Long-Term Toxicological Monitoring of a Multibarrier Advanced Wastewater Treatment Plant Comprising Ozonation and Granular Activated Carbon with In Vitro Bioassays
Abstract
:1. Introduction
2. Materials and Methods
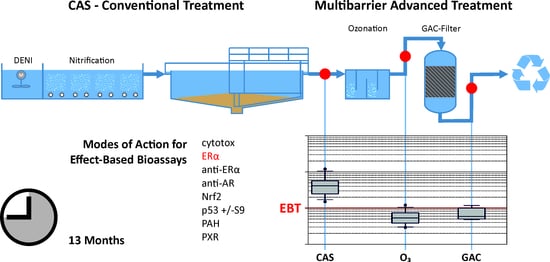
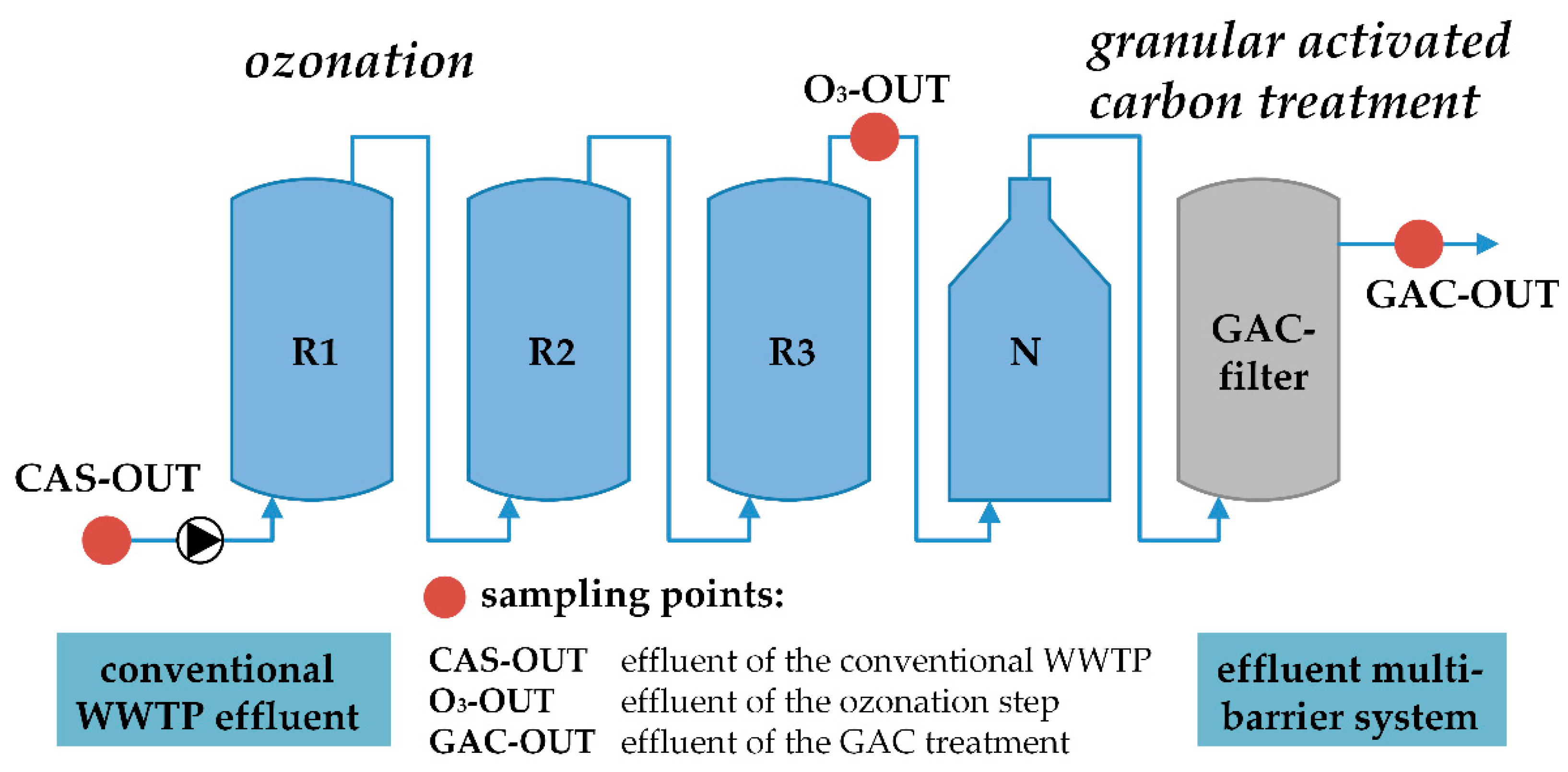
2.1. Sampling Site and Advanced Wastewater Treatment Plant
2.2. Sampling Campaigns
2.3. Sample Extraction for Bioanalysis
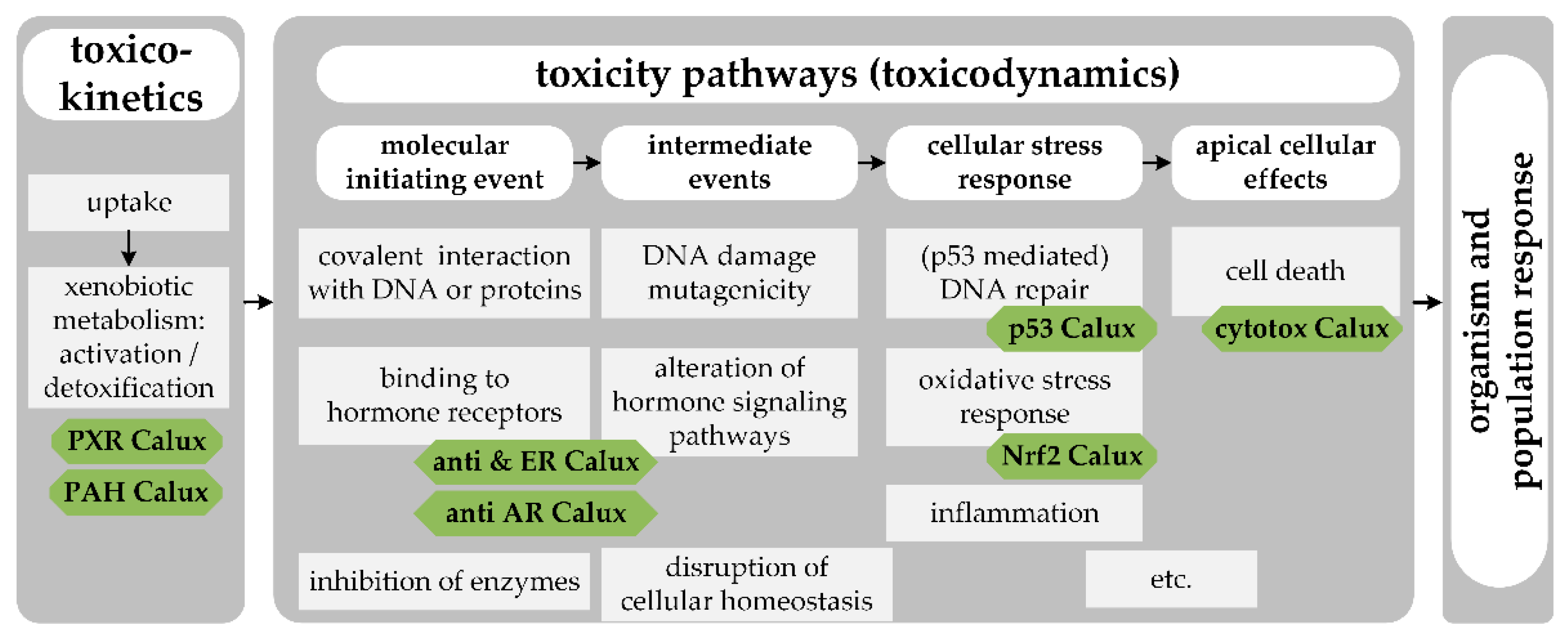
2.4. Bioassays
2.5. Data Interpretation
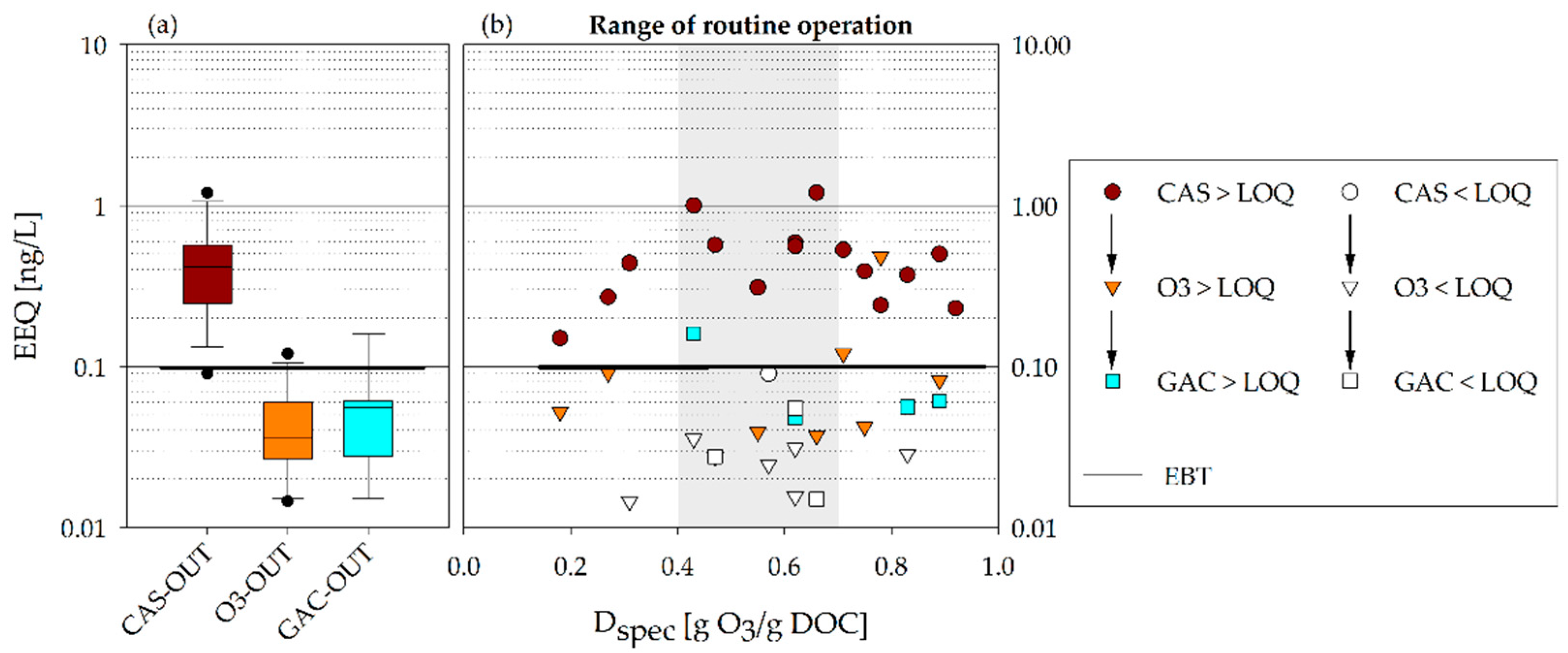
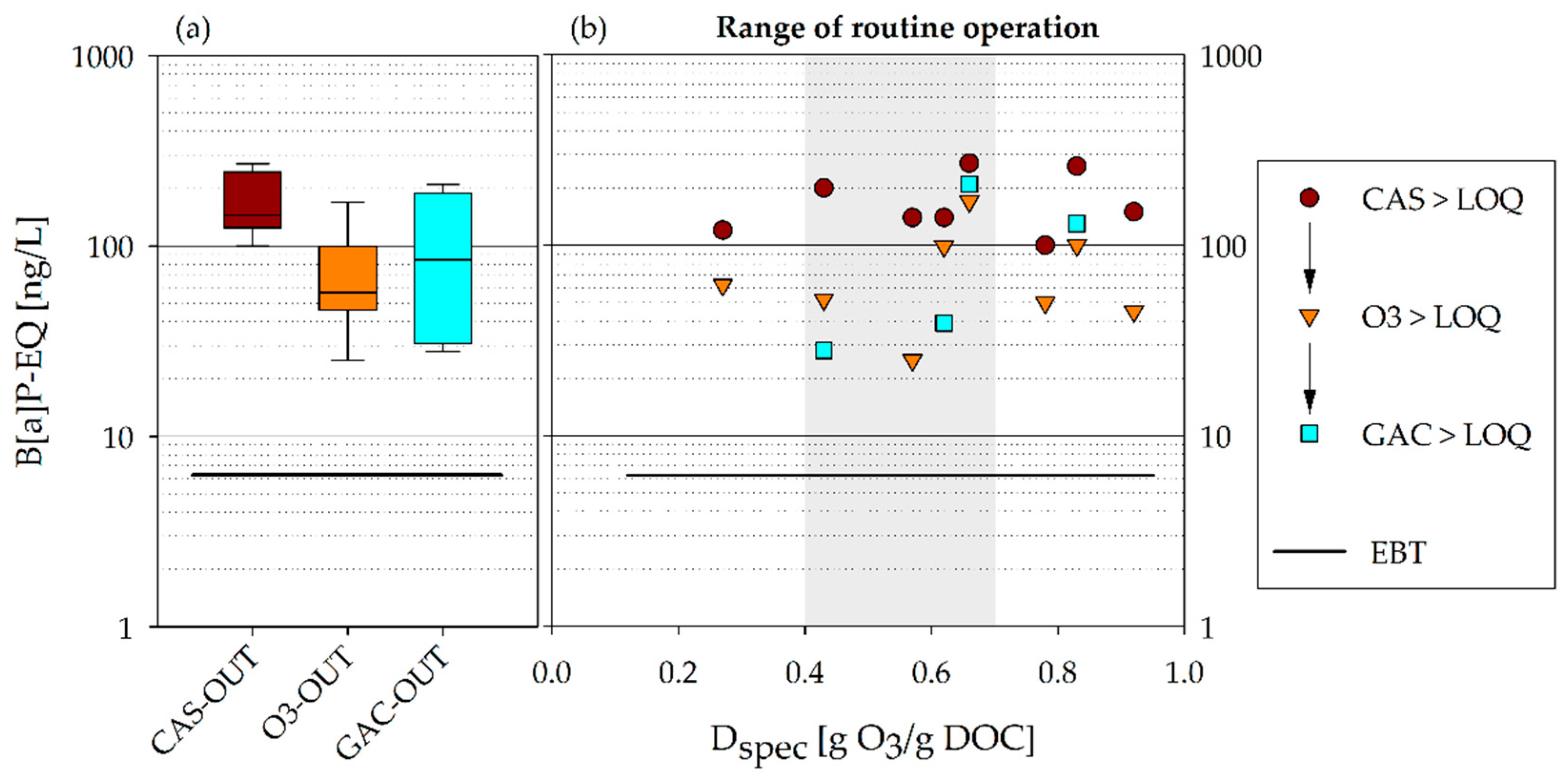
3. Results
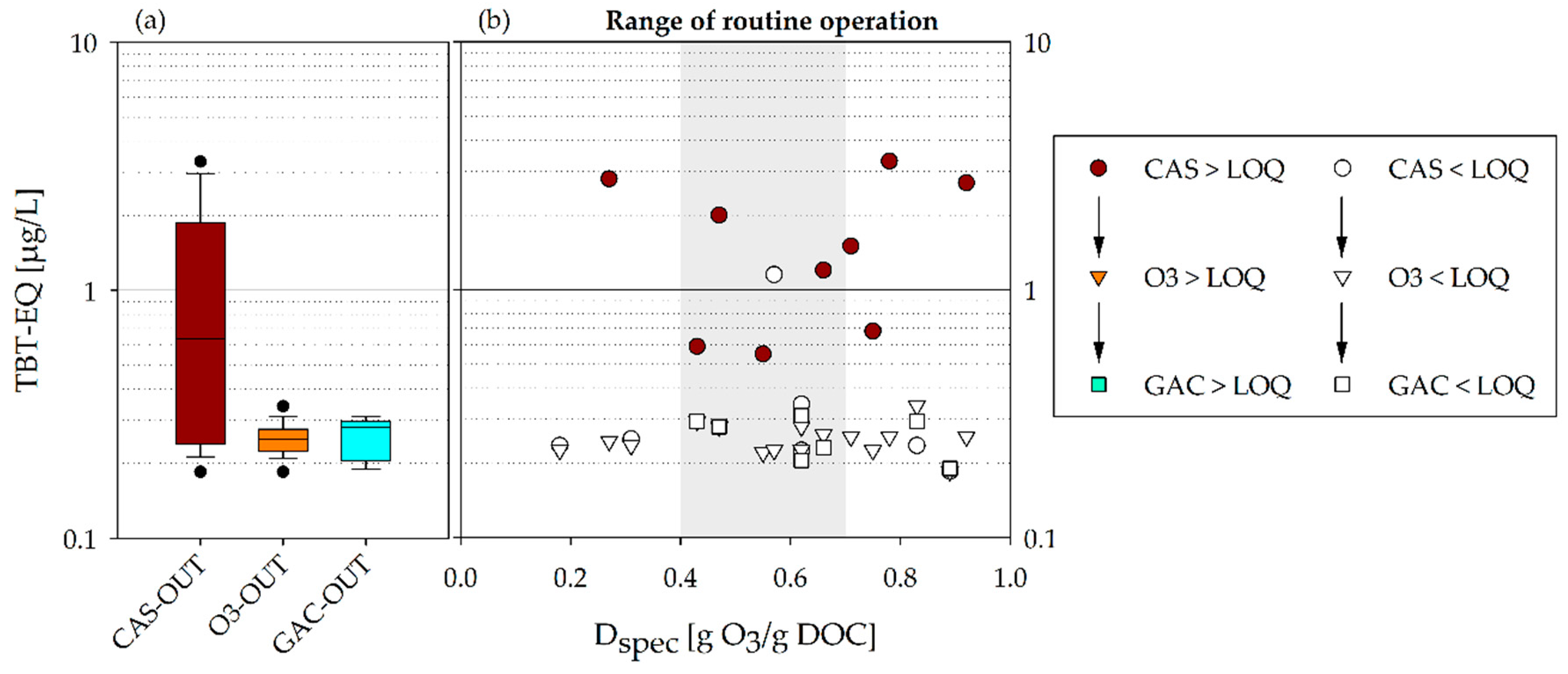
- The BEQ decrease was determined for the various steps of the multibarrier system (CAS-OUT, O3-OUT, and GAC-OUT).
- The BEQ were compared to currently discussed MOA-specific EBTs to identify the impact of advanced treatment.
4. Discussion
5. Conclusions
- Toxicological long-term monitoring delivered a valid basis for assessing the applicability and the performance of a multibarrier system for an advanced treatment.
- The combination of the two approaches applied in the present study, namely the quantification of BEQ decline and comparison of BEQ with currently discussed EBTs, represented a solid means of assessing the final effluent quality of the multibarrier system combining ozonation and granular activated carbon treatment.
- Despite natural variations in wastewater characteristics and other factors influencing CAS treatment efficiency over the 13-month monitoring, the overall removal pattern for various modes of action covering endpoints along the toxicity pathway revealed a decrease in BEQ.
- Even though the positive effect of ozonation resulting in signals below LOQ for some MOA impeded the assessment of the GAC treatment, a combination of O3 and GAC is strongly recommended for advanced treatment in order to follow the multibarrier approach and guarantee a high level of treatment.
- Since the presented toxicological results did not reveal significant differences within the recommended ozone dose range of 0.4–0.7 g O3/g DOC, it can be concluded that potential toxicological requirements should not be limiting for the implementation and operation of multibarrier systems for CEC abatement.
- Even though measures like implementing advanced treatment at WWTPs do not result in a complete removal, advanced treatment represents a relevant step in reducing the toxicological burden for the aquatic environment.
- Effect-based bioassays with their linked EBTs should be used as treatment goals and quality criteria for design, operation and the evaluation of advanced wastewater treatment.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Escher, B.I.; Stapleton, H.M.; Schymanski, E.L. Tracking complex mixtures of chemicals in our changing environment. Science 2020, 367, 388–392. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Bester, K.; Kümmerer, K. Xenobiotics in the Urban Water Cycle, 1st ed.; Springer: Dordrecht, The Netherlands; Heidelberg, Germany; London, UK; New York, NY, USA, 2010. [Google Scholar]
- Krzeminski, P.; Tomei, M.C.; Karaolia, P.; Langenhoff, A.; Almeida, C.M.R.; Felis, E.; Gritten, F.; Andersen, H.R.; Fernandes, T.; Manaia, C.M.; et al. Performance of secondary wastewater treatment methods for the removal of contaminants of emerging concern implicated in crop uptake and antibiotic resistance spread: A review. Sci. Total Environ. 2019, 648, 1052–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Directive 2000/60/EC. Directive of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for the Community Action in the Field of Water Policy. OJ L 327, 22 December 2000. [Google Scholar]
- Directive 91/271/EEC. Directive concerning Urban Waste Water Treatment (UWWTD). OJ L 135, 30 May 1991. [Google Scholar]
- Commission Implementing Decision (EU) 2015/495. Commission Implementing Decision establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council. OJ L 78, 2015. [Google Scholar]
- Commission Implementing Decision (EU) 2018/840. Commission Implementing Decision of 5 June 2018 Establishing a Watch List of Substances for Union-Wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and Repealing Commission Implementing Decision (EU) 2015/495 (Notified under Document C(29018) 3362). OJ L 141, 7 June 2018. [Google Scholar]
- Directive 2008/105/EC. Directive of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. OJ L 348, 24 December 2008. [Google Scholar]
- Directive 2013/39/EU. Directive of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. OJ L 226, 24 August 2013. [Google Scholar]
- Escher, B.I.; Aït-Aïssa, S.; Behnisch, P.A.; Brack, W.; Brion, F.; Brouwer, A.; Buchinger, S.; Crawford, S.E.; Du Pasquier, D.; Hamers, T.; et al. Effect-based trigger values for in vitro and in vivo bioassays performed on surface water extracts supporting the environmental quality standards (EQS) of the European Water Framework Directive. Sci. Total Environ. 2018, 628–629, 748–765. [Google Scholar] [CrossRef] [PubMed]
- Eggen, R.I.L.; Hollender, J.; Joss, A.; Schärer, M.; Stamm, C. Reducing the Discharge of Micropollutants in the Aquatic Environment: The Benefits of Upgrading Wastewater Treatment Plants. Environ. Sci. Technol. 2014, 48, 7683–7689. [Google Scholar] [CrossRef] [PubMed]
- Connon, R.E.; Geist, J.; Werner, I. Effect-Based Tools for Monitoring and Predicting the Ecotoxicological Effects of Chemicals in the Aquatic Environment. Sensors 2012, 12, 12741–12771. [Google Scholar] [CrossRef] [Green Version]
- Escher, B.I.; Leusch, F.D.L. Bioanalytical Tools in Water Quality Assessment; IWA Publishing: London, UK, 2012. [Google Scholar]
- Neale, P.A.; Altenburger, R.; Aït-Aïssa, S.; Brion, F.; Busch, W.; de Aragão Umbuzeiro, G.; Denison, M.S.; Du Pasquier, D.; Hilscherová, K.; Hollert, H.; et al. Development of a bioanalytical test battery for water quality monitoring: Fingerprinting identified micropollutants and their contribution to effects in surface water. Water Res. 2017, 123, 734–750. [Google Scholar] [CrossRef] [Green Version]
- Altenburger, R.; Ait-Aissa, S.; Antczak, P.; Backhaus, T.; Barceló, D.; Seiler, T.-B.; Brion, F.; Busch, W.; Chipman, K.; de Alda, M.L.; et al. Future water quality monitoring—Adapting tools to deal with mixtures of pollutants in water resource management. Sci. Total Environ. 2015, 512–513, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Brack, W.; Ait-Aissa, S.; Burgess, R.M.; Busch, W.; Creusot, N.; Di Paolo, C.; Escher, B.I.; Mark Hewitt, L.; Hilscherova, K.; Hollender, J.; et al. Effect-directed analysis supporting monitoring of aquatic environments—An in-depth overview. Sci. Total Environ. 2016, 544, 1073–1118. [Google Scholar] [CrossRef]
- Escher, B.I.; Allinson, M.; Altenburger, R.; Bain, P.A.; Balaguer, P.; Busch, W.; Crago, J.; Denslow, N.D.; Dopp, E.; Hilscherova, K.; et al. Benchmarking Organic Micropollutants in Wastewater, Recycled Water and Drinking Water with In Vitro Bioassays. Environ. Sci. Technol. 2014, 48, 1940–1956. [Google Scholar] [CrossRef]
- Könemann, S.; Kase, R.; Simon, E.; Swart, K.; Buchinger, S.; Schlüsener, M.; Hollert, H.; Escher, B.I.; Werner, I.; Aït-Aïssa, S.; et al. Effect-based and chemical analytical methods to monitor estrogens under the European Water Framework Directive. TrAC Trends Anal. Chem. 2018, 102, 225–235. [Google Scholar] [CrossRef]
- Kase, R.; Javurkova, B.; Simon, E.; Swart, K.; Buchinger, S.; Könemann, S.; Escher, B.I.; Carere, M.; Dulio, V.; Ait-Aissa, S.; et al. Screening and risk management solutions for steroidal estrogens in surface and wastewater. TrAC Trends Anal. Chem. 2018, 102, 343–358. [Google Scholar] [CrossRef]
- Pop, C.-E.; Draga, S.; Măciucă, R.; Niță, R.; Crăciun, N.; Wolff, R. Bisphenol A Effects in Aqueous Environment on Lemna minor. Processes 2021, 9, 1512. [Google Scholar] [CrossRef]
- Wernersson, A.S.; Carere, M.; Maggi, C.; Tusil, P.; Soldan, P.; James, A.; Sanchez, W.; Dulio, V.; Broeg, K.; Reifferscheid, G.; et al. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ. Sci. Eur. 2015, 27, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Brack, W.; Dulio, V.; Ågerstrand, M.; Allan, I.; Altenburger, R.; Brinkmann, M.; Bunke, D.; Burgess, R.M.; Cousins, I.; Escher, B.I.; et al. Towards the review of the European Union Water Framework Directive: Recommendations for more efficient assessment and management of chemical contamination in European surface water resources. Sci. Total Environ. 2017, 576, 720–737. [Google Scholar] [CrossRef] [PubMed]
- Escher, B.I.; Neale, P.A.; Leusch, F.D.L. Effect-based trigger values for in vitro bioassays: Reading across from existing water quality guideline values. Water Res. 2015, 81, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Van der Oost, R.; Sileno, G.; Suárez-Muñoz, M.; Nguyen, M.T.; Besselink, H.; Brouwer, A. SIMONI (Smart Integrated Monitoring) as a novel bioanalytical strategy for water quality assessment: Part I—Model design and effect-based trigger values. Environ. Toxicol. Chem. 2017, 36, 2385–2399. [Google Scholar] [CrossRef]
- NORMAN Network; Water Europe. Contaminants of Emerging Concern in Urban Wastewater. Joint NORMAN and Water Europe Position Paper. 2019. Available online: https://www.normandata.eu/sites/default/files/files/Publications/Position%20paper_CECs%20UWW_NORMAN_WE_2019_Final_20190910_public.pdf (accessed on 26 September 2021).
- Kienle, C.; Kase, R.; Werner, I. Evaluation of bioassays and wastewater quality. In In Vitro and In Vivo Bioassays for the Performance Review in the Project “Strategy MicroPoll”; Swiss Centre for Applied Ecotoxicology, Eawag-EPFL: Duebendorf, Switzerland, 2011. [Google Scholar]
- Bain, P.A.; Williams, M.; Kumar, A. Assessment of multiple hormonal activities in wastewater at different stages of treatment. Environ. Toxicol. Chem. 2014, 33, 2297–2307. [Google Scholar] [CrossRef]
- Völker, J.; Stapf, M.; Miehe, U.; Wagner, M. Systematic Review of Toxicity Removal by Advanced Wastewater Treatment Technologies via Ozonation and Activated Carbon. Environ. Sci. Technol. 2019, 53, 7215–7233. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Besselink, H.; Paulus, G.K.; Oswald, P.; Hornstra, L.M.; Oswaldova, M.; Medema, G.; Thomaidis, N.S.; Behnisch, P.A.; Slobodnik, J. Characterization of wastewater effluents in the Danube River Basin with chemical screening, in vitro bioassays and antibiotic resistant genes analysis. Environ. Int. 2019, 127, 420–429. [Google Scholar] [CrossRef]
- Ankley, G.T.; Bennett, R.S.; Erickson, R.J.; Hoff, D.J.; Hornung, M.W.; Johnson, R.D.; Mount, D.R.; Nichols, J.W.; Russom, C.L.; Schmieder, P.K.; et al. Adverse outcome pathways: A conceptual framework to support ecotoxicology research and risk assessment. Environ. Toxicol. Chem. 2010, 29, 730–741. [Google Scholar] [CrossRef]
- Escher, B.I.; Dutt, M.; Maylin, E.; Tang, J.Y.M.; Toze, S.; Wolf, C.R.; Lang, M. Water quality assessment using the AREc32 reporter gene assay indicative of the oxidative stress response pathway. J. Environ. Monit. 2012, 14, 2877–2885. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Lado Ribeiro, A.R.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total. Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef] [Green Version]
- Braun, R.; Hartmann, C.; Kreuzinger, N.; Lenz, K.; Schaar, H.; Scheffknecht, C. Untersuchung von Abwässern und Gewässern auf Unterschiedliche Toxikologische Endpunkte; Bundesministerium für Landwirtschaft, Regionen und Tourismus Vienna: Vienna, Austria, 2021; p. 248.
- Escher, B.I.; Fenner, K. Recent Advances in Environmental Risk Assessment of Transformation Products. Environ. Sci. Technol. 2011, 45, 3835–3847. [Google Scholar] [CrossRef]
- Dopp, E.; Pannekens, H.; Gottschlich, A.; Schertzinger, G.; Gehrmann, L.; Kasper-Sonnenberg, M.; Richard, J.; Joswig, M.; Grummt, T.; Schmidt, T.C.; et al. Effect-based evaluation of ozone treatment for removal of micropollutants and their transformation products in waste water. J. Toxicol. Environ. Health Part A 2021, 84, 418–439. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.A.; O’Brien, J.W.; Glauch, L.; König, M.; Krauss, M.; Mueller, J.F.; Tscharke, B.; Escher, B.I. Wastewater treatment efficacy evaluated with in vitro bioassays. Water Res. X 2020, 9, 100072. [Google Scholar] [CrossRef]
- Clara, M.; Kreuzinger, N.; Strenn, B.; Gans, O.; Kroiss, H. The solids retention time—A suitable design paramter to evaluate the capacity of wastewater treatment plants to remove micropollutants. Water Res. 2005, 39, 97–106. [Google Scholar] [CrossRef] [PubMed]
- NEREUS. Deliverable 13. White Paper on the existing knowledge with regard to wastewater and biological hazards. In NEREUS COST Action ES 1403; Deliverable of WG3; 2018; 20p, Available online: http://www.nereus-cost.eu/wp-content/uploads/2019/12/D13.pdf (accessed on 26 September 2021).
- Wolf, Y.; Oster, S.; Shuliakevich, A.; Brückner, I.; Dolny, R.; Linnemann, V.; Pinnekamp, J.; Hollert, H.; Schiwy, S. Improvement of wastewater and water quality via a full-scale ozonation plant?—A comprehensive analysis of the endocrine potential using effect-based methods. Sci. Total Environ. 2022, 803, 149756. [Google Scholar] [CrossRef]
- Escher, B.I.; Bramaz, N.; Ort, C. JEM Spotlight: Monitoring the treatment efficiency of a full scale ozonation on a sewage treatment plant with a mode-of-action based test battery. J. Environ. Monit. 2009, 11, 1836–1846. [Google Scholar] [CrossRef]
- Huber, M.M.; Göbel, A.; Joss, A.; Hermann, N.; Löffler, D.; McArdell, C.S.; Ried, A.; Siegrist, H.R.; Ternes, T.A.; von Gunten, U. Oxidation of Pharmaceuticals during Ozonation of Municipal Wastewater Effluents: A Pilot Study. Environ. Sci. Technol. 2005, 39, 4290–4299. [Google Scholar] [CrossRef]
- Neale, P.A.; Ait-Aissa, S.; Brack, W.; Creusot, N.; Denison, M.S.; Deutschmann, B.; Hilscherová, K.; Hollert, H.; Krauss, M.; Novák, J.; et al. Linking in Vitro Effects and Detected Organic Micropollutants in Surface Water Using Mixture-Toxicity Modeling. Environ. Sci. Technol. 2015, 49, 14614–14624. [Google Scholar] [CrossRef] [Green Version]
- Lemaire, G.R.; Mnif, W.; Pascussi, J.-M.; Pillon, A.; Rabenoelina, F.; Fenet, H.l.N.; Gomez, E.; Casellas, C.; Nicolas, J.-C.; Cavaillès, V.; et al. Identification of New Human Pregnane X Receptor Ligands among Pesticides Using a Stable Reporter Cell System. Toxicol. Sci. 2006, 91, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- König, M.; Escher, B.I.; Neale, P.A.; Krauss, M.; Hilscherová, K.; Novák, J.; Teodorović, I.; Schulze, T.; Seidensticker, S.; Kamal Hashmi, M.A.; et al. Impact of untreated wastewater on a major European river evaluated with a combination of in vitro bioassays and chemical analysis. Environ. Pollut. 2017, 220, 1220–1230. [Google Scholar] [CrossRef]
- Mišík, M.; Ferk, F.; Schaar, H.; Yamada, M.; Jaeger, W.; Knasmueller, S.; Kreuzinger, N. Genotoxic activities of wastewater after ozonation and activated carbon filtration: Different effects in liver-derived cells and bacterial indicators. Water Res. 2020, 186, 116328. [Google Scholar] [CrossRef] [PubMed]
- Neale, P.A.; Munz, N.A.; Aït-Aïssa, S.; Altenburger, R.; Brion, F.; Busch, W.; Escher, B.I.; Hilscherová, K.; Kienle, C.; Novák, J.; et al. Integrating chemical analysis and bioanalysis to evaluate the contribution of wastewater effluent on the micropollutant burden in small streams. Sci. Total Environ. 2017, 576, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Bioassay | Effect | Measured Endpoint or Molecular Target | Reference Compound | EBT )** [BEQ] |
|---|---|---|---|---|
| Cytotox | Cytotoxicity | Repression of constitutive transcriptional activation | Tributyltin acetate | - |
| ERα )* | Estrogenicity | Estrogen receptor α-mediated signalling | 17β-Estradiol | 0.1 ng/L |
| anti-ERα | Anti-estrogenicity | Repression of estrogen receptor α-mediated signalling | Tamoxifen | not available |
| anti-AR )* | Anti-androgenicity | Repression of androgen receptor activation | Flutamide | 14 µg/L |
| Nrf2 )* | Oxidative stress response | Activation of the Nrf2 pathway | Curcumin | 10 µg/L |
| p53 + S9 | Genotoxicity response +S9 | p53-dependent pathway activation with S9 | Cyclo-phosphamide | - |
| p53 − S9 | Genotoxicity response -S9 | p53-dependent pathway activation without S9 | Actinomycin | - |
| PAH )* | Toxic PAH-xenobiotics metabolism | Aryl-hydrocarbon receptor activation | Benzo[a]pyrene | 6.2 ng/L |
| PXR )* | Xenobiotic metabolism and sensing | Activation of pregnane X receptor | Nicardipine | 3 µg/L |
| Bioassay | EBT | CAS-OUT | O3-OUT | GAC-OUT | CAS-OUT | O3-OUT | GAC-OUT | |
|---|---|---|---|---|---|---|---|---|
| Frequency of Exceedance [n/All] | ||||||||
| ERα | 0.1 | 4 | 0.4 | 0.6 | 14/16 | 1/14 | 1/7 | |
| anti-AR | 14 | 0.1 | 0.2 | 0.2 | 2/16 | 0/16 | 0/7 | |
| Nrf2 | 10 | 9 | 7 | 9 | 13/13 | 13/13 | 5/5 | |
| PAH | 6.2 | 23 | 9 | 14 | 8/8 | 8/8 | 4/4 | |
| PXR | 3 | 13 | 6 | 5 | 3/3 | 2/3 | 2/2 | |
| BEQ/EBT < 1 | 1 ≤ BEQ/EBT < 3 | 3 ≤ BEQ/EBT < 10 | 10 ≤ BEQ/EBT < 100 | BEQ/EBT > 100 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phan, L.T.; Schaar, H.; Reif, D.; Weilguni, S.; Saracevic, E.; Krampe, J.; Behnisch, P.A.; Kreuzinger, N. Long-Term Toxicological Monitoring of a Multibarrier Advanced Wastewater Treatment Plant Comprising Ozonation and Granular Activated Carbon with In Vitro Bioassays. Water 2021, 13, 3245. https://doi.org/10.3390/w13223245
Phan LT, Schaar H, Reif D, Weilguni S, Saracevic E, Krampe J, Behnisch PA, Kreuzinger N. Long-Term Toxicological Monitoring of a Multibarrier Advanced Wastewater Treatment Plant Comprising Ozonation and Granular Activated Carbon with In Vitro Bioassays. Water. 2021; 13(22):3245. https://doi.org/10.3390/w13223245
Chicago/Turabian StylePhan, Lam T., Heidemarie Schaar, Daniela Reif, Sascha Weilguni, Ernis Saracevic, Jörg Krampe, Peter A. Behnisch, and Norbert Kreuzinger. 2021. "Long-Term Toxicological Monitoring of a Multibarrier Advanced Wastewater Treatment Plant Comprising Ozonation and Granular Activated Carbon with In Vitro Bioassays" Water 13, no. 22: 3245. https://doi.org/10.3390/w13223245