Dry-Season Fog Water Utilization by Epiphytes in a Subtropical Montane Cloud Forest of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
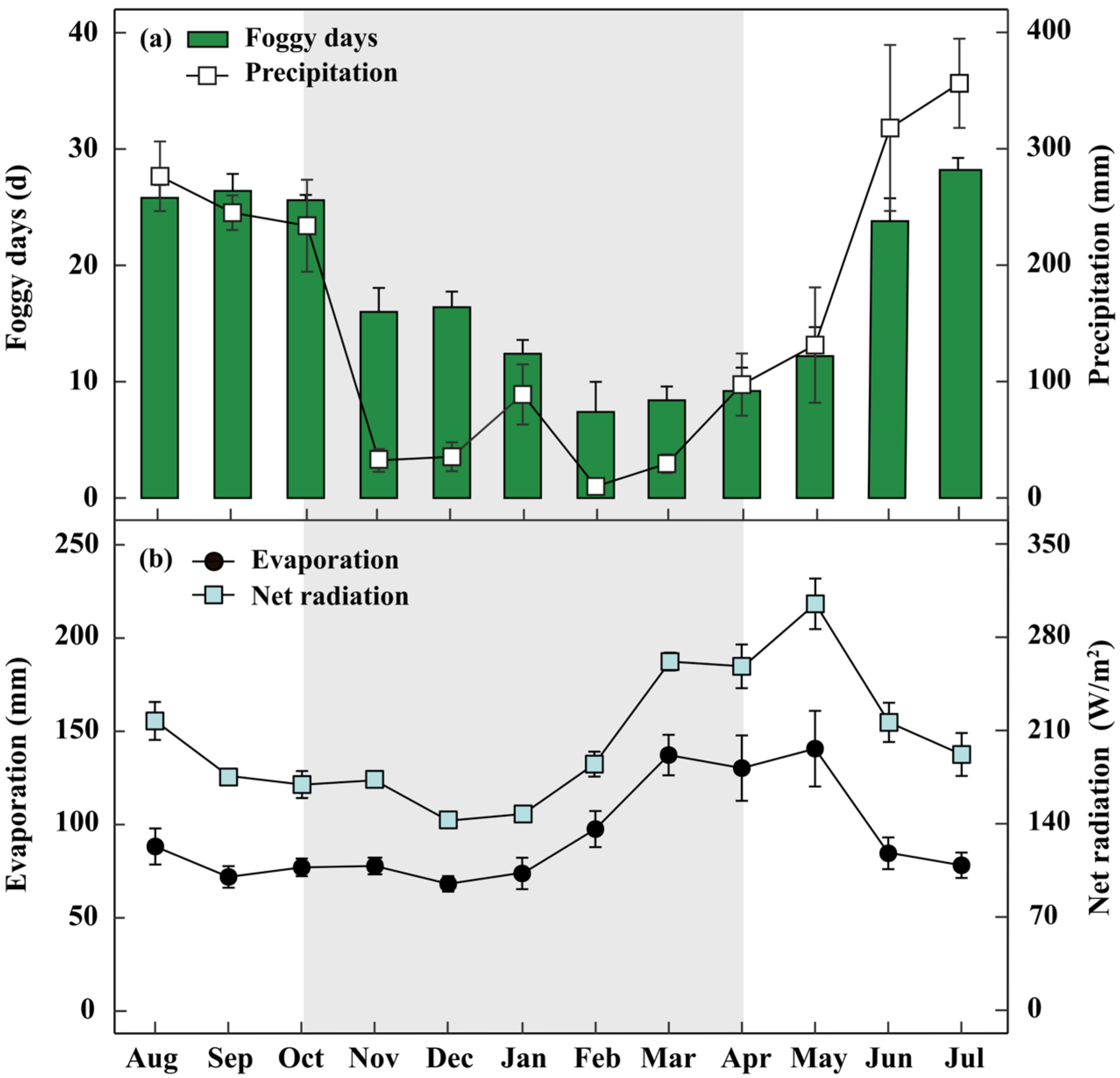
2.1. Study Site
2.2. Isotopic Sampling
2.3. Isotope Measurements
2.4. Statistical Analysis
3. Results
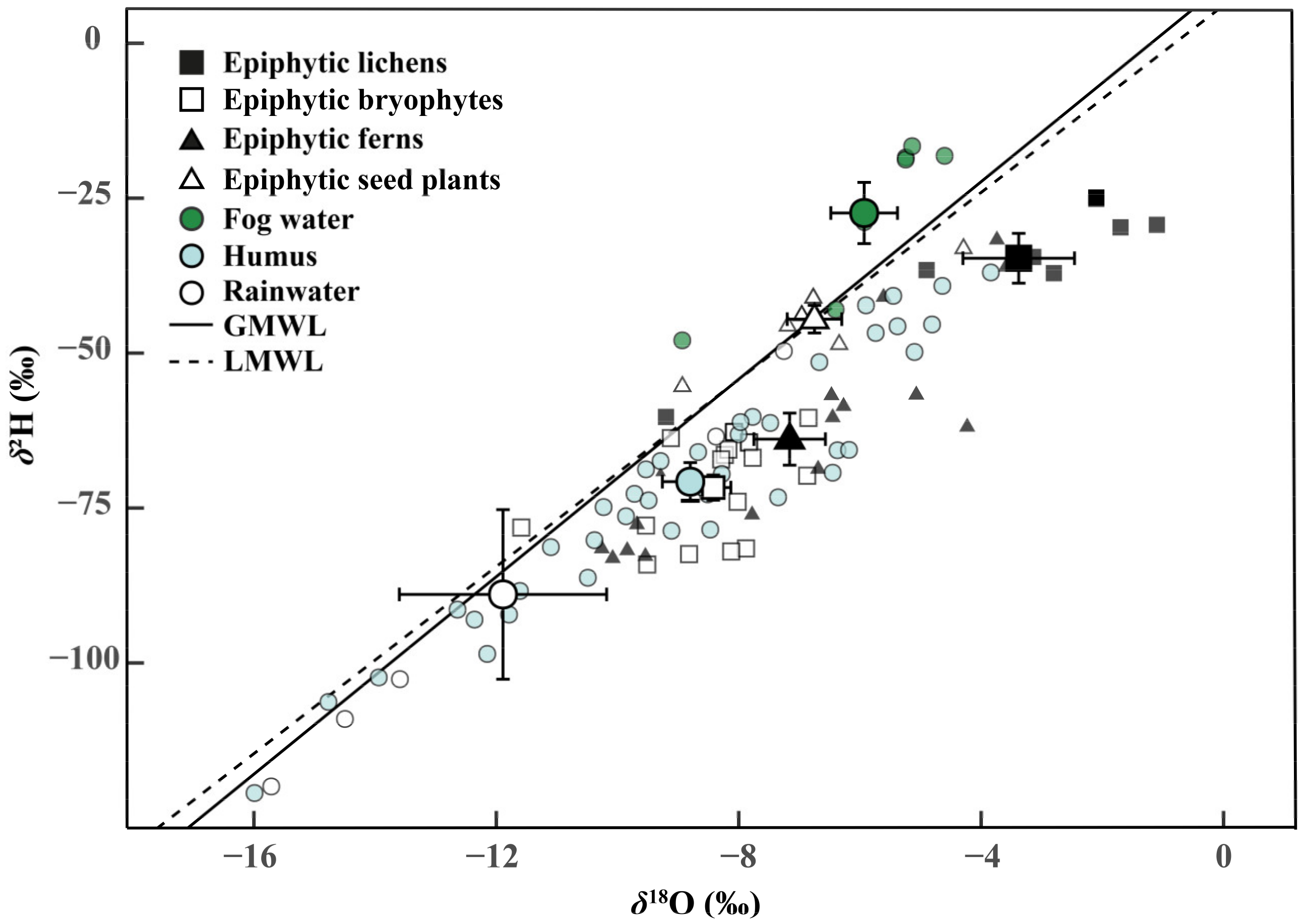
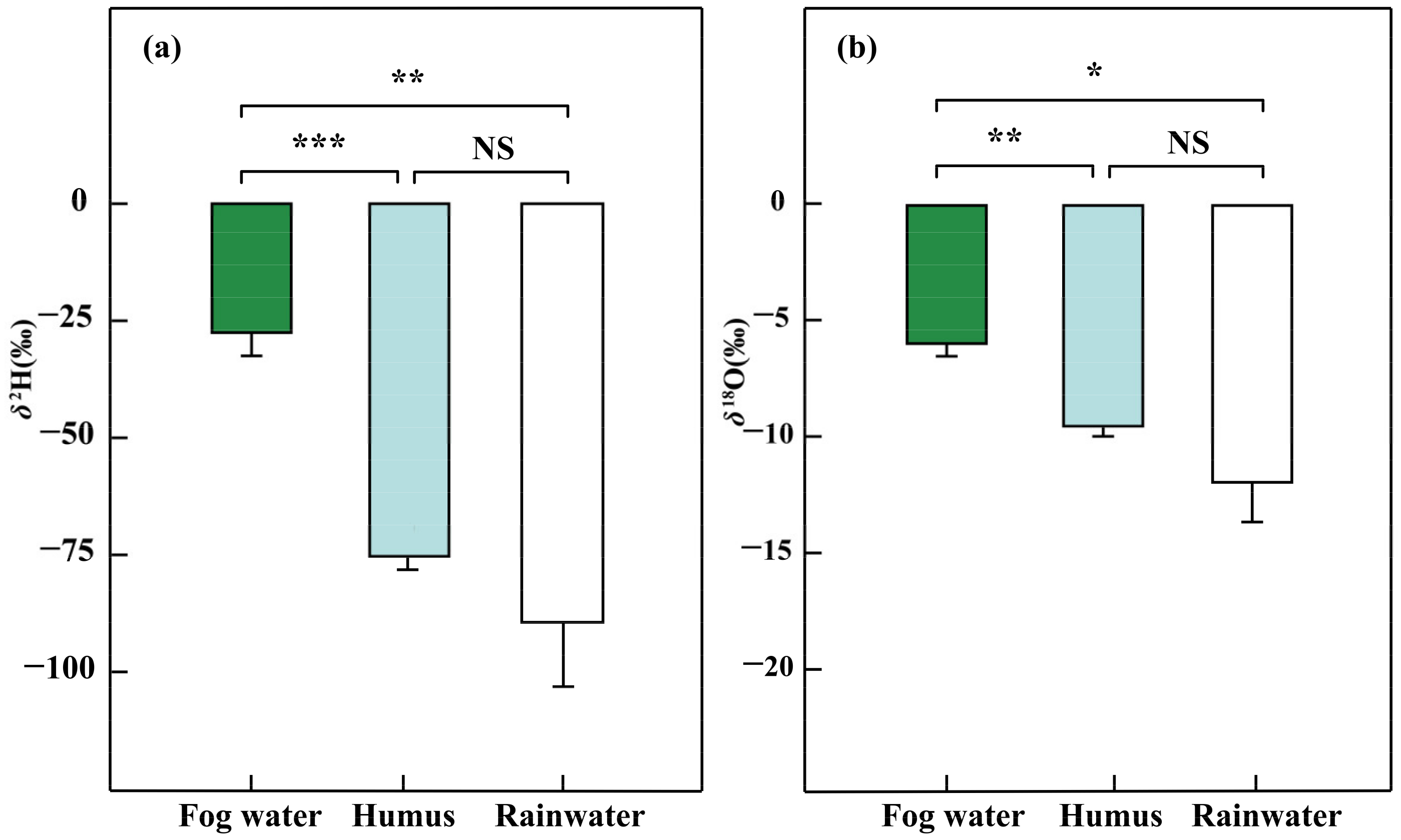
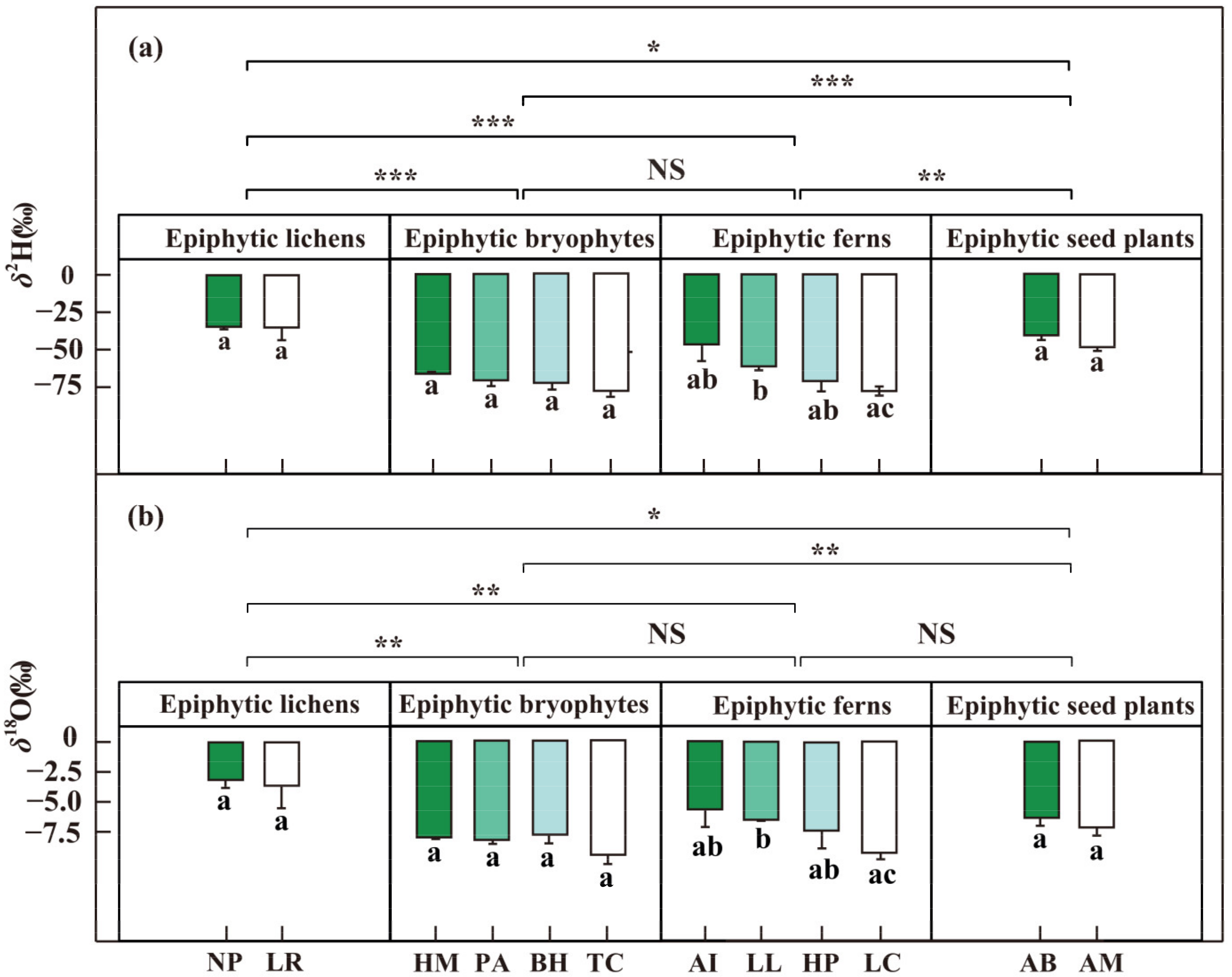
3.1. Isotopic Compositions of Water Sources and Epiphytes
3.2. Partitioning of Water Sources for Epiphytes
3.3. Leaf Carbon Isotope Ratios
4. Discussion
4.1. Water Conditions in the Subtropical MCF
4.2. Water Partitioning of the Four Epiphyte Groups
4.3. Ecological Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Ault, T.R. On the essentials of drought in a changing climate. Science 2020, 368, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Corlett, R.T. The Impacts of Droughts in Tropical Forests. Trends Plant Sci. 2016, 21, 584–593. [Google Scholar] [CrossRef]
- Wanek, W.; Pörtl, K. Phyllosphere nitrogen relations: Reciprocal transfer of nitrogen between epiphyllous liverworts and host plants in the understorey of a lowland tropical wet forest in Costa Rica. New Phytol. 2005, 166, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Bartels, S.F.; Chen, H. Mechanisms Regulating Epiphytic Plant Diversity. Crit. Rev. Plant Sci. 2012, 31, 391–400. [Google Scholar] [CrossRef]
- Hsu, R.C.-C.; Tamis, W.L.M.; Raes, N.; De Snoo, G.R.; Wolf, J.H.D.; Oostermeijer, G.; Lin, S.-H. Simulating climate change impacts on forests and associated vascular epiphytes in a subtropical island of East Asia. Divers. Distrib. 2012, 18, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Benzing, D.H. Vulnerabilities of tropical forests to climate change: The significance of resident epiphytes. In Potential Impacts of Climate Change on Tropical Forest Ecosystems; Springer: Dordrecht, The Netherlands, 1998; pp. 379–400. [Google Scholar]
- Gehrig-Downie, C.; Obregón, A.; Bendix, J.; Gradstein, S.R. Epiphyte Biomass and Canopy Microclimate in the Tropical Lowland Cloud Forest of French Guiana. Biotropica 2011, 43, 591–596. [Google Scholar] [CrossRef]
- Song, L.; Zhang, Y.-J.; Chen, X.; Li, S.; Lu, H.-Z.; Wu, C.-S.; Tan, Z.-H.; Liu, W.-Y.; Shi, X.-M. Water relations and gas exchange of fan bryophytes and their adaptations to microhabitats in an Asian subtropical montane cloud forest. J. Plant Res. 2015, 128, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, N.M. Epiphyte Biomass and Nutrient Capital of a Neotropical Elfin Forest. Biotropica 1984, 16, 249–256. [Google Scholar] [CrossRef]
- Cardelús, C.L.; Mack, M.C. The nutrient status of epiphytes and their host trees along an elevational gradient in Costa Rica. Plant Ecol. 2009, 207, 25–37. [Google Scholar] [CrossRef]
- Mendieta-Leiva, G.; Porada, P.; Bader, M.Y. Interactions of epiphytes with precipitation partitioning. In Precipitation Partitioning by Vegetation; Springer International Publishing: New York, NY, USA, 2020; pp. 133–146. [Google Scholar]
- Liu, W.-J.; Wang, P.-Y.; Li, J.-T.; Liu, W.-Y.; Li, H.-M. Plasticity of source-water acquisition in epiphytic, transitional and terrestrial growth phases of Ficus tinctoria. Ecohydrology 2014, 7, 1524–1533. [Google Scholar] [CrossRef]
- Yoder, J.A.; Zettler, L.W.; Stewart, S.L. Water requirements of terrestrial and epiphytic orchid seeds and seedlings, and evidence for water uptake by means of mycotrophy. Plant Sci. 2000, 156, 145–150. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Liu, W.-Y. Species diversity and distribution of epiphytes in the montane moist evergreen broad-leaved forest in Ailao Mountain, Yunnan. Biodivers. Sci. 2005, 13, 137–147. [Google Scholar] [CrossRef]
- Ma, W.-Z. The Composition and Biomass of Epiphytic Materials and Their Relationships with Ecological Factors in Xujiaba Region from Ailao Mountain, Yunnan. Ph.D. Thesis, Graduate School of the Chinese Academy of Sciences, Beijing, China, 2009. [Google Scholar]
- Zotz, G.; Hietz, P. The physiological ecology of vascular epiphytes: Current knowledge, open questions. J. Exp. Bot. 2001, 52, 2067–2078. [Google Scholar] [CrossRef] [PubMed]
- Watkins, J.E., Jr.; Mack, M.C.; Sinclair, T.R.; Mulkey, S.S. Ecological and evolutionary consequences of desiccation tolerance in tropical fern gametophytes. New Phytol. 2007, 176, 708–717. [Google Scholar] [CrossRef]
- Benzing, D.H. Air Plants: Epiphytes and Aerial Gardens; Cornell University Press: Ithaca, NY, USA, 2012. [Google Scholar]
- Reyes-García, C.; Mejia-Chang, M.; Griffiths, H. High but not dry: Diverse epiphytic bromeliad adaptations to exposure within a seasonally dry tropical forest community. New Phytol. 2011, 193, 745–754. [Google Scholar] [CrossRef]
- Querejeta, J.I.; Prieto, I.; Torres, P.; Campoy, M.; Alguacil, M.M.; Roldán, A. Water-spender strategy is linked to higher leaf nutrient concentrations across plant species colonizing a dry and nutrient-poor epiphytic habitat. Environ. Exp. Bot. 2018, 153, 302–310. [Google Scholar] [CrossRef]
- Zotz, G. The systematic distribution of vascular epiphytes—A critical update. Bot. J. Linn. Soc. 2013, 171, 453–481. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Long, W.; Zhang, H.; Huang, J.; Cheng, Y.; Jiang, H.; Liao, L.; Tan, Z. Divergent Strategies of Epiphytic Pteridophytes and Angiosperms Responding to Dry and Wet Seasons in a Tropical Cloud Forest. Trop. Conserv. Sci. 2020, 13. [Google Scholar] [CrossRef]
- Villegas, J.C.; Tobón, C.; Breshears, D.D. Fog interception by non-vascular epiphytes in tropical montane cloud forests: De-pendencies on gauge type and meteorological conditions. Hydrol. Process. 2008, 22, 2484–2492. [Google Scholar] [CrossRef]
- Darby, A.; Draguljić, D.; Glunk, A.; Gotsch, S.G. Habitat moisture is an important driver of patterns of sap flow and water balance in tropical montane cloud forest epiphytes. Oecologia 2016, 182, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.; Turton, S.M. Importance of drought on the distribution of the birds nest fern, Asplenium nidus, in the canopy of a lowland tropical rainforest in north-eastern Australia. Austral Ecol. 2007, 32, 70–76. [Google Scholar] [CrossRef]
- Wu, Y.; Song, L.; Liu, W.; Liu, W.; Li, S.; Fu, P.; Shen, Y.; Wu, J.; Wang, P.; Chen, Q.; et al. Fog Water Is Important in Maintaining the Water Budgets of Vascular Epiphytes in an Asian Tropical Karst Forests during the Dry Season. Forests 2018, 9, 260. [Google Scholar] [CrossRef] [Green Version]
- Watkins, J.E.; Rundel, P.W.; Cardelús, C.L. The influence of life form on carbon and nitrogen relationships in tropical rainforest ferns. Oecologia 2007, 153, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kranner, I.; Beckett, R.; Hochman, A.; Nash, T.H. Desiccation-Tolerance in Lichens: A Review. Bryologist 2008, 111, 576–593. [Google Scholar] [CrossRef]
- Green, T.G.A.; Sancho, L.G.; Pintado, A. Ecophysiology of desiccation/rehydration cycles in mosses and lichens. In Plant Desiccation Tolerance; Springer: Berlin/Heidelberg, Germany, 2011; pp. 89–120. [Google Scholar]
- Ah-Peng, C.; Cardoso, A.W.; Flores, O.; West, A.; Wilding, N.; Strasberg, D.; Hedderson, T.A. The role of epiphytic bryophytes in interception, storage, and the regulated release of atmospheric moisture in a tropical montane cloud forest. J. Hydrol. 2017, 548, 665–673. [Google Scholar] [CrossRef]
- Horwath, A.B.; Royles, J.; Tito, R.; Gudiño, J.A.; Allen, N.S.; Farfan-Rios, W.; Rapp, J.M.; Silman, M.R.; Malhi, Y.; Swamy, V.; et al. Bryophyte stable isotope composition, diversity and biomass define tropical montane cloud forest extent. Proc. R. Soc. B Boil. Sci. 2019, 286, 20182284. [Google Scholar] [CrossRef] [Green Version]
- Proctor, M.C.F. The diversification of bryophytes and vascular plants in evolving terrestrial environments. In Photosynthesis in Bryophytes and Early Land Plants; Hanson, D.T., Rice, S.K., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 59–77. [Google Scholar]
- Tsutsumi, C.; Kato, M. Evolution of epiphytes in Davalliaceae and related ferns. Bot. J. Linn. Soc. 2006, 151, 495–510. [Google Scholar] [CrossRef] [Green Version]
- Zotz, G. Plants on Plants: The Biology of Vascular Epiphytes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Yuan, G.-D.; Liu, W.-Y.; Shi, X.-M.; Fan, X.-Y. Effects of water content change on photosynthetic and fluorescence parameters of four bole epiphytic bryophytes in montane moist evergreen broad-leaved forest in the Ailao Mountains. Plant Sci. J. 2018, 36, 603–611. [Google Scholar]
- Zhao, C.; Deng, X.; Yuan, Y.; Yan, H.; Liang, H. Prediction of Drought Risk Based on the WRF Model in Yunnan Province of China. Adv. Meteorol. 2013, 2013, 295856. [Google Scholar] [CrossRef]
- Williams, C.B.; Murray, J.G.; Glunk, A.; Dawson, T.E.; Nadkarni, N.M.; Gotsch, S.G. Vascular epiphytes show low physiological resistance and high recovery capacity to episodic, short-term drought in Monteverde, Costa Rica. Funct. Ecol. 2020, 34, 1537–1550. [Google Scholar] [CrossRef]
- Song, L.; Lu, H.-Z.; Xu, X.-L.; Li, S.; Shi, X.-M.; Chen, X.; Wu, Y.; Huang, J.-B.; Chen, Q.; Liu, S.; et al. Organic nitrogen uptake is a significant contributor to nitrogen economy of subtropical epiphytic bryophytes. Sci. Rep. 2016, 6, 30408. [Google Scholar] [CrossRef] [Green Version]
- Goswami, P.; Sarkar, S. Analysis and quantification of contrasts in observed meteorological fields for foggy and non-foggy days. Meteorol. Atmos. Phys. 2015, 127, 605–623. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Cao, K.-F.; Goldstein, G. Winter photosynthesis of evergreen broadleaf trees from a montane cloud forest in Subtropical China. In Advanced Topics in Science and Technology in China; Springer: Berlin/Heidelberg, Germany, 2013; pp. 812–817. [Google Scholar]
- Li, S.; Liu, W.-Y.; Li, D.-W. Epiphytic lichens in subtropical forest ecosystems in Southwest China: Species diversity and implications for conservation. Biol. Conserv. 2013, 159, 88–95. [Google Scholar] [CrossRef]
- Lu, H.; Brooker, R.; Song, L.; Liu, W.; Sack, L.; Zhang, J.; Yu, F. When facilitation meets clonal integration in forest canopies. New Phytol. 2020, 225, 135–142. [Google Scholar] [CrossRef]
- Tang, D.-D.; Wu, Y.; Liu, W.-Y.; Chen, Q.; Li, D.-F.; Zhang, T.-T. Diversity and floristic characteristics of vascular epiphytes in montane forests in the Ailao Mountains, Yunnan. Plant Sci. J. 2018, 36, 658–666. [Google Scholar]
- Ehleringer, J.R.; Dawson, T.E. Water uptake by plants: Perspectives from stable isotope composition. Plant Cell Environ. 1992, 15, 1073–1082. [Google Scholar] [CrossRef]
- Dawson, T.E.; Simonin, K.A. The roles of stable isotopes in forest hydrology and biogeochemistry. In Forest Hydrology and Biogeochemistry; Springer: Dordrecht, The Netherlands, 2011; pp. 137–161. [Google Scholar]
- Hietz, P.; Wanek, W.; Popp, M. Stable isotopic composition of carbon and nitrogen and nitrogen content in vascular epiphytes along an altitudinal transect. Plant Cell Environ. 1999, 22, 1435–1443. [Google Scholar] [CrossRef]
- Moreno-Gutierrez, C.; Dawson, T.E.; Nicolas, E.; Querejeta, J.I. Isotopes reveal contrasting water use strategies among coex-isting plant species in a Mediterranean ecosystem. New Phytol. 2012, 196, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.S.O.; Dawson, T.E. The contribution of fog to the water relations of Sequoia sempervirens (D. Don): Foliar uptake and prevention of dehydration. Plant Cell Environ. 2004, 27, 1023–1034. [Google Scholar] [CrossRef]
- Emery, N.C. Foliar uptake of fog in coastal California shrub species. Oecologia 2016, 182, 731–742. [Google Scholar] [CrossRef]
- Eller, C.B.; Lima, A.L.; Oliveira, R.S. Foliar uptake of fog water and transport belowground alleviates drought effects in the cloud forest tree species, Drimys brasiliensis (Winteraceae). New Phytol. 2013, 199, 151–162. [Google Scholar] [CrossRef]
- Hietz, P.; Wanek, W. Size-Dependent Variation of Carbon and Nitrogen Isotope Abundances in Epiphytic Bromeliads. Plant Biol. 2003, 5, 137–142. [Google Scholar] [CrossRef]
- Yang, B.; Meng, X.; Zhu, X.; Zakari, S.; Singh, A.K.; Bibi, F.; Mei, N.; Song, L.; Liu, W. Coffee performs better than amomum as a candidate in the rubber agroforestry system: Insights from water relations. Agric. Water Manag. 2021, 244, 106593. [Google Scholar] [CrossRef]
- Stock, B.C.; Semmens, B.X. MixSIAR: Bayesian Mixing Models in R 2020. [R Package Version 3.1.12]. Available online: https://github.com/brianstock/MixSIAR (accessed on 12 November 2021).
- Wang, J.; Lu, N.; Fu, B. Inter-comparison of stable isotope mixing models for determining plant water source partitioning. Sci. Total Environ. 2019, 666, 685–693. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.-Y.; Li, J.; Zhang, C.; He, B.; Zhang, S.; Sun, W. Age-related water uptake patterns of alpine plantation shrubs in reforestation region of Qinghai-Tibetan Plateau based on stable isotopes. Ecohydrology 2019, 12, e2049. [Google Scholar] [CrossRef] [Green Version]
- Phillips, D.L.; Newsome, S.D.; Gregg, J.W. Combining sources in stable isotope mixing models: Alternative methods. Oecologia 2005, 144, 520–527. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; p. 2. [Google Scholar]
- Li, S.; Liu, S.; Shi, X.-M.; Liu, W.-Y.; Song, L.; Lu, H.-Z.; Chen, X.; Wu, C.-S. Forest Type and Tree Characteristics Determine the Vertical Distribution of Epiphytic Lichen Biomass in Subtropical Forests. Forests 2017, 8, 436. [Google Scholar] [CrossRef] [Green Version]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. lme4: Linear Mixed-Effects Models Using Eigen and S4. [R Package Version 1.1–27.1.]. 2021. Available online: https://cran.r-project.org/web/packages/lme4 (accessed on 12 November 2021).
- Brooks, J.R.; Barnard, H.R.; Coulombe, R.; Mcdonnell, J. Ecohydrologic separation of water between trees and streams in a Mediterranean climate. Nat. Geosci. 2009, 3, 100–104. [Google Scholar] [CrossRef]
- Scholl, M.; Eugster, W.; Burkard, R. Understanding the role of fog in forest hydrology: Stable isotopes as tools for determining input and partitioning of cloud water in montane forests. Hydrol. Process. 2011, 25, 353–366. [Google Scholar] [CrossRef]
- Limm, E.B.; Dawson, T.E. Polystichum munitum (Dryopteridaceae) varies geographically in its capacity to absorb fog water by foliar uptake within the redwood forest ecosystem. Am. J. Bot. 2010, 97, 1121–1128. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.; Rossman, A.; Rosentreter, R.; Ponzetti, J. Lichen ecology and diversity of a sagebrush steppe in Oregon: 1977 to the present. North Am. Fungi 2011, 6, 1–14. [Google Scholar]
- Krömer, T.; Gradstein, S. Vascular epiphytes. In Core Standardized Methods for Rapid Biological Field Assessment; Conservation International: Arlington, VA, USA, 2016; pp. 25–36. [Google Scholar]
- Gauslaa, Y.; Coxson, D. Interspecific and intraspecific variations in water storage in epiphytic old forest foliose lichens. Botany 2011, 89, 787–798. [Google Scholar] [CrossRef]
- Mediavilla, S.; Escudero, A.; Heilmeier, H. Internal leaf anatomy and photosynthetic resource-use efficiency: Interspecific and intraspecific comparisons. Tree Physiol. 2001, 21, 251–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weathers, K.C.; Ponette-González, A.G.; Dawson, T.E. Medium, vector, and connector: Fog and the maintenance of ecosystems. Ecosystems 2020, 23, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Feng, Y. Fog water absorption by the leaves of epiphytes and non-epiphytes in Xishuangbanna. J. Appl. Ecol. 2006, 17, 977–981. [Google Scholar]
- Goldsmith, G.R.; Matzke, N.; Dawson, T.E. The incidence and implications of clouds for cloud forest plant water relations. Ecol. Lett. 2013, 16, 307–314. [Google Scholar] [CrossRef]
- Yang, B.; Wen, X.; Sun, X. Seasonal variations in depth of water uptake for a subtropical coniferous plantation subjected to drought in an East Asian monsoon region. Agric. For. Meteorol. 2015, 201, 218–228. [Google Scholar] [CrossRef]
- Fischer, B.M.; van Meerveld, H.J.; Seibert, J. Spatial variability in the isotopic composition of rainfall in a small headwater catchment and its effect on hydrograph separation. J. Hydrol. 2017, 547, 755–769. [Google Scholar] [CrossRef] [Green Version]
- Bedaso, Z.K.; DeLuca, N.M.; Levin, N.; Zaitchik, B.; Waugh, D.W.; Wu, S.-Y.; Harman, C.J.; Shanko, D. Spatial and temporal variation in the isotopic composition of Ethiopian precipitation. J. Hydrol. 2020, 585, 124364. [Google Scholar] [CrossRef]
- Limm, E.B.; Simonin, K.A.; Bothman, A.G.; Dawson, T.E. Foliar water uptake: A common water acquisition strategy for plants of the redwood forest. Oecologia 2009, 161, 449–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Querejeta, J.I.; Estrada-Medina, H.; Allen, M.F.; Jiménez-Osornio, J.J. Water source partitioning among trees growing on shallow karst soils in a seasonally dry tropical climate. Oecologia 2007, 152, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, P.; You, D.; Liu, W. Coupling evapotranspiration partitioning with root water uptake to identify the water consumption characteristics of winter wheat: A case study in the North China Plain. Agric. For. Meteorol. 2018, 259, 296–304. [Google Scholar] [CrossRef]
- Nadkarni, N.M.; Solano, R. Potential effects of climate change on canopy communities in a tropical cloud forest: An experimental approach. Oecologia 2002, 131, 580–586. [Google Scholar] [CrossRef] [PubMed]
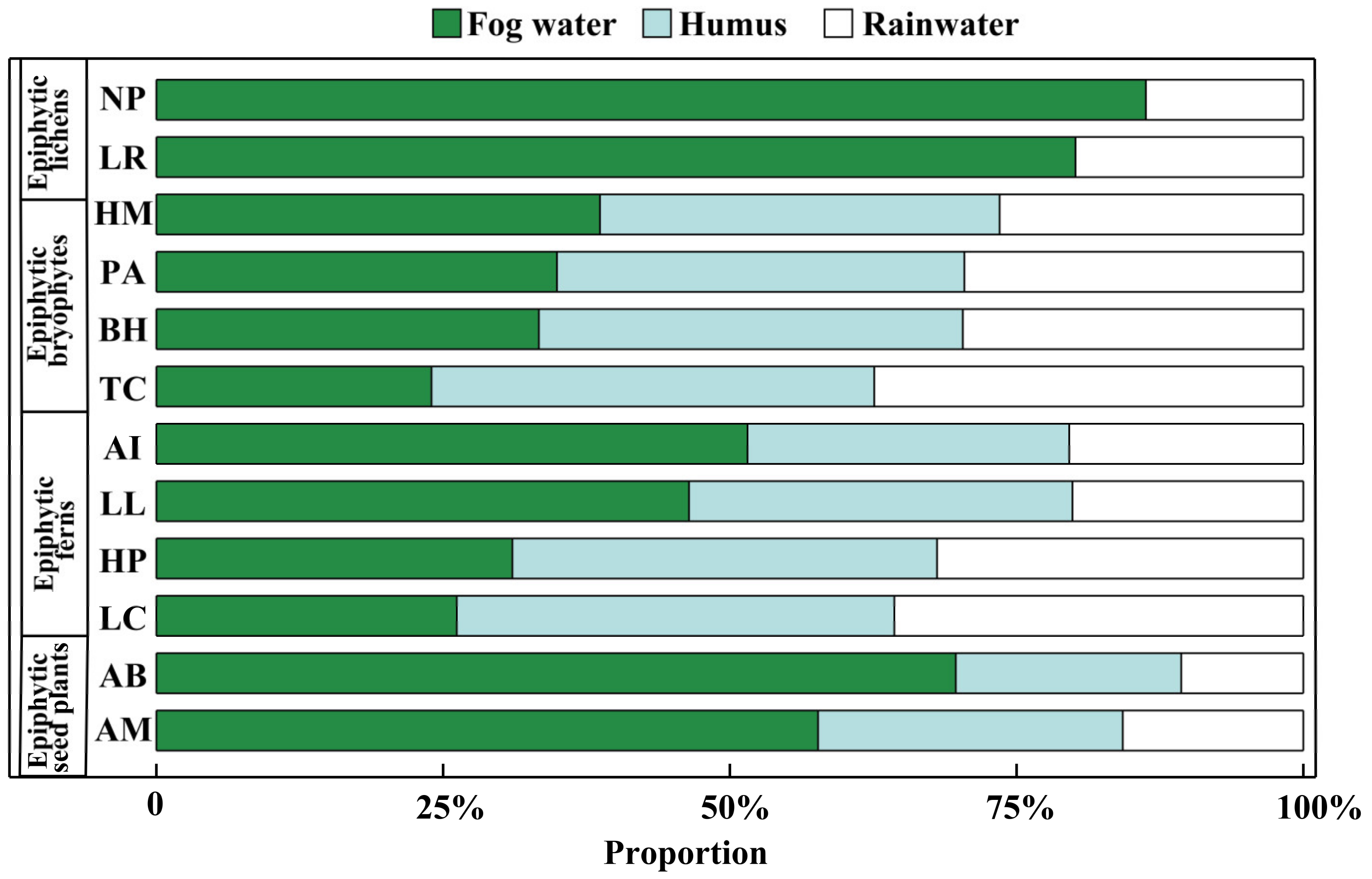
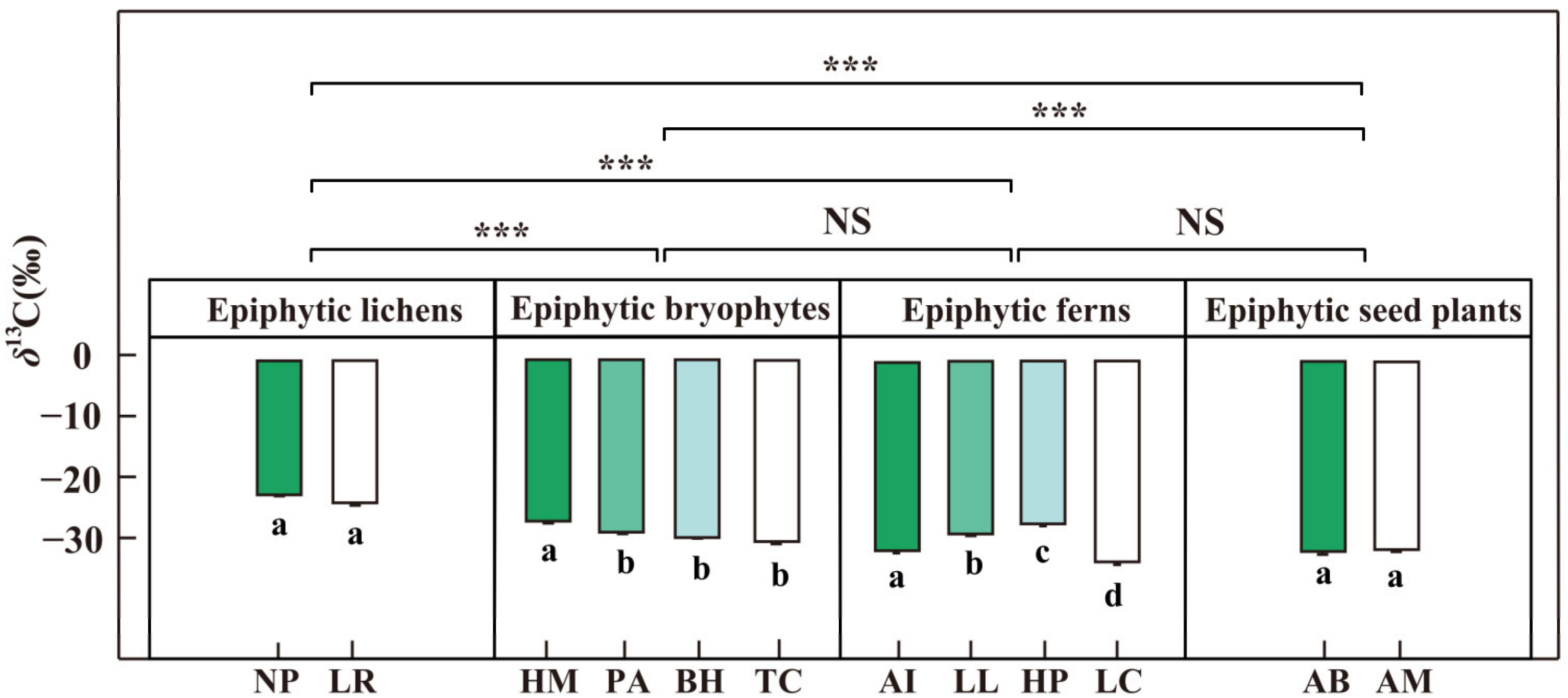
| Fixed Effect | Epiphytic Lichens | Epiphytic Bryophytes | Epiphytic Ferns | Epiphytic Seed Plants | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SE | χ2 | p | SE | χ2 | p | SE | χ2 | p | SE | χ2 | p | |
| δ2H | 0.115 | 4.624 | 0.032 * | 0.177 | 0.188 | 0.665 | 0.091 | 1.406 | 0.236 | 0.300 | 1.459 | 0.227 |
| δ18O | 0.091 | 2.394 | 0.122 | 0.197 | 0.002 | 0.965 | 0.077 | 0.065 | 0.065 | 0.194 | 3.644 | 0.056 |
| δ2H × δ18O | 1.283 | 9.236 | 0.026 * | 2.935 | 4.258 | 0.235 | 0.601 | 0.139 | 0.139 | 1.698 | 5.048 | 0.168 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.-L.; Yang, B.; Lu, H.-Z.; Wu, Y.; Meng, X.-J.; Zhang, Y.-J.; Song, L. Dry-Season Fog Water Utilization by Epiphytes in a Subtropical Montane Cloud Forest of Southwest China. Water 2021, 13, 3237. https://doi.org/10.3390/w13223237
Liu L-L, Yang B, Lu H-Z, Wu Y, Meng X-J, Zhang Y-J, Song L. Dry-Season Fog Water Utilization by Epiphytes in a Subtropical Montane Cloud Forest of Southwest China. Water. 2021; 13(22):3237. https://doi.org/10.3390/w13223237
Chicago/Turabian StyleLiu, Lu-Lu, Bin Yang, Hua-Zheng Lu, Yi Wu, Xian-Jing Meng, Yong-Jiang Zhang, and Liang Song. 2021. "Dry-Season Fog Water Utilization by Epiphytes in a Subtropical Montane Cloud Forest of Southwest China" Water 13, no. 22: 3237. https://doi.org/10.3390/w13223237







