1. Introduction
In 2015, the United Nations proposed the Sustainable Development Goals to improve the world’s social, environmental, and economic sustainability. The effects of contaminated water are related to Goal 6, clean water and sanitation [
1]. Heavy metals are currently the most common pollutants, with a toxic effect on water environments [
2]. Point sources such as sewage treatment plants, drainage systems, storm overflows, and surface runoff are responsible for the pollutants introduced [
3]. The amount of wastewater discharged from sewage treatment plants and drainage systems is closely monitored. Due to the unpredictability and irregularity of pollutant production, in most cases the amount of wastewater released by rain runoff cannot be controlled or monitored [
4]. The most unmanageable source of pollutants that could damage water is rainwater. This rainwater contains 650 different types of organic substances, 30 metals, and several inorganic compounds [
3]. Heavy metals, especially zinc, copper, lead, and cadmium, are often discovered [
5]. The main sources of these heavy metals are wear and tear and maintenance of vehicles and roads, road asphalt, fuel combustion, car exhaust fumes, and parking dust [
6,
7,
8]. The presence of heavy metals in precipitation depends on the type of surface, the type and nature of precipitation, and the degree of pollution of the land. Many types of heavy metals are found on urban roads, including cadmium (Cd), copper (Cu), lead (Pb), chromium (Cr), nickel (Ni), and arsenic (As), etc. They enter the rainwater runoff in the form of particles or in a dissolved state, and the heavy metals in the dissolved state have the properties of expansion, concealment, hysteresis, and non-degradability. Heavy metals dissolved in water not only destroy the aquatic ecosystem but can also endanger human health by enriching the food chain. Some heavy metals can be toxic to human health, and the risk ratio has been observed in the order Pb > Cd > Cu > Zn [
9].
Heavy metals can be removed using a variety of methods, including bioretention processes [
9,
10], froth flotation separation processes [
11], microbial remediation [
12], chemical processing [
13], flocculant processes [
14,
15], and adsorption processes [
16,
17]. Bioretention, for example, has a significant influence on the removal of heavy metals from rainwater runoff and is often used in the design of green infrastructure [
18]. The bioretention system comprises four main steps: sedimentation, filtration, adsorption, and plant uptake [
9,
19]. Heavy metals in particulate form can be easily removed by sedimentation and filtration processes. The adsorption process, on the other hand, removes dissolved heavy metals from rainwater [
20]. Dissolved heavy metals are more bioavailable than particulate heavy metals [
9,
21]. Therefore, it is critical to modernize bio-retention techniques to remove dissolved heavy metals. It has been reported that trees and soil under the pavement have high levels of heavy metal removal in the traditional bioretention system. [
4,
9]. The presence of heavy metals in the bioretention systems is concentrated in the top 10 cm [
10]. Fom this point of view, the development or modification of the top 10 cm of the bioretention system can improve the removal capacity.
In addition, many laboratory and pilot scale bioretention systems have shown that the overall efficiency of removing Zn, Cu, and Pb from synthetic runoff was typically greater than 95% [
22]. The results obtained suggest that bioretention can be an effective method for improving the quality of urban stormwater runoff [
23]. Now the question arises for the case of the mass balance perspective. There are three possible options for optimized removal efficiency [
23]. The first is to incorporate Fe or Al oxide (sparingly soluble substances) to sequester metals over a long period of time to control their mobility. The second option is to remove the surface layer of the bioretention system, and the third and most important is to promote uptake by selecting appropriate vegetation and harvesting the vegetation regularly to remove heavy metal uptake. There are two types of plants: hyper-accumulator plants and non-accumulator plants [
24]. Hyper-accumulators can accumulate 100 times more heavy metals than non-accumulators [
24]. In the case of non-accumulator plants, plants with high biomass are coupled with soil conditioning with chelating agents to increase plant uptake [
25]. From the above point of view, the importance of the selectivity of the surface layer and the vegetation selectivity is clear.
To develop soil bioaccumulation, organosilanes are often used to produce organically modified soil and clay in order to improve heavy metal binding sites and to adjust the hydrophobicity of soil/clay [
26]. In the case of clay, the amount of organosilane loading is generally determined by the cation exchange of the clay and, in the case of soil, by the reactive chemical sites of the soil [
27,
28]. However, a chemical modifier containing benzyl substitutes is chosen in order to obtain desired properties such as hydrophobicity [
27]. According to previously published clay chemistry research, the benzyl ring acts as a flat block or tile structure in clay galleries and prevents water from penetrating clay sites [
27,
29]. Hydrophobic surfaces are created by the type of organic substituents, the degree of surface coverage, unreacted groups, and silane dispersion.
In the current study, aliphatic silane and benzyl-substituted silane were used to modify bentonite clay and red earth. To analyze the changes in physical properties, Flourier transform infrared absorption spectroscopy, X-ray diffraction, scanning electron microscopy, and wettability tests using contact angle measurements and water penetration experiments were carried out. In addition, this silane-modified clay/soil, as well as sand, soil, and gravel, were used as bioretention media to investigate the removal of Cd (II) heavy metals in synthetic rainwater runoff. Finally, we carried out preliminary tests to determine the influence of silane-modified clay/soils on the yield and quality of Cyperus Corps. To the best of our knowledge, no studies have looked at the effects of wettable clay/soil on heavy metal removal in a bioretention system.
2. Materials and Methods
2.1. Materials
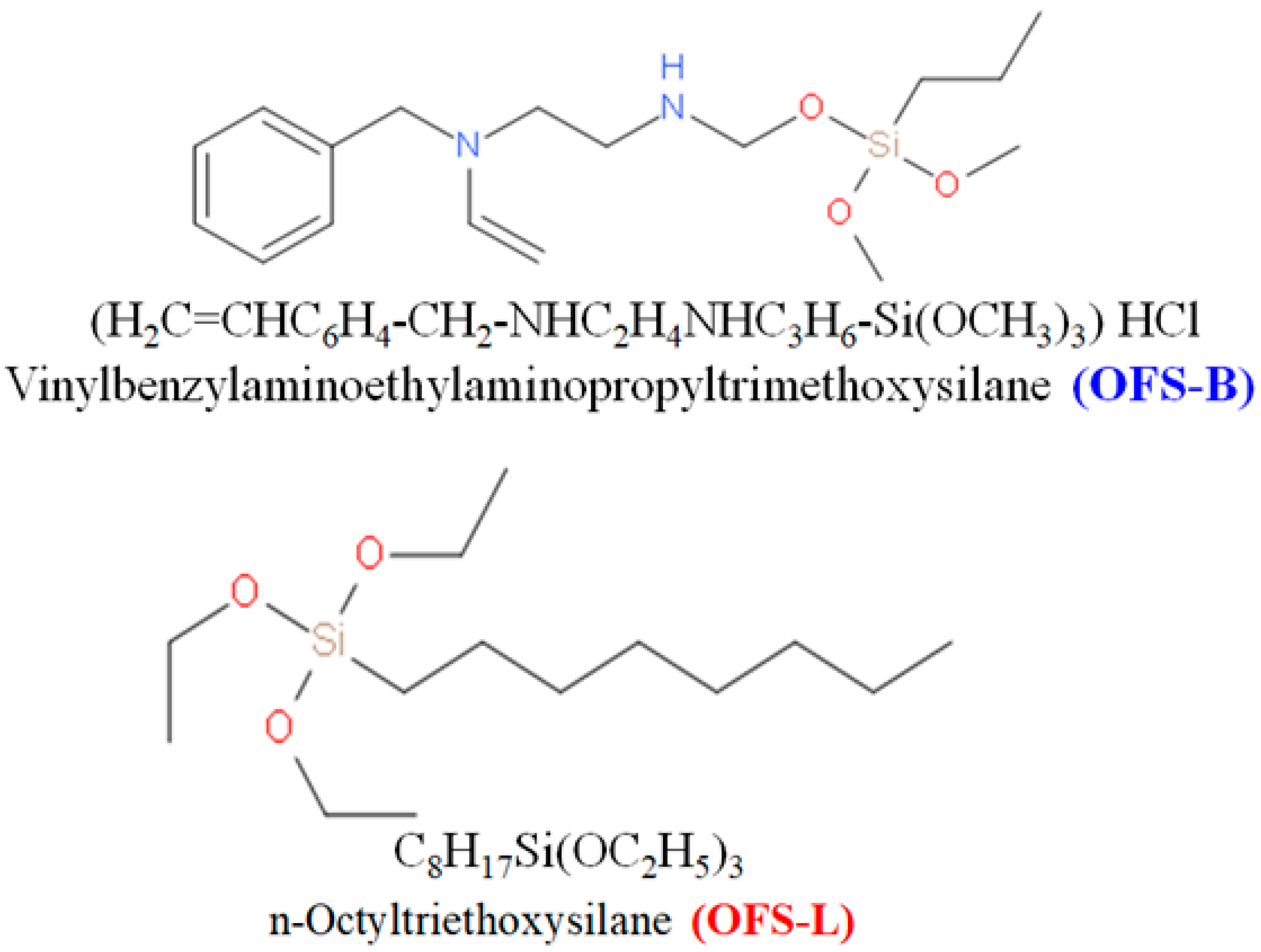
Silane-modified clay and soil for the bioretention system were mixed at high temperature and acidic conditions with or without benzyl substituted silane. Bentonite clay and red soil were purchased from Yuanyuan water purification Co., Ltd. Nanning, China And SanXi mountain soil, respectively. N-Octyltriethoxysilane (OFS-L) (C14H32O3Si) and vinylbenzylaminoethylaminopropyltrimethoxysilane (OFS-B) (C17H31ClN2O3Si) were obtained from Nanjing golden chemical Co., Ltd., Nanjing, China. Basic reaction chemicals such as AgNO3, concentrated HCl, HF, H2O2, and Cadmium nitrate tetrahydrate, Cd(NO3)2∙4H2O, were purchased from Sinoptico group chemical reagents Co., Ltd., Shanghai, China. All of the chemicals employed in the study were of commercial grade and did not require further purification.
2.2. Instrumentation and Techniques
The FT-IR study was carried out using the KBr pellets technique with the range of 4000 to 600 cm−1 on IRPrestige-21, Shimadzu. To accurately determine d-spacing, clay samples were analyzed using X-ray diffractometer analysis (Bruker D-8 advance). An optical tensiometer was utilized to measure the contact angle of clay and soil samples (Theta attention, Biolin Scientific). A SEM (SU1510, Hitachi) was utilized to examine the surface topography, and EDAX analysis was utilized to determine elemental arrangements (C, K, Al, Si). The presence of Cd (II) was determined by atomic absorption spectroscopy (AAS) (Z-1000, Enduro) with a detection limit of 9µg/L. Samples (clay/soil) were digested with concentrated HCl, HNO3, HF, and H2O2 for the heavy metal detection experiments. Finally, an ultraviolet absorption spectrophotometer was utilized to measure chlorophyll in the plant (UV 5500).
2.3. Preparation of Silane Modify Clay/Soil
Silane modified clay and soil were prepared as in previous work by Shah et al. [
27,
29] and Gupta et al. [
28]. In short, the organoclays/soils were synthesized in the laboratory by condensing clay and soil particles with an aqueous silane material solution (OFS-B and OFS-L) (see
Figure 1). The organic silane was dispersed in water at a ratio of 1:10 (silane (ml): water (ml)). Clay and soil were added to disperse silane materials and allowed to react for 45 min at 40 °C, followed by pH adjustment to 3–4 with HCl and constant stirring for 30 min. The reaction was cooled and stirred for an additional 3 h to homogenize it. Finally, after allowing the modified samples to sit for 10 h at room temperature, the supernatant slurry was decanted into separate vessels. The remaining slurry was centrifuged at 2000 rpm for 10 min, and the sediment was dried in an oven at 80 °C overnight. The bioretention system was built from the silane-modified clay and soil obtained.
2.4. Set Up of Bioretention Column
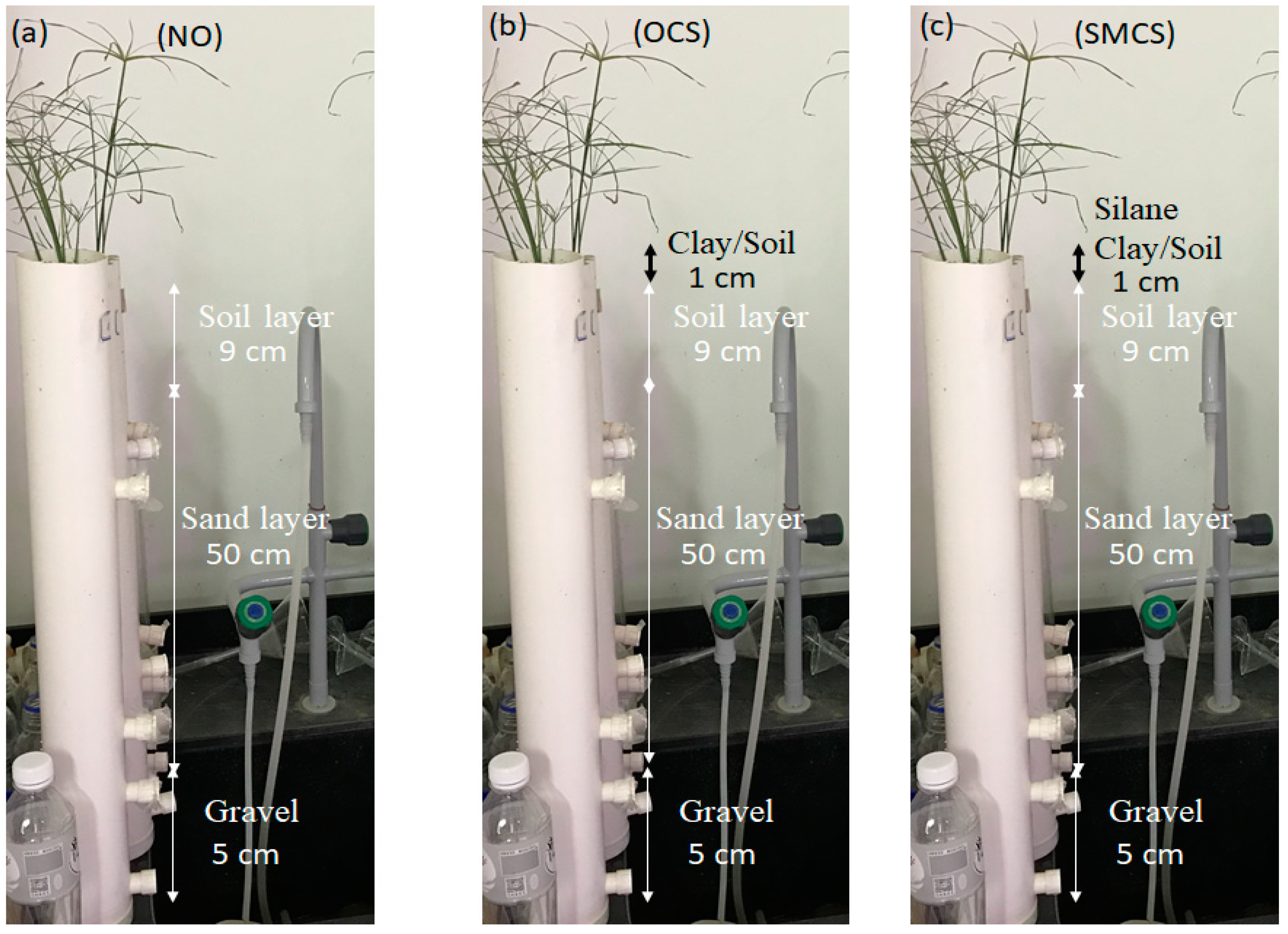
PVC pipe, 7.5 cm in diameter and 60 cm height, was used to prepare the bioretention columns (see
Figure 2). The layers of gravel, sand, soil, and an additional upper soil/clay layer were conserved to 5, 40, 9, and 1 cm, respectively in this study. The gravel, sand, and soil layers were filled gravel 4.75 to 6.3 mm in size, 1.18 mm sand: soil (6:4), and 2 mm sand layer, respectively. The gravel used in this experiment was the type of gravel commonly used in construction. Peat soil, perlite, vermiculite, and carbonized rice hulls make up the soil composition.
Cyperus alternifolius is commonly referred to as umbrella grass, which has a grass like clumps of 2–3 fit height and was the type of vegetation used on the superficial bioretention column. This is one of those houseplants that overwatering cannot kill.
2.5. Rainfall Simulation and Runoff Calculation
The primary pollutant in urban runoff are SS, heavy metals, COD, nitrogen, phosphorus, chloride, oil, and pathogenic bacteria. In order to obtain stable raw water, an artificial allocation of simulated raw water was carried out according to the calculation described by You et al. [
30].
Table 1 shows the composition and dosage of synthetic water.
In general, Nanjing has an annual rainfall of 1256 mm and an average temperature of 16.1 °C [
31]. There is significant rainfall throughout the year in Nanjing. We simulated rainfall patterns using the Nanjing region rainfall intensity formula (Equation (1)). The runoff coefficient was set to 0.3, the repetition period to two years, the rainfall duration to 120 min, the catchment area ratio to 5%, and the repetition period to two years (road runoff coefficient).
where
q = 83.02 L/(s hm
2) is the rainfall intensity,
p is the return period (a), and
t is the rainfall duration (min) (s hm
2).
The design rainwater flow is calculated by following formula:
where
Qs is the design discharge (L/s), q is rainfall intensity (L/(s hm
2)),
ψ is the runoff coefficient,
F is the catchment area (hm), and
f is the plant surface area (hm). The experimental column in this experiment was 7.5 cm in diameter;
F = 883.58 cm
2 and
Qs = 2.57×10
4 L/s were computed, resulting in a total volume of influent water of 1.15 L after two hours.
After calculating the water, three systems were installed (see
Figure 2), one without an additional layer (
Figure 2a), one with untreated clay and soil (
Figure 2b), and one with a silane-modified clay and soil incorporated system (
Figure 2c). After all the systems were installed, the test columns were rinsed with tap water (each test column was reported by 2 L at a time, twice a day, for 4 days) to allow the water to flow from the top down in the test column and between the layers. After the scour was complete, the bio-retention column was flushed with simulated rainwater (three times a day) to obtain saturation.
The experimental water intake was then carried out on the first and third days, with 1.15 L of simulated rainfall being fed to the experimental column for 2 h using a peristaltic pump. On the seventh day, after a period of three days, water was again injected into the sample. Finally, a seven-day drying period was framed, with water intake, water sampling, and soil sampling taking place on the fifteenth day. After each flow, water samples were collected for the analysis. In addition, through the sampling holes, soil samples from each layer were collected. Finally, after a 15-day experiment, plant samples were taken to determine heavy metal uptakes and chlorophyll content.
2.6. Yield and Quality of Cyperus Alternifolius
Finally, the presence of Cd (II) and Chlorophyll-b tests were performed on
Cyperus alternifolius to identify initializing plant quality experiments, Cyperus was divided into three different components, i.e., leaves, stem, and roots. All three components were carefully cleaned with deionized water numerous times before being dried in the oven at 70 °C. The dried materials were crushed and digested with concentrated HNO
3 and H
2O
2 before being subjected to the heavy metal identification procedures described by Chen et al. [
32]. The content of the chlorophyll-b test of leaves, as described by Arnon’s method [
33], can be utilized to determine the plants ‘survival. A 50 mL colorimetric tube was filled with 0.1 g of dried leaf sample. Then, 25 mL of acetone was added and shaken thoroughly for 10 min before filtration. An ultraviolet spectrophotometer was utilized to measure the filtrate at a wavelength of 645 nm (chlorophyll b).
3. Results
Silane-modified clay and soils for the bioretention system were mixed at high temperature and acidic conditions, with or without benzyl substituted silane. The physico-chemical properties after modification and their applicability for bio-retention systems were determined in this section.
3.1. Characterization of Silane Modified Clay and Soil
FT-IR analysis can demonstrate the presence of modifiers on the clay and soil surfaces.
Figure S1 shows the FT-IR absorption spectra of pristine and silane-modified clay and soils. For soil and clay samples, bands in the 3700–3500 cm
−1 and 1034 cm
−1 ranges were ascribed to the −OH stretching vibration area of the Me-OH (Me = Si, Mg, or Al) group and the stretching vibration of structural silicate groups, respectively, for soil and clay samples [
27]. The −OH bending mode is represented by a band at 1635 cm
−1, whereas the Si-O-Si stretching vibration and the vibration mode of Me-O-Me′ (Me and Me′ = Si, Mg, or Al) are represented by bands at 1114 and 907 cm
−1, respectively. The peak at 700 cm
−1 represents quartz, with a higher intensity in the soil sample indicating a higher amount of quartz in the soil sample [
34].
All characteristic bands of clay and soil samples were retained after the silane conversion. Additional bands were found at 2924 and 2848 cm
−1, which were attributed to the silane modifiers −CH
2 stretching vibration [
35]. In addition, an extra silicate group of silane modifiers [
36] boosted the intensity of the peak 1034 cm
−1. The presence of a silane group on the surface of the soil and clay is confirmed by this increase. An additional spectral peak at 1470 cm
−1 in the FT-IR spectra of silane-modified clay and soil, OFS-B modified clay and soil, and soil shows the existence of benzyl substituents [
37]. However, no additional peak of the N-H group was observed owing to the weak intensity of the present group. The FT-IR results obtained confirm the presence of silane modifiers on the surfaces of soil and clay. In order to identify silane modifiers in the layer spacing (d-spacing) of clay, XRD analysis was performed. The d-spacing values of original clay and silane-modified clay samples are shown in
Table 2. The addition of a silane modifier resulted in an increase in d-spacing values, as expected. Benzyl substituted silane (OFS-B) exhibited a higher d-spacing because the benzyl group was present inside the clay layers. The presence of a silane modifier in the clay interlayer is confirmed by the d-spacing data.
Basic physico-chemical properties can be altered by changing the geometry of clay and soil after the change. The contact angle of the samples was measured, and the findings are shown in
Table 2. The contact angles of the original clay and the original soil were 78° and 120°, respectively. According to the results, the soil is more hydrophobic than clay because of its spear structure. Clay, on the other hand, has a layered structure that traps water within the interlayer. When benzyl substituted (OFS-B) silane was compared to liner (OFS-L) silane, the contact angle increased dramatically. The benzyl substitution resisted water in the clay structure by preventing interlayer (d-spacing) spacing and soil structure by higher-stacking (stearic) effects [
29]. The results indicate that, after using OFS-B and OFS-L silane modifiers, variation in clay and soil physicochemical parameters occur. In addition, when compared to soil-OFS-L, the super-hydrophobic character of soil-OFS-B was detected.
3.2. Removal of Cd (II) from Bioretention System
In order to investigate the applicability of organoclay for the Cd (II) removal, adsorption experiments were carried out with different rainfall intervals in order to simulate the Cd (II) uptakes.
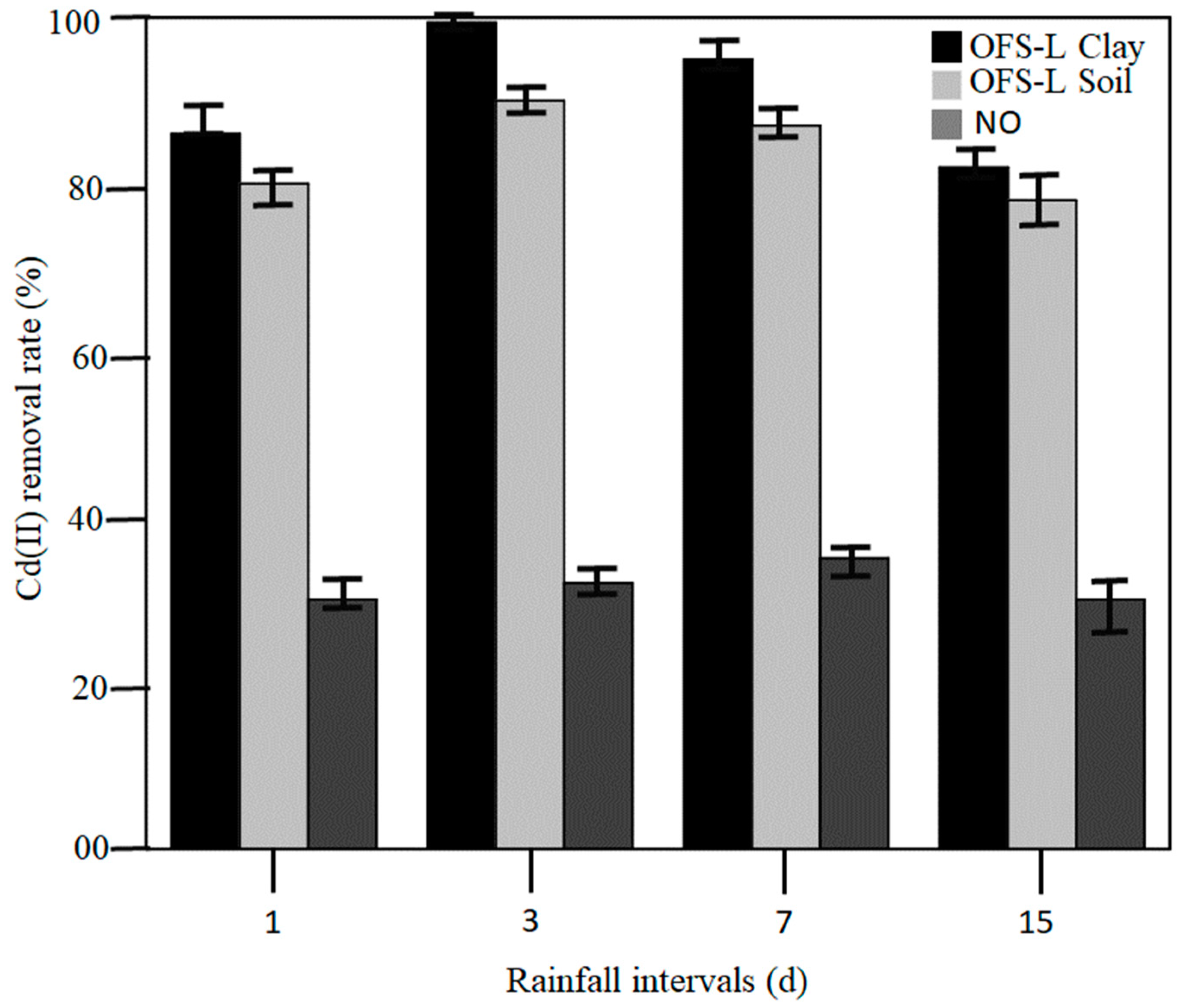
Figure 3 shows the Cd (II) removal rate in the bioretention system utilizing OFS-L modified clay and soil samples over rainfall intervals. NO represents a typical bioretention system without adding any clay or soil. The clearance rate of Cd (II) increased at first and then declined after 7 days, as seen in the graph. On the third day, the removal rate of Cd (II) by OFS-L modified clay reached a maximum of 98.3%, then stabilized at above 80% in the samples. The OFS-L modified soil displayed the second highest adsorption on the third day. Meanwhile, the removal rate for bioretention systems with no extra layer, i.e., NO, was the lowest. Furthermore, the removal rate of NO samples remained nearly constant when compared to the other two types of systems. The reason for this is that the OFS-L modified clay and soil system has a larger interlayer spacing, implying more interaction space. For the same reason, clay and soil samples are analyzed. Clay has a comparatively larger layer space than soil. The reason for the increased amount of removal for day 3 is the occupation of available free sites. Bioretention systems were dried on the first day but were wet on the third day due to rain. Swelling may be visible in different samples throughout this time. This swelling allows Cd (II) to be removed from the clay [
27]. The binding sites for the Cd (II) in soil are provided by the arrangement of modifiers on surfaces.
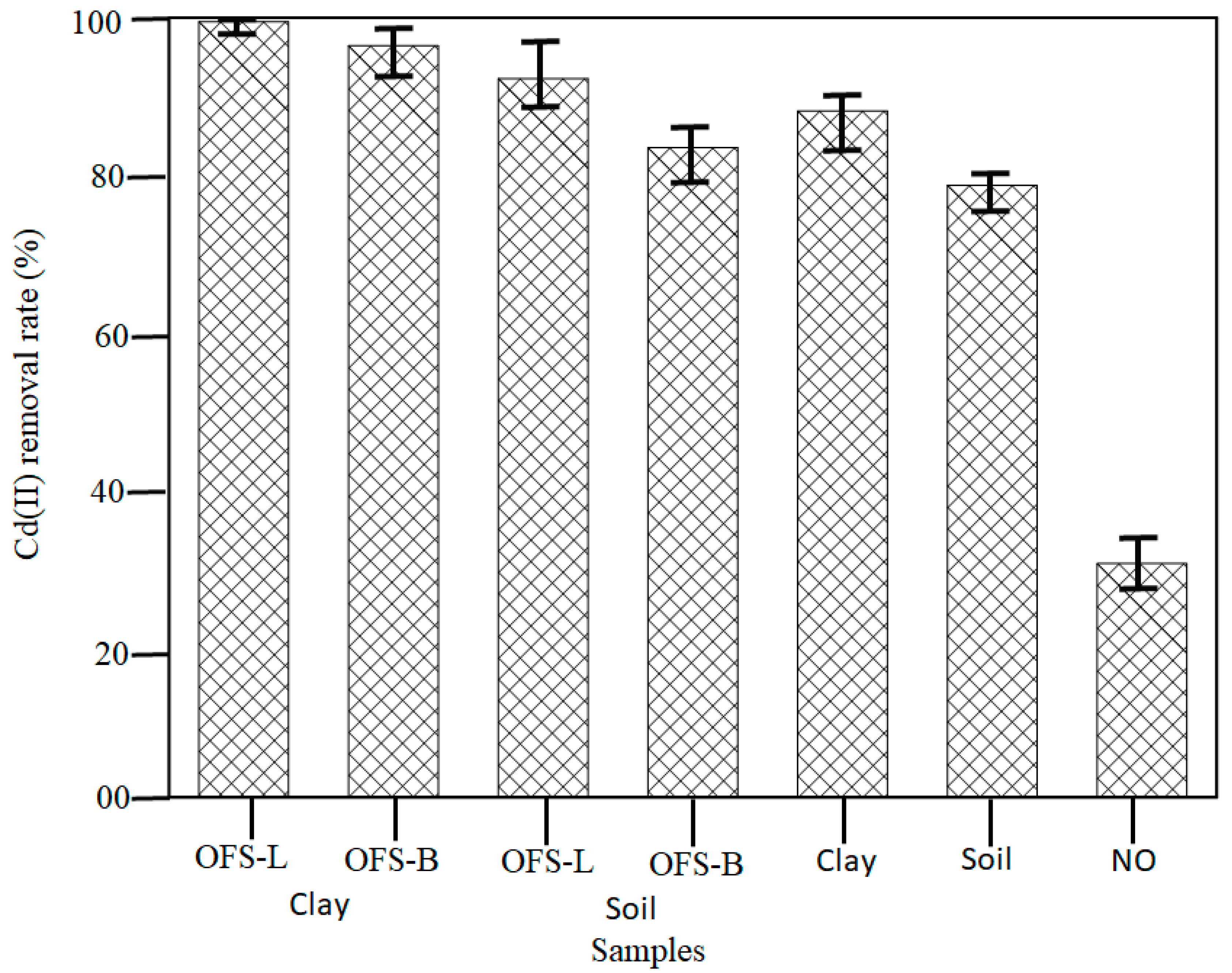
The tendency of each bioretention system was studied after confirming the optimal day for adsorption, and Cd (II) removal rates of all systems on day 3 are shown in
Figure 4. The removal rates of Cd (II) of the seven bioretention systems are separated into four groups, as shown in the
Figure 4 modified clay, modified soil, original clay/soil, and NO (no additional layer). Cd (II) removal capability was the highest among them in the case of a clay system. Modified clay > modified soil > original clay/soil > NO is the order of adsorption rate. The reason for this is that the modified clay used in bioretention systems contains a liner silane modifier with a greater interlayer spacing than soil and original clay samples. This finding demonstrates that adding clay or modified clay to a bioretention system improves the adsorption and removal of Cd (II). The OFS-L modified clay has a higher adsorption capacity than the OFS-B modified clay when the silane modifier effect is compared. In the case of changed soil systems, a similar occurrence was also seen. The phenomenon is noticed when Cd (II) enters the system. Wettable (hydrophilic) clay begins adsorption in its interlayer before moving on to the next layer. The penetration rate of non-wettable (hydrophobic) clay is prolonged in the competition. As a result, it can be inferred that simply adding a modified clay layer to a bioretention system is a superior application strategy and has a better effect on Cd (II) removal. Furthermore, hydrophilic clay is found to be more suited for bioretention systems.
3.3. Mobility and Adsorption of Cd (II) in Each Layer of Bioretention System
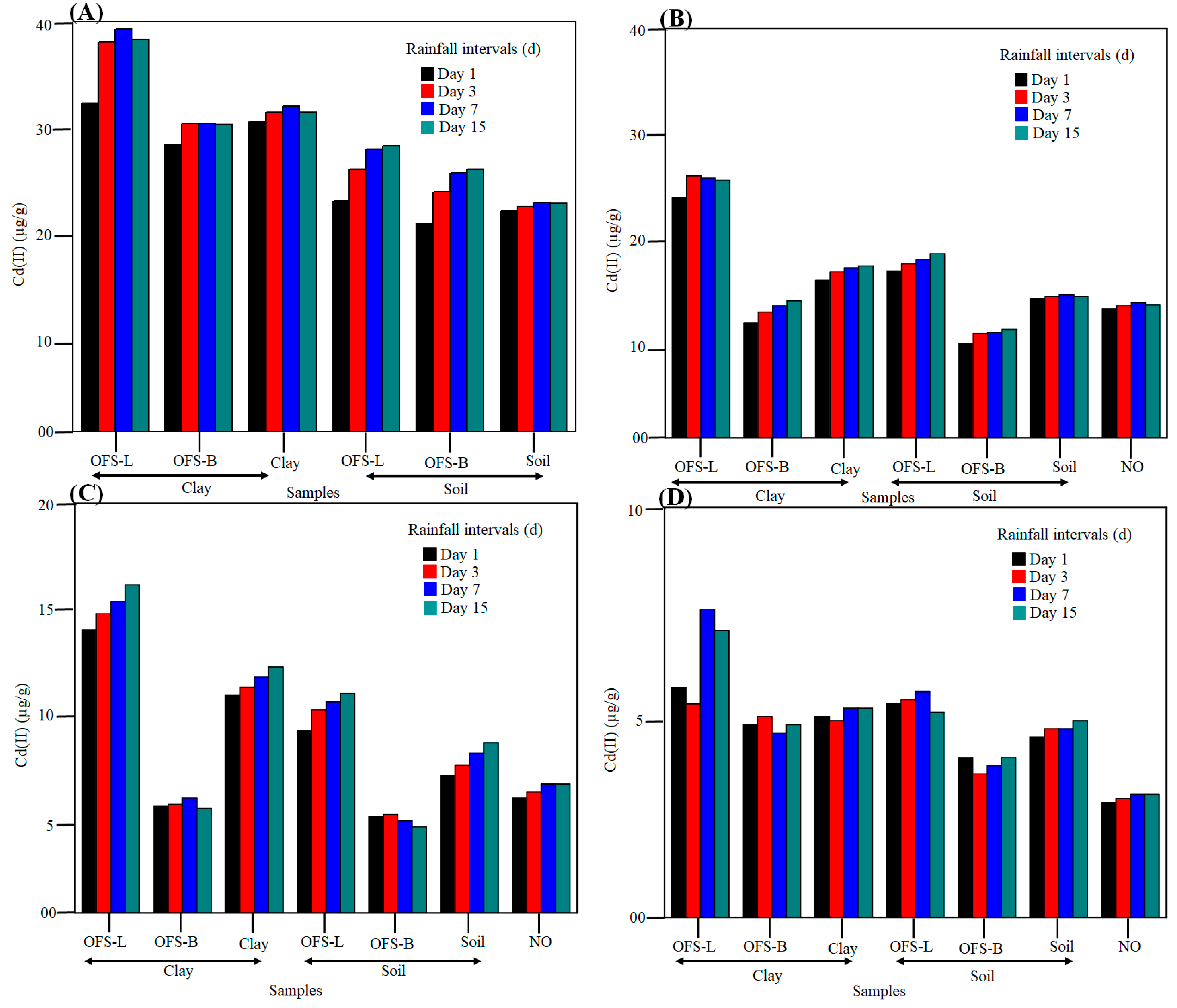
In order to determine the amount of Cd (II) adsorption in each layer, soil, clay, sand, and gravel samples were tested. Changes in Cd (II) content were seen in each section of the bioretention layer (a) extra layer (1 cm), (b) soil layer (9 cm), (c) sand layer (50 cm), and (d) gravel layer (5 cm) (as shown in
Figure 5). When comparing all four layers, the Cd (II) adsorption can be found to be in the order layer I > layer II > layer III > layer IV. The acquired result confirms that binding sites in each layer play a critical role. The sand, soil, and clay layers have a larger surface area and more binding sites than the gravel layer. Furthermore, silane-modified clay/soil contains more binding sites and possibly more adsorption capacity than unmodified clay/soil. There were six systems in the case of the additional layer. The “NO” system is missing because it lacks an additional layer. OFS-L clay > clay > OFS-B clay > OFS-L soil > soil > OFS-B soil is the order of Cd (II) adsorption. The findings demonstrate that adsorption occurs in the order of their wettability. The hydrophobic behavior of OFS-B modified clay/soil prevents water from penetrating the bioretention system, causing agglomeration.
Hydrophilic clay/soil, on the other hand, displays water penetration in the bioretention system, resulting in heavy metal leaching in the interlayer, and also downwards in the bioretention system. The soil layer is layer II. We may compare 7 bio-retention systems, including NO, in this article. OFS-L clay > OFS-L soil > Clay > Soil > NO > OFS-B clay > OFS-B soil is the order of Cd (II) removal. In comparison to the hydrophobic nature, the acquired data support the hydrophilic nature. The transmission or exchange of heavy metal from each layer is the primary reason for this. Furthermore, because of the larger interlayer gap, hydrophilic clay has a higher propensity for passing Cd (II). Layer III and layer IV followed a similar pattern. Furthermore, the rate of Cd (II) elimination on day 7 is larger than on other days. The reason for this is because most Cd (II) will be leached from layer I to layer III during the next two wet days, namely days 1 and 3. The majority of Cd (II) has time to settle over the layer surfaces during a four-day interval, however. Higher drying times, on the other hand, cause agglomerates and, as a result, creaks in the bio-retention system, resulting in lower Cd (II) removal capacity.
Beyond all of these studies, an additional layer of wettable OFS-L clay was found to be the most effective modifier for removing Cd (II) from the bio-retention system. The preceding findings demonstrate that the OFS-L and OFS-B modifiers have a considerable impact on the removal’s final attributes.
3.4. Cd (II) Uptakes and Accumulation in Cyperus Alternifolius Plants
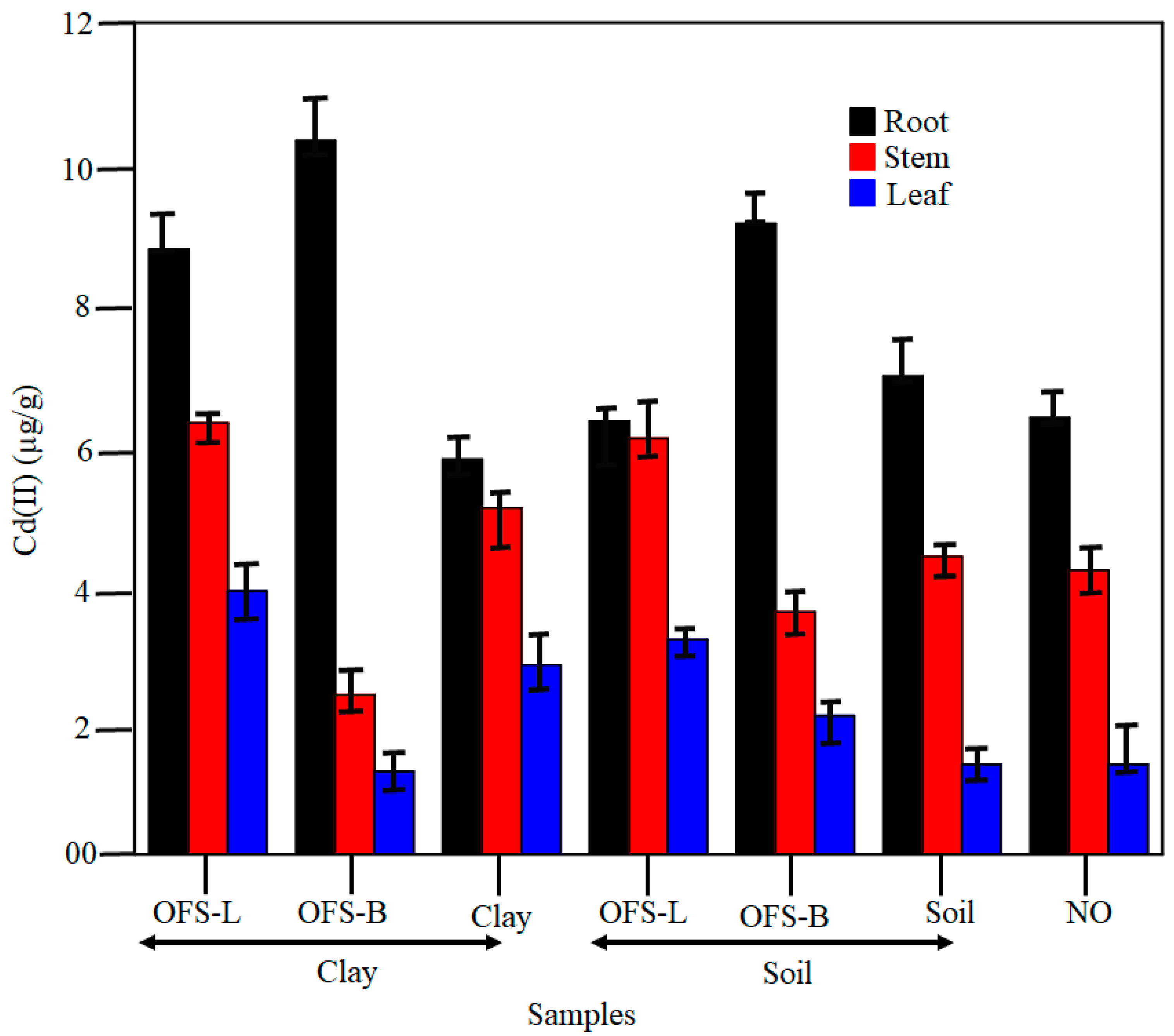
Figure 6 shows the effect of silane-modified clay/soil on Cd (II) uptake and accumulation in plant tissue. The base of the plant had the highest concentration of Cd (II) compared to the stem and leaf. Meanwhile, the order of Cd (II) adsorption is clay > soil > NO. The lack of binding sites or extra sites can be blamed for the lower Cd (II) concentration. When compared to soil, clay has a larger surface area and more binding sites. There is no extra layer for NO, which clearly demonstrates the influence of accessible binding sites for Cd adsorption (II). Cd (II) adsorption is significantly improved after the hydrophilic OFS-L and hydrophobic OFS-B silane-modifiers are added to the clay and soil samples. In the case of Root, the adsorption is modified Clay > modified soil in that sequence. OFS-B clay > BFS-B soil > soil > OFS-L clay > OFS-L soil > clay. According to the findings, the OFS-L are responsible for increased Cd (II) adsorption in the stem and leaf. Meanwhile, the capillary effect is thought to be responsible for the vegetative uptakes, which indicates OFS-B could restrict the edges of plants. The capillary effect and Cd (II) adsorption in the stem and leaf could be reduced as a result. Due to the presence of OFS-B near the margins, agglomeration or colloid formation is common.
3.5. Cyperus Alternifolius Plant Analysis
The purpose of this study was to investigate how a silane-modified clay/soil layer compared to no additional layer affected plant tolerance by assessing biomass, chlorophyll b content, and observing plant height. The silane-modified clay/soil layer had a substantial impact on the growth of
Cyperus alternifolius plants, as shown in
Table 3. The amount of biomass in the plant leaf is on the order of Clay OFS-L > Soil OFS-L > Clay > Clay OFS-B > Soil > Soil OFS-B > NO > Fresh. The presence of the heavy metal CD (II) in the system is responsible for the increased amount of dry biomass. Furthermore, the presence of heavy metal in plants with hydrophilic clay and soil is significantly higher than hydrophobic clay and soil samples, according to biomass order. Dry biomass in the stems of
Cyperus alternifolius plants followed a similar pattern i.e., Clay OFS-L > Soil OFS-L > Soil OFS-B > Soil > Clay OFS-B > Clay > NO > Fresh. The biomass of the root, on the other hand, is in the order Clay OFS-B > Clay OFS-L > Soil OFS-B > Soil OFS-L = Soil = Clay > NO = Fresh. This indicates that the presence of Cd (II) is higher in the hydrophobic samples. Plant height, on the other hand, is in the same Clay OFS-L > Soil OFS-L > Clay > Soil > Fresh > Clay OFS-B > Soil OFS-B > NO. Plant height is lower in silane-modified clay or soil with OFS-B (benzyl substituents) than in fresh plants. Furthermore, the concentration of chlorophyll b follows the order of Fresh > Clay OFS-L > Clay OFS-B = Clay > Soil OFS-L > Soil OFS-B > Soil > NO. The chlorophyll content of other samples was measured after 15 days of experiments; therefore, fresh plants contain higher chlorophyll b. When comparing 15-day plant chlorophyll b, the OFS-L modified samples have a higher chlorophyll b level. The results clearly demonstrate the effect of hydrophobic and hydrophilic silane-modifiers on adsorption properties. With rainfall, hydrophilic clays can migrate downwards from the first layer to the second till the third layer, whereas hydrophobic clay or soil cannot. Meanwhile, the Cd (II) and their binding sites are carried by the migrating clay or soil. When compared to OFS-B modified clay or soil, this may aid plant root, stem, and leaf uptake, resulting in higher biomass and plant height. The results are consistent with heavy metal adsorption results in bioretention clay/soil samples and are in line with the obtained results of heavy metal adsorption in bioretention clay/soil samples.
4. Mechanism Analysis
According to the findings of the above study, the aspect of the additional layer added to the bioretention system has an impact on the Cd (II) removal rate. Furthermore, the wettability of clay and soil is affected by silane alteration, as evident by the above-mentioned data. However, scanning electron microcopy with EDAX was performed on OFS-L and OFS-B modified clay samples to prove structural property correlations.
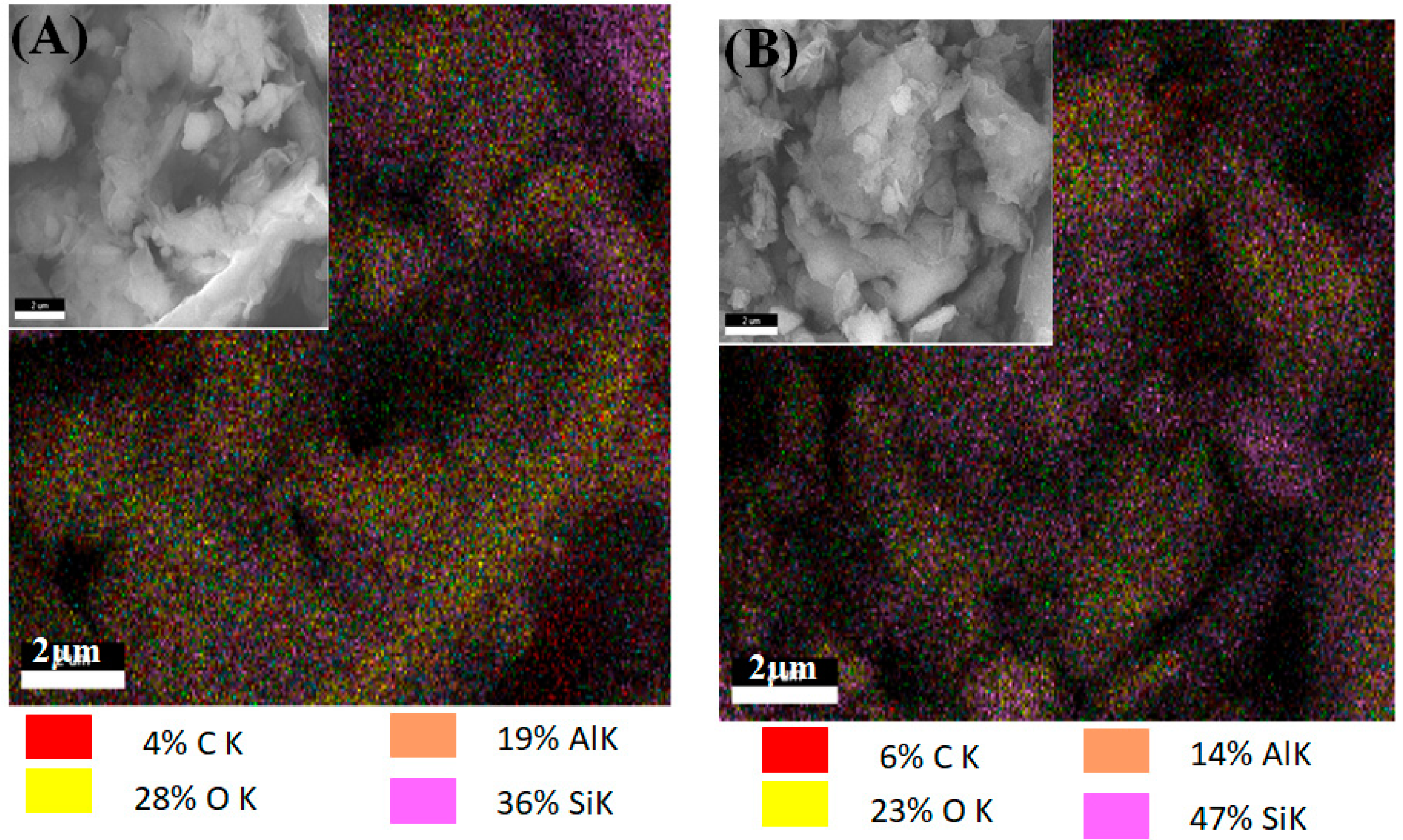
Figure 7A,B shows SEM images of two clays, Clay OFS-L and Clay OFS-B, as well as EDAX mapping of the clays. OFS-B clay has more carbon than OFS-L clay in terms of silane modifier dispersion on the surface. This conclusion is due to the molecular carbon number ration, as OFS-B has more carbon (structural) than OFS-L. However, there is a noticeable variation in certain Al and O components. The findings point to the presence of clay structural Al on the surfaces. Long-chain quaternary ammonium salt modifier enters the interlayer mostly over the surface, according to earlier research. Benzyl substituted quaternary ammonium salt modifier, on the other hand, is mostly left on the surface [
27]. In the case of silane-modified clays, similar behavior has been found. EDAX backs up these findings with Si elemental measurement. The OFS-B had a higher amount of Si on the surfaces than OFS-L, as can be observed.
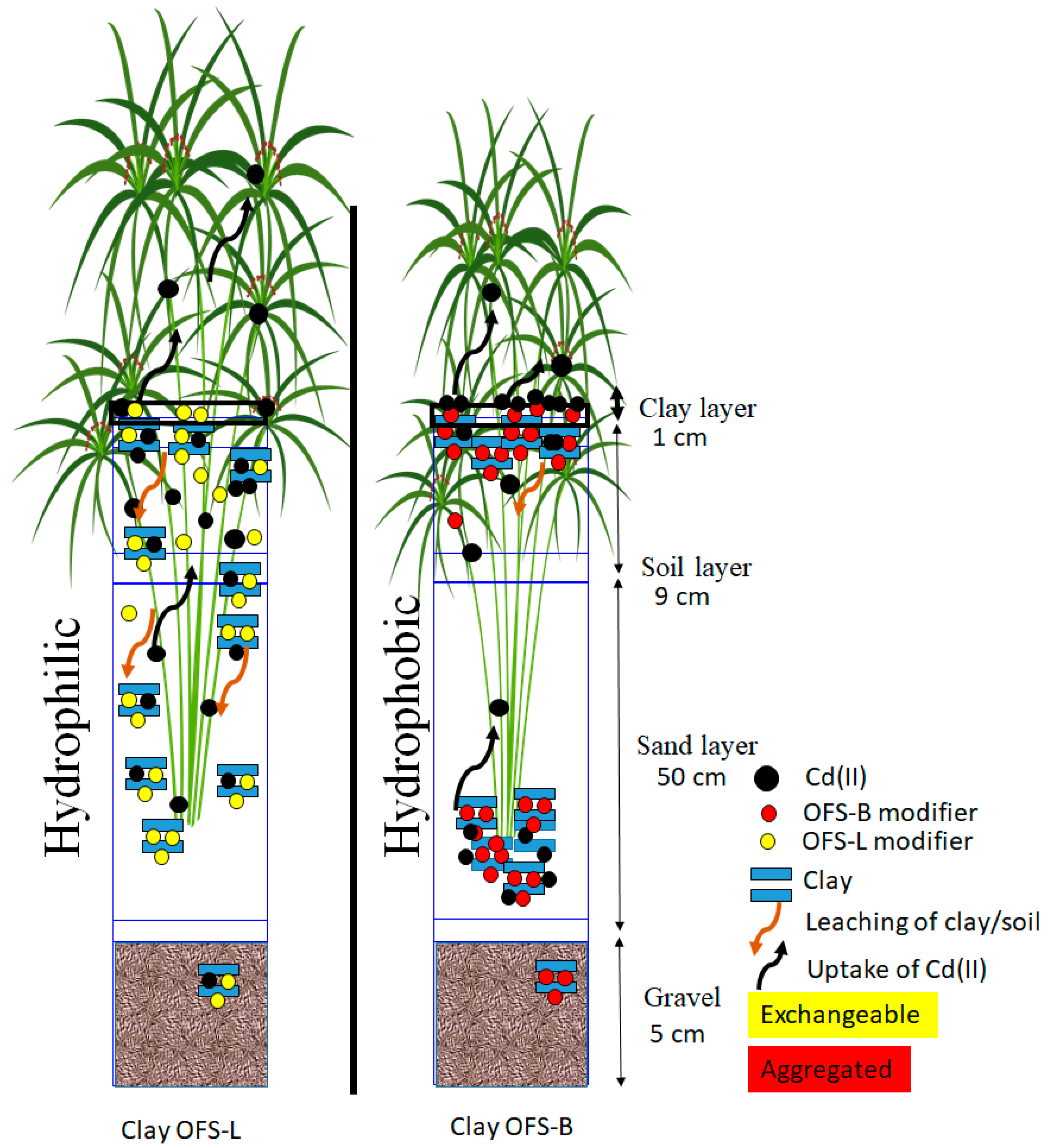
The bioretention system mechanism has been examined and is schematically represented in
Figure 8 after the structural arrangements in clay were completed. Cd (II) adsorption in layer I of the bioretention system is higher in OFS-L modified clay or soil. The stacking of benzyl rings on the clay surfaces, on the other hand, resulted in steric hindrance and steric effects in OFS-B modified clay or soil. The presence of stearic hindrance influences Cd (II) adsorption, causing Cd (II) to move through the interlayer more slowly. As a result, OFS-B modified clay allows water to pass through while remaining non-wettable. At the same time, due to loose stearic force, the clay was leached from layer I to another layer, increasing Cd (II) adsorption in each layer. To put it another way, wettable materials act as a Cd (II) transmission medium or carrier. In non-wettable clay and soil, this process was missing, resulting in aggregation on the surfaces. This is a major reason why OFS-L modified clay removes more Cd (II) than OFS-B modified clay.
Plant uptake has been shown to have similar effects. In general, the plant exhibited capillary rise adsorption, and loosely bound Cd (II) in wettable clay or soil samples had higher uptake, as seen by plant Cd (II) removal and biomass data. Meanwhile, aggregation formed near plant roots with OFS-B modified clay or soil, resulting in a larger amount of Cd (II) in the root than in other plants. Because of their carrier transforming role in the bioretention system, wettable OFS-A modified clay demonstrated high Cd (II) removal capability, according to the results.
5. Conclusions
In this study, a bioretention system made with an additional clay/soil and silane-modified clay/soil layer removes Cd (II) from simulated rainwater flow. In this study, clay and soil were treated with the linear silane modifier OFS-L and the benzyl-substituted silane modifier OFS-B. Physicochemical studies such as FT-IR, XRD, and contact angle analysis were used to determine the improvement in silane-modified clay and soil properties. Clay and soil modified with the OFS-L modifier had a higher wettability than clay and soil modified with the OFS-B modifier. Therefore, the hydrophobic or non-wettable properties of silane modified clay and soil cannot be guaranteed. On the other hand, an increased d-spacing may be required in order to improve the wettability. The silane modifier OFS-B, on the other hand, prevented this d-spacing, which led to a hydrophobic or non-wettable behavior.
These altered clays and soils are now being used in a bioretention system. To investigate the removal of Cd (II), seven different systems were used including clay, soil, OFS-L clay, OFS-B clay, OFS-L soil, OFS-B soil, and NO. The NO represents a basic bioretention system with no additional layers, whereas the other six systems have a layer of 1cm or more. The optimal clearance rate of Cd (II) was recorded on the 3rd day of the rain interval, compared to the 7th and 15th days, after 15 days of testing. Additionally, when compared to OFS-B clay and OFS-L soil, OFS-L had the highest Cd (II) removal capacity. The obtained results clearly reveal that by improving d-spacing, the adsorption capacity of OFS-L clay was improved. For soil samples, these potential binding sites were absent, and OFS-B prevents them through steric hindrance between existing benzyl substituents. While analyzing the layer samples, it was discovered that OFS-L modified clay improved Cd (II) adsorption in each layer. The leaching properties are responsible for the increased adsorption amount. It is considered that the hydrophilic clay was leached based on the findings. Adsorption in each layer and Cyperus alternifolius plants is improved by adsorbed Cd (II) in the interlayer or outer surface of the clay. The adsorbed Cd (II) improves. Because of the steric effects, benzyl substituted silane (OFS-B) produced agglomerates and did not exhibit this behavior. OFS-L clay improves the quality of Cyperus alternifolius plants by increasing height and preserving chlorophyll b levels in the leaves, in addition to removing Cd (II). Due to their steric effects, benzyl substituted silane (OFS-B) did not exhibit this behavior. Of the seven bioretention systems, OFS-L modified clay was more effective than OFS-L modified soil due to its better wettability, availability of binding sites, and d-spacing for Cd (II) removal. Finally, all results suggest that the selective loading of the material and the configurations of the surface modifier play a significant role in the Cd (II) removal rates.