A Strontium and Hydro-Geochemical Perspective on Human Impacted Tributary of the Mekong River Basin: Sources Identification, Fluxes, and CO2 Consumption
Abstract
:1. Introduction
2. Materials and Methods
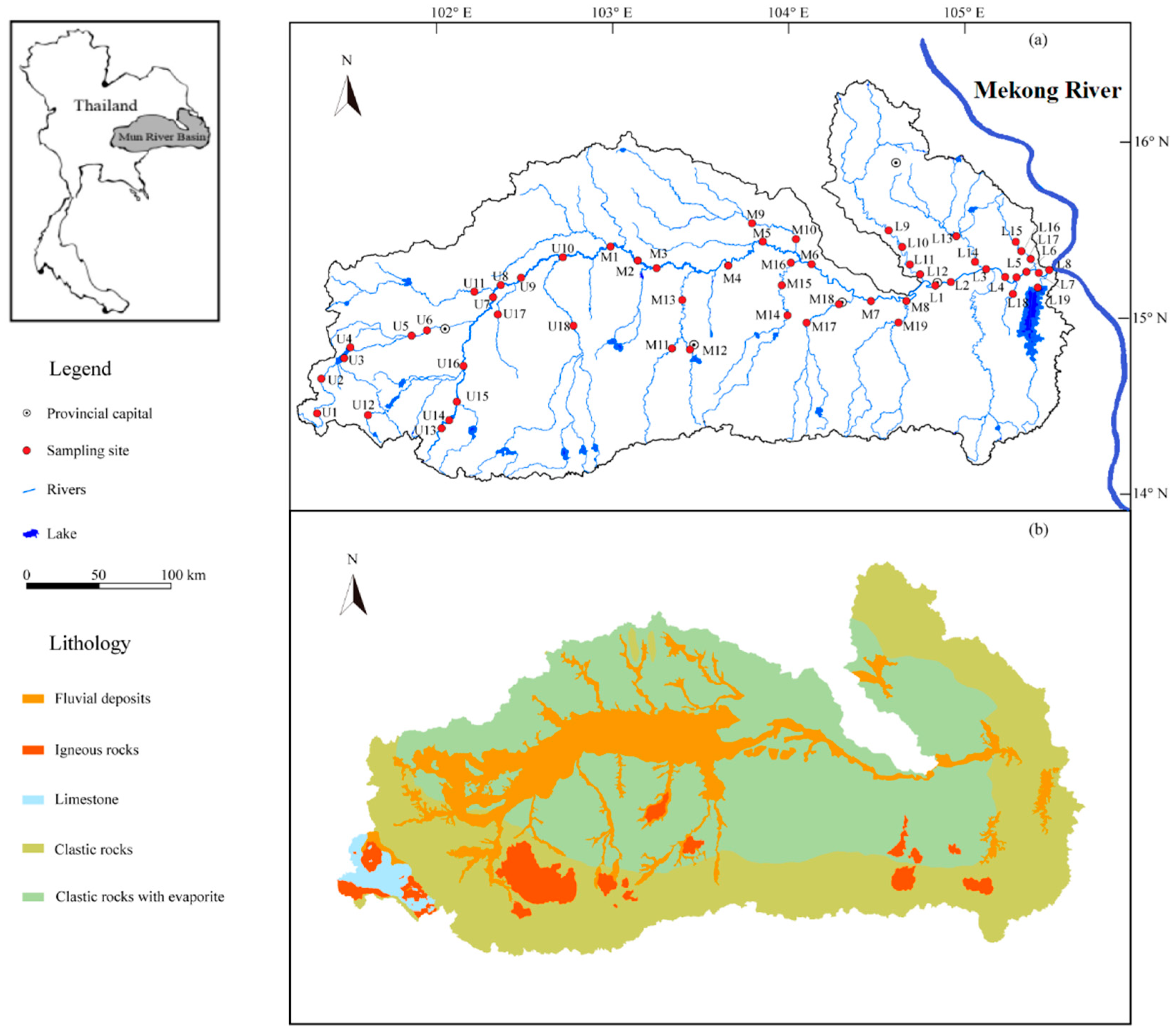
2.1. Study Region
2.2. Sampling and Chemical Analysis
2.3. Data Processing
2.4. Software
3. Results
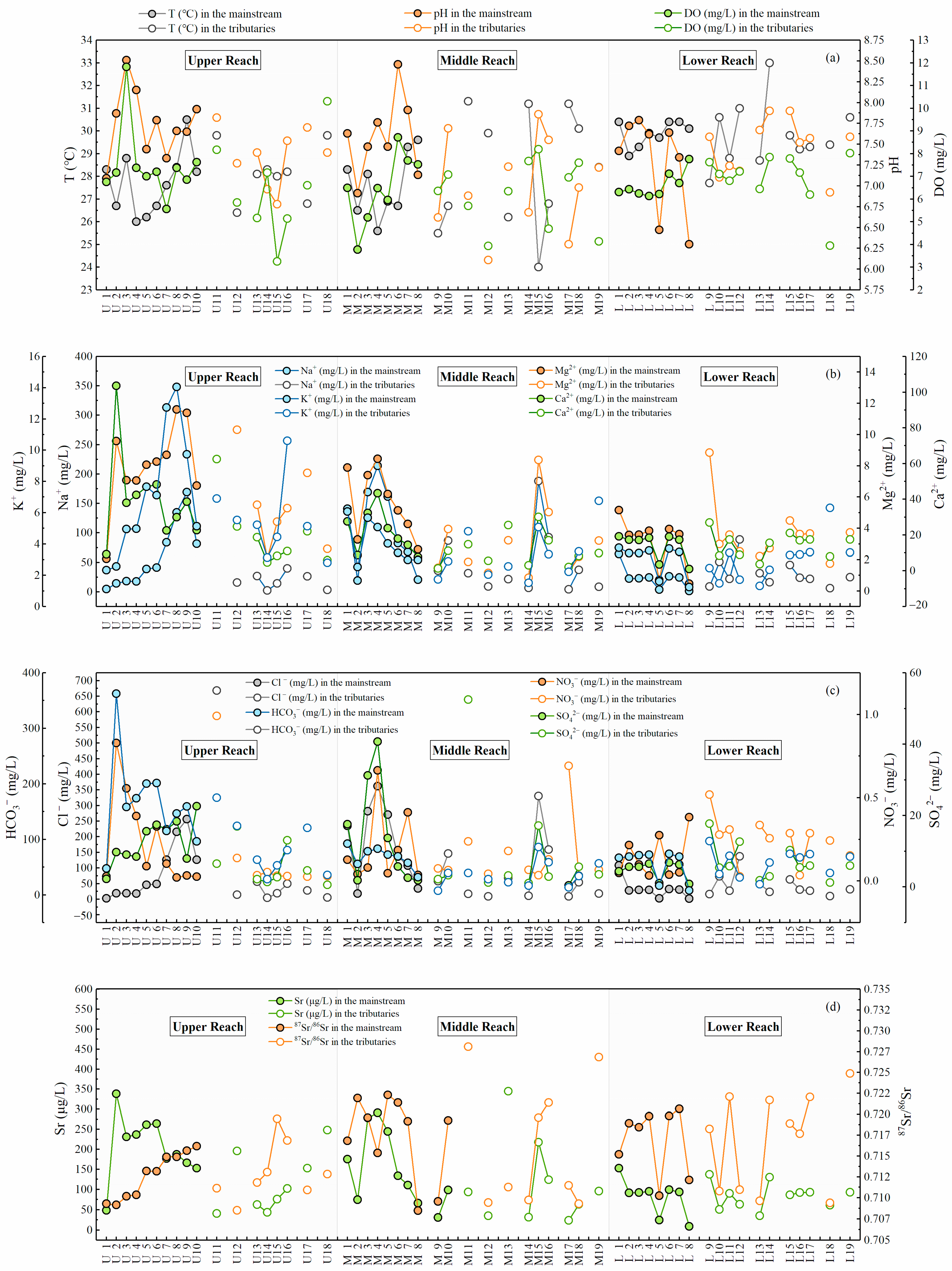
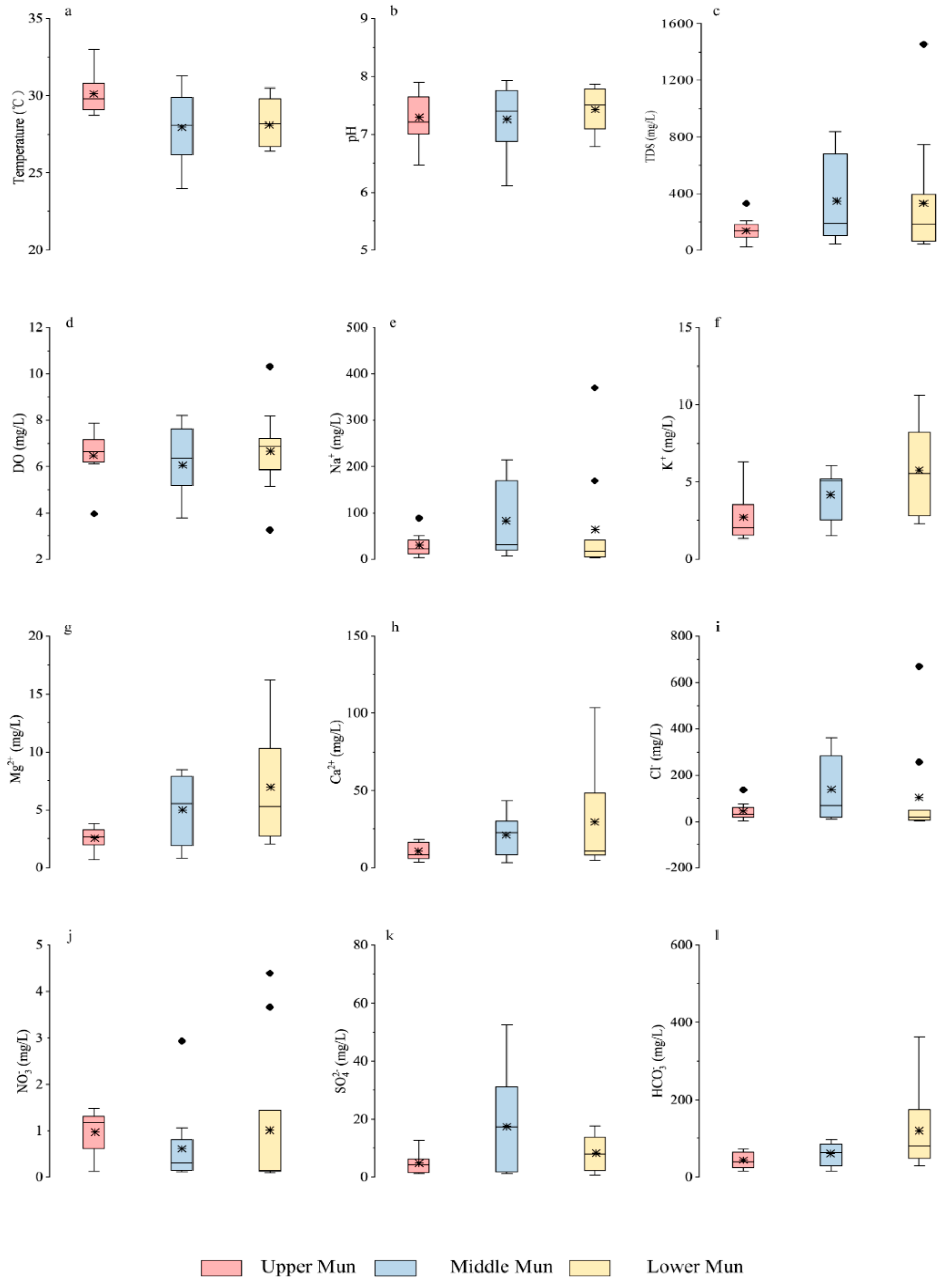
3.1. The Spatial Distribution of Dissolved Ions and Strontium Isotopes
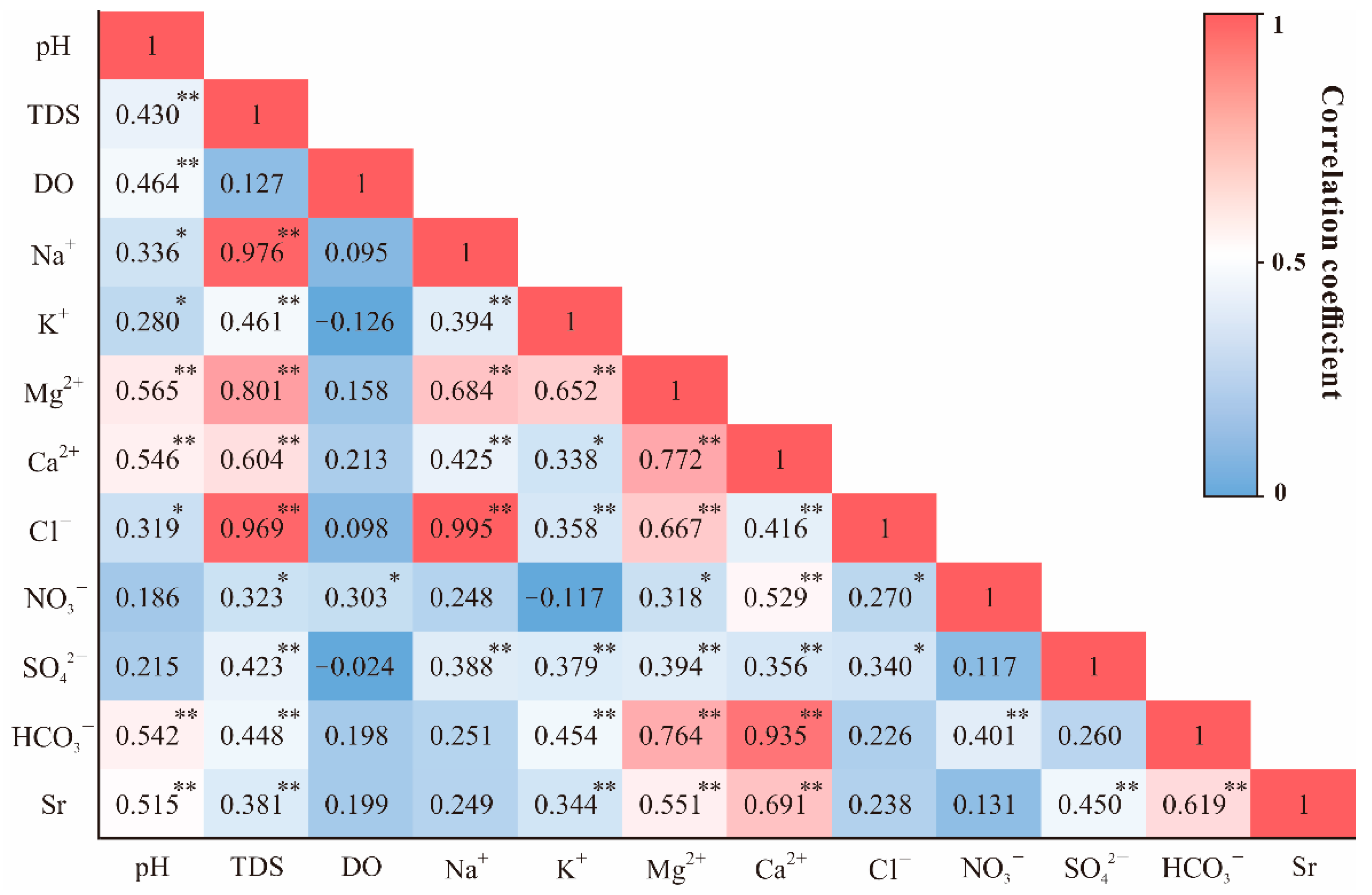
3.2. The Source of Dissolved Ions and Strontium Isotopes
3.3. The Sr Flux, Chemcial Weathering and Related CO2 Consumption Rate
4. Discussion
4.1. Contributions of Chemical Weathering to Dissolved Ions
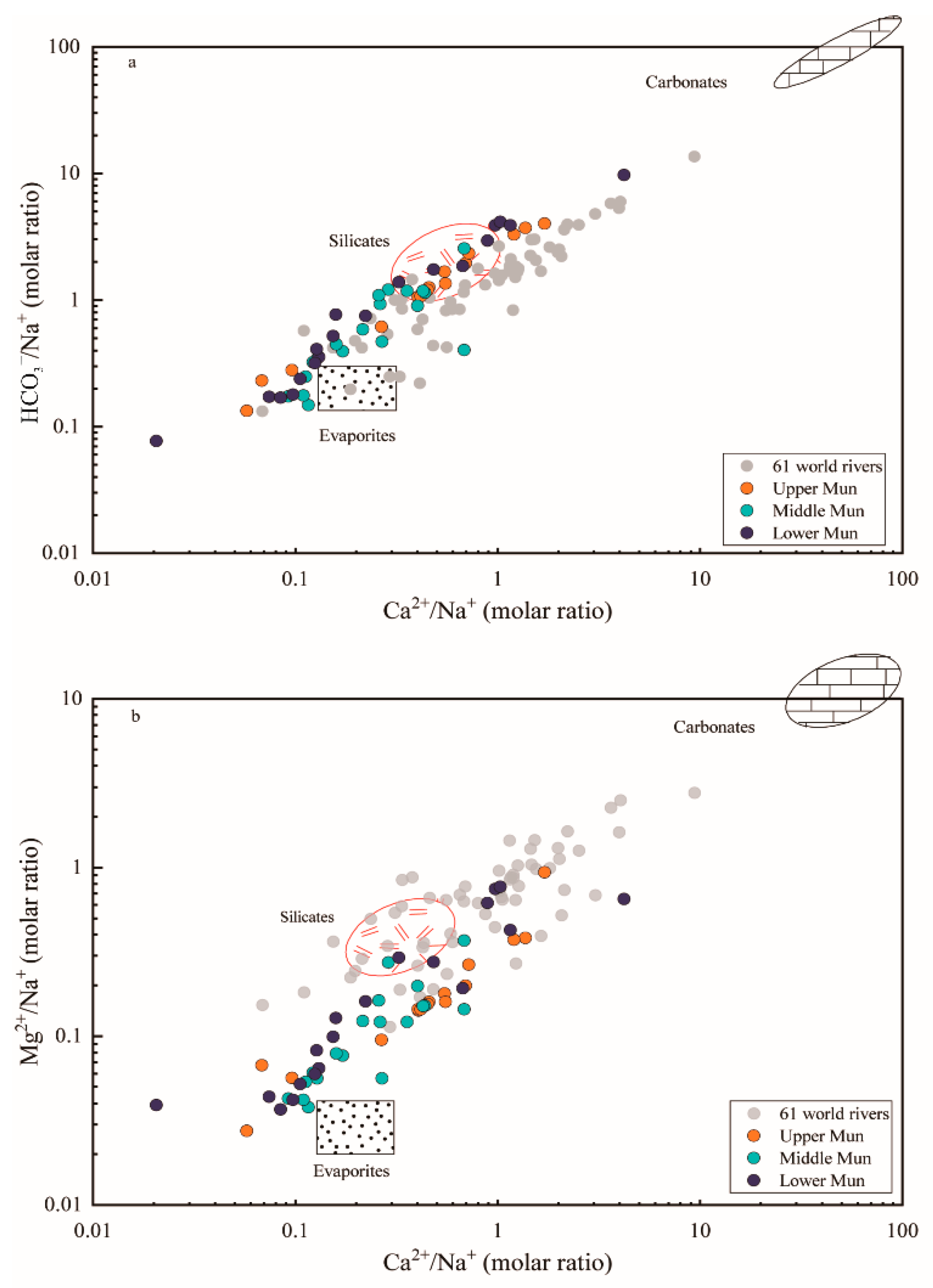
4.1.1. Weathering Source Identification of Dissolved Ions
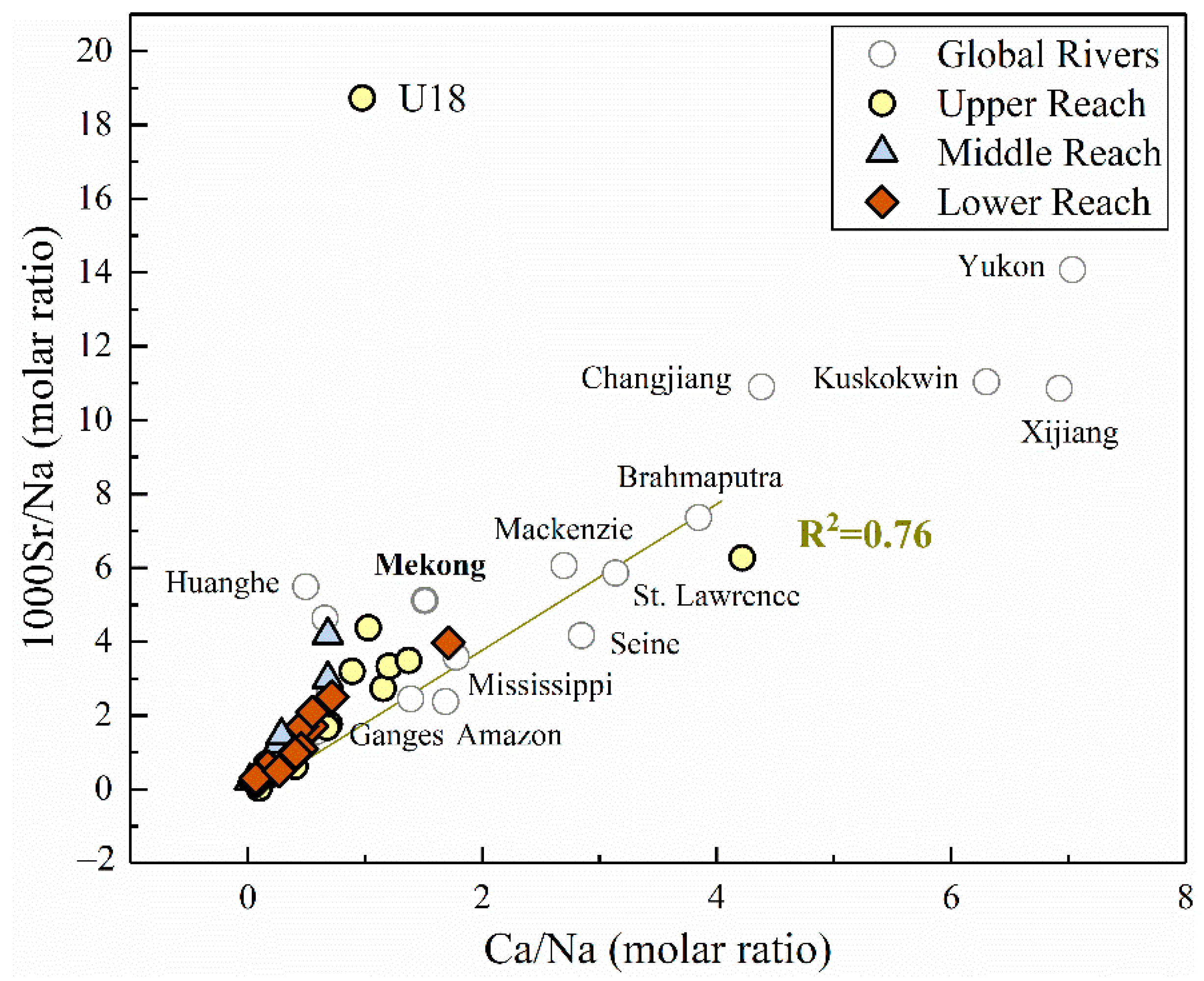
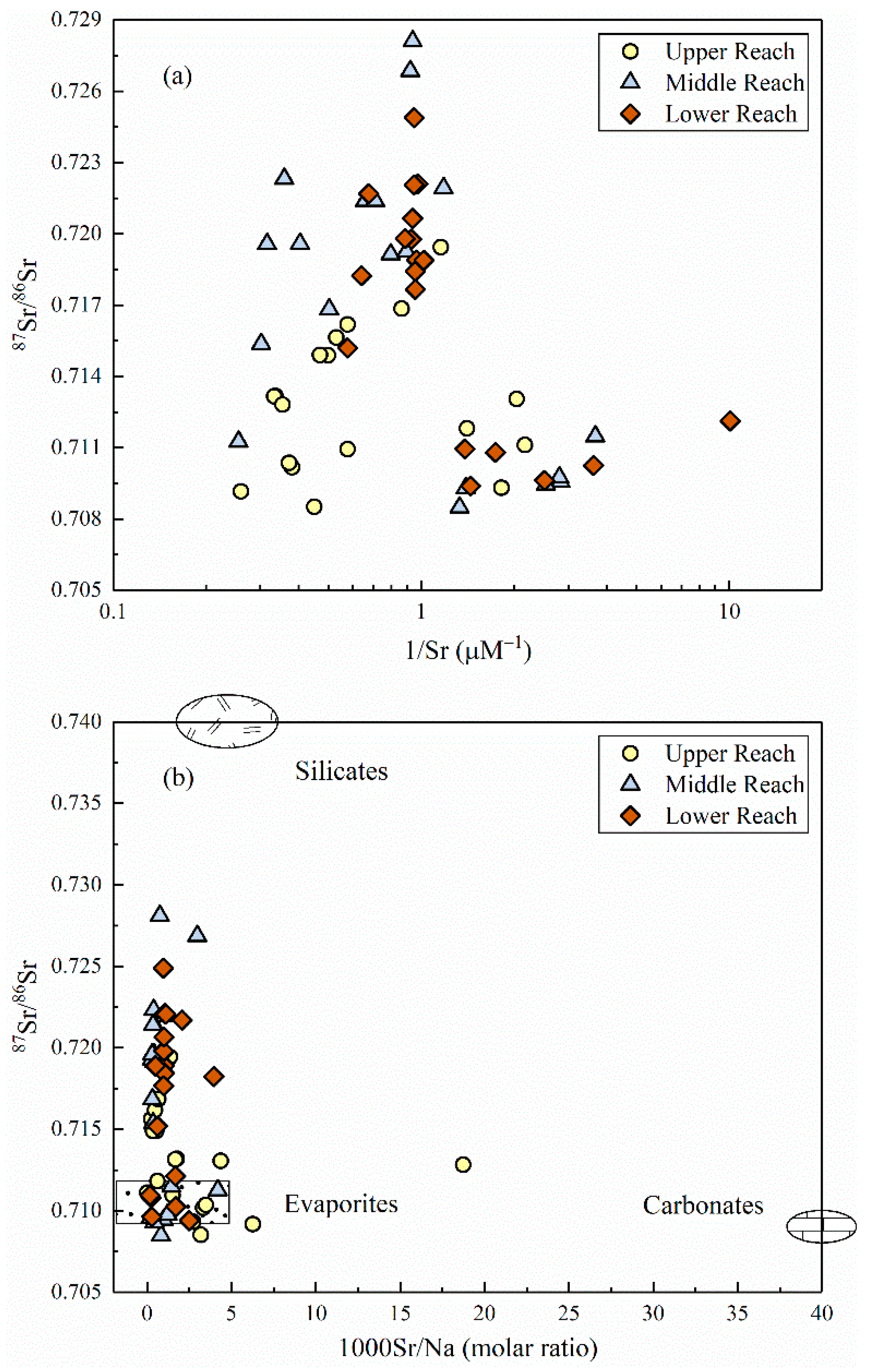
4.1.2. Potential End-Member Contribution of Strontium
4.1.3. Estimation of Dissolved Sr Flux
4.2. Influencing Factors of Chemical Weathering and Related CO2 Consumption Rate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gaillardet, J.; Dupré, B.; Louvat, P.; Allegre, C. Global silicate weathering and CO2 consumption rates deduced from the chemistry of large rivers. Chem. Geol. 1999, 159, 3–30. [Google Scholar] [CrossRef]
- Roy, S.; Gaillardet, J.; Allegre, C. Geochemistry of dissolved and suspended loads of the Seine river, France: Anthropogenic impact, carbonate and silicate weathering. Geochim. Cosmochim. Acta 1999, 63, 1277–1292. [Google Scholar] [CrossRef]
- Dalai, T.K.; Krishnaswami, S.; Kumar, A. Sr and 87Sr/86Sr in the Yamuna River System in the Himalaya: Sources, fluxes, and controls on Sr isotope composition. Geochim. Cosmochim. Acta 2003, 67, 2931–2948. [Google Scholar] [CrossRef]
- Millot, R.; Gaillardet, J.; Dupré, B.; Allègre, C.J. The global control of silicate weathering rates and the coupling with physical erosion: New insights from rivers of the Canadian Shield. Earth Planet. Sci. Lett. 2002, 196, 83–98. [Google Scholar] [CrossRef]
- Boral, S.; Peucker-Ehrenbrink, B.; Hemingway, J.D.; Sen, I.S.; Galy, V.; Fiske, G.J. Controls on short-term dissolved 87Sr/86Sr variations in large rivers: Evidence from the Ganga–Brahmaputra. Earth Planet. Sci. Lett. 2021, 566, 116958. [Google Scholar] [CrossRef]
- Uiga, K.; Tenno, T.; Zekker, I.; Tenno, T. Dissolution modeling and potentiometric measurements of the SrS–H2O–gas system at normal pressure and temperature at salt concentrations of 0.125–2.924 mM. J. Sulfur Chem. 2011, 32, 137–149. [Google Scholar] [CrossRef]
- Chen, L.; Liu, J.; Zhang, W.; Zhou, J.; Luo, D.; Li, Z. Uranium (U) source, speciation, uptake, toxicity and bioremediation strategies in soil-plant system: A review. J. Hazard. Mater. 2021, 413, 125319. [Google Scholar] [CrossRef]
- Zeng, J.; Han, G. Tracing zinc sources with Zn isotope of fluvial suspended particulate matter in Zhujiang River, southwest China. Ecol. Indic. 2020, 118, 106723. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wang, Y.; Tsang, D.C.W.; Yang, X.; Beiyuan, J.; Yin, M.; Xiao, T.; Jiang, Y.; Lin, W.; et al. Emerging risks of toxic metal(loid)s in soil-vegetables influenced by steel-making activities and isotopic source apportionment. Environ. Int. 2021, 146, 106207. [Google Scholar] [CrossRef]
- Qin, C.; Li, S.-L.; Waldron, S.; Yue, F.-J.; Wang, Z.-J.; Zhong, J.; Ding, H.; Liu, C.-Q. High-frequency monitoring reveals how hydrochemistry and dissolved carbon respond to rainstorms at a karstic critical zone, Southwestern China. Sci. Total Environ. 2020, 714, 136833. [Google Scholar] [CrossRef]
- Mortatti, J.; Probst, J.-L. Silicate rock weathering and atmospheric/soil CO2 uptake in the Amazon basin estimated from river water geochemistry: Seasonal and spatial variations. Chem. Geol. 2003, 197, 177–196. [Google Scholar] [CrossRef] [Green Version]
- Chetelat, B.; Liu, C.Q.; Zhao, Z.Q.; Wang, Q.L.; Li, S.L.; Li, J.; Wang, B.L. Geochemistry of the dissolved load of the Changjiang Basin rivers: Anthropogenic impacts and chemical weathering. Geochim. Cosmochim. Acta 2008, 72, 4254–4277. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Liu, M.; Van Zwieten, L.; Yang, X.; Yu, C.; Wang, H.; Song, Z. Carbon-nitrogen isotope coupling of soil organic matter in a karst region under land use change, Southwest China. Agric. Ecosyst. Environ. 2020, 301, 107027. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Su, J.; Yue, F.; Zhong, J.; Chen, S. Oxidation of pyrite and reducing nitrogen fertilizer enhanced the carbon cycle by driving terrestrial chemical weathering. Sci. Total Environ. 2021, 768, 144343. [Google Scholar] [CrossRef]
- Moon, S.; Huh, Y.; Qin, J.; van Pho, N. Chemical weathering in the Hong (Red) River basin: Rates of silicate weathering and their controlling factors. Geochim. Cosmochim. Acta 2007, 71, 1411–1430. [Google Scholar] [CrossRef]
- Liu, J.; Han, G. Controlling factors of riverine CO2 partial pressure and CO2 outgassing in a large karst river under base flow condition. J. Hydrol. 2021, 593, 125638. [Google Scholar] [CrossRef]
- Wang, W.; Li, S.-L.; Zhong, J.; Wang, L.; Yang, H.; Xiao, H.; Liu, C.-Q. CO2 emissions from karst cascade hydropower reservoirs: Mechanisms and reservoir effect. Environ. Res. Lett. 2021, 16, 044013. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, A.; France-Lanord, C. Sr and 87Sr/86Sr in waters and sediments of the Brahmaputra river system: Silicate weathering, CO2 consumption and Sr flux. Chem. Geol. 2006, 234, 308–320. [Google Scholar] [CrossRef]
- Stevenson, R.; Pearce, C.R.; Rosa, E.; Hélie, J.-F.; Hillaire-Marcel, C. Weathering processes, catchment geology and river management impacts on radiogenic (87Sr/86Sr) and stable (δ88/86Sr) strontium isotope compositions of Canadian boreal rivers. Chem. Geol. 2018, 486, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Bickle, M.J.; Bunbury, J.; Chapman, H.J.; Harris, N.B.; Fairchild, I.J.; Ahmad, T. Fluxes of Sr into the headwaters of the Ganges. Geochim. Cosmochim. Acta 2003, 67, 2567–2584. [Google Scholar] [CrossRef]
- Oliver, L.; Harris, N.; Bickle, M.; Chapman, H.; Dise, N.; Horstwood, M. Silicate weathering rates decoupled from the 87Sr/86Sr ratio of the dissolved load during Himalayan erosion. Chem. Geol. 2003, 201, 119–139. [Google Scholar] [CrossRef]
- Zieliński, M.; Dopieralska, J.; Belka, Z.; Walczak, A.; Siepak, M.; Jakubowicz, M. Sr isotope tracing of multiple water sources in a complex river system, Noteć River, central Poland. Sci. Total Environ. 2016, 548–549, 307–316. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Song, C.; Zhang, Q.; Yang, K.; Liu, J. Hydrochemistry and Dissolved Inorganic Carbon (DIC) Cycling in a Tropical Agricultural River, Mun River Basin, Northeast Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3410. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Han, G.; Liu, X.; Liu, M.; Song, C.; Yang, K.; Li, X.; Zhang, Q. Distributive Characteristics of Riverine Nutrients in the Mun River, Northeast Thailand: Implications for Anthropogenic Inputs. Water 2019, 11, 954. [Google Scholar] [CrossRef] [Green Version]
- Liang, B.; Han, G.; Liu, M.; Li, X.; Song, C.; Zhang, Q.; Yang, K. Spatial and Temporal Variation of Dissolved Heavy Metals in the Mun River, Northeast Thailand. Water 2019, 11, 380. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Han, G.; Yang, K. Assessment and sources of heavy metals in suspended particulate matter in a tropical catchment, northeast Thailand. J. Clean. Prod. 2020, 265, 121898. [Google Scholar] [CrossRef]
- Yang, K.; Han, G. Controls over hydrogen and oxygen isotopes of surface water and groundwater in the Mun River catchment, northeast Thailand: Implications for the water cycle. Hydrogeol. J. 2020, 28, 1021–1036. [Google Scholar] [CrossRef]
- Zhou, W.; Han, G.; Liu, M.; Li, X. Effects of soil pH and texture on soil carbon and nitrogen in soil profiles under different land uses in Mun River Basin, Northeast Thailand. PeerJ 2019, 7, e7880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Han, G. Tracing riverine sulfate source in an agricultural watershed: Constraints from stable isotopes. Environ. Pollut. 2021, 288, 117740. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Han, G.; Li, X. Contributions of soil erosion and decomposition to SOC loss during a short-term paddy land abandonment in Northeast Thailand. Agric. Ecosyst. Environ. 2021, 321, 107629. [Google Scholar] [CrossRef]
- Han, G.; Yang, K.; Zeng, J.; Zhao, Y. Dissolved iron and isotopic geochemical characteristics in a typical tropical river across the floodplain: The potential environmental implication. Environ. Res. 2021, 200, 111452. [Google Scholar] [CrossRef]
- Liu, M.; Han, G.; Li, X. Using stable nitrogen isotope to indicate soil nitrogen dynamics under agricultural soil erosion in the Mun River basin, Northeast Thailand. Ecol. Indic. 2021, 128, 107814. [Google Scholar] [CrossRef]
- Prabnakorn, S.; Maskey, S.; Suryadi, F.; de Fraiture, C. Rice yield in response to climate trends and drought index in the Mun River Basin, Thailand. Sci. Total Environ. 2018, 621, 108–119. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Liu, G.; Liu, Q.; Huang, C.; Li, H. Studies on the spatiotemporal variability of river water quality and its relationships with soil and precipitation: A case study of the Mun River Basin in Thailand. Int. J. Environ. Res. Public Health 2018, 15, 2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akter, A.; Babel, M.S. Hydrological modeling of the Mun River basin in Thailand. J. Hydrol. 2012, 452–453, 232–246. [Google Scholar] [CrossRef]
- Liang, B.; Han, G.; Liu, M.; Li, X. Zn isotope fractionation during the development of low-humic gleysols from the Mun River Basin, northeast Thailand. Catena 2021, 206, 105565. [Google Scholar] [CrossRef]
- Liu, X.-L.; Han, G.; Zeng, J.; Liu, M.; Li, X.-Q.; Boeckx, P. Identifying the sources of nitrate contamination using a combined dual isotope, chemical and Bayesian model approach in a tropical agricultural river: Case study in the Mun River, Thailand. Sci. Total Environ. 2021, 760, 143938. [Google Scholar] [CrossRef]
- Li, X.; Han, G. One-step chromatographic purification of K, Ca, and Sr from geological samples for high precision stable and radiogenic isotope analysis by MC-ICP-MS. J. Anal. At. Spectrom. 2021, 36, 676–684. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Yang, K.; Liu, J. Hydro-Geochemistry of the River Water in the Jiulongjiang River Basin, Southeast China: Implications of Anthropogenic Inputs and Chemical Weathering. Int. J. Environ. Res. Public Health 2019, 16, 440. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zheng, H.; Yang, J.; Luo, C.; Zhou, B. Chemical weathering, atmospheric CO2 consumption, and the controlling factors in a subtropical metamorphic-hosted watershed. Chem. Geol. 2013, 356, 141–150. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zhang, J.; Liu, C.Q. Strontium isotopic compositions of dissolved and suspended loads from the main channel of the Yangtze River. Chemosphere 2007, 69, 1081–1088. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Liu, C.-Q. Water geochemistry controlled by carbonate dissolution: A study of the river waters draining karst-dominated terrain, Guizhou Province, China. Chem. Geol. 2004, 204, 1–21. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum. 2017. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 5 July 2021).
- Palmer, M.; Edmond, J. The strontium isotope budget of the modern ocean. Earth Planet. Sci. Lett. 1989, 92, 11–26. [Google Scholar] [CrossRef]
- Karim, A.; Veizer, J. Weathering processes in the Indus River Basin: Implications from riverine carbon, sulfur, oxygen, and strontium isotopes. Chem. Geol. 2000, 170, 153–177. [Google Scholar] [CrossRef]
- Negrel, P.; Allègre, C.J.; Dupré, B.; Lewin, E. Erosion sources determined by inversion of major and trace element ratios and strontium isotopic ratios in river water: The Congo Basin case. Earth Planet. Sci. Lett. 1993, 120, 59–76. [Google Scholar] [CrossRef]
- Li, X.; Han, G.; Liu, M.; Liu, J.; Zhang, Q.; Qu, R. Potassium and its isotope behaviour during chemical weathering in a tropical catchment affected by evaporite dissolution. Geochim. Cosmochim. Acta 2021, 316, 105–121. [Google Scholar] [CrossRef]
- Millot, R.; érôme Gaillardet, J.; Dupré, B.; Allègre, C.J. Northern latitude chemical weathering rates: Clues from the Mackenzie River Basin, Canada. Geochim. Cosmochim. Acta 2003, 67, 1305–1329. [Google Scholar] [CrossRef]
- Palmer, M.; Edmond, J. Controls over the strontium isotope composition of river water. Geochim. Cosmochim. Acta 1992, 56, 2099–2111. [Google Scholar] [CrossRef]
- Han, G.; Tang, Y.; Xu, Z. Fluvial geochemistry of rivers draining karst terrain in Southwest China. J. Asian Earth Sci. 2010, 38, 65–75. [Google Scholar] [CrossRef]
- Li, R.; Huang, H.; Yu, G.; Yu, H.; Bridhikitti, A.; Su, T. Trends of runoff variation and effects of main causal factors in Mun River, Thailand During 1980–2018. Water 2020, 12, 831. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Han, G. Major ions and δ34SSO4 in Jiulongjiang River water: Investigating the relationships between natural chemical weathering and human perturbations. Sci. Total Environ. 2020, 724, 138208. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, C.-Q. Chemical weathering in the upper reaches of Xijiang River draining the Yunnan–Guizhou Plateau, Southwest China. Chem. Geol. 2007, 239, 83–95. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Han, G.; Zeng, J.; Xiao, X.; Malem, F. A Strontium and Hydro-Geochemical Perspective on Human Impacted Tributary of the Mekong River Basin: Sources Identification, Fluxes, and CO2 Consumption. Water 2021, 13, 3137. https://doi.org/10.3390/w13213137
Zhang S, Han G, Zeng J, Xiao X, Malem F. A Strontium and Hydro-Geochemical Perspective on Human Impacted Tributary of the Mekong River Basin: Sources Identification, Fluxes, and CO2 Consumption. Water. 2021; 13(21):3137. https://doi.org/10.3390/w13213137
Chicago/Turabian StyleZhang, Shitong, Guilin Han, Jie Zeng, Xuhuan Xiao, and Fairda Malem. 2021. "A Strontium and Hydro-Geochemical Perspective on Human Impacted Tributary of the Mekong River Basin: Sources Identification, Fluxes, and CO2 Consumption" Water 13, no. 21: 3137. https://doi.org/10.3390/w13213137





