Observation and Analysis of Water Temperature in Ice-Covered Shallow Lake: Case Study in Qinghuahu Lake
Abstract
:1. Introduction
2. Study Area and Methods
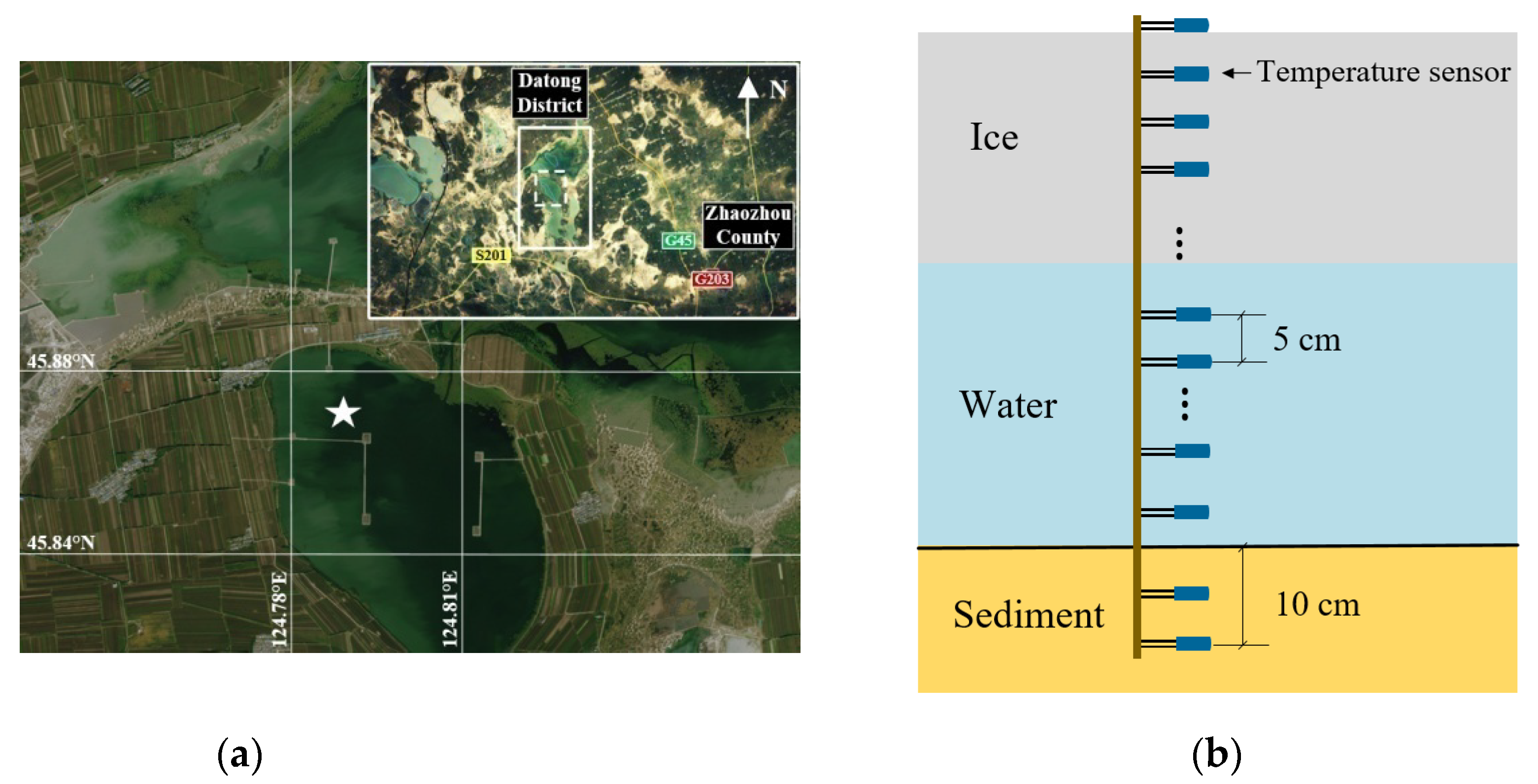
2.1. Overview of the Observation Site
2.2. Observational Devices and Methods
2.3. Calculation Method of Heat Flux and Thermal Diffusivity
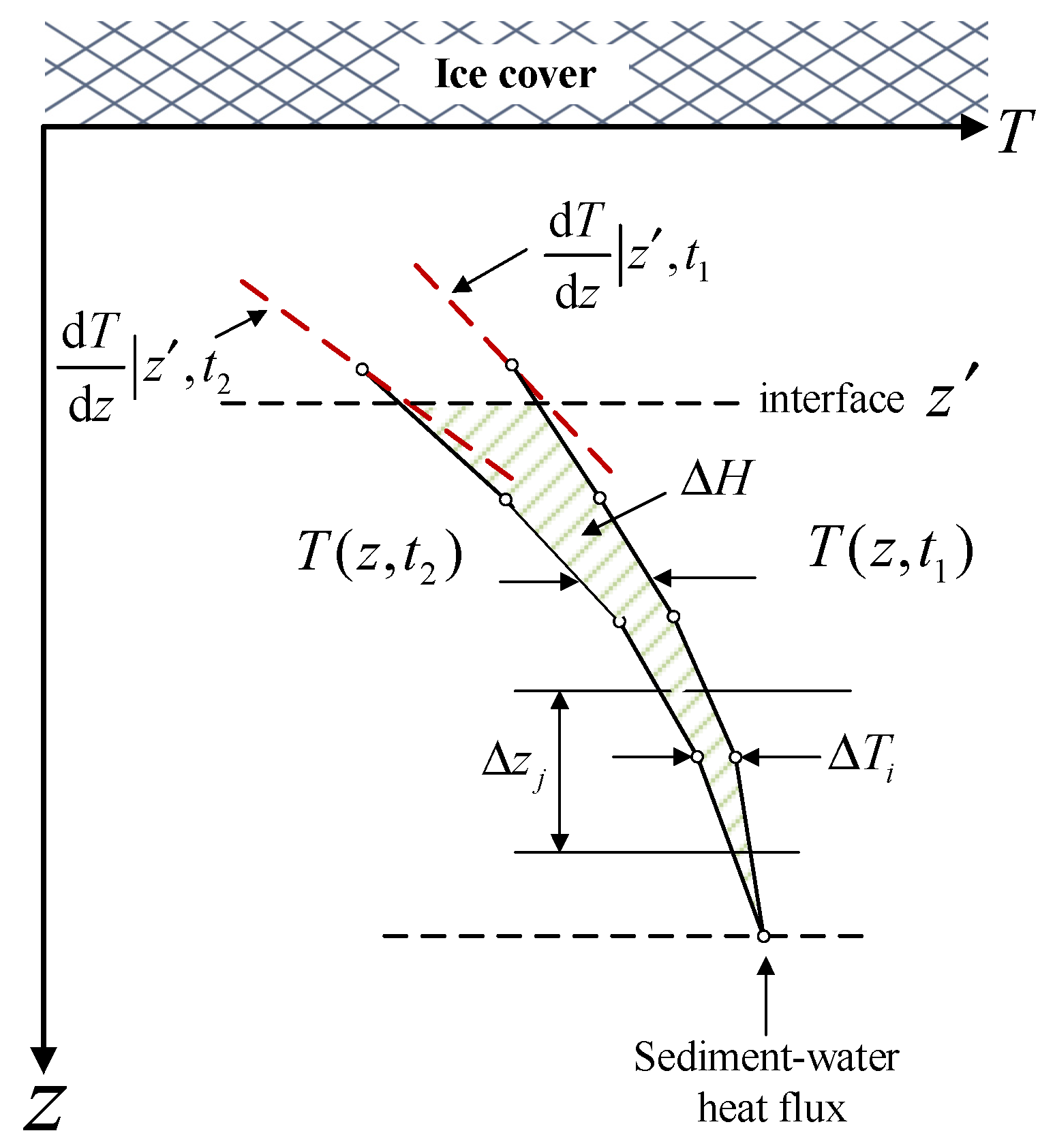
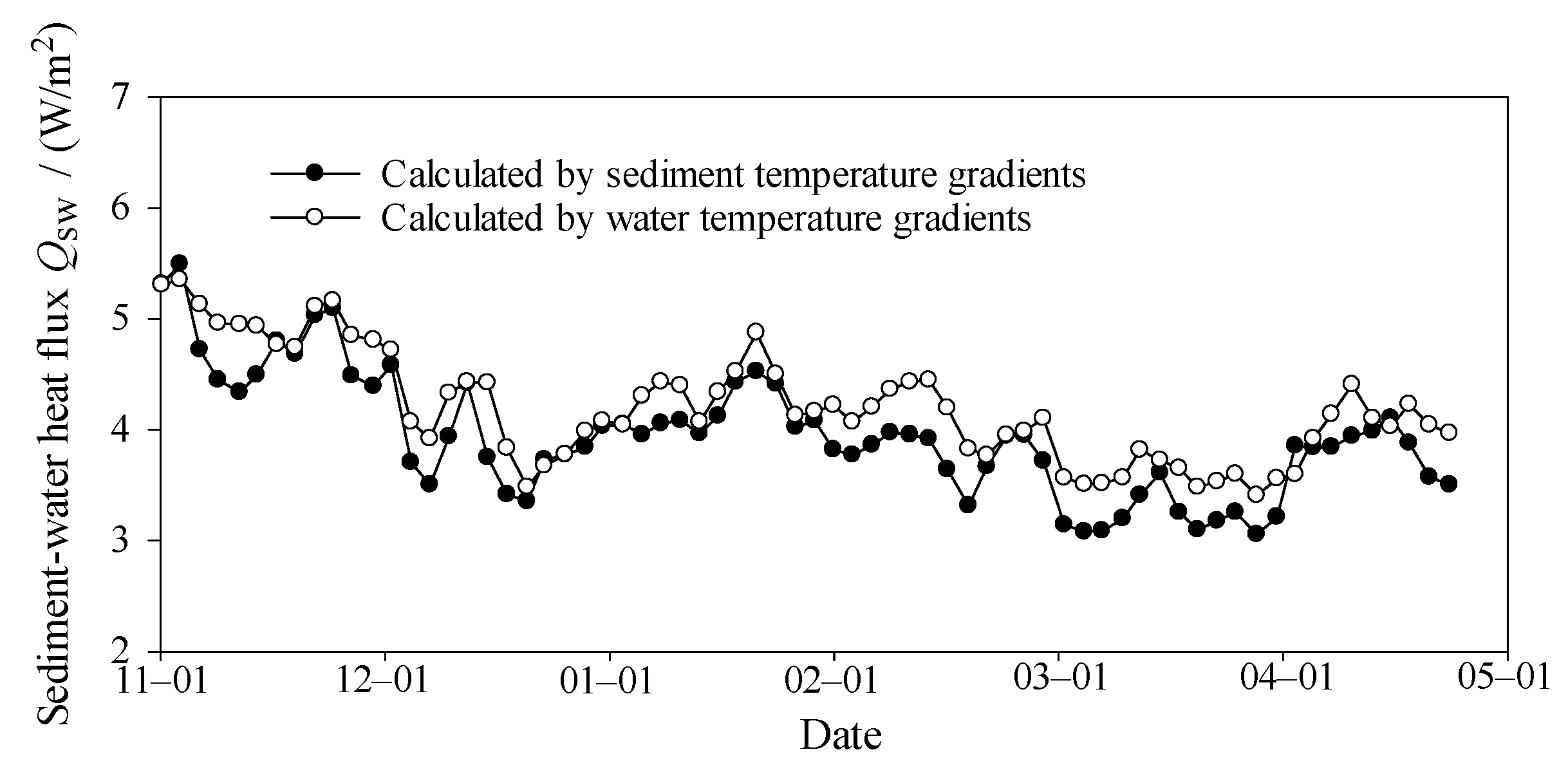
2.3.1. Sediment–Water Heat Flux
2.3.2. Water–Ice Heat Flux
2.3.3. Effective Thermal Diffusivity
3. Results and Discussion
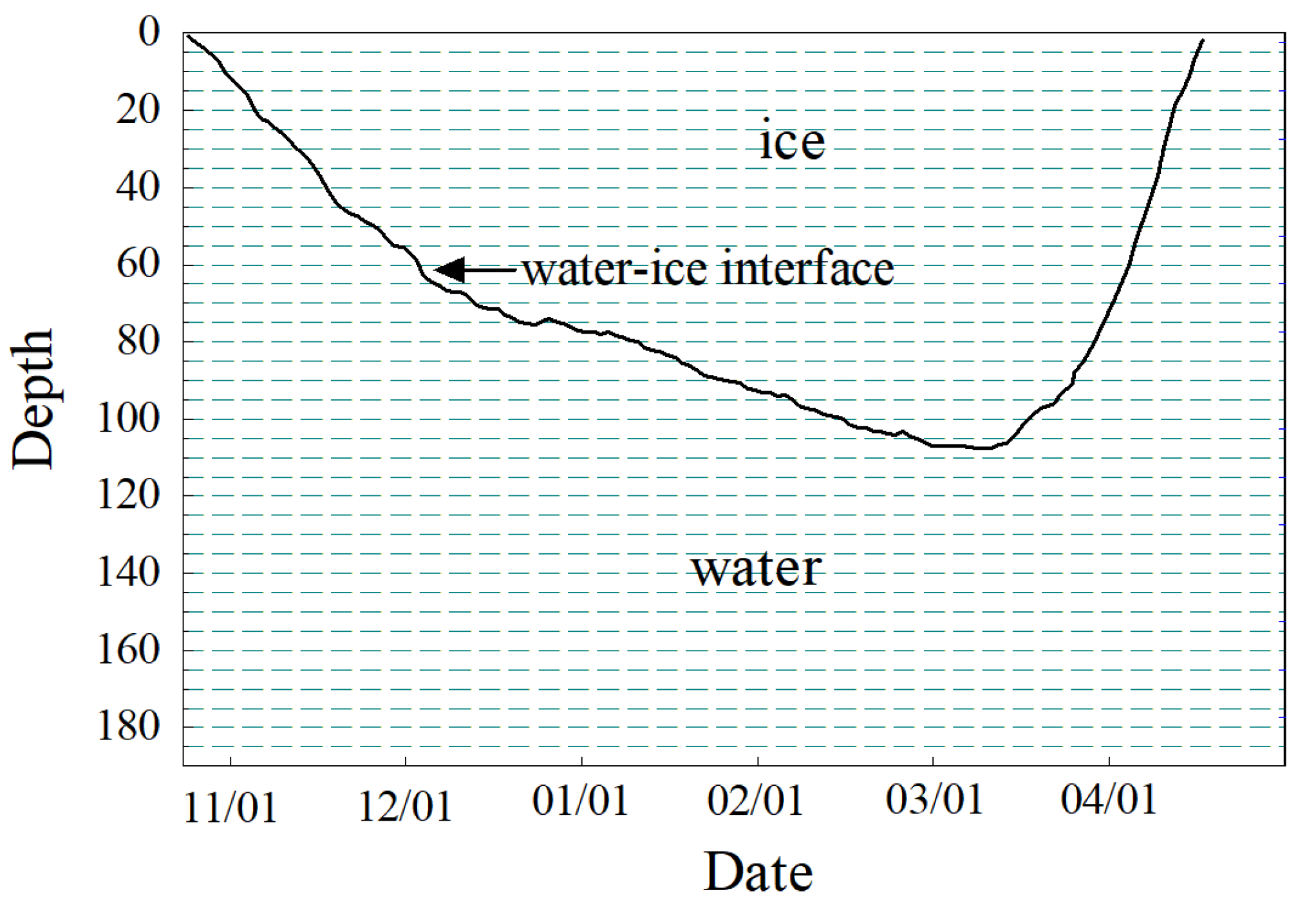
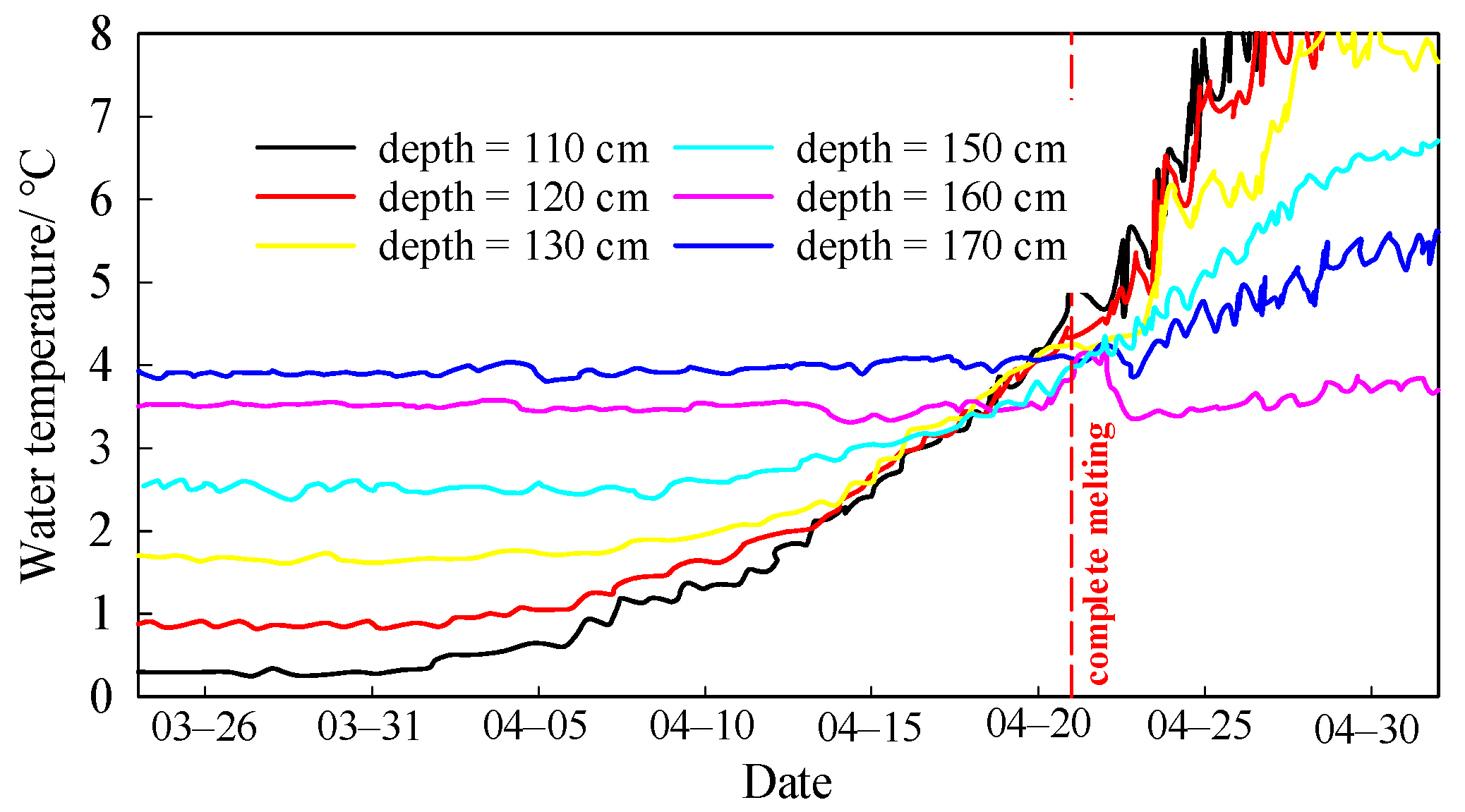
3.1. Ice Formation and Melting
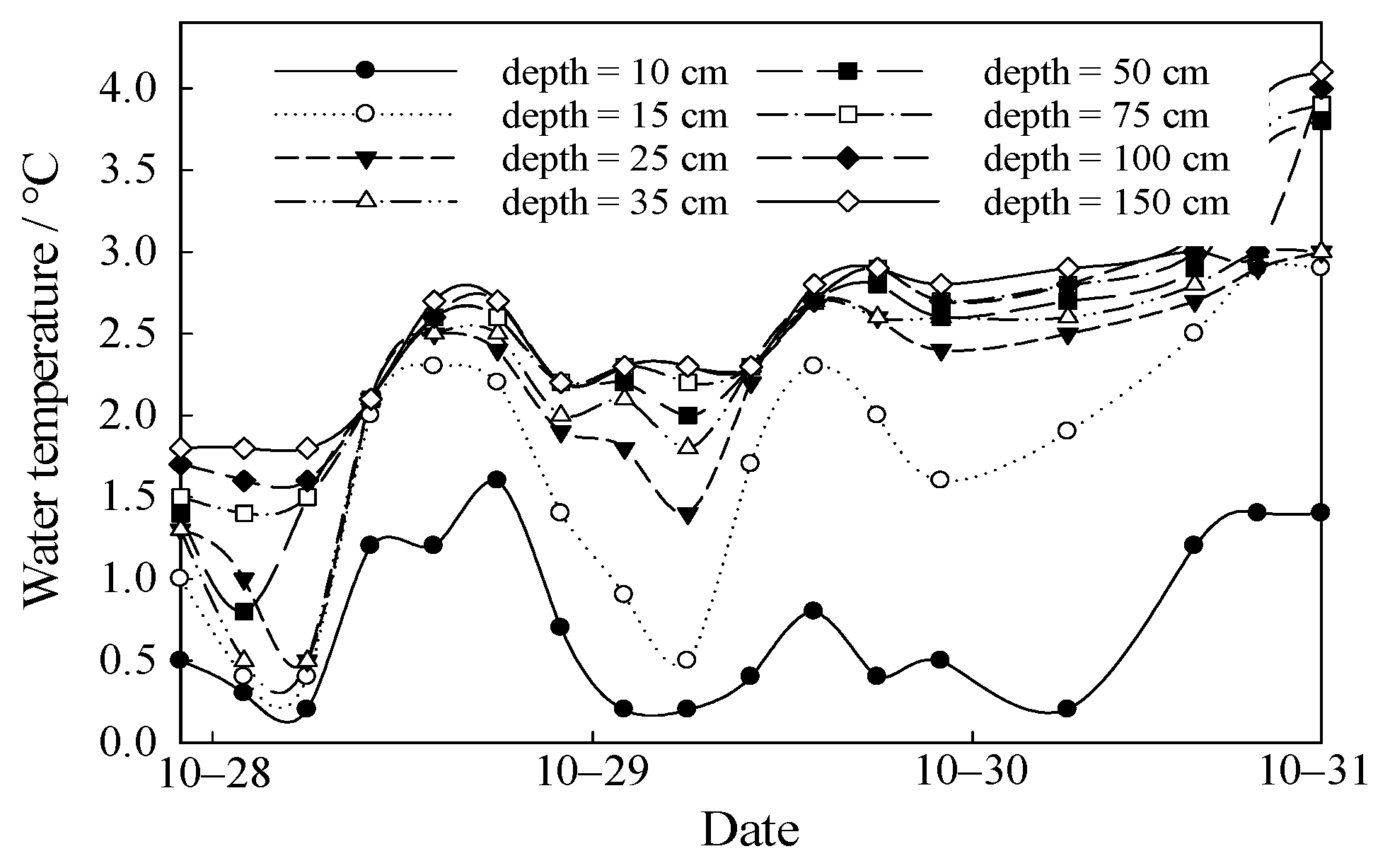
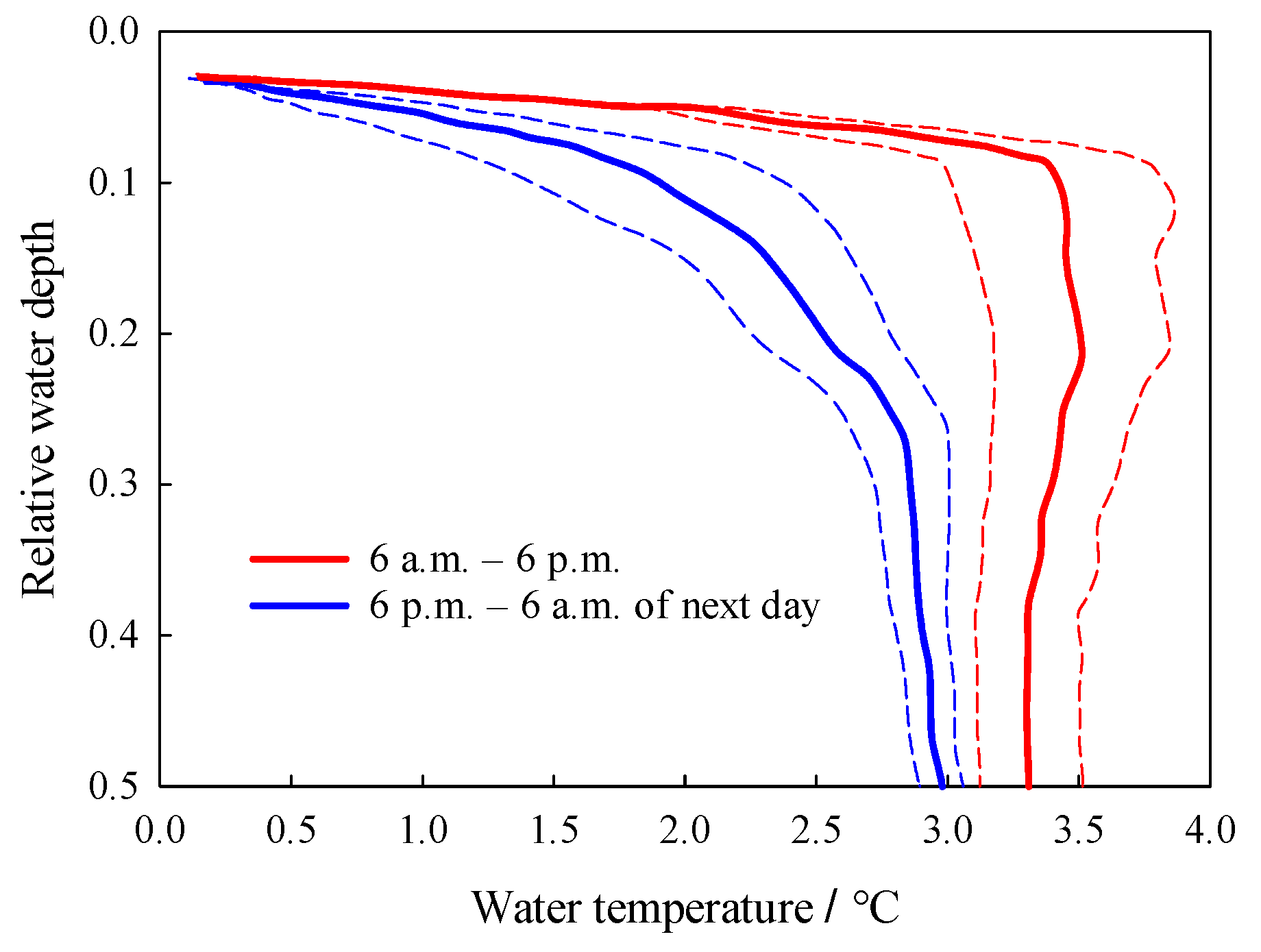
3.2. Temporal Variations of Water Temperature
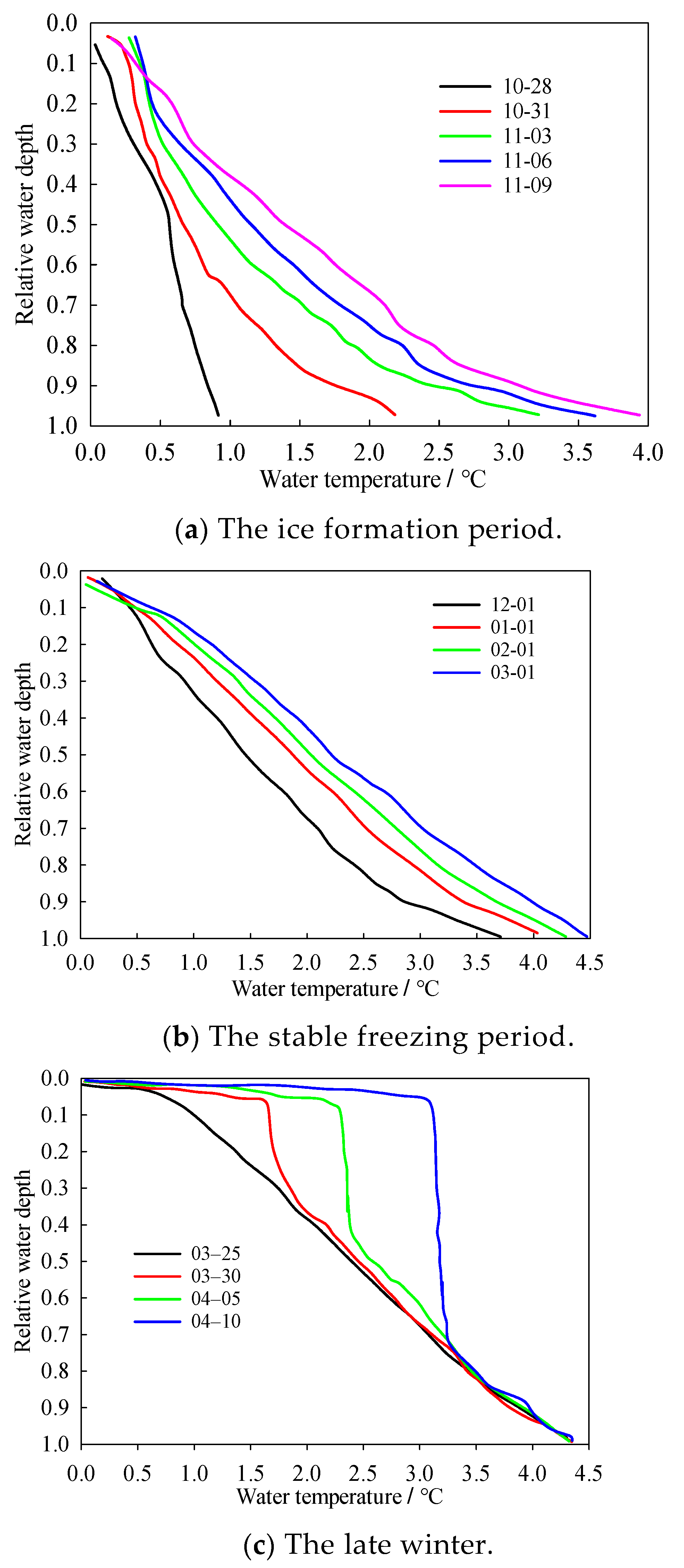
3.3. Vertical Profiles of Water Temperature
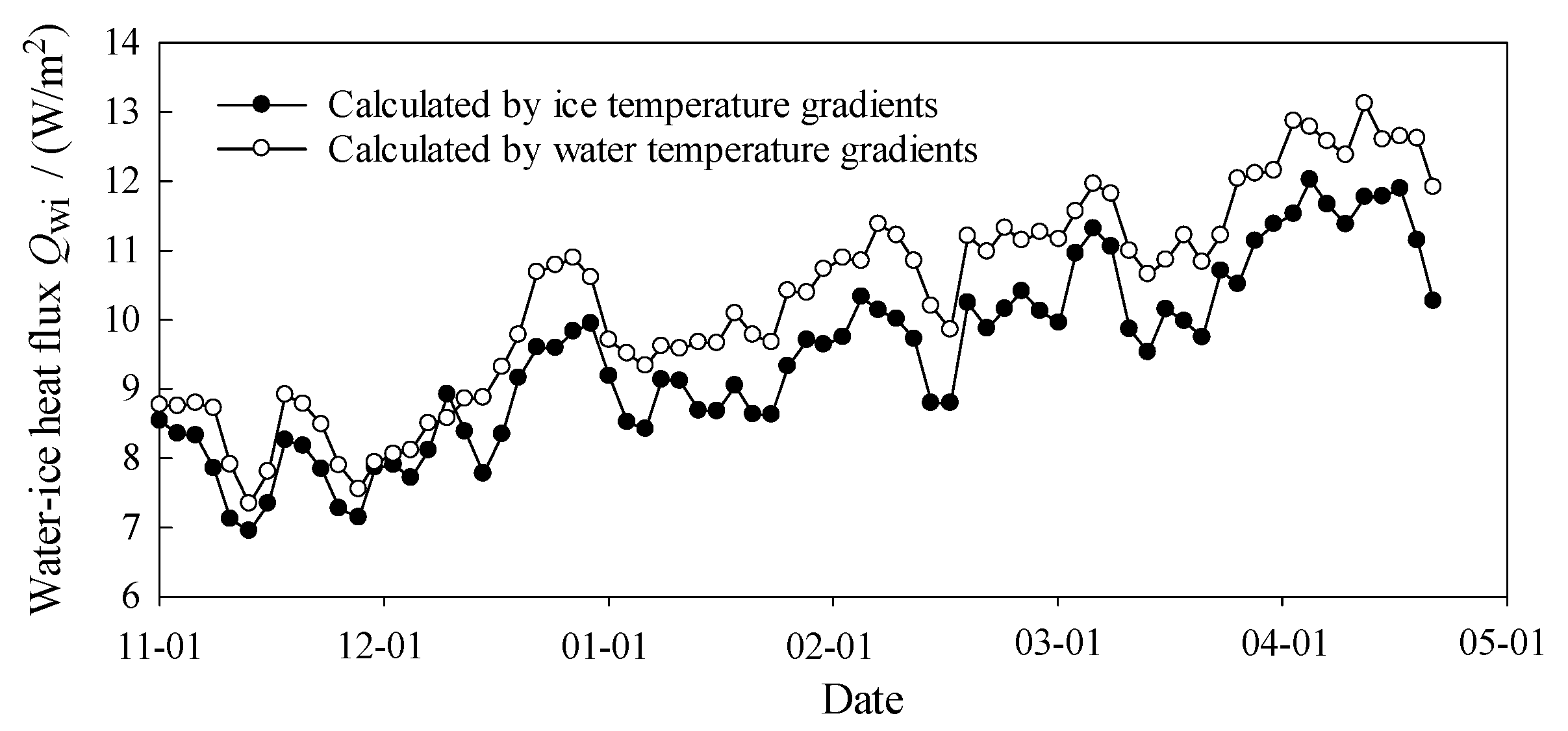
3.4. Heat Flux at Interfaces
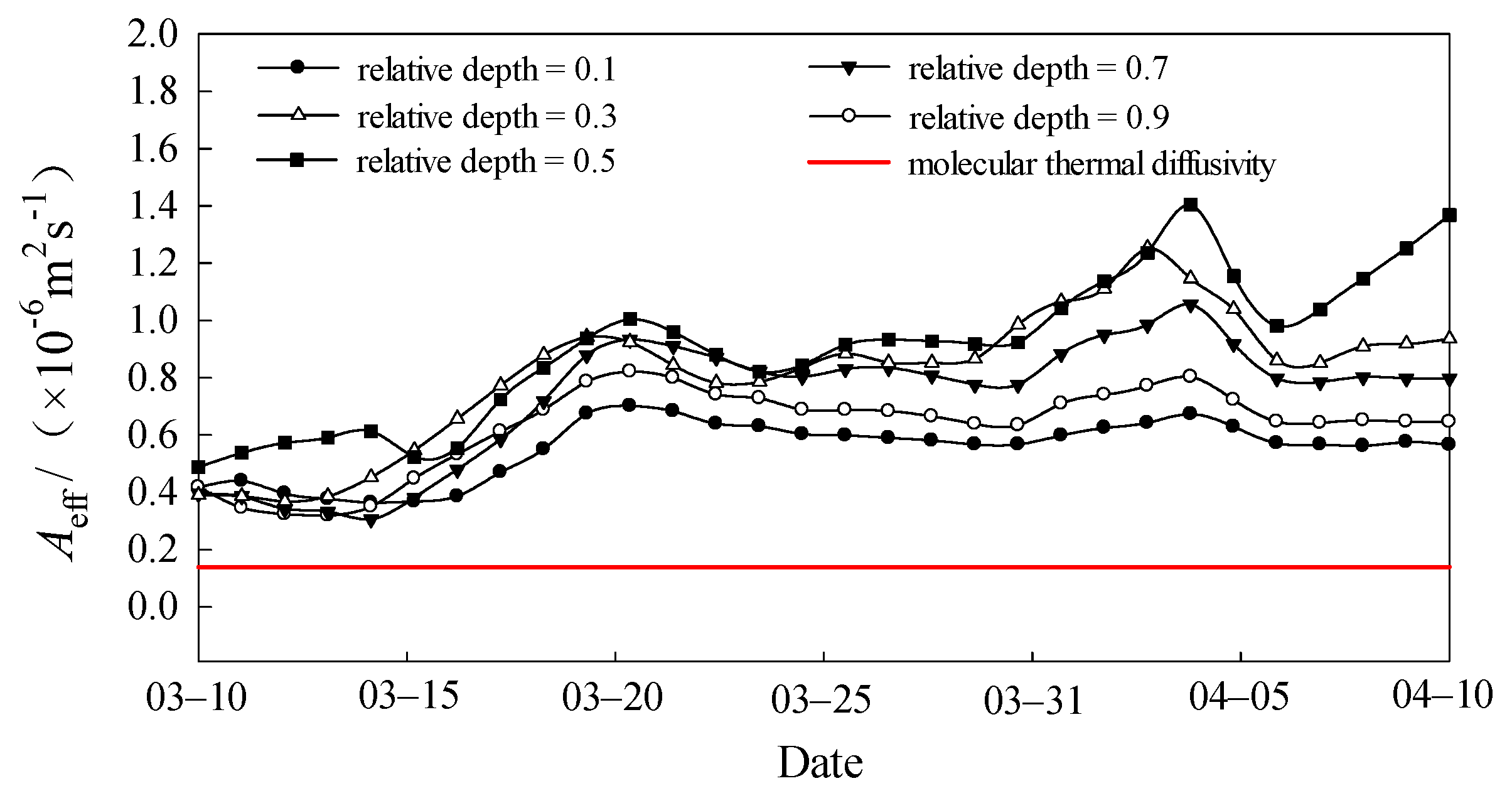
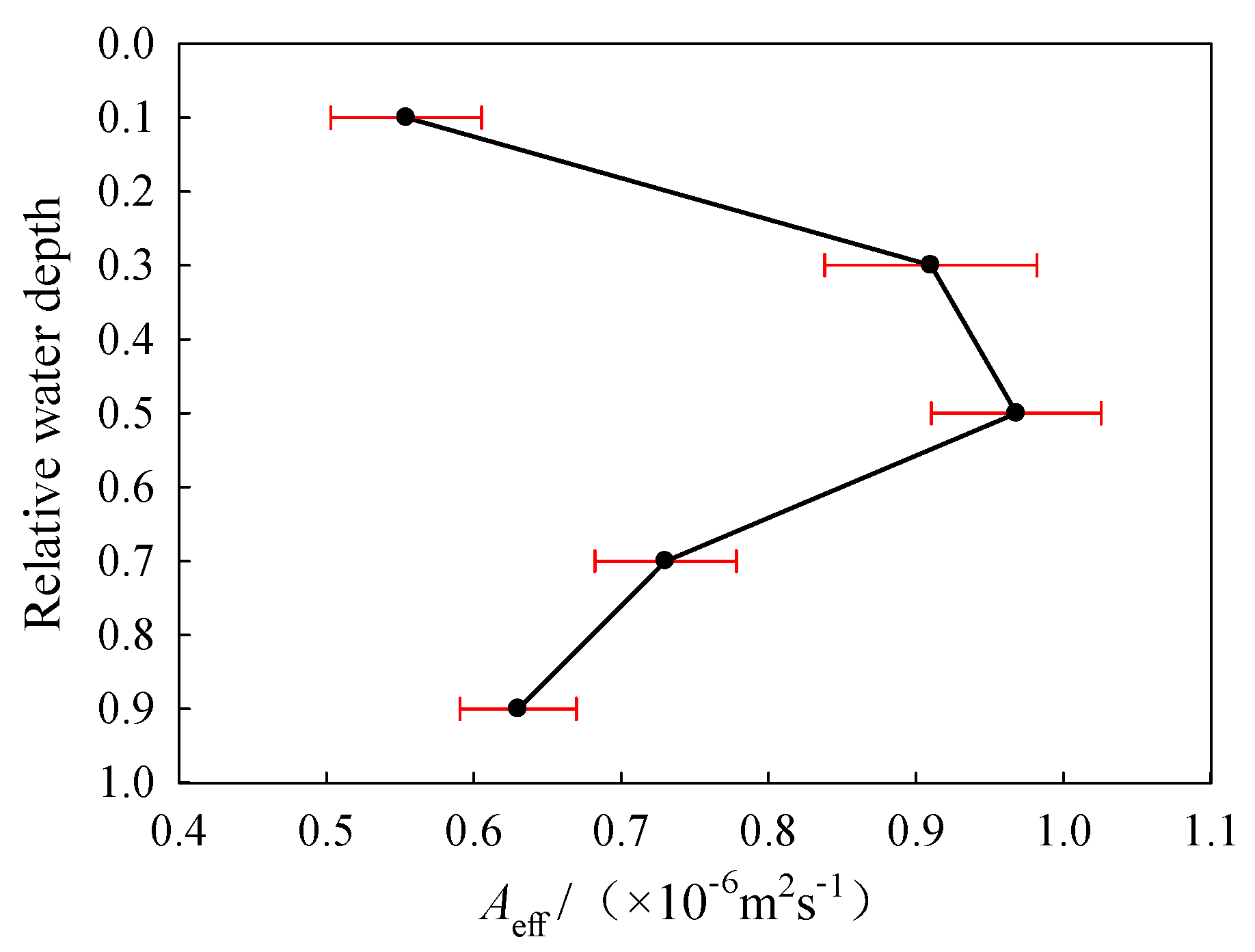
3.5. Effective Thermal Diffusivity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.L.; Chen, W.M.; Yang, D.T.; Huang, W.Y.; Jiang, J. Monitoring and analysis of thermodynamics in Tianmuhu Lake. ShuikexueJinzhan/Adv. Water Sci. 2004, 15, 61–67. [Google Scholar] [CrossRef]
- Zhu, S.; Ptak, M.; Yaseen, Z.M.; Dai, J.; Sivakumar, B. Forecasting surface water temperature in lakes: A comparison of approaches. J. Hydrol. 2020, 585, 124809–124818. [Google Scholar] [CrossRef]
- Bachmann, R.W.; Canfield, D.E.; Sharma, S.; Lecours, V. Warming of near-surface summer water temperatures in lakes of the conterminous united states. Water 2020, 12, 3381. [Google Scholar] [CrossRef]
- Ptak, M.; Sojka, M.; Choiński, A.; Nowak, B. Effect of environmental conditions and morphometric parameters on surface water temperature in Polish lakes. Water 2018, 10, 580. [Google Scholar] [CrossRef] [Green Version]
- Ptak, M.; Tomczyk, A.M.; Wrzesinski, D. Effect of teleconnection patterns on changes in water temperature in Polish lakes. Atmosphere 2018, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; Yu, Z.; Luo, Y.; Yang, Y.; Zhao, L.; Zhou, X. Spatial and temporal variations in the relationship between lake water surface temperatures and water quality—A case study of Dianchi Lake. Sci. Total Environ. 2018, 624, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, S. Freezing Temperature Controls Winter Water Discharge for Cold Region Watershed. Water Resour. Res. 2019, 55, 10051–11354. [Google Scholar] [CrossRef] [Green Version]
- Gronewold, A.D.; Anderson, E.J.; Lofgren, B.; Blanken, P.D.; Wang, J.; Smith, J.; Hunter, T.; Lang, G.; Stow, C.A.; Beletsky, D.; et al. Impacts of extreme 2013–2014 winter conditions on Lake Michigan’s fall heat content, surface temperature, and evaporation. Geophys. Res. Lett. 2015, 42, 3364–3370. [Google Scholar] [CrossRef] [Green Version]
- Iestyn Woolway, R.; Merchant, C.J. Amplified surface temperature response of cold, deep lakes to inter-annual air temperature variability. Sci. Rep. 2017, 7, 4130–4138. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Li, C.; Shi, X.; Zhao, S.; Hao, Y. Impact of seasonal ice structure characteristics on ice cover impurity distributions in Lake Ulansuhai. HupoKexue/J. Lake Sci. 2016, 28, 455–462. [Google Scholar] [CrossRef] [Green Version]
- Kirillin, G.; Leppäranta, M.; Terzhevik, A.; Granin, N.; Bernhardt, J.; Engelhardt, C.; Efremova, T.; Golosov, S.; Palshin, N.; Sherstyankin, P.; et al. Physics of seasonally ice-covered lakes: A review. Aquat. Sci. 2012, 74, 659–682. [Google Scholar] [CrossRef]
- Zhou, C.; Liang, R.; Xiao, Y.; Li, K. Water temperature and ice conditions of high-dam reservoirs in northwestern cold and arid regions. ShuiliFadianXuebao/J. Hydroelectr. Eng. 2016, 35, 31–39. [Google Scholar] [CrossRef]
- Teng, H.; Deng, Y.; Huang, F.B.; Tuo, Y.C. Experimental study on the simulation of freezing processes in calm waters and thermal changes on reservoir ice cover. ShuikexueJinzhan/Adv. Water Sci. 2011, 22, 720–726. [Google Scholar] [CrossRef] [Green Version]
- Ulloa, H.N.; Winters, K.B.; Wüest, A.; Bouffard, D. Differential Heating Drives Downslope Flows that Accelerate Mixed-Layer Warming in Ice-Covered Waters. Geophys. Res. Lett. 2019, 46, 13872–13882. [Google Scholar] [CrossRef] [Green Version]
- Frassl, M.A.; Boehrer, B.; Holtermann, P.L.; Hu, W.; Klingbeil, K.; Peng, Z.; Zhu, J.; Rinke, K. Opportunities and limits of using meteorological reanalysis data for simulating seasonal to sub-daily water temperature dynamics in a large shallow lake. Water 2018, 10, 594. [Google Scholar] [CrossRef] [Green Version]
- Williams, P.; Whitfield, M.; Biggs, J.; Bray, S.; Fox, G.; Nicolet, P.; Sear, D. Comparative biodiversity of rivers, streams, ditches and ponds in an agricultural landscape in Southern England. Biol. Conserv. 2004, 115, 329–341. [Google Scholar] [CrossRef]
- Downing, J.A. Emerging global role of small lakes and ponds: Little things mean a lot. Limnetica 2010, 29, 9–24. [Google Scholar] [CrossRef]
- Huang, W.; Han, H.; Niu, F.; Li, Z. Field observations on water temperature and stratification in a seasonally ice-covered shallow thermokarst lake. ShuikexueJinzhan/Adv. Water Sci. 2016, 27, 280–289. [Google Scholar] [CrossRef]
- de la Fuente, A.; Meruane, C. Dimensionless numbers for classifying the thermodynamics regimes that determine water temperature in shallow lakes and wetlands. Environ. Fluid Mech. 2017, 17, 1081–1098. [Google Scholar] [CrossRef]
- Torma, P.; Wu, C.H. Temperature and circulation dynamics in a small and shallow lake: Effects of weak stratification and littoral submerged macrophytes. Water 2019, 11, 128. [Google Scholar] [CrossRef] [Green Version]
- Bouffard, D.; Zdorovennov, R.E.; Zdorovennova, G.E.; Pasche, N.; Wüest, A.; Terzhevik, A.Y. Ice-covered Lake Onega: Effects of radiation on convection and internal waves. Hydrobiologia 2016, 780, 1–16. [Google Scholar] [CrossRef]
- Hamilton, D.P.; Magee, M.R.; Wu, C.H.; Kratz, T.K. Ice cover and thermal regime in a dimictic seepage lake under climate change. Inl. Waters 2018, 8, 381–398. [Google Scholar] [CrossRef]
- Zhao, L.L.; Zhu, G.W.; Chen, Y.F.; Li, W.; Zhu, M.Y.; Yao, X.; Cai, L.L. Thermal stratification and its influence factors in a large-sized and shallow Lake Taihu. ShuikexueJinzhan/Adv. Water Sci. 2011, 22, 844–850. [Google Scholar] [CrossRef]
- Huang, W.; Cheng, B.; Zhang, J.; Zhang, Z.; Vihma, T.; Li, Z.; Niu, F. Modeling experiments on seasonal lake ice mass and energy balance in Qinghai-Tibet Plateau: A case study. Hydrol. Earth Syst. Sci. Discuss. 2018, 23, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Feng, W.; Matti, L.; Yang, Y.; Merkouriadi, I.; Cen, R.; Bai, Y.; Li, C.; Liao, H. Simulation and seasonal characteristics of the intra-annual heat exchange process in a shallow ice-covered lake. Sustainability 2020, 12, 7832. [Google Scholar] [CrossRef]
- Song, K.; Wen, Z.; Jacinthe, P.A.; Zhao, Y.; Du, J. Dissolved carbon and CDOM in lake ice and underlying waters along a salinity gradient in shallow lakes of Northeast China. J. Hydrol. 2019, 571, 545–558. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Bao, K.; Yao, S.; Yang, F.; Wang, X. Ecological risk assessment and distribution of potentially harmful trace elements in lake sediments of Songnen Plain, NE China. Ecotoxicol. Environ. Saf. 2018, 163, 117–124. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, H.G. Dynamics of heat exchange between sediment and water in a lake. Water Resour. Res. 1996, 32, 1719–1727. [Google Scholar] [CrossRef]
- Birge, E.; Juday, C.; March, H. The Temperature of the Bottom Deposits of Lake Mendota, A Chapter in the Heat Exchanges of the Lake. Trans. Wis. Acad. Sci. Arts Lett. 1927, 23, 187–231. [Google Scholar]
- Pivovarov, A.A. Thermal Conditions in Freezing Lakes and Rivers; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Ryanzhin, S.V. Thermophysical Properties of Lake Sediments and Water-Sediments Heat Interaction; Repo~No. 3214; Department of Water Resources Engineering, Institute of Technology, University of Lund: Lund, Sweden, 1997. [Google Scholar]
- Yang, S.M.; Tao, W.Q. Heat Transfer, 4th ed.; Higher Education Press: Beijing, China, 2006; p. 272. [Google Scholar]
- Petrov, M.P.; Terzhevik, A.Y.; Zdorovennov, R.E.; Zdorovennova, G.E. The thermal structure of a shallow lake in early winter. Water Resour. 2006, 33, 135–143. [Google Scholar] [CrossRef]
- Aslamov, I.A.; Kozlov, V.V.; Misandrontsev, I.B.; Kucher, K.M.; Granin, N.G. Estimate of heat flux at the ice-water interface in Lake Baikal from experimental data. Dokl. Earth Sci. 2014, 457, 982–985. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, J.; Wang, Y.; Hu, W. Changes of water temperature and heat flux at water-sediment interface, East Lake Taihu. HupoKexue/J. Lake Sci. 2018, 30, 1599–1609. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.J. Heat transfer. Magn. Reson. Mater. Biol. Phys. Med. 1974, 9, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Jakkila, J.; Leppäranta, M.; Kawamura, T.; Shirasawa, K.; Salonen, K. Radiation transfer and heat budget during the ice season in Lake Pääjärvi, Finland. Aquat. Ecol. 2009, 43, 681–692. [Google Scholar] [CrossRef]
- Kirillin, G.; Terzhevik, A. Thermal instability in freshwater lakes under ice: Effect of salt gradients or solar radiation? Cold Reg. Sci. Technol. 2011, 65, 184–190. [Google Scholar] [CrossRef]
- Leppäranta, M.; Terzhevik, A.; Shirasawa, K. Solar radiation and ice melting in Lake vendyurskoe, Russian Karelia. Hydrol. Res. 2010, 41, 50–62. [Google Scholar] [CrossRef]
- Aslamov, I.A.; Kozlov, V.V.; Kirillin, G.B.; Mizandrontsev, I.B.; Kucher, K.M.; Makarov, M.M.; Gornov, A.Y.; Granin, N.G. Ice-water heat exchange during ice growth in Lake Baikal. J. Great Lakes Res. 2014, 40, 599–607. [Google Scholar] [CrossRef]
- Bengtsson, L.; Svensson, T. Thermal regime of ice covered Swedish lakes. Hydrol. Res. 1996, 27, 39–56. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Stephenson, D.B. Forecast Verification: A Practitioner’s Guide in Atmospheric Science, 2nd ed.; Forecast Verification; Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
| Date | d | Qrad (W/m2) | Qrad-ice (W/m2) | z (m) | Qrad-water (W/m2) |
|---|---|---|---|---|---|
| 11/1 | −61 | 183.34 | 91.67 | 0.07 | 76.95 |
| 11/6 | −56 | 167.73 | 83.87 | 0.15 | 57.64 |
| 11/11 | −51 | 154.14 | 77.07 | 0.24 | 42.30 |
| 11/16 | −46 | 142.47 | 71.23 | 0.31 | 32.82 |
| 11/21 | −41 | 132.65 | 66.33 | 0.43 | 22.64 |
| 11/26 | −36 | 124.62 | 62.31 | 0.47 | 19.24 |
| 12/1 | −31 | 118.29 | 59.14 | 0.54 | 15.33 |
| 12/6 | −26 | 113.59 | 56.80 | 0.63 | 11.76 |
| 12/11 | −21 | 110.45 | 55.22 | 0.67 | 10.34 |
| 12/16 | −16 | 108.78 | 54.39 | 0.70 | 9.45 |
| 12/21 | −11 | 108.52 | 54.26 | 0.74 | 8.53 |
| 12/26 | −6 | 109.59 | 54.80 | 0.75 | 8.40 |
| 1/1 | 1 | 113.19 | 56.59 | 0.76 | 8.46 |
| 1/6 | 6 | 117.14 | 58.57 | 0.76 | 8.76 |
| 1/11 | 11 | 122.17 | 61.09 | 0.79 | 8.48 |
| 1/16 | 16 | 128.21 | 64.10 | 0.82 | 8.25 |
| 1/21 | 21 | 135.16 | 67.58 | 0.85 | 8.07 |
| 1/26 | 26 | 142.97 | 71.49 | 0.88 | 7.92 |
| 2/1 | 32 | 153.36 | 76.68 | 0.92 | 7.69 |
| 2/6 | 37 | 162.77 | 81.38 | 0.94 | 7.76 |
| 2/11 | 42 | 172.79 | 86.40 | 0.96 | 7.84 |
| 2/16 | 47 | 183.35 | 91.67 | 1.01 | 7.34 |
| 2/21 | 52 | 194.37 | 97.18 | 1.03 | 7.40 |
| 2/26 | 57 | 205.77 | 102.89 | 1.04 | 7.64 |
| 3/1 | 60 | 212.77 | 106.38 | 1.05 | 7.71 |
| 3/6 | 65 | 224.63 | 112.31 | 1.05 | 8.14 |
| 3/11 | 70 | 236.68 | 118.34 | 1.05 | 8.57 |
| 3/16 | 75 | 248.85 | 124.43 | 1.02 | 9.72 |
| 3/21 | 80 | 261.06 | 130.53 | 0.94 | 12.45 |
| 3/26 | 85 | 273.23 | 136.62 | 0.86 | 15.91 |
| 4/1 | 91 | 287.69 | 143.84 | 0.71 | 24.38 |
| 4/6 | 96 | 299.52 | 149.76 | 0.52 | 40.81 |
| 4/11 | 101 | 311.08 | 155.54 | 0.24 | 85.36 |
| 4/16 | 106 | 322.28 | 161.14 | 0.08 | 131.93 |
| 4/21 | 111 | 333.06 | 166.53 | 0.01 | 162.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, F.; Mao, Z. Observation and Analysis of Water Temperature in Ice-Covered Shallow Lake: Case Study in Qinghuahu Lake. Water 2021, 13, 3139. https://doi.org/10.3390/w13213139
Ding F, Mao Z. Observation and Analysis of Water Temperature in Ice-Covered Shallow Lake: Case Study in Qinghuahu Lake. Water. 2021; 13(21):3139. https://doi.org/10.3390/w13213139
Chicago/Turabian StyleDing, Falong, and Zeyu Mao. 2021. "Observation and Analysis of Water Temperature in Ice-Covered Shallow Lake: Case Study in Qinghuahu Lake" Water 13, no. 21: 3139. https://doi.org/10.3390/w13213139






