Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Water Sampling and Characteristics
2.2. Chemicals and Instruments
2.3. Jar Test Procedure
2.4. Chemometric Methods
3. Results and Discussion
3.1. Water Characterization
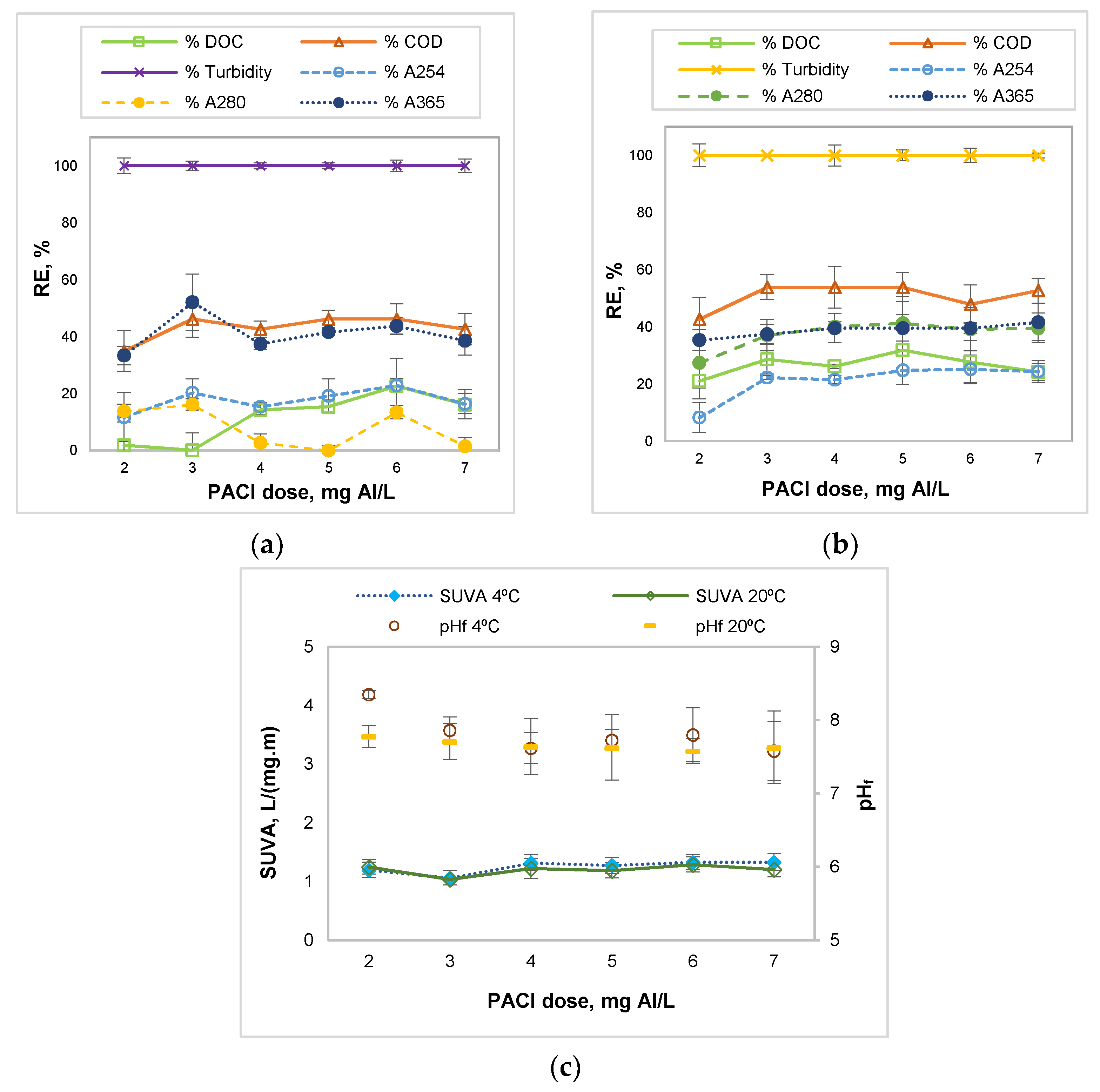
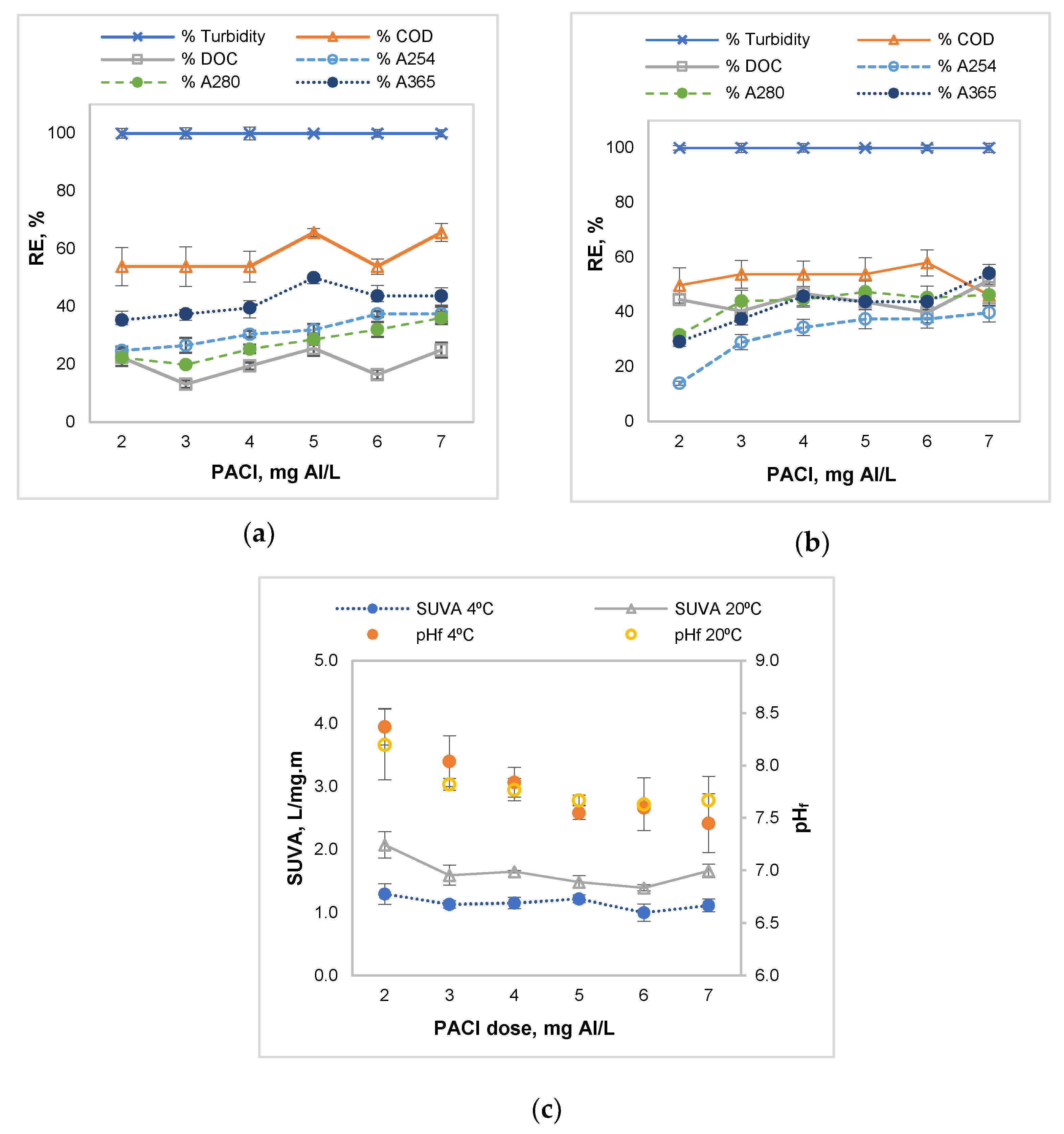
3.2. Influence of the Coagulant Dose
3.3. Influence of the Polyelectrolyte Addition
3.4. Influence of Mixing Conditions
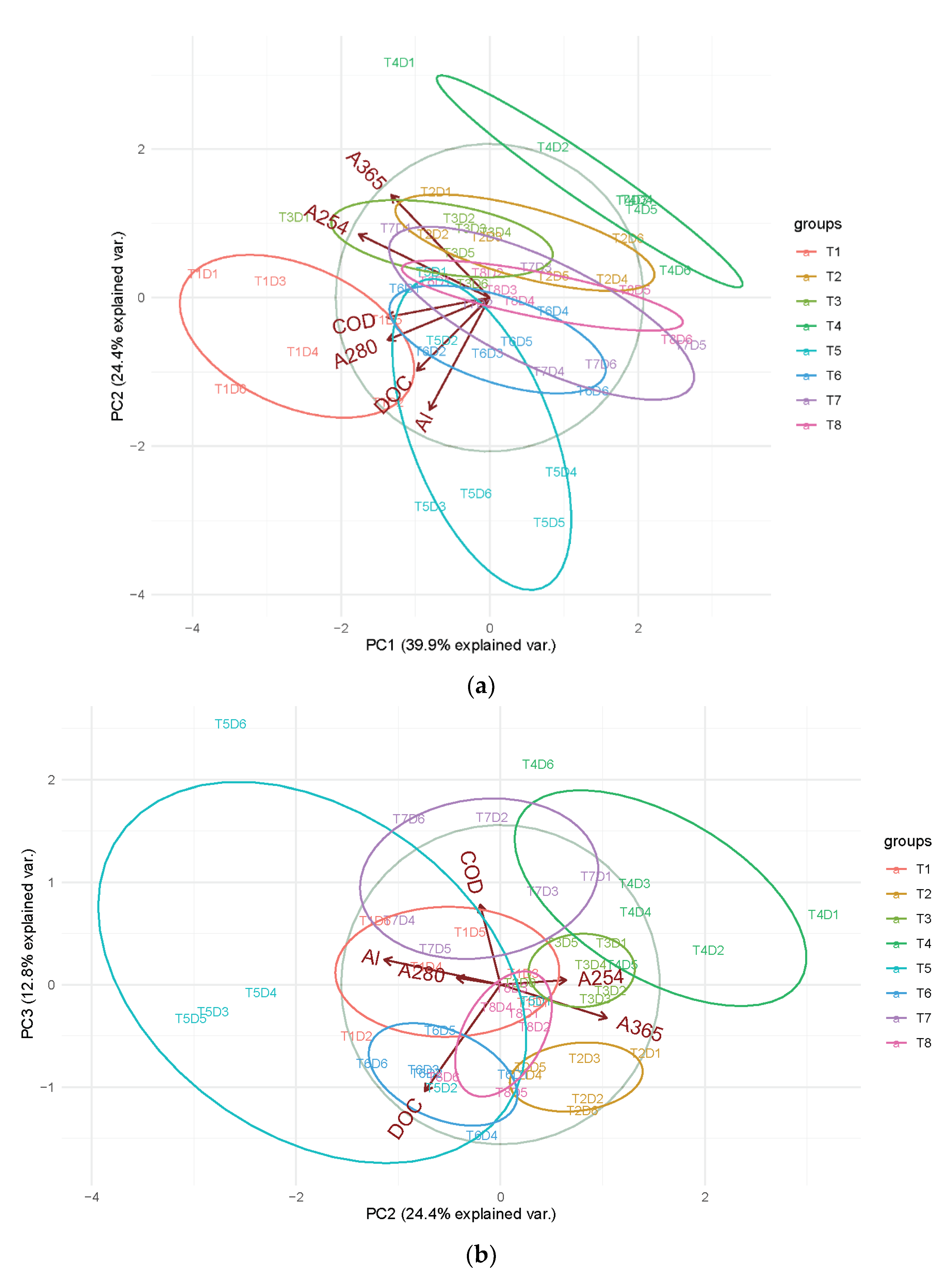
3.5. Principal Component Analysis
3.6. Selecting the Optimum Coagulant Dose
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sillanpää, M.; Matilainen, A.; Lahtinen, T. Characterization of NOM. In Natural Organic Matter in Water; Elsevier: Amsterdam, The Netherlands, 2015; pp. 17–53. ISBN 9780128015032. [Google Scholar]
- Adusei-Gyamfi, J.; Ouddane, B.; Rietveld, L.; Cornard, J.-P.; Criquet, J. Natural organic matter-cations complexation and its impact on water treatment: A critical review. Water Res. 2019, 160, 130–147. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.N.; Bridgeman, J. A fluorescence-based assessment of the fate of organic matter in water treated using crude/purified Hibiscus seeds as coagulant in drinking water treatment. Sci. Total Environ. 2019, 646, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Dayarathne, H.N.; Angove, M.J.; Aryal, R.; Abuel-Naga, H.; Mainali, B. Removal of natural organic matter from source water: Review on coagulants, dual coagulation, alternative coagulants, and mechanisms. J. Water Process Eng. 2021, 40, 101820. [Google Scholar] [CrossRef]
- Brezinski, K.; Gorczyca, B. Multi-spectral characterization of natural organic matter (NOM) from Manitoba surface waters using high performance size exclusion chromatography (HPSEC). Chemosphere 2019, 225, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Sillanpää, M.; Ncibi, M.C.; Matilainen, A.; Vepsäläinen, M. Removal of natural organic matter in drinking water treatment by coagulation: A comprehensive review. Chemosphere 2018, 190, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Gilca, A.F.; Teodosiu, C.; Fiore, S.; Musteret, C.P. Emerging disinfection byproducts: A review on their occurrence and control in drinking water treatment processes. Chemosphere 2020, 259, 127476. [Google Scholar] [CrossRef]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption (Recast), OJ L 435, 23.12.2020, pp. 1–62. Available online: https://eur-lex.europa.eu/eli/dir/2020/2184/oj (accessed on 10 June 2021).
- Xiao, F.; Ma, J.; Yi, P.; Huang, J.-C.H. Effects of low temperature on coagulation of kaolinite suspensions. Water Res. 2008, 42, 2983–2992. [Google Scholar] [CrossRef]
- Go, R.J.C.; Yang, H.L.; Kan, C.C.; Ong, D.C.; Garcia-Segura, S.; de Luna, M.D.G. Natural organic matter removal from raw surface water: Benchmarking performance of chemical coagulants through excitation-emission fluorescence matrix spectroscopy analysis. Water 2021, 13, 146. [Google Scholar] [CrossRef]
- Wang, C.; Alpatova, A.; McPhedran, K.N.; Gamal El-Din, M. Coagulation/flocculation process with polyaluminum chloride for the remediation of oil sands process-affected water: Performance and mechanism study. J. Environ. Manag. 2015, 160, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Wang, D.; Ni, J.; Qu, J.; Chow, C.W.K.; Liu, H. Mechanism of natural organic matter removal by polyaluminum chloride: Effect of coagulant particle size and hydrolysis kinetics. Water Res. 2008, 42, 3361–3370. [Google Scholar] [CrossRef]
- Lin, J.-L.; Huang, C.; Chin, C.-J.M.; Pan, J.R. Coagulation dynamics of fractal flocs induced by enmeshment and electrostatic patch mechanisms. Water Res. 2008, 42, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jing, R.; He, S.; Qian, J.; Zhang, K.; Ma, G.; Chang, X.; Zhang, M.; Li, Y. Coagulation of low temperature and low turbidity water: Adjusting basicity of polyaluminum chloride (PAC) and using chitosan as coagulant aid. Sep. Purif. Technol. 2018, 206, 131–139. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Phuntsho, S.; Gao, B.Y.; Yang, Y.Z.; Kim, J.-H.; Shon, H.K. Comparison of a novel polytitanium chloride coagulant with polyaluminium chloride: Coagulation performance and floc characteristics. J. Environ. Manag. 2015, 147, 194–202. [Google Scholar] [CrossRef]
- Yan, M.; Wang, D.; Yu, J.; Ni, J.; Edwards, M.; Qu, J. Enhanced coagulation with polyaluminum chlorides: Role of pH/Alkalinity and speciation. Chemosphere 2008, 71, 1665–1673. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, B.; Cao, B.; Xu, W.; Yue, Q. Effect of OH−/Al3+ ratio on the coagulation behavior and residual aluminum speciation of polyaluminum chloride (PAC) in surface water treatment. Sep. Purif. Technol. 2011, 80, 59–66. [Google Scholar] [CrossRef]
- Pan, Y.; Li, H.; Zhang, X.; Li, A. Characterization of natural organic matter in drinking water: Sample preparation and analytical approaches. Trends Environ. Anal. Chem. 2016, 12, 23–30. [Google Scholar] [CrossRef]
- Masu, S.; Burtica, G.; Manea, F.; Pisoi, I. Spectrophotometric parameters for organic matter characterization in raw and treated surface water. Environ. Eng. Manag. J. 2011, 10, 1451–1457. [Google Scholar] [CrossRef]
- Matilainen, A.; Gjessing, E.T.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, M. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Law 458/2002 on Drinking Water Quality, Modified and Republished in Romanian Official Monitor No. 705, Bucharest, Romania. Available online: http://legislatie.just.ro/Public/DetaliiDocument/37723 (accessed on 26 May 2021).
- RStudio. RStudio: Integrated Development for R; RStudio: Boston, MA, USA, 2021. [Google Scholar]
- Boubchir, M.; Aourag, H. Materials genome project: The application of principal component analysis to the formability of perovskites and inverse perovskites. Comput. Condens. Matter 2020, 24, e00495. [Google Scholar] [CrossRef]
- Iriarte-Velasco, U.; Álvarez-Uriarte, J.I.; González-Velasco, J.R. Enhanced coagulation under changing alkalinity-hardness conditions and its implications on trihalomethane precursors removal and relationship with UV absorbance. Sep. Purif. Technol. 2007, 55, 368–380. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Tobiason, J.E. Enhanced coagulation: Us requirements and a broader view. Water Sci. Technol. 1999, 40, 63–70. [Google Scholar] [CrossRef]
- Zouboulis, A.; Traskas, G.; Samaras, P. Comparison of Efficiency between Poly-aluminium Chloride and Aluminium Sulphate Coagulants during Full-scale Experiments in a Drinking Water Treatment Plant. Sep. Sci. Technol. 2008, 43, 1507–1519. [Google Scholar] [CrossRef]
- Qiao, G.; Zhang, J.; Zhang, Q.; Feng, X.; Lu, Y.; Feng, W. The Influence of Temperature on the Bulk Settling of Cohesive Sediment in Still Water with the Lattice Boltzmann Method. Water 2019, 11, 945. [Google Scholar] [CrossRef] [Green Version]
- Fitzpatrick, C.S.B.; Fradin, E.; Gregory, J. Temperature effects on flocculation, using different coagulants. Water Sci. Technol. 2004, 50, 171–175. [Google Scholar] [CrossRef]
- Maćczak, P.; Kaczmarek, H.; Ziegler-Borowska, M. Recent Achievements in Polymer Bio-Based Flocculants for Water Treatment. Materials 2020, 13, 3951. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Hu, D.; Li, D.; Ren, X.; Cheng, Y.; Luan, Z. Effects of slow-mixing on the coagulation performance of polyaluminum chloride (PACI). Chin. J. Chem. Eng. 2013, 21, 318–323. [Google Scholar] [CrossRef]
- Lapointe, M.; Papineau, I.; Peldszus, S.; Peleato, N.; Barbeau, B. Identifying the best coagulant for simultaneous water treatment objectives: Interactions of mononuclear and polynuclear aluminum species with different natural organic matter fractions. J. Water Process Eng. 2021, 40, 101829. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Gao, B.Y.; Shon, H.K.; Cao, B.C.; Kim, J.-H. Coagulation characteristics of titanium (Ti) salt coagulant compared with aluminum (Al) and iron (Fe) salts. J. Hazard. Mater. 2011, 185, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Ytow, N.; Utsumi, M.; Seki, H. UV spectrometry for measuring particulate organic matter in natural water. Aquat. Sci. 1996, 58, 91–102. [Google Scholar] [CrossRef]
- Huang, X.; Sun, S.; Gao, B.; Yue, Q.; Wang, Y.; Li, Q. Coagulation behavior and floc properties of compound bioflocculant–polyaluminum chloride dual-coagulants and polymeric aluminum in low temperature surface water treatment. J. Environ. Sci. 2015, 30, 215–222. [Google Scholar] [CrossRef]
- Hidayah, E.N.; Chou, Y.-C.; Yeh, H.-H. Characterization and removal of natural organic matter from slow sand filter effluent followed by alum coagulation. Appl. Water Sci. 2018, 8, 3. [Google Scholar] [CrossRef] [Green Version]
- Gao, B.-Y.; Chu, Y.-B.; Yue, Q.-Y.; Wang, B.-J.; Wang, S.-G. Characterization and coagulation of a polyaluminum chloride (PAC) coagulant with high Al13 content. J. Environ. Manag. 2005, 76, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.-X.; Xu, H.; Xiao, F.; Wang, D.-S.; Ye, C.-Q.; Jiao, R.-Y.; Liu, Y.-G. Effects of Al species on coagulation efficiency, residual Al and floc properties in surface water treatment. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 14–21. [Google Scholar] [CrossRef]
- Song, W.C.; Li, X.; Sun, S.H.; Yang, Y.L.; Jia, R.B. Test Study of Enhanced Coagulation for Conventional Treatment with Low Temperature and Low Turbidity. J. Residuals Sci. Technol. 2015, S39–S46. [Google Scholar] [CrossRef] [Green Version]
- Lou, I.; Gong, S.; Huang, X.; Liu, Y. Coagulation optimization for low temperature and low turbidity source water using combined coagulants: A case study. Desalin. Water Treat. 2012, 46, 107–114. [Google Scholar] [CrossRef]
- Lin, J.-L.; Ika, A.R. Effect of Al speciation on residual turbidity and Al minimization by coagulation with single and dual dosing. J. Water Supply Res. Technol. 2019, 68, 51–62. [Google Scholar] [CrossRef]
| Quality Indicator | pH | Conductivity (at 20 °C) | Turbidity | COD (Permanganate Index) | Total Hardness | NH4⁺ | NO3− | NO2− | SO42− | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unit | pH Units | µS/cm | UNT | mg O2/L | °Ge | mg/L | mg/L | mg/L | mg/L | μg/L | |
| 2013 | min | 7.1 | 556 | 0.8 | 1.8 | 13.2 | 0.06 | 1.2 | 0.0 | 66.1 | 43 |
| avg | 7.5 | 848 | 7.02 | 2.1 | 21.25 | 0.21 | 2.0 | 0.02 | 126.2 | 412.4 | |
| max | 7.9 | 1020 | 24.0 | 2.4 | 27.2 | 0.43 | 3.0 | 0.08 | 156.2 | 885 | |
| 2014 | min | 7.0 | 569 | 2.03 | 1.7 | 14.21 | 0.0 | 1.1 | 0 | 77.2 | 87 |
| avg | 7.3 | 841 | 9.19 | 2.3 | 21.47 | 0.18 | 2.3 | 0.02 | 122 | 538 | |
| max | 8.0 | 1094 | 20.4 | 2.7 | 30.8 | 0.47 | 3.4 | 0.06 | 185.8 | 1139 | |
| 2015 | min | 7.3 | 510 | 1.3 | 1.8 | 13.2 | 0.04 | 1.7 | 0.01 | 53.4 | 28 |
| avg | 7.5 | 676 | 4.9 | 2.2 | 21.45 | 0.08 | 2.3 | 0.01 | 94.6 | 355 | |
| max | 8.0 | 973 | 13.7 | 2.5 | 31.6 | 0.15 | 3.0 | 0.02 | 167.2 | 846 | |
| 2016 | min | 7.6 | 227 | 1.98 | 2.0 | 8.81 | 0.0 | 1.4 | 0.0 | 20.4 | 37 |
| avg | 7.8 | 515 | 7.86 | 2.2 | 14.72 | 0.04 | 2.2 | 0.004 | 68.8 | 241 | |
| max | 8.3 | 817 | 20.4 | 2.8 | 21.35 | 0.1 | 3.0 | 0.01 | 126.7 | 905 | |
| 2017 | min | 7.2 | 401 | 1.02 | 1.8 | 11.2 | 0 | 2.2 | 0.002 | 35.5 | 43 |
| avg | 7.8 | 595 | 6.45 | 2.5 | 14.13 | 0.03 | 3.1 | 0.01 | 67.6 | 139 | |
| max | 8.3 | 807 | 25.1 | 3.0 | 20.1 | 0.08 | 3.9 | 0.01 | 113.4 | 319 | |
| 2018 | min | 7.4 | 447 | 0.66 | 1.6 | 11.23 | 0.0 | 1.4 | 0.01 | 23.9 | 19 |
| avg | 7.8 | 618 | 5.38 | 2.5 | 14.55 | 0.05 | 3.1 | 0.01 | 58.7 | 78 | |
| max | 8.3 | 860 | 13.2 | 3.9 | 23.68 | 0.13 | 6.9 | 0.03 | 119.5 | 187 | |
| 2019 | min | 6.9 | 488 | 2.8 | 2.1 | 11.67 | 0.05 | 2.6 | 0.009 | 29.8 | 116 |
| avg | 7.7 | 885 | 8.56 | 2.3 | 22.8 | 0.13 | 4.6 | 0.03 | 99.4 | 301.5 | |
| max | 8.4 | 1400 | 40 | 2.7 | 40.9 | 0.24 | 6.7 | 0.2 | 166.9 | 546 | |
| 2020 | min | 7.44 | 419 | 1.46 | 2.0 | 11.45 | 0.02 | 2.9 | 0.006 | 55.5 | 60 |
| avg | 8.04 | 743 | 5.29 | 2.2 | 16.62 | 0.11 | 5.1 | 0.02 | 92.0 | 142.4 | |
| max | 8.4 | 1182 | 28.8 | 2.4 | 29.87 | 0.18 | 7.8 | 0.05 | 175.5 | 267 | |
| MAC * (Law 458/2002) [21] | ≥6.5; ≤9.5 | 2500 | ≤5 | 5 | Min 5 | 0.5 | 50 | 0.5 | 250 | 200 | |
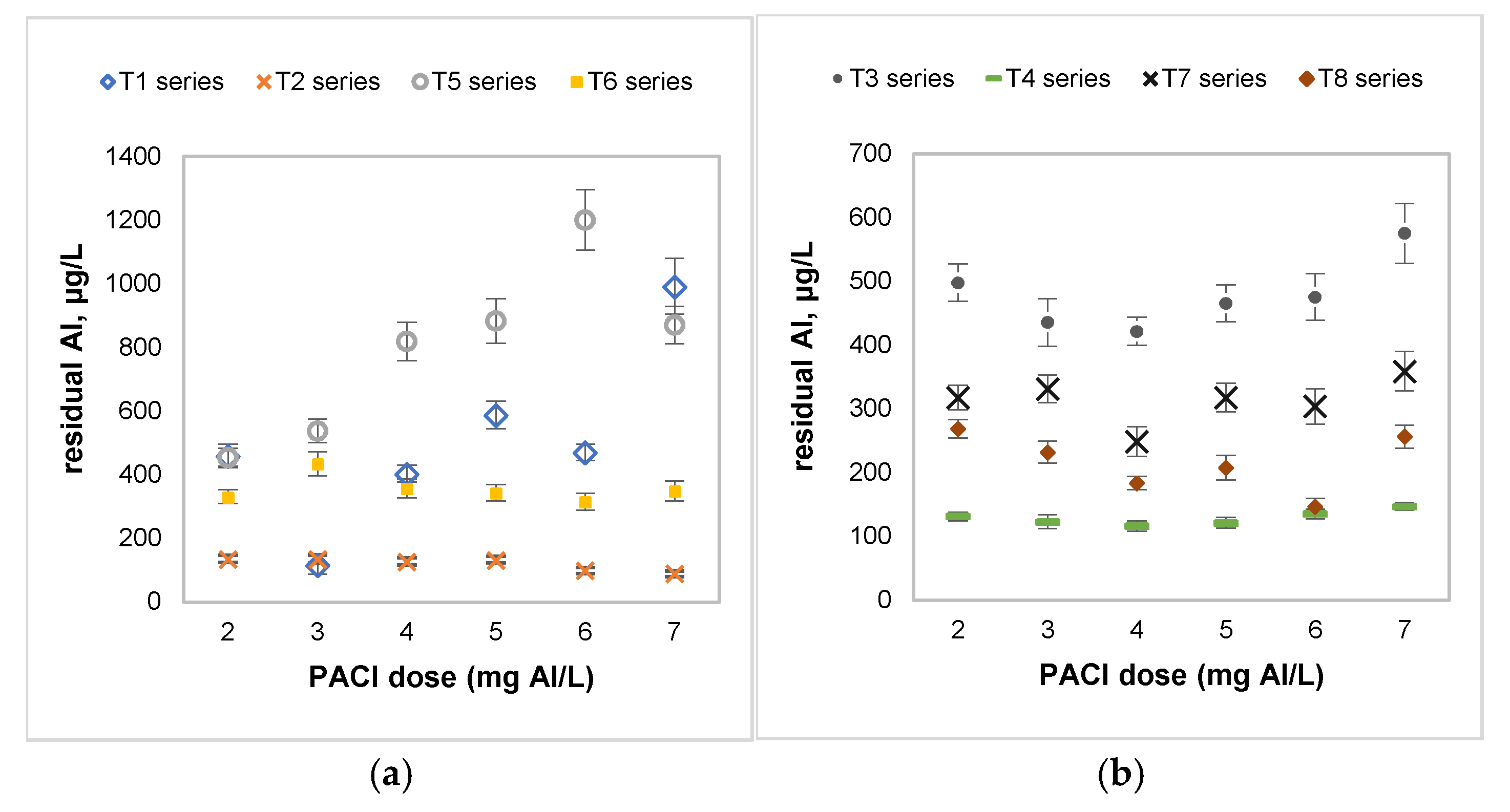
| Tests Series | PACl Dose (mg Al/L) | T (°C) | Coagulant Aid (mL/L) | Stirring Conditions | |
|---|---|---|---|---|---|
| Time of Rapid Mixing | Time of Slow Mixing | ||||
| T1 | 2–7 | 4 | 0 | 3 min at 300 rpm | 15 min at 60 rpm |
| T2 | 2–7 | 4 | 0.05 | 3 min at 300 rpm | 15 min at 60 rpm |
| T3 | 2–7 | 20 | 0 | 3 min at 300 rpm | 15 min at 60 rpm |
| T4 | 2–7 | 20 | 0.05 | 3 min at 300 rpm | 15 min at 60 rpm |
| T5 | 2–7 | 4 | 0.05 | 1.5 min at 300 rpm | 10 min at 60 rpm |
| T6 | 2–7 | 4 | 0.05 | 3 min at 200 rpm | 15 min at 25 rpm |
| T7 | 2–7 | 20 | 0.05 | 1.5 min at 300 rpm | 10 min at 60 rpm |
| T8 | 2–7 | 20 | 0.05 | 3 min at 200 rpm | 15 min at 25 rpm |
| Quality Indicator | Unit | Value |
|---|---|---|
| Conventional Parameters | ||
| pH | unit. pH | 8.17 (at 21.5 °C) |
| Turbidity | UNT | 1.27 |
| Conductivity (at 20 °C) | µS/cm | 561.0 |
| COD (permanganate index) | mg O2/L | 1.69 |
| TOC | mg/L | 2.49 |
| Total suspended solids | mg/L | 49.0 |
| NH4⁺ | mg/L | <0.05 |
| NO2‾ | mg/L | 0.03 |
| NO3‾ | mg/L | 1.39 |
| Total nitrogen | mg/L | <1.0 |
| Sulphates | mg/L | 70.40 |
| Chloride | mg/L | 48.99 |
| Total hardness | °G | 10.86 |
| Fe | μg/L | 78.16 |
| Al | μg/L | 125.20 |
| Unconventional Parameters | ||
| A254 | cm−1 | 0.0333 |
| A280 | cm−1 | 0.0239 |
| A365 | cm−1 | 0.0048 |
| SUVA | L/(mg∙m) | 1.39 |
| A254/A280 | - | 1.39 |
| A254/A365 | - | 6.93 |
| Coagulant/Flocculant | Raw Water Characteristics | Optimum Coagulant Dose | Efficiency | Ref. |
|---|---|---|---|---|
| PACl | Low turbidity and DOC, hydrophilic NOM, and low temperature | 10 mg Al/L | 30% DOC, 40% A254, and 34% turbidity | [34] |
| PACl/compound bioflocculant | Low turbidity and DOC, hydrophilic NOM, and low temperature | 10 mg Al/L | 29% DOC, 38% A254, and 40% turbidity | [34] |
| PACl | Acid pH and turbidity = 9.5 NTU | 40 mg Al/L | 78.5% humic acids, 75.6% fulvic acids, and 80.9% turbidity | [10] |
| Alum | High DOC and hydrophilic NOM | 8 mg Al/L | 23.5% non-purgedable DOC, 25% A254, 31.9% trihalomethanes formation potential, and 25.4% haloacetic acid formation potential | [35] |
| AlCl3 | River water with turbidity = 151 NTU and pH = 8.12 | 2.6 mg Al/L | 63% turbidity | [36] |
| PACl | River water with turbidity = 151 NTU and pH = 8.12 | 1.3 mg Al/L | 93% turbidity | [36] |
| PACl-Al13 | River water with turbidity = 151 NTU and pH = 8.12 | 1.3 mg Al/L | 95% turbidity | [36] |
| AlCl3 | Low turbidity and DOC, and low temperature | 2.16 mg Al/L | 17.5% DOC, residual dissolved Al = 0.4 mg/L | [37] |
| Al13 | Low turbidity and DOC, and low temperature | 2.16 mg Al/L | 19% DOC, residual dissolved Al = 0.2 mg/L | [37] |
| Al30 | Low turbidity and DOC, and low temperature | 2.16 mg Al/L | 17% DOC, residual dissolved Al = 0.3 mg/L | [37] |
| Composite potassium permanganate with composite polymeric aluminum ferric chloride/poly-dimethyl-diallyl- ammonium chloride coagulant | River water, low turbidity, and low temperature | - | 76.8% turbidity, 14.2% oxygen consumption, 60.8% THM, and 19.4% absorbable organic halogens | [38] |
| FeCl3 | River water, low turbidity, and low temperature | 40 mg/L | 72.4% A254, 11.5% COD, and residual Fe = 0.08 mg/L | [39] |
| Polyaluminum ferric chloride/FeCl3 (3:1 by mass) | River water, low turbidity, and low temperature | 20 mg coagulant/L | 84% A254, 43% COD, and turbidity < 0.5 NTU, residual Al = 0.09 mg/L, and residual Fe = 0.01 mg/L | [39] |
| Commercial PACl (8% Alpolymeric)/FeCl3 (2:1) | Low turbidity | 18.8 mg Al/L | 81.28% turbidity, and residual dissolved Al = 0.08 mg/L | [40] |
| Commercial PACl(35% Alpolymeric)/FeCl3 (2:1) | Low turbidity | 18.8 mg Al/L | 81.92% turbidity, and residual dissolved Al = 0.05 mg/L | [40] |
| PACl (90.3% basicity)/chitosan | Reservoir water, low temperature, and low turbidity | 8 mg Al2O3/L | 87% turbidity, 63% DOC, 82% A254, and residual Al < 0.15 mg/L | [14] |
| PACl/anionic polyacrilamide | River water, low temperature, low turbidity, and hydrophilic NOM | 7 mg Al/L | 100% turbidity, 65.68% COD, 24.94% DOC, 37.44% A254, 36.06% A280, 43.75% A365, and residual Al = 0.09 mg/L | This study |
| PACl/anionic polyacrilamide | River water, high temperature, low turbidity, and hydrophilic NOM | 4 mg Al/L | 100% turbidity, 53.85% COD, 46.79% DOC, 34.44% A254, 44.65% A280, 45.83% A365, and residual Al = 0.12 mg/L | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustereț, C.P.; Morosanu, I.; Ciobanu, R.; Plavan, O.; Gherghel, A.; Al-Refai, M.; Roman, I.; Teodosiu, C. Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment. Water 2021, 13, 3073. https://doi.org/10.3390/w13213073
Mustereț CP, Morosanu I, Ciobanu R, Plavan O, Gherghel A, Al-Refai M, Roman I, Teodosiu C. Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment. Water. 2021; 13(21):3073. https://doi.org/10.3390/w13213073
Chicago/Turabian StyleMustereț, Corina Petronela, Irina Morosanu, Ramona Ciobanu, Oana Plavan, Andreea Gherghel, Malek Al-Refai, Ioana Roman, and Carmen Teodosiu. 2021. "Assessment of Coagulation–Flocculation Process Efficiency for the Natural Organic Matter Removal in Drinking Water Treatment" Water 13, no. 21: 3073. https://doi.org/10.3390/w13213073






