S.; Moscôso, J.S.C.; Sarfaraz, Q.; Júnior, L.F.R.; Lopes, A.F.; Marzari, L.B.; Molin, G.D. Chemical and physical characterization of rice husk biochar and ashes and their iron adsorption capacity. SN Appl. Sci. 2020, 2, 1286. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, Z.; Wang, J.; Yang, Y.; Luo, X. Comparison of the Physicochemical Characteristics of Bio-char Pyrolyzed from Moso Bamboo and Rice Husk with Different Pyrolysis Temperatures. BioResources 2017, 12, 4652–4669. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Li, J.; Liu, Y.; Yan, Z.; Tan, X.; Liu, S.; Zeng, G.; Gu, Y.; Hu, X.; Jiang, L. Titanium dioxide-coated biochar composites as adsorptive and photocatalytic degradation materials for the removal of aqueous organic pollutants. J. Chem. Technol. Biotechnol. 2018, 93, 783–791. [Google Scholar] [CrossRef]
- Coleman, H.; Vimonses, V.; Leslie, G.; Amal, R. Degradation of 1,4-dioxane in water using TiO2 based photocatalytic and H2O2/UV processes. J. Hazard. Mater. 2007, 146, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Lea, J.; Adesina, A.A. The photo-oxidative degradation of sodium dodecyl sulphate in aerated aqueous TiO2 suspension. J. Photochem. Photobiol. A Chem. 1998, 118, 111–122. [Google Scholar] [CrossRef]
- Moma, J.; Baloyi, J. Modified Titanium Dioxide for Photocatalytic Applications. In Photocatalysts-Applications and Attributes; IntechOpen: London, UK, 2019; Volume 18. [Google Scholar]
- Garcia, J.C.; Takashima, K. Photocatalytic degradation of imazaquin in an aqueous suspension of titanium dioxide. J. Photochem. Photobiol. A Chem. 2003, 155, 215–222. [Google Scholar] [CrossRef]
- Muneer, M.; Boxall, C. Photocatalyzed Degradation of a Pesticide Derivative Glyphosate in Aqueous Suspensions of Titanium Dioxide. Int. J. Photoenergy 2008, 2008, 197346. [Google Scholar] [CrossRef] [Green Version]
- Lund-Høie, K.; Friestad, H.O. Photodegradation of the herbicide glyphosate in water. Bull. Environ. Contam. Toxicol. 1986, 36, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Trinelli, M.A.; Cantera, C.G.; Areco, M.M.; Afonso, M.D.S. Glyphosate photodegradation: Stoichiometry, kinetic and catalytic effects. Int. J. Environ. Health 2019, 9, 306. [Google Scholar] [CrossRef]
- Lesueur, C.; Pfeffer, M.; Fuerhacker, M. Photodegradation of phosphonates in water. Chemosphere 2005, 59, 685–691. [Google Scholar] [CrossRef]
- O’Shea, K.E.; Beightol, S.; Garcia, I.; Aguilar, M.; Kalen, D.V.; Cooper, W.J. Photocatalytic decomposition of organophosphonates in irradiated TiO2 suspensions. J. Photochem. Photobiol. A Chem. 1997, 107, 221–226. [Google Scholar] [CrossRef]
- Manassero, A.; Passalia, C.; Negro, A.; Cassano, A.; Zalazar, C. Glyphosate degradation in water employing the H2O2/UVC process. Water Res. 2010, 44, 3875–3882. [Google Scholar] [CrossRef] [PubMed]



| % Weight | RHB | TiO2/RHB |
|---|---|---|
| C O Si Ti Others | 40.25 44.92 14.83 0 0 | 40.19 31.98 11.14 14.09 2.60 |
| Total | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.T.; Le, D.N.; Nguyen, T.H.; Bui, H.T.; Pham, L.A.; Nguyen, L.L.; Nguyen, Q.S.; Nguyen, T.P.; Dang, T.H.; Duong, T.T.; et al. On the Degradation of Glyphosate by Photocatalysis Using TiO2/Biochar Composite Obtained from the Pyrolysis of Rice Husk. Water 2021, 13, 3326. https://doi.org/10.3390/w13233326
Le PT, Le DN, Nguyen TH, Bui HT, Pham LA, Nguyen LL, Nguyen QS, Nguyen TP, Dang TH, Duong TT, et al. On the Degradation of Glyphosate by Photocatalysis Using TiO2/Biochar Composite Obtained from the Pyrolysis of Rice Husk. Water. 2021; 13(23):3326. https://doi.org/10.3390/w13233326
Chicago/Turabian StyleLe, Phuong Thu, Duy Ngoc Le, Thi Hue Nguyen, Huyen Thuong Bui, Le Anh Pham, Luong Lam Nguyen, Quoc Son Nguyen, Thu Phuong Nguyen, Thu Hien Dang, Thi Thuy Duong, and et al. 2021. "On the Degradation of Glyphosate by Photocatalysis Using TiO2/Biochar Composite Obtained from the Pyrolysis of Rice Husk" Water 13, no. 23: 3326. https://doi.org/10.3390/w13233326






