Discharge and Temperature Controls of Dissolved Organic Matter (DOM) in a Forested Coastal Plain Stream
Abstract
:1. Introduction
2. Materials and Methods
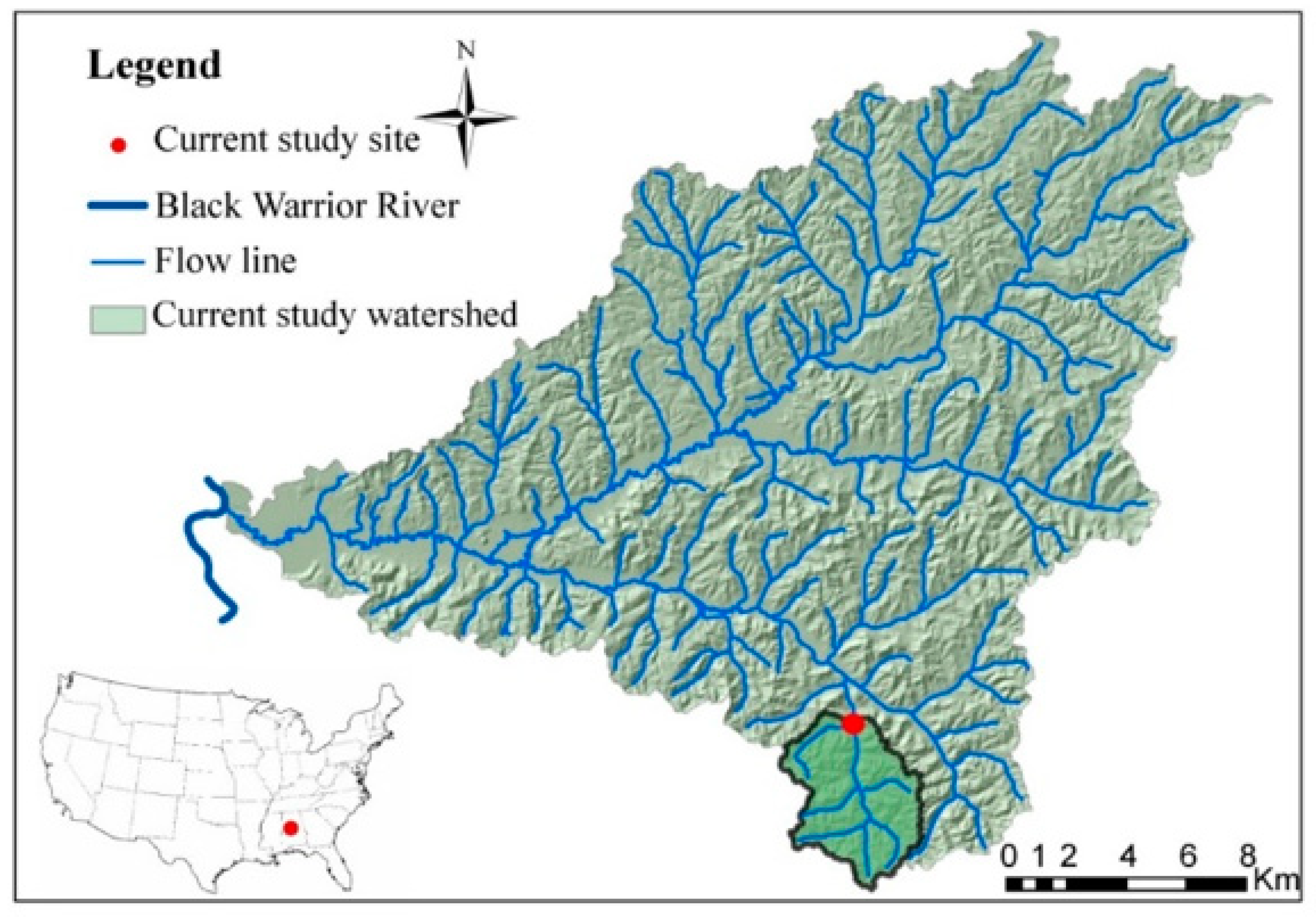
2.1. Study Site
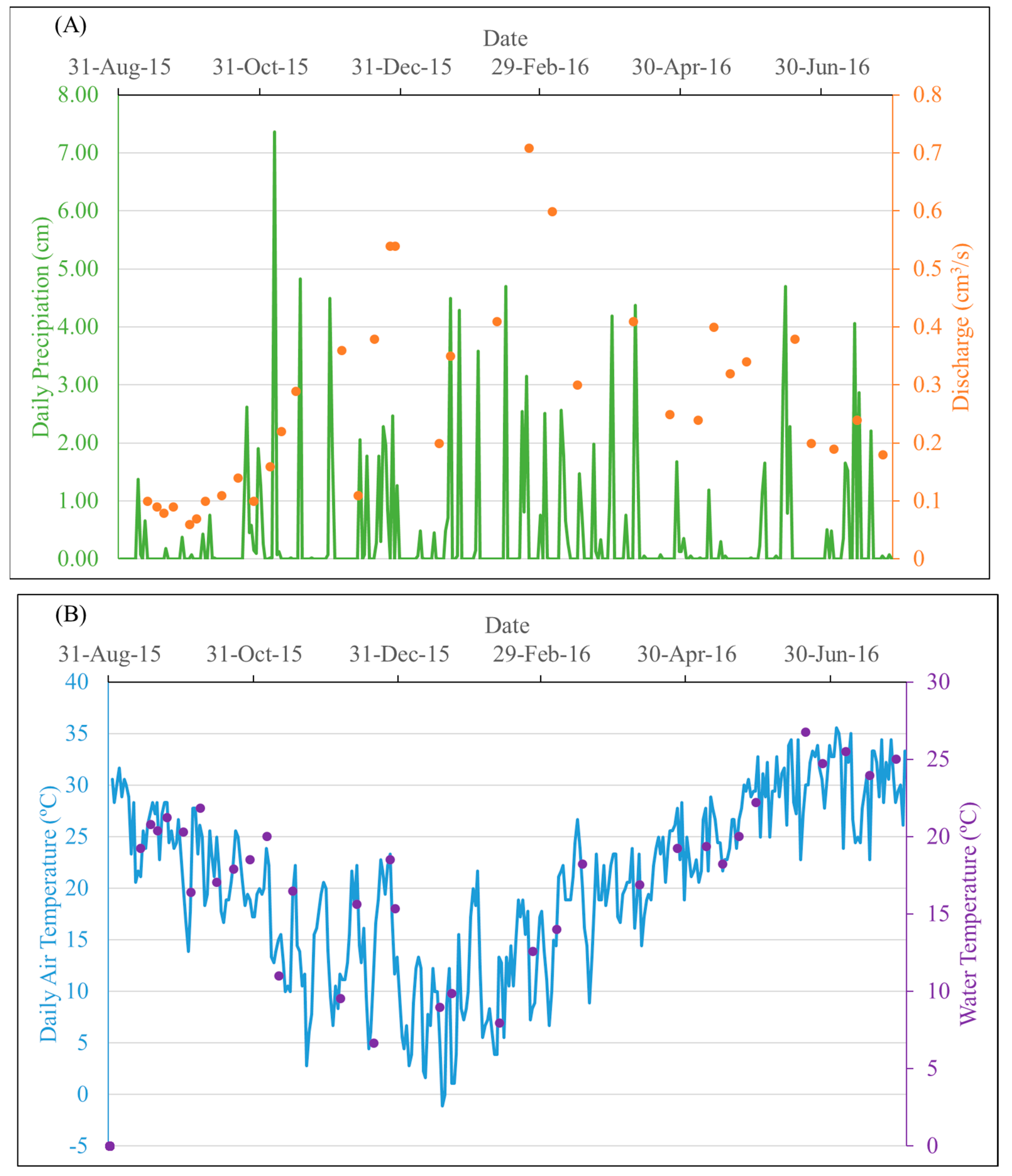
2.2. Flow Characterization and Sample Collection
2.3. DOC Concentration and DOM Optical Property
2.4. Laboratory Incubations to Evaluate DOC Bioreactivity
2.5. Ancillary Hydrological and Biogeochemical Parameters: Cations, Inorganic Nutrients, and Stable Oxygen and Hydrogen Isotopes of Water
2.6. Statistical Analysis
3. Results
3.1. Physiochemical Parameters
3.2. DOC and DOM Optical Indices
3.3. Predictors of DOM Indices: Linear Regression and RDA Models
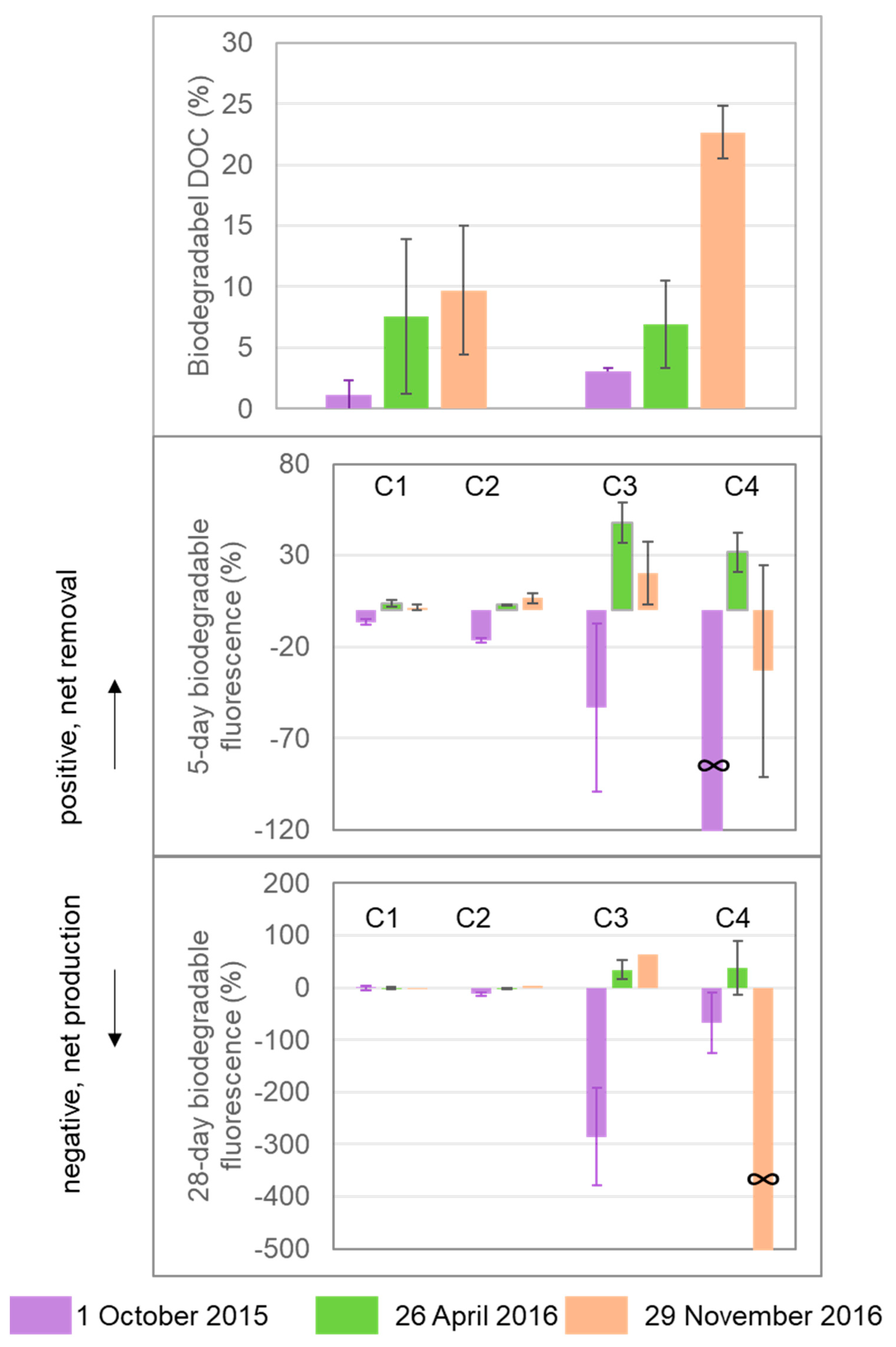
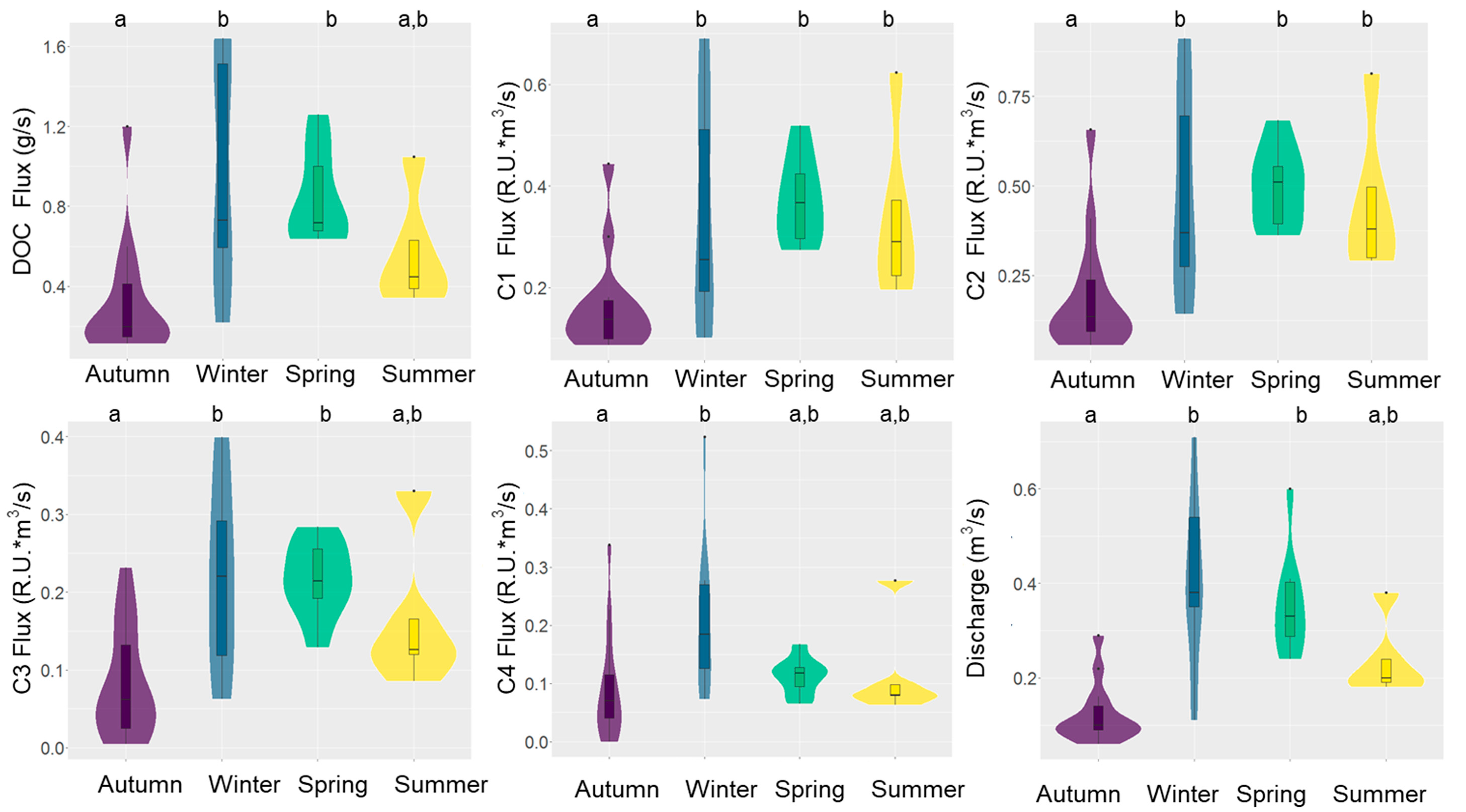
3.4. DOM Yield
4. Discussion
4.1. Source-Composition Characteristics of DOM Exported by Coast Plain Forested Streams
4.2. Temperature and Discharge Controls of DOM Source and Composition
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Jaffé, R. Characterizing the interactions between trace metals and dissolved organic matter using excitation-emission matrix and parallel factor analysis. Environ. Sci. Technol. 2008, 42, 7374–7379. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yang, H.; Li, S.; Yu, X. Tracking and analysis of DBP precursors’ properties by fluorescence spectrometry of dissolved organic matter. Chemosphere 2020, 239, 124790. [Google Scholar] [CrossRef]
- Jian, Q.; Boyer, T.H.; Yang, X.; Xia, B.; Yang, X. Characteristics and DBP formation of dissolved organic matter from leachates of fresh and aged leaf litter. Chemosphere 2016, 152, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Seitzinger, S.P.; Sanders, R.W.; Styles, R. Bioavailability of DON from natural and anthropogenic sources to estuarine plankton. Limnol. Oceanogr. 2002, 47, 353–366. [Google Scholar] [CrossRef]
- Villafañe, V.E.; Paczkowska, J.; Andersson, A.; Durán Romero, C.; Valiñas, M.S.; Helbling, E.W. Dual role of DOM in a scenario of global change on photosynthesis and structure of coastal phytoplankton from the South Atlantic Ocean. Sci. Total Environ. 2018, 634, 1352–1361. [Google Scholar] [CrossRef]
- Chen, S.; Lu, Y.H.; Dash, P.; Das, P.; Li, J.; Capps, K.; Majidzadeh, H.; Elliott, M. Hurricane pulses: Small watershed exports of dissolved nutrients and organic matter during large storms in the Southeastern USA. Sci. Total Environ. 2019, 689, 232–244. [Google Scholar] [CrossRef]
- Hansen, A.M.; Kraus, T.E.C.; Pellerin, B.A.; Fleck, J.A.; Downing, B.D.; Bergamaschi, B.A. Optical properties of dissolved organic matter (DOM): Effects of biological and photolytic degradation. Limnol. Oceanogr. 2016, 61, 1015–1032. [Google Scholar] [CrossRef] [Green Version]
- Kaushal, S.S.; Gold, A.J.; Bernal, S.; Johnson, T.A.N.; Addy, K.; Burgin, A.; Burns, D.A.; Coble, A.A.; Hood, E.; Lu, Y.H.; et al. Watershed ‘chemical cocktails’: Forming novel elemental combinations in Anthropocene fresh waters. Biogeochemistry 2018, 141, 281–305. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Wollheim, W.M.; Bernal, S.; Burns, D.A.; Czuba, J.A.; Driscoll, C.T.; Hansen, A.T.; Hensley, R.T.; Hosen, J.D.; Inamdar, S.; Kaushal, S.S.; et al. River network saturation concept: Factors influencing the balance of biogeochemical supply and demand of river networks. Biogeochemistry 2018, 141, 503–521. [Google Scholar] [CrossRef]
- Gold, A.C.; Thompson, S.P.; Magel, C.L.; Piehler, M.F. Urbanization alters coastal plain stream carbon export and dissolved oxygen dynamics. Sci. Total Environ. 2020, 747, 141132. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, X.; Xu, X.; Asrar, G.R. An Analysis of Terrestrial and Aquatic Environmental Controls of Riverine Dissolved Organic Carbon in the Conterminous United States. Water 2017, 9, 383. [Google Scholar] [CrossRef] [Green Version]
- Kalbitz, K.; Schwesig, D.; Rethemeyer, J.; Matzner, E. Stabilization of dissolved organic matter by sorption to the mineral soil. Soil Biol. Biochem. 2005, 37, 1319–1331. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.H.; Michalzik, B.; Matzner, E. Controls on the dynamics of dissolved organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Wilson, H.F.; Raymond, P.A.; Saiers, J.E.; Sobczak, W.V.; Xu, N. Increases in humic and bioavailable dissolved organic matter in a forested New England headwater stream with increasing discharge. Mar. Freshw. Res. 2016, 67, 1279–1292. [Google Scholar] [CrossRef]
- Xu, N.; Saiers, J.E.; Wilson, H.F.; Raymond, P.A. Simulating streamflow and dissolved organic matter export from a forested watershed. Water Resour. Res. 2012, 48, 5519. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Lu, Y.; Edmonds, J.W.; Liu, C.; Wang, S.; Das, O.; Liu, J.; Zheng, C. Hydrological and land use control of watershed exports of dissolved organic matter in a large arid river basin in northwestern China. J. Geophys. Res. Biogeosci. 2016, 121, 466–478. [Google Scholar] [CrossRef] [Green Version]
- Lynch, L.M.; Sutfin, N.A.; Fegel, T.S.; Boot, C.M.; Covino, T.P.; Wallenstein, M.D. River channel connectivity shifts metabolite composition and dissolved organic matter chemistry. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Mao, R.; Li, S. Hydrological seasonality largely contributes to riverine dissolved organic matter chemical composition: Insights from EEM-PARAFAC and optical indicators. J. Hydrol. 2021, 595, 125993. [Google Scholar] [CrossRef]
- Sankar, M.S.; Dash, P.; Singh, S.; Lu, Y.H.; Mercer, A.E.; Chen, S. Effect of photo-biodegradation and biodegradation on the biogeochemical cycling of dissolved organic matter across diverse surface water bodies. J. Environ. Sci. (China) 2019, 77, 130–147. [Google Scholar] [CrossRef]
- Carter, L.M.; Jones, J.W.; Berry, L.; Burkett, V.; Murley, J.F.; Obeysekera, J.; Schramm, P.J.; Wear, D. Ch. 17: Southeast and the Caribbean. Climate Change Impacts in the United States: The Third National Climate Assessment. U.S. Glob. Chang. Res. Progr. 2014, 396–417. [Google Scholar] [CrossRef]
- Otero, M.; Mendonça, A.; Válega, M.; Santos, E.B.H.; Pereira, E.; Esteves, V.I.; Duarte, A. Fluorescence and DOC contents of estuarine pore waters from colonized and non-colonized sediments: Effects of sampling preservation. Chemosphere 2007, 67, 211–220. [Google Scholar] [CrossRef]
- Spencer, R.G.M.; Bolton, L.; Baker, A. Freeze/thaw and pH effects on freshwater dissolved organic matter fluorescence and absorbance properties from a number of UK locations. Water Res. 2007, 41, 2941–2950. [Google Scholar] [CrossRef]
- Shang, P.; Lu, Y.; Du, Y.; Jaffé, R.; Findlay, R.H.; Wynn, A. Climatic and watershed controls of dissolved organic matter variation in streams across a gradient of agricultural land use. Sci. Total Environ. 2018, 612, 1442–1453. [Google Scholar] [CrossRef]
- Cory, R.M.; McKnight, D.M. Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 2005, 39, 8142–8149. [Google Scholar] [CrossRef]
- Murphy, K.R.; Stedmon, C.A.; Graeber, D.; Bro, R. Fluorescence spectroscopy and multi-way techniques. PARAFAC. Anal. Methods 2013, 5, 6557–6566. [Google Scholar] [CrossRef] [Green Version]
- Weishaar, J.L.; Aiken, G.R.; Bergamaschi, B.A.; Fram, M.S.; Fujii, R.; Mopper, K. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 2003, 37, 4702–4708. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, R.; Blough, N.V. Photobleaching of chromophoric dissolved organic matter in natural waters: Kinetics and modeling. Mar. Chem. 2002, 78, 231–253. [Google Scholar] [CrossRef]
- Helms, J.R.; Stubbins, A.; Ritchie, J.D.; Minor, E.C.; Kieber, D.J.; Mopper, K. Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 2008, 53, 955–969. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T. Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter. Environ. Sci. Technol. 2002, 36, 742–746. [Google Scholar] [CrossRef]
- Huguet, A.; Vacher, L.; Relexans, S.; Saubusse, S.; Froidefond, J.-M.; Parlanti, E. Properties of fluorescent dissolved organic matter in the Gironde Estuary. Org. Geochem. 2009, 40, 706–719. [Google Scholar] [CrossRef]
- Servais, P.; Anzil, A.; Ventresque, C. Simple method for determination of biodegradable dissolved organic carbon in water. Appl. Environ. Microbiol. 1989, 55, 2732–2734. [Google Scholar] [CrossRef] [Green Version]
- Jaffé, R.; McKnight, D.; Maie, N.; Cory, R.; McDowell, W.H.; Campbell, J.L. Spatial and temporal variations in DOM composition in ecosystems: The importance of long-term monitoring of optical properties. J. Geophys. Res. Biogeosci. 2008, 113, G04032. [Google Scholar] [CrossRef]
- Peuravuori, J.; Pihlaja, K. Molecular size distribution and spectroscopic properties of aquatic humic substances. Anal. Chim. Acta 1997, 337, 133–149. [Google Scholar] [CrossRef]
- Zsolnay, A.; Baigar, E.; Jimenez, M.; Steinweg, B.; Saccomandi, F. Differentiating with fluorescence spectroscopy the sources of dissolved organic matter in soils subjected to drying. Chemosphere 1999, 38, 45–50. [Google Scholar] [CrossRef]
- Parlanti, E.; Wörz, K.; Geoffroy, L.; Lamotte, M. Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 2000, 31, 1765–1781. [Google Scholar] [CrossRef]
- Coble, P.G. Marine Optical Biogeochemistry: The Chemistry of Ocean Color. Chem. Rev. 2007, 107, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Ishii, S.K.L.; Boyer, T.H. Behavior of Reoccurring PARAFAC Components in Fluorescent Dissolved Organic Matter in Natural and Engineered Systems: A Critical Review. Environ. Sci. Technol. 2012, 46, 2006–2017. [Google Scholar] [CrossRef] [PubMed]
- Shutova, Y.; Baker, A.; Bridgeman, J.; Henderson, R.K. Spectroscopic characterisation of dissolved organic matter changes in drinking water treatment: From PARAFAC analysis to online monitoring wavelengths. Water Res. 2014, 54, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, L.; Stedmon, C.A.; Kragh, T.; Markager, S.; Middelboe, M.; Søndergaard, M. Global trends in the fluorescence characteristics and distribution of marine dissolved organic matter. Mar. Chem. 2011, 126, 139–148. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Thomas, D.N.; Papadimitriou, S.; Granskog, M.A.; Dieckmann, G.S. Using fluorescence to characterize dissolved organic matter in Antarctic sea ice brines. J. Geophys. Res. 2011, 116, G03027. [Google Scholar] [CrossRef] [Green Version]
- Yamashita, Y.; Scinto, L.J.; Maie, N.; Jaffé, R. Dissolved Organic Matter Characteristics Across a Subtropical Wetland’s Landscape: Application of Optical Properties in the Assessment of Environmental Dynamics. Ecosystems 2010, 13, 1006–1019. [Google Scholar] [CrossRef]
- Lu, Y.H.; Bauer, J.E.; Canuel, E.A.; Chambers, R.M.; Yamashita, Y.; Jaffé, R.; Barrett, A. Effects of land use on sources and ages of inorganic and organic carbon in temperate headwater streams. Biogeochemistry 2014, 119, 275–292. [Google Scholar] [CrossRef]
- Chen, M.; Kim, S.H.; Jung, H.J.; Hyun, J.H.; Choi, J.H.; Lee, H.J.; Huh, I.A.; Hur, J. Dynamics of dissolved organic matter in riverine sediments affected by weir impoundments: Production, benthic flux, and environmental implications. Water Res. 2017, 121, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Boyer, J.N.; Jaffé, R. Evaluating the distribution of terrestrial dissolved organic matter in a complex coastal ecosystem using fluorescence spectroscopy. Cont. Shelf Res. 2013, 66, 136–144. [Google Scholar] [CrossRef] [Green Version]
- Fellman, J.B.; D’Amore, D.V.; Hood, E.; Boone, R.D. Fluorescence characteristics and biodegradability of dissolved organic matter in forest and wetland soils from coastal temperate watersheds in southeast Alaska. Biogeochemistry 2008, 88, 169–184. [Google Scholar] [CrossRef]
- Lu, Y.; Bauer, J.E.; Canuel, E.A.; Yamashita, Y.; Chambers, R.M.; Jaffé, R. Photochemical and microbial alteration of dissolved organic matter in temperate headwater streams associated with different land use. J. Geophys. Res. Biogeosci. 2013, 118, 566–580. [Google Scholar] [CrossRef] [Green Version]
- Cory, R.M.; Kaplan, L.A. Biological lability of streamwater fluorescent dissolved organic matter. Limnol. Oceanogr. 2012, 57, 1347–1360. [Google Scholar] [CrossRef]
- Fellman, J.B.; Hood, E.; Spencer, R.G.M. Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: A review. Wiley Online Libr. 2010, 55, 2452–2462. [Google Scholar] [CrossRef]
- Stedmon, C.A.; Markager, S. Tracing the production and degradation of autochthonous fractions of dissolved organic matter by fluorescence analysis. Limnol. Oceanogr. 2005, 50, 1415–1426. [Google Scholar] [CrossRef]
- Atkinson, C.L.; van Ee, B.C.; Lu, Y.H.; Zhong, W. Wetland floodplain flux: Temporal and spatial availability of organic matter and dissolved nutrients in an unmodified river. Biogeochemistry 2019, 142, 395–411. [Google Scholar] [CrossRef]
- Dosskey, M.G.; Bertsch, P.M. Forest sources and pathways of organic matter transport to a blackwater stream: A hydrologic approach. Biogeochemistry 1994, 24, 1–19. [Google Scholar] [CrossRef]
- Hosen, J.D.; Armstrong, A.W.; Palmer, M.A. Dissolved organic matter variations in coastal plain wetland watersheds: The integrated role of hydrological connectivity, land use, and seasonality. Hydrol. Process. 2018, 32, 1664–1681. [Google Scholar] [CrossRef] [Green Version]
- Meyer, J.L. A blackwater perspective on riverine ecosystems. Bioscience 1990, 40, 643–651. [Google Scholar] [CrossRef]
- Meyer, J.L. Seasonal Patterns of Water Quality in Blackwater Rivers of the Coastal Plain, Southeastern United States; Neitzel, D.A., Becker, C.D., Eds.; Battelle Press: Columbus, OH, USA, 1992. [Google Scholar]
- Bano, N.; Moran, M.; Hodson, R. Bacterial utilization of dissolved humic substances from a freshwater swamp. Aquat. Microb. Ecol. 1997, 12, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Koh, D.-C.; Kim, Y.S.; Jeen, S.-W.; Lee, J. Stable Isotopes of Water and Nitrate for the Identification of Groundwater Flowpaths: A Review. Water 2020, 12, 138. [Google Scholar] [CrossRef] [Green Version]
- D’Arcy, P.; Carignan, R. Influence of catchment topography on water chemistry in southeastern Québec Shield lakes. Can. J. Fish. Aquat. Sci. 1997, 54, 2215–2227. [Google Scholar] [CrossRef]
- Stewart, M.K.; Mehlhorn, J.; Elliott, S. Hydrometric and natural tracer (oxygen-18, silica, tritium and sulphur hexafluoride) evidence for a dominant groundwater contribution to Pukemanga Stream, New Zealand. Hydrol. Process. 2007, 21, 3340–3356. [Google Scholar] [CrossRef]
- Inamdar, S.; Singh, S.; Dutta, S.; Levia, D.; Mitchell, M.; Scott, D.; Bais, H.; McHale, P. Fluorescence characteristics and sources of dissolved organic matter for stream water during storm events in a forested mid-Atlantic watershed. J. Geophys. Res. Biogeosci. 2011, 116, G03043. [Google Scholar] [CrossRef] [Green Version]
- Vidon, P.; Wagner, L.E.; Soyeux, E. Changes in the character of DOC in streams during storms in two Midwestern watersheds with contrasting land uses. Biogeochemistry 2008, 88, 257–270. [Google Scholar] [CrossRef]
- Christ, M.J.; David, M.B. Temperature and moisture effects on the production of dissolved organic carbon in a Spodosol. Soil Biol. Biochem. 1996, 28, 1191–1199. [Google Scholar] [CrossRef]
- Roth, V.-N.; Dittmar, T.; Gaupp, R.; Gleixner, G. The Molecular Composition of Dissolved Organic Matter in Forest Soils as a Function of pH and Temperature. PLoS ONE 2015, 10, e0119188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stutter, M.I.; Lumsdon, D.G.; Cooper, R.J. Temperature and soil moisture effects on dissolved organic matter release from a moorland Podzol O horizon under field and controlled laboratory conditions. Eur. J. Soil Sci. 2007, 58, 1007–1016. [Google Scholar] [CrossRef]
- Benke, A.C.; Chaubey, I.; Milton Ward, G.; Dunn, E.L. Flood pulse dynamics of an unregulated river floodplain in the southeastern U.S. coastal plain. Ecology 2000, 81, 2730–2741. [Google Scholar] [CrossRef]
- Raymond, P.A.; Saiers, J.E.; Sobczak, W.V. Hydrological and biogeochemical controls on watershed dissolved organic matter transport: Pulse-shunt concept. Ecology 2016, 97, 5–16. [Google Scholar] [CrossRef]
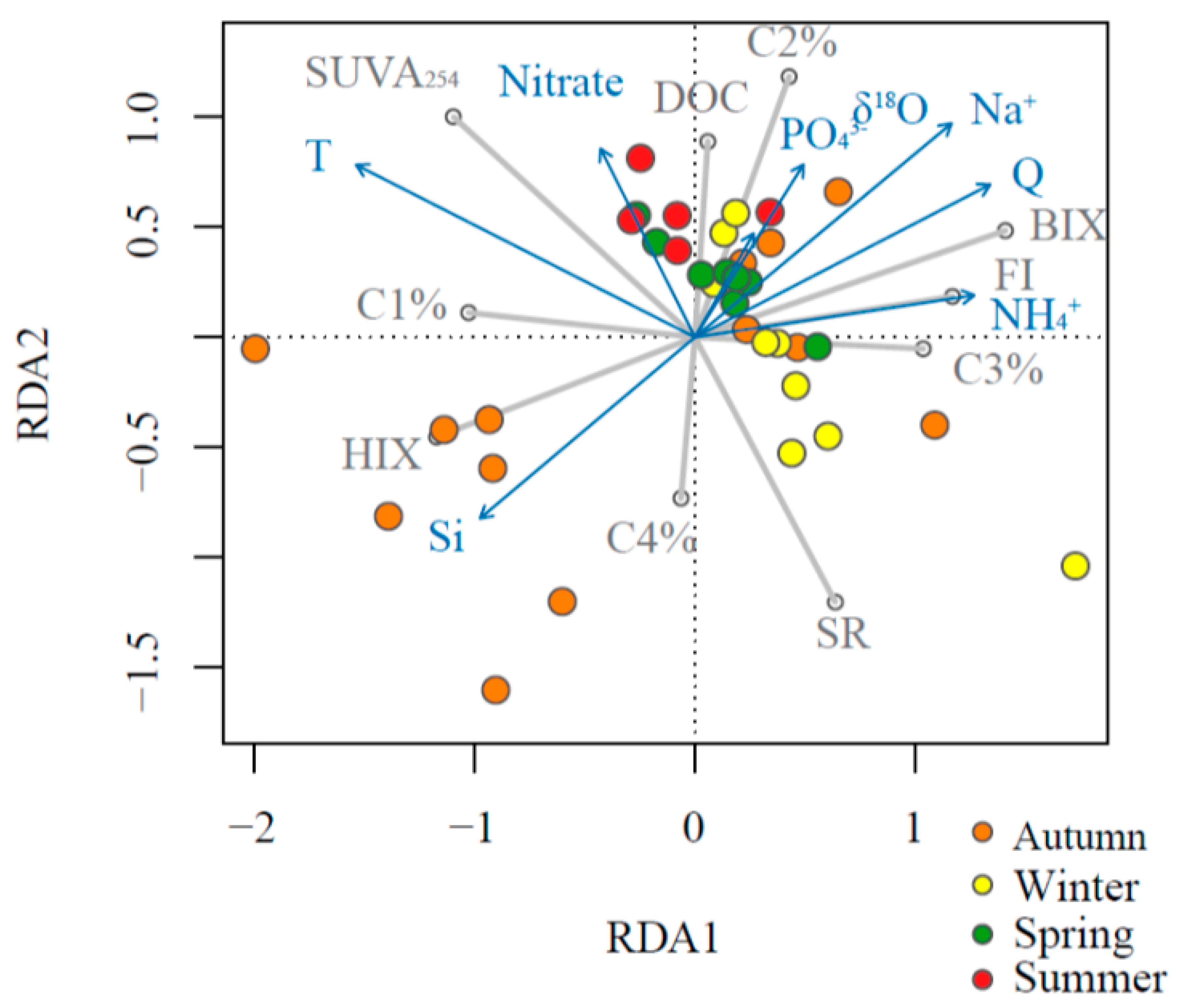
| SUVA254 | HIX | SR | BIX | FI | |
|---|---|---|---|---|---|
| %C1 a | 0.35, p = 0.04 | 0.76, p < 0.001 | −0.23, p = 0.17 | −0.73, p < 0.001 | −0.49, p < 0.001 |
| %C2 | −0.03, p = 0.87 | −0.37, p = 0.03 | −0.13, p = 0.44 | 0.49, p < 0.001 | 0.56, p < 0.001 |
| %C3 | −0.35, p = 0.04 | −0.76, p < 0.001 | 0.53, p < 0.001 | 0.74, p < 0.001 | 0.67, p < 0.001 |
| %C4 | −0.05, p = 0.78 | 0.08, p = 0.66 | −0.02, p = 0.91 | −0.15, p = 0.39 | −0.32, p = 0.06 |
| DOM Indices | Generalized Linear Model a | R-Square |
|---|---|---|
| DOC | −0.5486 × Si + 0.3104 × Nitrate − 0.4945 × δ18O + 2.070e−17 | 0.20 |
| SUVA254 | 0.511 × T + 0.1988 × Si + 0.3608 × Nitrate + 3.243e−16 | 0.61 |
| SR | −0.4901 × T + 0.8060 × Si + 0.4645 × Q − 3.675e−16 | 0.39 |
| HIX | 0.2585 × T − 0.2211 × Q − 0.4335 × Na+ + 1.930e−16 | 0.40 |
| FI | 0.5681 × Q − 0.5096 × δ18O + 0.4041 × Na+ + 5.934e−16 | 0.32 |
| BIX | 0.4812 × Q − 0.2253 × T − 0.3443 × d18O + 0.5079 × Na+ + 1.79e−16 | 0.51 |
| Percentage C1 | 0.4093 × T − 0.3043 × Na+ − 1.184e−16 | 0.25 |
| Percentage C2 | 0.3766 × Q + 0.2392 × T + 0.2567 × Na+ − 1.808e−16 | 0.25 |
| Percentage C3 | −0.3650 × T + 0.3537 × Na+ − 1.387e−16 | 0.25 |
| Percentage C4 | No reasonable model can be established from these predictors; the model with the lowest AIC has only intercept and R2 = 0 | 0 |
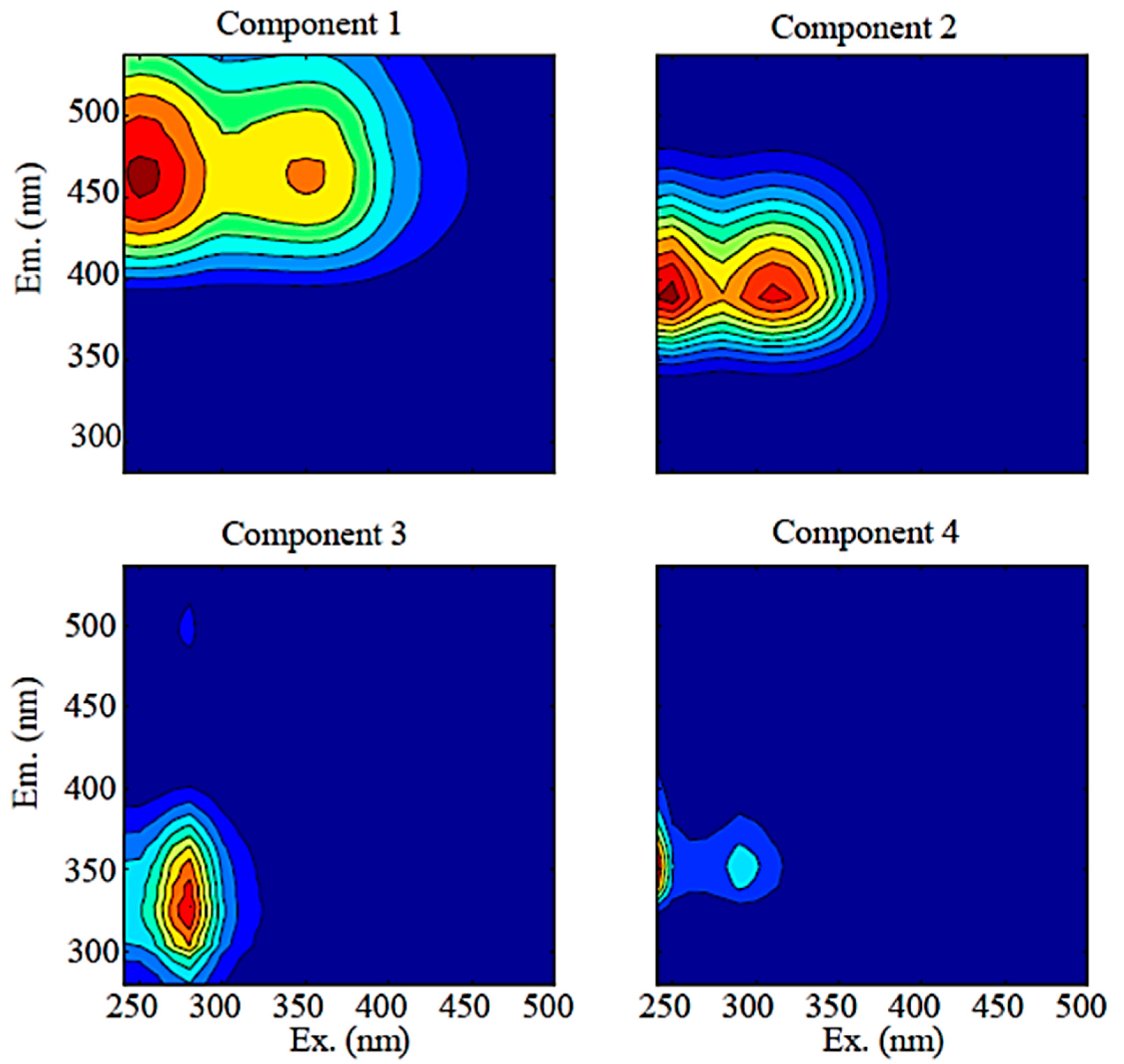
| Component | Excitation Maximum Wavelength | Emission Maximum Wavelength | Similar Fluorescence Components Identified in Previous Studies | Present Study | |||
|---|---|---|---|---|---|---|---|
| Ref. [38] | Ref. [26] | Ref. [43] | Ref. [44] | ||||
| C1 | 250, 350 | 466 | A, C | C1 or SQ2 | C1 (Terrestrial) | C1 (Terrestrial Fulvic acid) | Terrestrial humic-like DOM |
| C2 | 250, 310 | 388 | M | C3 or Q3 | C4 (Microbial) | C3 (Microbial humic-like) | Microbial humic-like DOM from soils |
| C3 | 280 | 328 | B | C8 | C7 (Protein) | C4 (Protein-like) | Tyrosine-like, protein-like, autochthonous |
| C4 | <240, 290 | 352 | T | C13 | C8 (Protein) | - | Tryptophan-like, protein-like, autochthonous |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Shang, P.; Chen, S.; Du, Y.; Bonizzoni, M.; Ward, A.K. Discharge and Temperature Controls of Dissolved Organic Matter (DOM) in a Forested Coastal Plain Stream. Water 2021, 13, 2919. https://doi.org/10.3390/w13202919
Lu Y, Shang P, Chen S, Du Y, Bonizzoni M, Ward AK. Discharge and Temperature Controls of Dissolved Organic Matter (DOM) in a Forested Coastal Plain Stream. Water. 2021; 13(20):2919. https://doi.org/10.3390/w13202919
Chicago/Turabian StyleLu, Yuehan, Peng Shang, Shuo Chen, Yingxun Du, Marco Bonizzoni, and Amelia K. Ward. 2021. "Discharge and Temperature Controls of Dissolved Organic Matter (DOM) in a Forested Coastal Plain Stream" Water 13, no. 20: 2919. https://doi.org/10.3390/w13202919






