Status of Freshwater Mussels (Unionidae) in the French Creek Watershed, USA at the Onset of Invasion by Round Goby, Neogobius melanostomus
Abstract
:1. Introduction
2. Materials and Methods
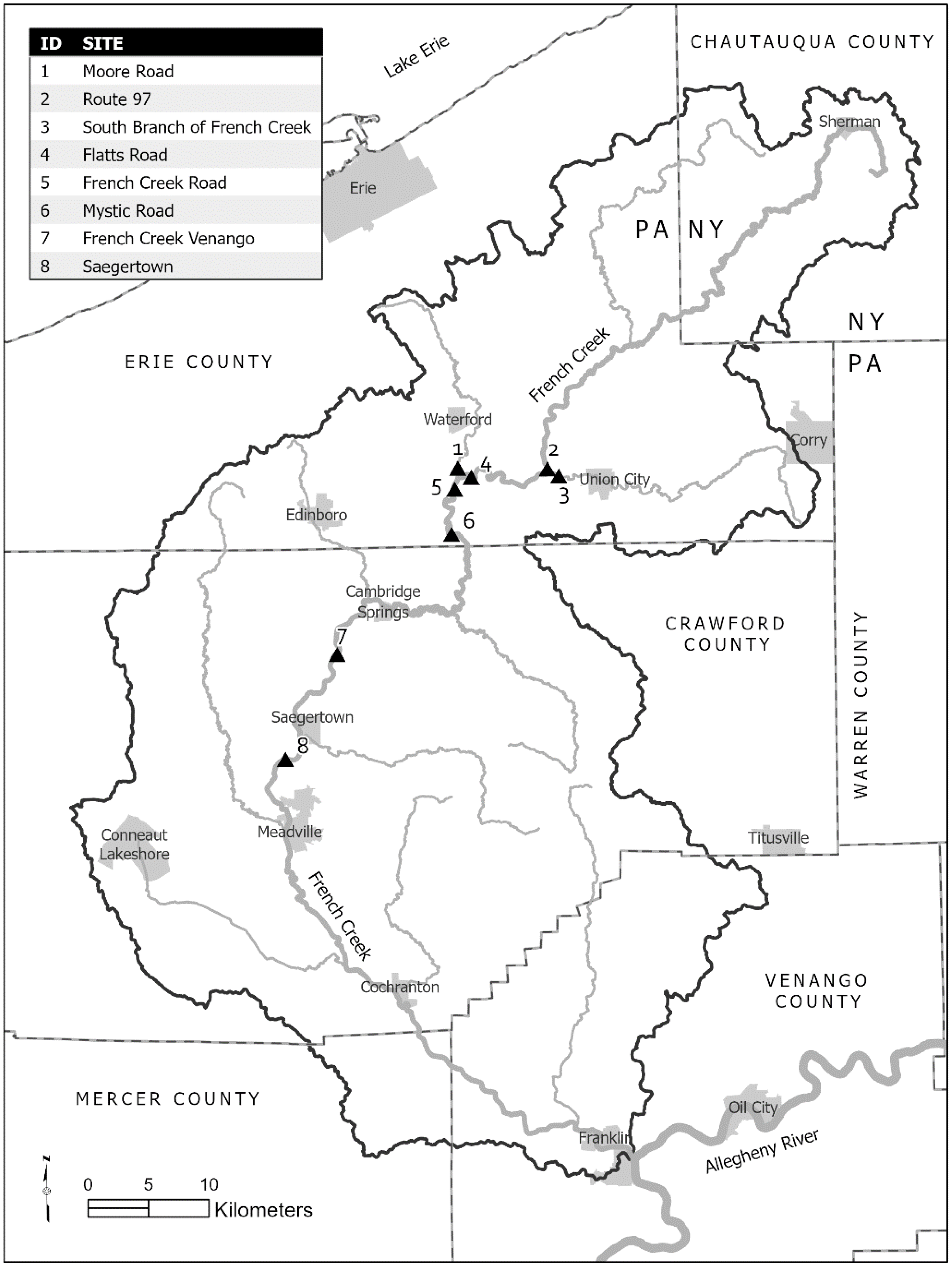
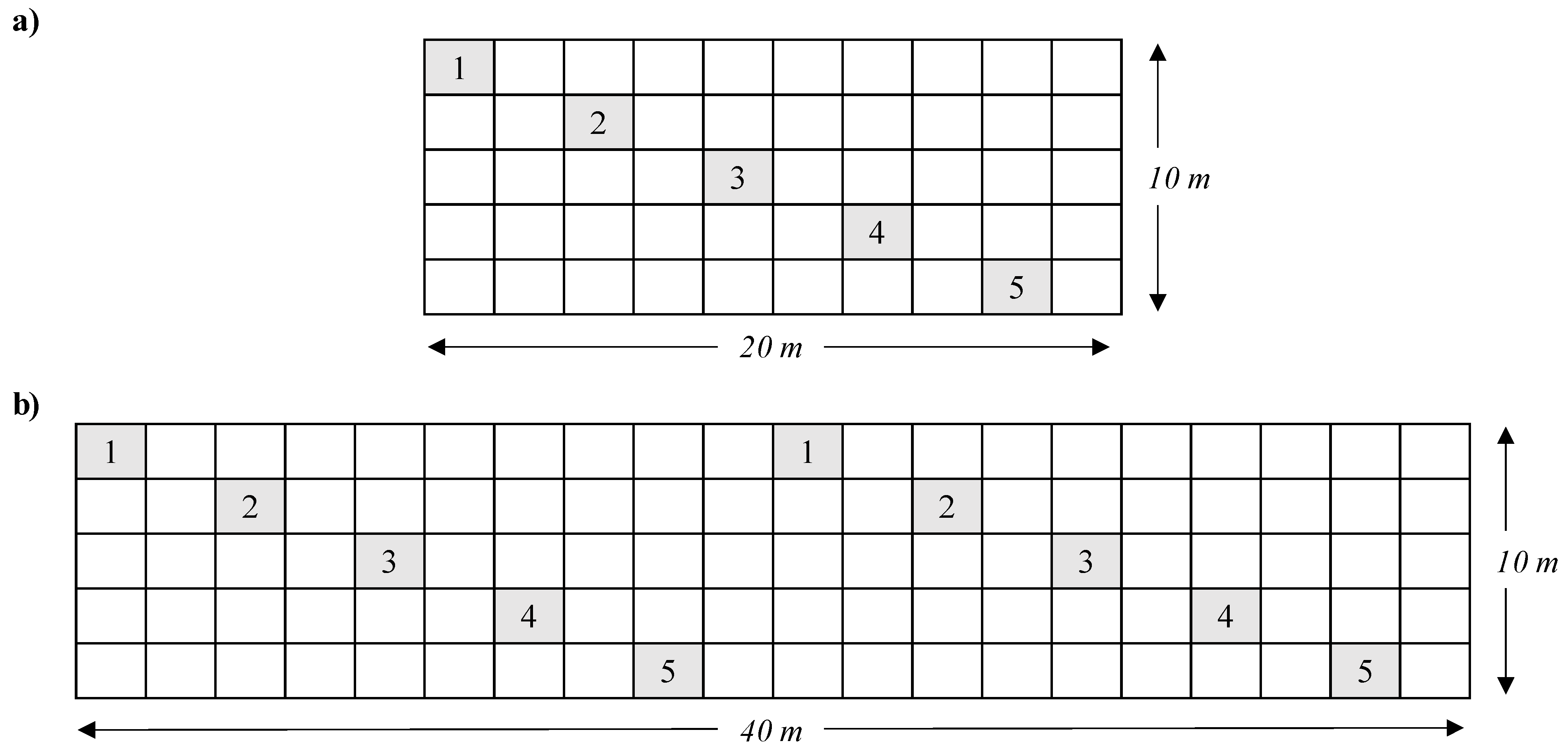
2.1. Field Site Sampling
2.2. Freshwater Mussel Surveys
2.3. Fish Surveys
2.4. Substrate Sampling
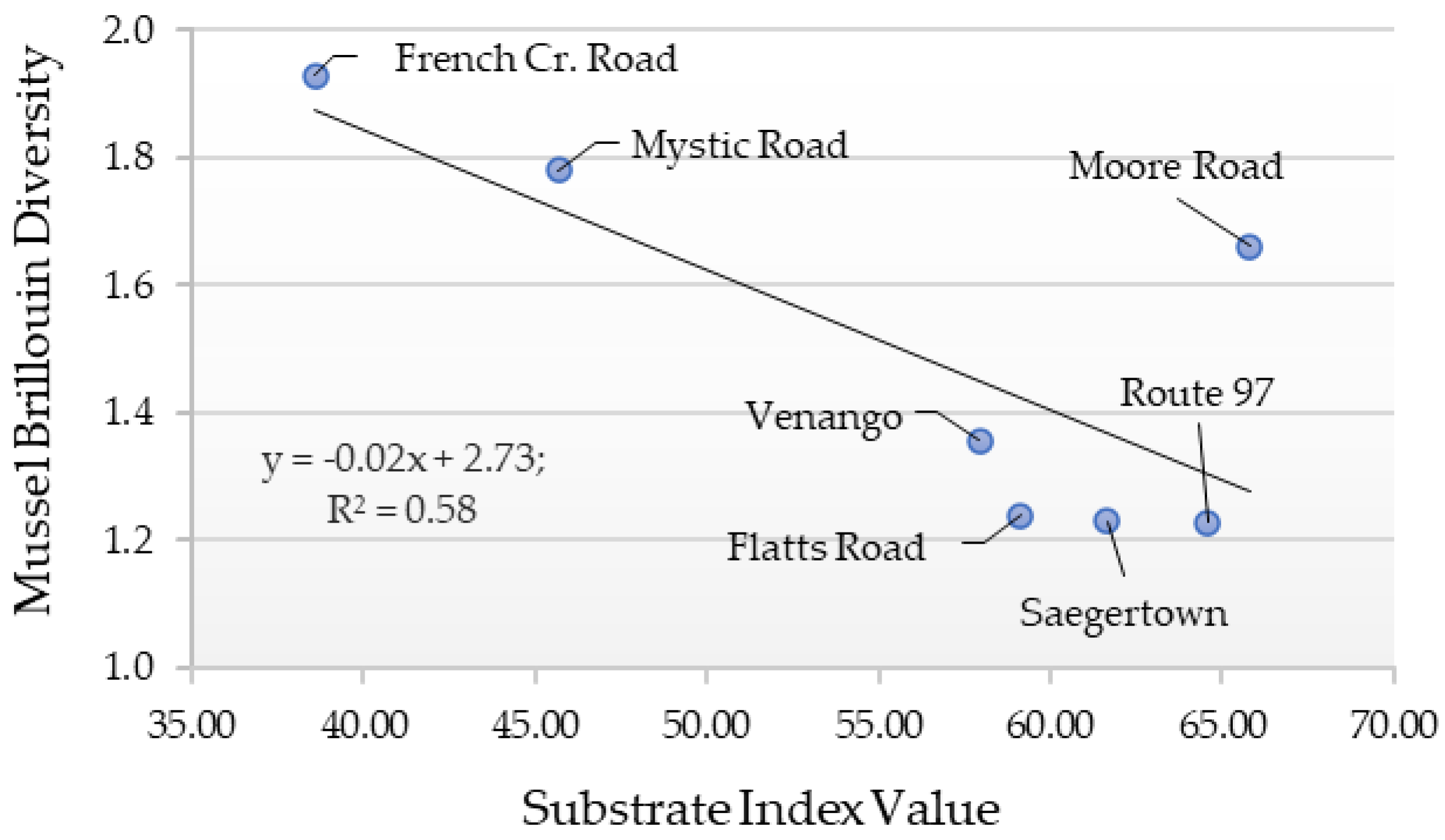
2.5. Diversity Index and Substrate Index Values
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pimentel, D.; Zuniga, R.; Morrison, D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol. Econ. 2005, 52, 273–288. [Google Scholar] [CrossRef]
- Gordon, D.R. Effects of invasive, non-indigenous plant species on ecosystem processes: Lessons from Florida. Ecol. Appl. 1998, 8, 975–989. [Google Scholar] [CrossRef]
- Kornis, M.S.; Mercado-Silva, N.; Zanden, M.J.V. Twenty years of invasion: A review of Round Goby Neogobius melanostomus biology, spread and ecological implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef] [PubMed]
- Strayer, D.L.; Eviner, V.T.; Jeschke, J.M.; Pace, M.L. Understanding the long-term effects of species invasions. Trends Ecol. Evol. 2006, 21, 645–651. [Google Scholar] [CrossRef]
- Strayer, D.L.; Cole, J.J.; Findlay, S.E.G.; Fischer, D.T.; Gephart, J.A.; Malcom, H.M.; Pace, M.L.; Rosi Marshall, J. Decadal-scale change in a large-river ecosystem. Bioscience 2014, 64, 496–510. [Google Scholar] [CrossRef] [Green Version]
- Charlebois, P.M.; Corkum, L.D.; Jude, D.J.; Knight, C. The Round Goby (Neogobius melanostomus) Invasion: Current Impacts and Future Needs. J. Great Lakes Res. 2001, 27, 263–266. [Google Scholar] [CrossRef]
- Stauffer, J.R., Jr.; Schnars, J.; Wilson, C.; Taylor, R.; Murray, C.K. Status of exotic round and tubenose gobies in Pennsylvania. Northeast. Nat. 2016, 23, 395–407. [Google Scholar] [CrossRef]
- Balshine, S.; Verma, A.; Chant, V.; Theysmeyer, T. Competitive interactions between round gobies and logperch. J. Great Lakes Res. 2005, 31, 68–77. [Google Scholar] [CrossRef]
- Bergstrom, M.A.; Mensinger, A.F. Interspecific resource competition between the invasive Round Goby and three native species: Logperch, slimy sculpin, and spoonhead sculpin. Trans. Am.Fish. Soc. 2009, 138, 1009–1017. [Google Scholar] [CrossRef]
- French, J.R.P., III; Jude, D.J. Diets and diet overlap of nonindigenous gobies and small benthic native fishes co-inhabiting the St. Clair River, Michigan. J. Great Lakes Res. 2001, 27, 300–311. [Google Scholar] [CrossRef]
- Lauer, T.E.; Allen, P.J. Changes in the mottled sculpin and johnny darter trawl catches after the appearance of Round Gobies in the Indiana waters of Lake Michigan. Trans. Am. Fish. Soc. 2011, 133, 185–189. [Google Scholar] [CrossRef] [Green Version]
- Chotkowski, M.A.; Marsden, J.E. Round Goby and mottled sculpin predation on lake trout eggs and fry: Field predictions from laboratory experiments. J. Great Lakes Res. 1999, 25, 26–35. [Google Scholar] [CrossRef]
- Steinhart, G.B.; Marschall, E.A.; Stein, R.A. Round Goby predation on Smallmouth Bass offspring in nests during simulated catch-and-release angling. Trans. Am. Fish. Soc. 2004, 133, 121–131. [Google Scholar] [CrossRef] [Green Version]
- Janssen, J. Recruitment failure of mottled sculpin (Cottus Bairdii) in Calumet Harbor, Southern Lake Michigan, induced by the newly introduced Round Goby (Neogobius melanostomus). J. Great Lakes Res. 2001, 27, 319–328. [Google Scholar] [CrossRef]
- Ray, W.J.; Corkum, L.D. Predation of zebra mussels by Round Gobies, Neogobius melanostomus. Environ. Biol. Fishes. 1997, 50, 267–273. [Google Scholar] [CrossRef]
- Bradshaw-Wilson, C.; Stauffer, J.; Wisor, J.; Clark, K.; Mueller, S. Documentation of freshwater mussels in the diet of Round Gobies (Neogobius melanostomus) within the French Creek watershed, Pennsylvania. Am. Midl. Nat. 2019, 181, 259–270. [Google Scholar] [CrossRef]
- Mueller, S.; Stauffer JR, J.R.; Wisor, J.; Bradshaw-Wilson, C. Expansion of the invasive Round Goby (Neogobius melanostomus) into the Allegheny River tributaries: LeBouef and French Creeks in Pennsylvania. J. Pa. Acad. Sci. 2017, 91, 105–111. [Google Scholar] [CrossRef]
- Di Maio, J.; Corkum, L.D. Relationship between the spatial distribution of freshwater mussels (Bivalvia: Unionidae) and the hydrological variability of rivers. Can. J. Zool. 1995, 73, 663–671. [Google Scholar] [CrossRef]
- Gangloff, M.M.; Feminella, J.W. Stream channel geomorphology influences mussel abundance in southern Appalachian streams, U.S.A. Freshwater Biol. 2007, 52, 64–74. [Google Scholar] [CrossRef]
- Hardison, B.S.; Layzer, J.B. Relations between complex hydraulics and the localized distribution of mussels in three regulated rivers. Regul. Rivers Res. Manag. 2001, 17, 77–84. [Google Scholar] [CrossRef]
- Morales, L.J.; Weber, A.; Mynett, E.; Newton, T.J. Effects of substrate and hydrodynamic conditions on the formation of mussel beds in a large river. J. N. Am. Benthol. Soc. 2006, 25, 664–676. [Google Scholar] [CrossRef] [Green Version]
- McRae, S.E.; Allan, J.D.; Burch, J.B. Reach- and catchment-scale determinants of the distribution of freshwater mussels (Bivalvia: Unionidae in south-eastern Michigan, U.S.A. Freshw. Biol. 2004, 49, 127–142. [Google Scholar] [CrossRef] [Green Version]
- Box, J.B.; Dorazio, R.M.; Liddell, W.D. Relationships between streambed substrate characteristics and freshwater mussels (Bivalvia: Unionidae) in Coastal Plain streams. J. N. Am. Benthol. Soc. 2002, 21, 253–260. [Google Scholar] [CrossRef] [Green Version]
- Huehner, M.K. Field and laboratory determination of substrate preferences of unionid mussels. Ohio J. Sci. 1987, 87, 29–32. [Google Scholar]
- Strayer, D.L. The effects of surface geology and stream size on freshwater mussel (Bivalvia, Unionidae) distribution in Southeastern Michigan, U.S.A. Freshw. Biol. 1983, 13, 253–264. [Google Scholar] [CrossRef]
- Strayer, D.L. Freshwater Mussel Ecology: A Multifactor Approach to Distribution and Abundance; Freshwater Ecology Series; University of California Press: Oakland, CA, USA, 2008; 216p. [Google Scholar]
- Smith, D.R. Survey design for detecting rare freshwater mussels. J. N. Am. Benthol. Soc. 2006, 25, 701–711. Available online: https://www.fws.gov/northeast/pafo/endangered/surveys.html (accessed on 25 August 2021). [CrossRef]
- Clark, K.H. Abundance and Distribution of Freshwater Mussels in French Creek Pennsylvania. Master’s Thesis, The Pennsylvania State University, University Park, PA, USA, 2018; 80p. [Google Scholar]
- Stauffer, J.R., Jr.; Criswell, R.W.; Fischer, D.P. Fishes of Pennsylvania; Cichlid Press: El Paso, TX, USA, 2016; 556p. [Google Scholar]
- Gray, E.V.S.; Stauffer, J.R. Comparative microhabitat use of ecologically similar benthic fishes. Environ. Biol. Fishes. 1998, 56, 443–453. [Google Scholar] [CrossRef]
- Gray, E.V.S.; Kellogg, K.A.; Stauffer, J.R. Habitat Shift of a Native Darter Etheostoma olmstedi (Teleostei: Percidae) in Sympatry with a Non-native Darter Etheostoma zonale. Am. Midl. Nat. 2005, 154, 166–177. [Google Scholar] [CrossRef]
- Ogle, D.H.; Wheeler, P.; Dinno, A. FSA: Fisheries Stock Analysis. R Package Version 0.8.32. 2021. Available online: https://github.com/droglenc/FSA (accessed on 25 August 2021).
- Jolly, S.M. Explicit estimates from capturerecapture data with both death and immigration-stochastic model. Biometrika 1965, 52, 225–247. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 25 August 2021).
- Seber, G.A.F. A note on the multiple-recapture census. Biometrika 1965, 52, 249–259. [Google Scholar] [CrossRef]
- Wickham, J.; Hester, J.; Chang, W. Devtools: Tools to Make Developing R Packages Easier. R Package Version 2.3.2. 2020. Available online: https://CRAN.R-project.org/package=devtools (accessed on 25 August 2021).
- Turgeon, D.D.; Quinn, J.F., Jr.; Bogan, A.E.; Coan, E.V.; Hochberg, F.G.; Lyons, W.G.; Mikkelsen, P.M.; Neves, R.J.; Roper, C.F.E.; Rosenberg, G.; et al. Common and Scientific Names of Aquatic Invertebrates from the United States and Canada: Mollusks, 2nd ed.; American Fisheries Society, Special Publication: Bethseda, MD, USA, 1998; p. 526. [Google Scholar]
- Strayer, D.L. Effects of alien species on freshwater mollusks in North America. J. N. Am. Benthol. Soc. 1999, 18, 74–98. [Google Scholar] [CrossRef]
- Smith, T.A.; Crabtree, D. Freshwater mussel (Unionidae: Bivalvia) distributions and densities in French Creek, Pennsylvania. Northeast. Nat. 2010, 17, 387–414. [Google Scholar] [CrossRef]
- Pennuto, C.; Krakowiak, P.; Janik, C. Seasonal abundance, diet and energy consumption of Round Gobies (Neogobius melanostomus) in Lake Erie tributary streams. Ecol. Freshw. Fish 2010, 19, 206215. [Google Scholar] [CrossRef]
- Krakowiak, P.; Pennuto, C. Fish and macroinvertebrate communities in tributary streams of Eastern Lake Erie with and without Round Gobies (Neogobius melanostomus, Pallas 1814). J. Great Lakes Res. 2008, 34, 675–689. [Google Scholar] [CrossRef]
- Mikl, L.; Adamek, Z.; Vsetickova, L.; Janac, M.; Roche, K.; Slapansky, L.; Jurajda, P. Repsonse of benthic macroinvertebrate assemblages to round (Neogobius melanostomus, Pallas 1814) and tubenose (Proterorhinus semilunaris, Keckel 1837) goby predation pressure. Hydrobiologica 2017, 785, 219–232. [Google Scholar] [CrossRef]
- Wilson, C.; Stauffer, J., Jr.; Schnars, J.; Taylor, R.; Murray, C. Research Summary: Impacts of Gobies on Native Fishes of the Lake Erie Drainage, Pennsylvania; Regional Science Consortium Ninth Annual Research Symposium: Erie, PA, USA, 2014. [Google Scholar]
- Hastie, L.C.; Cooksley, S.L.; Scougall, F.; Young, M.R.; Boon, P.J.; Gaywood, M.J. Characterization of freshwater pearl mussel (Margaritifera margaritifera) riverine habitat using River Habitat Survey data. Aquatic. Conserv. Mar. Freshw. Ecosyst. 2003, 13, 213–224. [Google Scholar] [CrossRef]
- Stauffer, J.R., Jr.; Beiles, H.A.; Cox, J.W.; Dickson, K.L.; Simonet, D.E. Colonization of macrobenthic communities on artificial substrates. Rev. Biol. 1976, 10, 49–61. [Google Scholar]
- Smith, T.A.; Crabtree, D. Population attributes of an endangered mussel, Epioblasma Torulosa Rangiana (northern riffleshell), in French Creek and implications for its recovery. Northeast. Nat. 2009, 16, 339–354. [Google Scholar]
- Clark, K.H.; Wisor, J.M.; Mueller, S.J.; Bradshaw-Wilson, C.; Boyer, E.W.; Stauffer, J.R. Supporting Information for “Status of Freshwater Mussels (Unionidae) in the French Creek Watershed, USA at the Onset of Invasion by Round Goby, Neogobius melanostomus”. CUAHSI HydroShare Data Repository. 2021. Available online: http://www.hydroshare.org/resource/e72f2afe5eb54dfda6fe4465279a2fb3 (accessed on 25 August 2021).
| Species | Route 97 | Flatts Road | Moore Road | French Creek Road | Mystic Road | Venango | Saegertown | Total Number Across Sites |
|---|---|---|---|---|---|---|---|---|
| Actinonaias ligamentina (Lamarck) | 4 | 9 | 40 | 72 | 440 | 928 | 12 | 1505 |
| Alasmidonta marginata (Say) | 5 | 2 | 34 | -- | 3 | 10 | -- | 54 |
| Amblema plicata (Say) | -- | -- | 9 | -- | 9 | -- | -- | 18 |
| Anodontoides ferussacianus (Lea) | -- | -- | -- | 1 | -- | -- | -- | 1 |
| Cyclonaias tuberculata (Rafinesque) | -- | -- | -- | -- | -- | -- | -- | 0 |
| Elliptio dilatata (Rafinesque) | 4 | -- | 17 | 7 | 91 | 563 | 19 | 701 |
| Epioblasma torulosa rangiana (Lea) 1,3 | -- | -- | -- | -- | 11 | 34 | -- | 45 |
| Epioblasma triquetra (Rafinesque) 1,3 | 8 | -- | 2 | 5 | 13 | 1 | -- | 29 |
| Fusconaia subrotunda (Lea) | -- | -- | -- | -- | 1 | -- | -- | 1 |
| Lampsilis cardium (Rafinesque) | 1 | -- | -- | 14 | 28 | 6 | -- | 49 |
| Lampsilis fasciola (Rafinesque) | 4 | 1 | -- | 6 | 13 | 1 | 2 | 27 |
| Lampsilis siliquoidea (Barnes) | -- | -- | 6 | -- | 1 | 11 | -- | 18 |
| Lasmigona complanata (Barnes) | -- | -- | -- | -- | -- | -- | -- | 0 |
| Lasmigona compressa (Lea) | -- | -- | -- | 1 | -- | -- | -- | 1 |
| Lasmigona costata (Rafinesque) | -- | -- | 6 | 3 | 13 | 20 | -- | 42 |
| Ligumia nasuta (Say) | -- | -- | -- | -- | -- | -- | -- | 0 |
| Ligumia recta (Lamarck) | 3 | -- | -- | 5 | 12 | 6 | 3 | 29 |
| Pleurobema clava (Lamarck) 1,3 | -- | 1 | -- | 1 | 17 | 2 | -- | 21 |
| Pleurobema sintoxia (Rafinesque) | -- | 1 | -- | 5 | 7 | 6 | -- | 19 |
| Ptychobranchus fasciolaris (Rafinesque) | 35 | 6 | 69 | 46 | 119 | 1124 | 43 | 1442 |
| Pyganodon grandis (Say) | -- | -- | 2 | 2 | -- | -- | -- | 4 |
| Quadrula cylindrica cylindrica (Say) 2,3 | -- | -- | -- | 6 | 4 | 1 | -- | 11 |
| Simpsonaias ambigua (Say) 3 | -- | -- | -- | -- | -- | -- | -- | 0 |
| Strophitus undulatus (Say) | -- | -- | -- | 1 | 3 | -- | -- | 4 |
| Taxolasma parvus (Barnes) | -- | -- | -- | -- | -- | -- | -- | 0 |
| Utterbackia imbecillis (Say) | -- | -- | -- | -- | -- | -- | -- | 0 |
| Villosa fabalis (Lea) 2,4 | -- | -- | 1 | 5 | 16 | -- | 1 | 23 |
| Villosa iris (Lea) | -- | 1 | 6 | 34 | 167 | 100 | 4 | 312 |
| Total number of mussels | 64 | 21 | 192 | 214 | 968 | 2813 | 84 | -- |
| Total number of species | 8 | 7 | 11 | 17 | 19 | 15 | 7 | -- |
| Brillouin Diversity Value | 1.23 | 1.23 | 1.66 | 1.93 | 1.78 | 1.36 | 1.23 | -- |
| July 2017 | August 2017 | September 2017 | Total | ||||
|---|---|---|---|---|---|---|---|
| French Creek Road Mussel Species | Total Captures | No. Re- Captured | Total Captures | No. Re- Captured | Total Captures | Population Estimate | Standard Error |
| Kidneyshell-Ptychobranchus fasciolaris (Rafinesque) * | 16 | 10 | 16 | 11 | 14 | 46 | -- |
| Spike mussel, Mucket- Actinonaias ligamentina (Lamarck) | 16 | 12 | 25 | 24 | 30 | 26 | 1.2 |
| Spike Mussel- Elliptio dilatata (Rafinesque) * | 2 | 1 | 2 | 2 | 3 | 7 | -- |
| Rainbow mussel- Villosa iris (Lea) | 12 | 9 | 13 | 7 | 9 | 15 | 2.4 |
| July 2017 | August 2017 | September 2017 | June 2018 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| LeBeouf Creek Moore Road Mussel Species | Total Captures | No. Re- Captured | Total Captures | No. Re- Captured | Total Captures | No. Re- Captured | Population Estimate | Standard Error |
| Kidneyshell-Ptychobranchus fasciolaris (Rafinesque) | 8 | 7 | 21 | 15 | 21 | 18 | 66.7 | 23 |
| Spike mussel, Mucket- Actinonaias ligamentina (Lamarck) | 6 | 4 | 8 | 7 | 8 | 15 | 18 | 2.5 |
| Spike Mussel- Elliptio dilatata (Rafinesque) * | 3 | 1 | 5 | 5 | 5 | 2 | 17 | -- |
| Rainbow mussel- Villosa iris (Lea) * | 2 | 0 | 3 | 1 | 1 | 0 | 6 | -- |
| July 2017 | August 2017 | September 2017 | Total | ||||
|---|---|---|---|---|---|---|---|
| French Creek Mystic Road Mussel Species | Total Captures | No. Re- Captured | Total Captures | No. Re- Captured | Total Captures | Population Estimate | Standard Error |
| Kidneyshell-Ptychobranchus fasciolaris (Rafinesque) | 44 | 25 | 40 | 31 | 35 | 48 | 3.6 |
| Spike mussel, Mucket- Actinonaias ligamentina (Lamarck) | 148 | 113 | 151 | 119 | 140 | 167 | 3.8 |
| Spike Mussel- Elliptio dilatata (Rafinesque) | 36 | 20 | 27 | 22 | 28 | 37 | 3.8 |
| Rainbow mussel- Villosa iris (Lea) | 102 | 34 | 49 | 10 | 16 | 56 | 7.1 |
| July 2017 | August 2017 | September 2017 | June 2018 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| French Creek Venango Mussel Species | Total Captures | No. Re- Captured | Total Captures | No. Re- captured | Total Captures | No. Re- Captured | Population Estimate | Standard Error |
| Kidneyshell-Ptychobranchus fasciolaris (Rafinesque) | 219 | 190 | 270 | 246 | 300 | 172 | 529.3 | 30.4 |
| Spike mussel, Mucket- Actinonaias ligamentina (Lamarck) | 169 | 175 | 225 | 209 | 251 | 162 | 443.2 | 25.4 |
| Spike Mussel- Elliptio dilatata (Rafinesque) * | 120 | 82 | 144 | 105 | 137 | 78 | 240.1 | 20.8 |
| Rainbow mussel- Villosa iris (Lea) * | 51 | 6 | 9 | 11 | 15 | 25 | 28 | 6.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, K.H.; Wisor, J.M.; Mueller, S.J.; Bradshaw-Wilson, C.; Boyer, E.W.; Stauffer, J.R., Jr. Status of Freshwater Mussels (Unionidae) in the French Creek Watershed, USA at the Onset of Invasion by Round Goby, Neogobius melanostomus. Water 2021, 13, 3064. https://doi.org/10.3390/w13213064
Clark KH, Wisor JM, Mueller SJ, Bradshaw-Wilson C, Boyer EW, Stauffer JR Jr. Status of Freshwater Mussels (Unionidae) in the French Creek Watershed, USA at the Onset of Invasion by Round Goby, Neogobius melanostomus. Water. 2021; 13(21):3064. https://doi.org/10.3390/w13213064
Chicago/Turabian StyleClark, Kyle H., Joshua M. Wisor, Sara J. Mueller, Casey Bradshaw-Wilson, Elizabeth W. Boyer, and Jay R. Stauffer, Jr. 2021. "Status of Freshwater Mussels (Unionidae) in the French Creek Watershed, USA at the Onset of Invasion by Round Goby, Neogobius melanostomus" Water 13, no. 21: 3064. https://doi.org/10.3390/w13213064






