Bioremediation of Uranium- and Nitrate-Contaminated Groundwater after the In Situ Leach Mining of Uranium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Culture of Clostridium sp. PXL2
2.3. Groundwater Treatments
2.4. Measurement of Growth
2.5. Analytical Methods
2.6. Chemicals
2.7. Statistical Methods
3. Results and Discussion
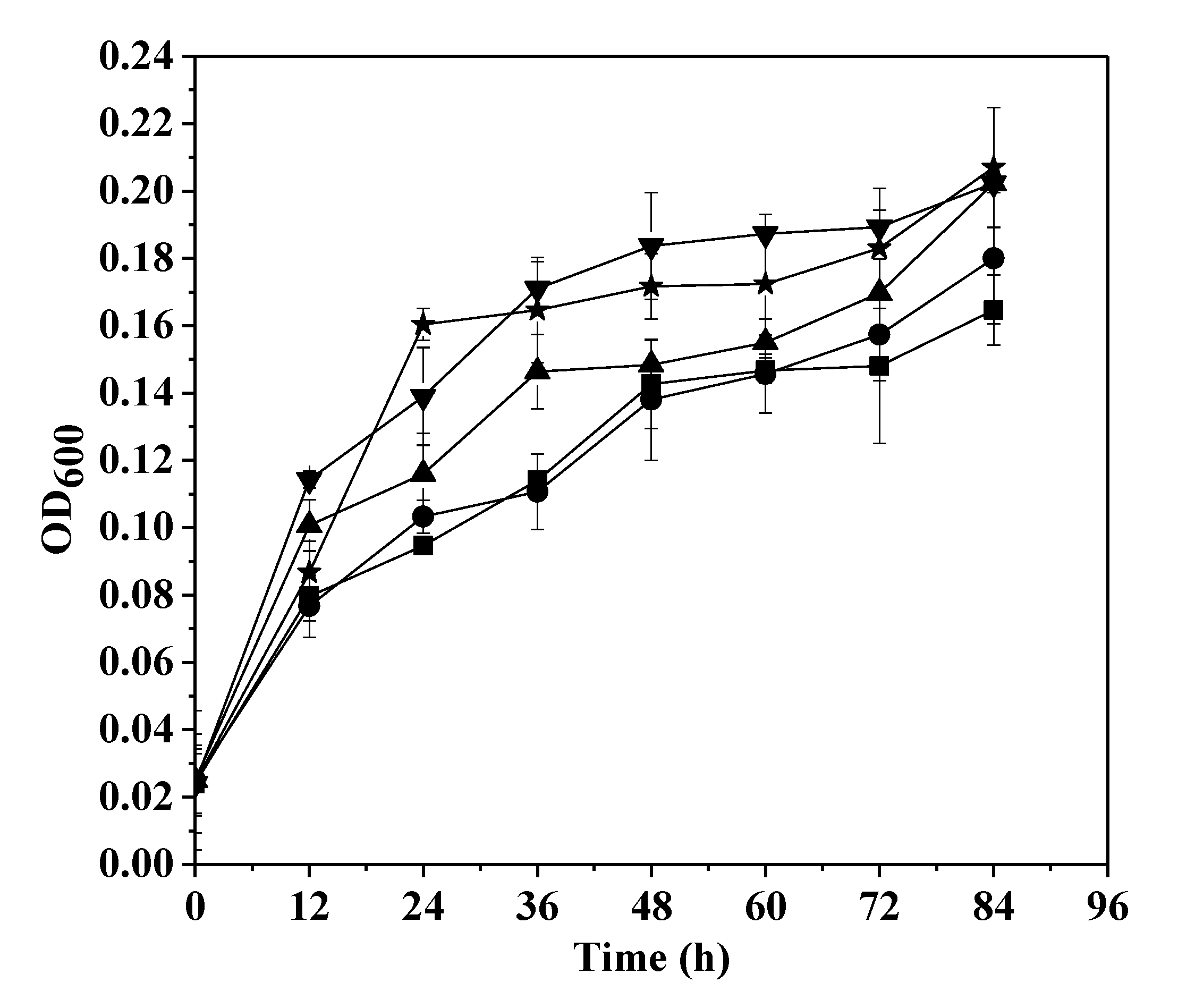
3.1. Growth in the Presence of U(VI)
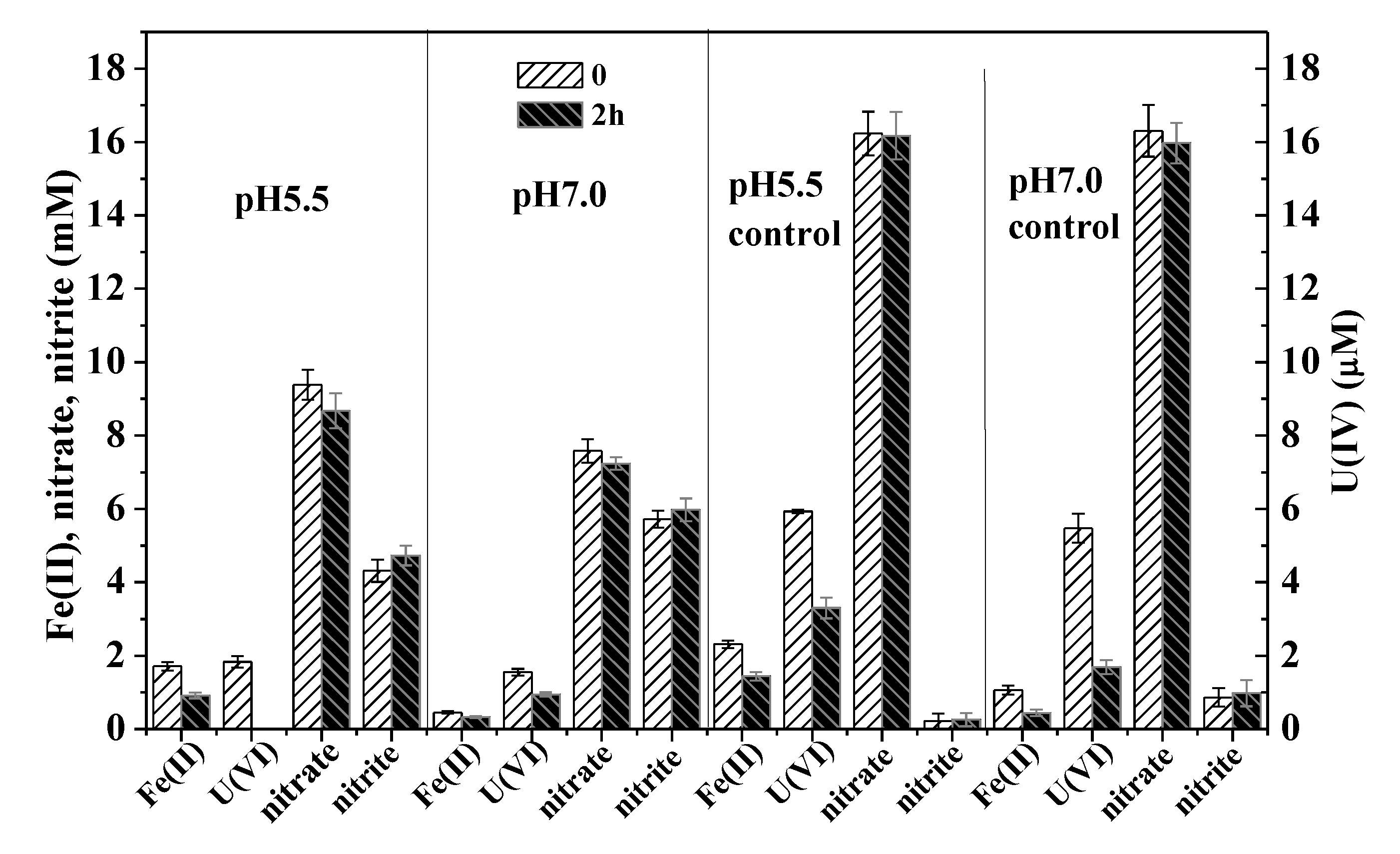
3.2. Influence of pH
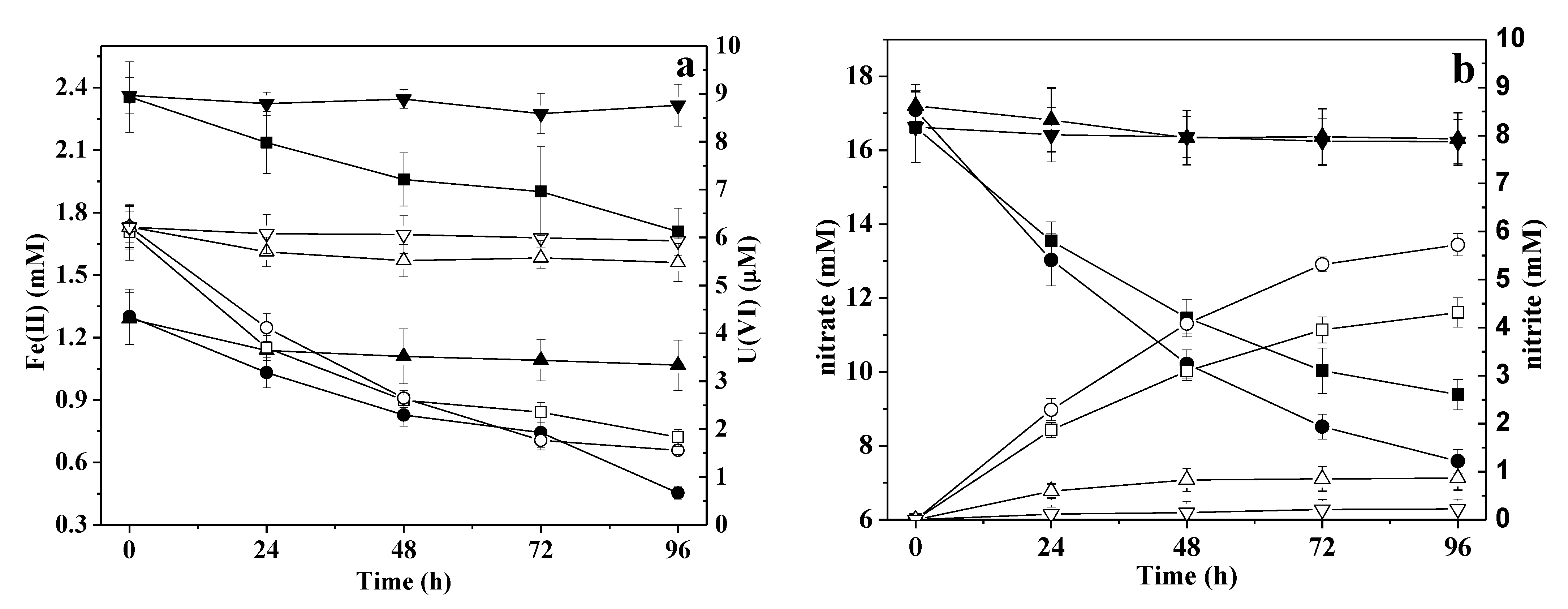
3.3. Influence of Oxidation
3.4. Effect of Fe(II)
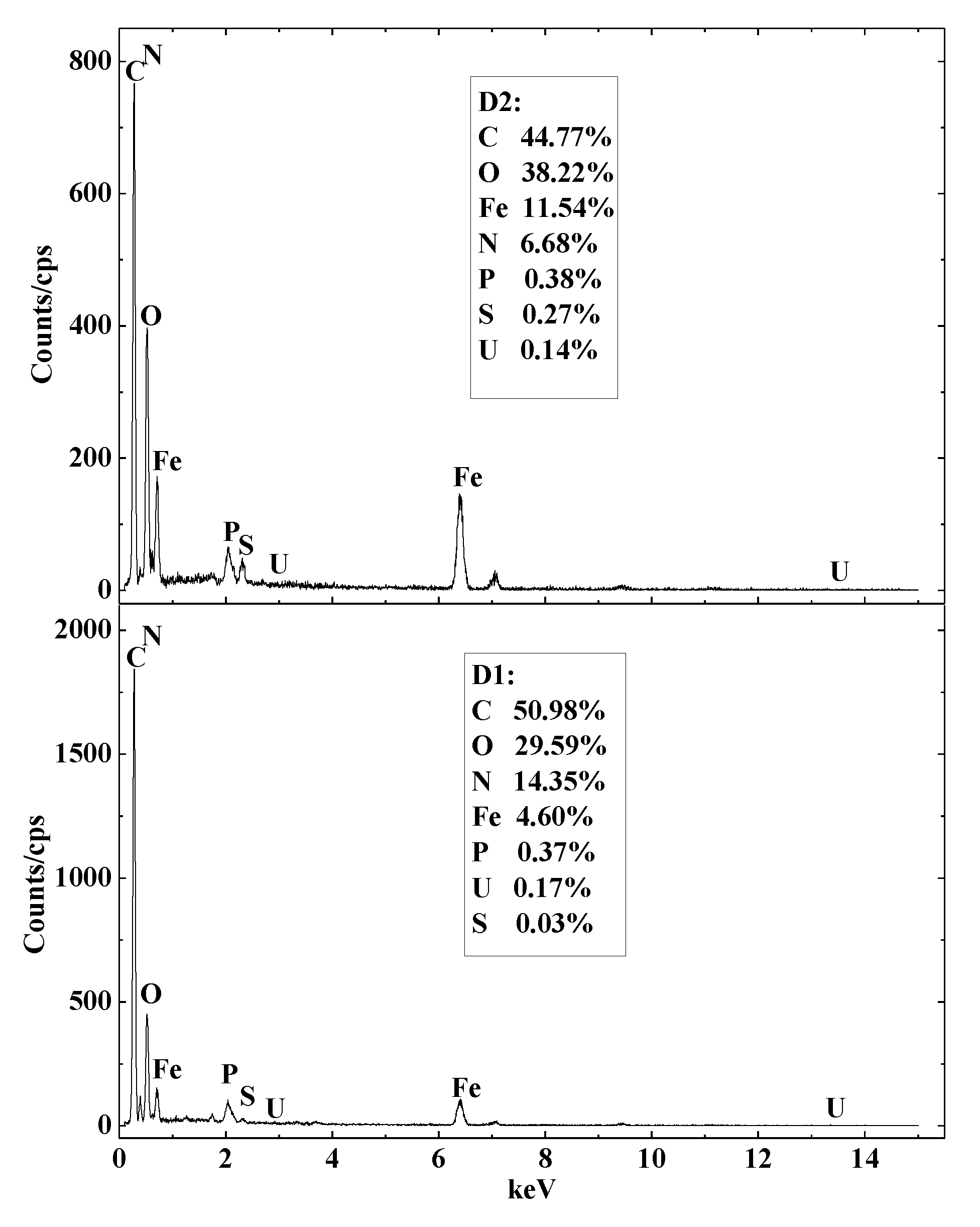
3.5. SEM-EDS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD-NEA; IAEA. Environmental Activities in Uranium Mining and Milling; OECD Publishing: Paris, France, 1999; p. 173. [Google Scholar]
- Wu, W.; Carley, J.; Fienen, M.; Mehlhorn, T.; Lowe, K.; Nyman, J.; Luo, J.; Gentile, M.; Rajan, R.; Wagner, D. Pilot-scale in situ bioremediation of uranium in a highly contaminated aquifer. 1. Conditioning of a treatment zone. Environ. Sci. Technol. 2006, 40, 3978–3985. [Google Scholar] [CrossRef] [PubMed]
- Wufuer, R.; Song, W.; Zhang, D.; Pan, X.; Gadd, G.M. A survey of uranium levels in urine and hair of people living in a coal mining area in Yili, Xinjiang, China. J. Environ. Radioact. 2018, 189, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ATSDR. Toxicological Profile for Uranium; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 1999; p. 462. [Google Scholar]
- Choy, C.C.; Korfiatis, G.P.; Meng, X. Removal of depleted uranium from contaminated soils. J. Hazard. Mater. 2006, 136, 53–60. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking Water Quality; The World Health Organisation: Geneva, Switzerland, 1998. [Google Scholar]
- USEPA. Drinking Water Contaminants; 1999. Available online: http://www.epa.gov/safewater/contaminants/index.html (accessed on 25 December 2015).
- Istok, J.D.; Senko, J.M.; Krumholz, L.R.; Watson, D.; Bogle, M.A.; Peacock, A.; Chang, Y.-J.; White, D.C. In Situ Bioreduction of Technetium and Uranium iii a Nitrate-Contaminated Aquifer. Environ. Sci. Technol. 2004, 38, 468–475. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Macaskie, L.E. Bioremediation of radionuclide-containing wastewaters. In Environmental Microbe-Metal Interactions; ASM Press: Washington, DC, USA, 2000; pp. 277–327. [Google Scholar]
- Abdelouas, A.; Lutze, W.; Gong, W.; Nuttall, E.H.; Travis, B.J. Biological reduction of uranium in groundwater and subsurface soil. Sci. Total Environ. 2000, 250, 21–35. [Google Scholar] [CrossRef]
- Fan, A.M.; Steinberg, V.E. Health Implications of Nitrate and Nitrite in Drinking Water: An Update on Methemoglobinemia Occurrence and Reproductive and Developmental Toxicity. Regul. Toxicol. Pharmacol. Rtp 1996, 23, 35. [Google Scholar] [CrossRef]
- Li, B.; Pan, X.; Zhang, D.; Lee, D.J.; Al-Misned, F.A.; Mortuza, M.G. Anaerobic nitrate reduction with oxidation of Fe(II) by Citrobacter Freundii strain PXL1–a potential candidate for simultaneous removal of As and nitrate from groundwater. Ecol. Eng. 2015, 77, 196–201. [Google Scholar] [CrossRef]
- Mudd, G.M. Critical review of acid in situ leach uranium mining: 1. USA and Australia. Environ. Geol. 2001, 41, 390–403. [Google Scholar] [CrossRef]
- Mudd, G.M. Critical review of acid in situ leach uranium mining: 2. Soviet Block and Asia. Environ. Geol. 2001, 41, 404–416. [Google Scholar] [CrossRef]
- Beazley, M.J.; Martinez, R.J.; Webb, S.M.; Sobecky, P.A.; Taillefert, M. The effect of pH and natural microbial phosphatase activity on the speciation of uranium in subsurface soils. Geochim. Cosmochim. Acta 2011, 75, 5648–5663. [Google Scholar] [CrossRef]
- Macaskie, L.E.; Bonthrone, K.M.; Yong, P.; Goddard, D.T. Enzymically mediated bioprecipitation of uranium by a Citrobacter sp.: A concerted role for exocellular lipopolysaccharide and associated phosphatase in biomineral formation. Microbiology 2000, 146, 1855–1867. [Google Scholar] [CrossRef] [Green Version]
- Kuippers, G.; Morris, K.; Townsend, L.T.; Bots, P.; Lloyd, J.R. Biomineralization of Uranium-Phosphates Fueled by Microbial Degradation of Isosaccharinic Acid (ISA). Environ. Sci. Technol. 2021, 55, 4597–4606. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.; Gorby, Y.A.; Landa ER Lovley, D.R.; Phillips, E.J.; Gorby, Y.A.; Landa, E.R. Microbial reduction of uranium. Nature 1991, 350, 413. [Google Scholar] [CrossRef]
- Williams, K.H.; Long, P.E.; Davis, J.A.; Wilkins, M.J.; N’Guessan, A.L.; Steefel, C.I.; Yang, L.; Newcomer, D.; Spane, F.A.; Kerkhof, L.J. Acetate availability and its influence on sustainable bioremediation of uranium-contaminated groundwater. Geomicrobiol. J. 2011, 28, 519–539. [Google Scholar] [CrossRef]
- Wang, G.; Yang, S.; Zhou, Y.; Yuan, H.; Shiyou, L.I.; Yijin, L.; Xie, S. Research Progress on the Bioremediation of Groundwater Polluted by Uranium via Bio-reduction. Environ. Sci. Technol. 2019, 42, 47–53. [Google Scholar]
- You, W.; Peng, W.; Tian, Z.; Zheng, M. Uranium bioremediation with U(VI)-reducing bacteria. Sci. Total Environ. 2021, 798, 1–15. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 2010, 84, 13–28. [Google Scholar] [CrossRef]
- Schiewer, S.; Volesky, B. Biosorption processes for heavy metal removal. Environ. Microbe-Met. Interact. 2000, 14, 329–362. [Google Scholar]
- Suzuki, Y.; Banfield, J.F. Geomicrobiology of uranium. Rev. Minera. Geochem. 1999, 38, 393–432. [Google Scholar]
- Coelho, E.; Reis, T.A.; Cotrim, M.; Rizzutto, M.; Corrêa, B. Bioremediation of water contaminated with uranium using Penicillium piscarium. Biotechnol. Prog. 2020, 36, e30322. [Google Scholar] [CrossRef]
- Choudhary, S.; Sar, P. Uranium biomineralization by a metal resistant Pseudomonas aeruginosa strain isolated from contaminated mine waste. J. Hazard. Mater. 2011, 186, 336–343. [Google Scholar] [CrossRef]
- Anderson, R.T.; Vrionis, H.A.; Ortiz-Bernad, I.; Resch, C.T.; Long, P.E.; Dayvault, R.; Karp, K.; Marutzky, S.; Metzler, D.R.; Peacock, A. Stimulating the in situ activity of geobacter species to remove uranium from the groundwater of a uranium-contaminated aquifer. Appl. Environ. Microbiol. 2003, 69, 5884–5891. [Google Scholar] [CrossRef] [Green Version]
- Duff, M.C.; Hunter, D.B.; Bertsch, P.M.; Amrhein, C. Factors influencing uranium reduction and solubility in evaporation pond sediments. Biogeochemistry 1999, 45, 95–114. [Google Scholar] [CrossRef]
- Wu, W.M.; Carley, J.; Gentry, T.; Ginder-Vogel, M.A.; Criddle, C.S. Pilot-scale in situ bioremedation of uranium in a highly contaminated aquifer. 2. Reduction of u(VI) and geochemical control of u(VI) bioavailability. Environ. Sci. Technol. 2006, 40, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Komlos, J.; Jaffe, P.R. Uranium reoxidation in previously bioreduced sediment by dissolved oxygen and nitrate. Environ. Sci. Technol. 2007, 41, 4587–4592. [Google Scholar] [CrossRef] [PubMed]
- Senko, J.M.; Mohamed, Y.; Dewers, T.A.; Krumholz, L.R. Role for Fe(III) minerals in nitrate-dependent microbial U(IV) oxidation. Environ. Sci. Technol. 2005, 39, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Senko, J.M.; Istok, J.D.; Suflita, J.M.; Krumholz, L.R. In-situ evidence for uranium immobilization and remobilization. Environ. Sci. Technol. 2002, 36, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Ferard, C. Significance, mechanisms and environmental implications of microbial biomineralization. Comptes Rendus-Géoscience 2011, 343, 160–167. [Google Scholar] [CrossRef]
- Straub, K.L.; Schonhuber, W.A.; Buchholz-Cleven, B.; Schink, B. Diversity of ferrous iron-oxidizing, nitrate-reducing bacteria and their involvement in oxygen-independent iron cycling. Geomicrobiol. J. 2004, 21, 371–378. [Google Scholar] [CrossRef]
- Weber, K.A.; Urrutia, M.M.; Churchill, P.F.; Kukkadapu, R.K.; Roden, E.E. Anaerobic redox cycling of iron by freshwater sediment microorganisms. Environ. Microbiol. 2010, 8, 100–113. [Google Scholar] [CrossRef] [Green Version]
- Means, J.L.; Crerar, D.A.; Borcsik, M.P.; Duguid, J.O. Radionuclide adsorption by manganese oxides and implications for radioactive waste disposal. Nature 1978, 274, 44–47. [Google Scholar] [CrossRef]
- Means, J.L.; Crerar, D.A.; Borcsik, M.P.; Duguid, J.O. Adsorption of Co and selected actinides by Mn and Fe oxides in soils and sediments. Geochim. Cosmochim. Acta 1978, 42, 1763–1773. [Google Scholar] [CrossRef]
- Slanina, J.; Lingerak, W.A.; Bergman, L. A fast determination of nitrate in rain and surface waters by means of UV spectrophotometry. Fresenius Z. Für Anal. Chem. 1976, 280, 365–368. [Google Scholar] [CrossRef]
- Gendel, Y.; Lahav, O. Accurate determination of Fe(II) concentrations in the presence of a very high soluble Fe(III) background. Appl. Geochem. 2008, 23, 2123–2129. [Google Scholar] [CrossRef]
- Klueglein, N.; Kappler, A. Abiotic oxidation of Fe(II) by reactive nitrogen species in cultures of the nitrate-reducing Fe(II) oxidizer Acidovorax sp. BoFeN1–questioning the existence of enzymatic Fe(II) oxidation. Geobiology 2013, 11, 180–190. [Google Scholar] [CrossRef]
- Jauberty, L.; Drogat, N.; Decossas, J.L.; Delpech, V.; Gloaguen, V.; Sol, V. Optimization of the arsenazo-III method for the determination of uranium in water and plant samples. Talanta 2013, 115, 751–754. [Google Scholar] [CrossRef]
- Geng, Y.J.; Qi, W.; Muszynski, M.; Hansson, G.K.; Libby, P. Apoptosis of vascular smooth muscle cells induced by in vitro stimulation with interferon-γ, tumor necrosis factor–α, and interleukin-1β. Arterioscler. Thromb. 2015, 7, 19–27. [Google Scholar] [CrossRef]
- Han, R.; Zou, W.; Wang, Y.; Lu, Z. Removal of uranium(VI) from aqueous solutions by manganese oxide coated zeolite: Discussion of adsorption isotherms and pH effect. J. Environ. Radioact. 2007, 93, 127–143. [Google Scholar] [CrossRef]
- Waite, T.D.; Davis, J.A.; Payne, T.E.; Waychunas, G.A.; Xu, N. Uranium(VI) adsorption to ferrihydrite: Application of a surface complexation model. Geochim. Et Cosmochim. Acta 1994, 58, 5465–5478. [Google Scholar] [CrossRef]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Hoboken, NJ, USA, 1997. [Google Scholar]
- Ulrich, K.U.; Ilton, E.S.; Veeramani, H.; Sharp, J.O.; Bernier-Latmani, R.; Schofield, E.J.; Bargar, J.R.; Giammar, D.E. Comparative dissolution kinetics of biogenic and chemogenic uraninite under oxidizing conditions in the presence of carbonate. Geochim. Cosmochim. Acta 2009, 73, 6065–6083. [Google Scholar] [CrossRef]
- Lack, J.G.; Chaudhuri, S.K.; Kelly, S.D.; Kemner, K.M.; O’Connor, S.M.; Coates, J.D. Immobilization of Radionuclides and Heavy Metals through Anaerobic Bio-Oxidation of Fe(II). Appl. Environ. Microbiol. 2002, 68, 2704–2710. [Google Scholar] [CrossRef] [Green Version]
- Benckiser, G.; Santiago, S.; Neue, H.U.; Watanabe, I.; Ottow, J.C.G. Effect of fertilization on exudation, dehydrogenase activity, iron-reducing populations and Fe++ formation in the rhizosphere of rice (Oryza sativa L.) in relation to iron toxicity. Plant Soil 1984, 79, 305–316. [Google Scholar] [CrossRef]
- Kappler, A. Geomicrobiological Cycling of Iron. Rev. Mineral. Geochem. 2005, 59, 85–108. [Google Scholar] [CrossRef]
- Li, B.; Tian, C.; Zhang, D.; Pan, X. Anaerobic Nitrate-Dependent Iron (II) Oxidation by a Novel Autotrophic Bacterium, Citrobacter freundii Strain PXL1. Geomicrobiology 2014, 31, 138–144. [Google Scholar] [CrossRef]


| Czech Rep. | Uzbekistan | Yining, China | |
|---|---|---|---|
| nitrate | 200–1400 | 750 | - |
| U(VI) | 20–500 | 86 | 75 |
| pH | U(VI) | Conductivity | Cl− | SO42− | Ca2+ | K+ | Mg2+ | Na+ | CO32− | HCO3− | Nitrate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| µg L−1 | us cm−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | mg L−1 | |
| 7.74 | 8.71 | 1238 | 113.1 | 357.9 | 175.6 | 3.4 | 36.6 | 116.0 | 0 | 55.9 | 49.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wufuer, R.; Duo, J.; Li, W.; Fan, J.; Pan, X. Bioremediation of Uranium- and Nitrate-Contaminated Groundwater after the In Situ Leach Mining of Uranium. Water 2021, 13, 3188. https://doi.org/10.3390/w13223188
Wufuer R, Duo J, Li W, Fan J, Pan X. Bioremediation of Uranium- and Nitrate-Contaminated Groundwater after the In Situ Leach Mining of Uranium. Water. 2021; 13(22):3188. https://doi.org/10.3390/w13223188
Chicago/Turabian StyleWufuer, Rehemanjiang, Jia Duo, Wenfeng Li, Jinglong Fan, and Xiangliang Pan. 2021. "Bioremediation of Uranium- and Nitrate-Contaminated Groundwater after the In Situ Leach Mining of Uranium" Water 13, no. 22: 3188. https://doi.org/10.3390/w13223188





