Possibility of Humid Municipal Wastes Hygienisation Using Gliding Arc Plasma Reactor
Abstract
:1. Introduction
2. Material and Methods
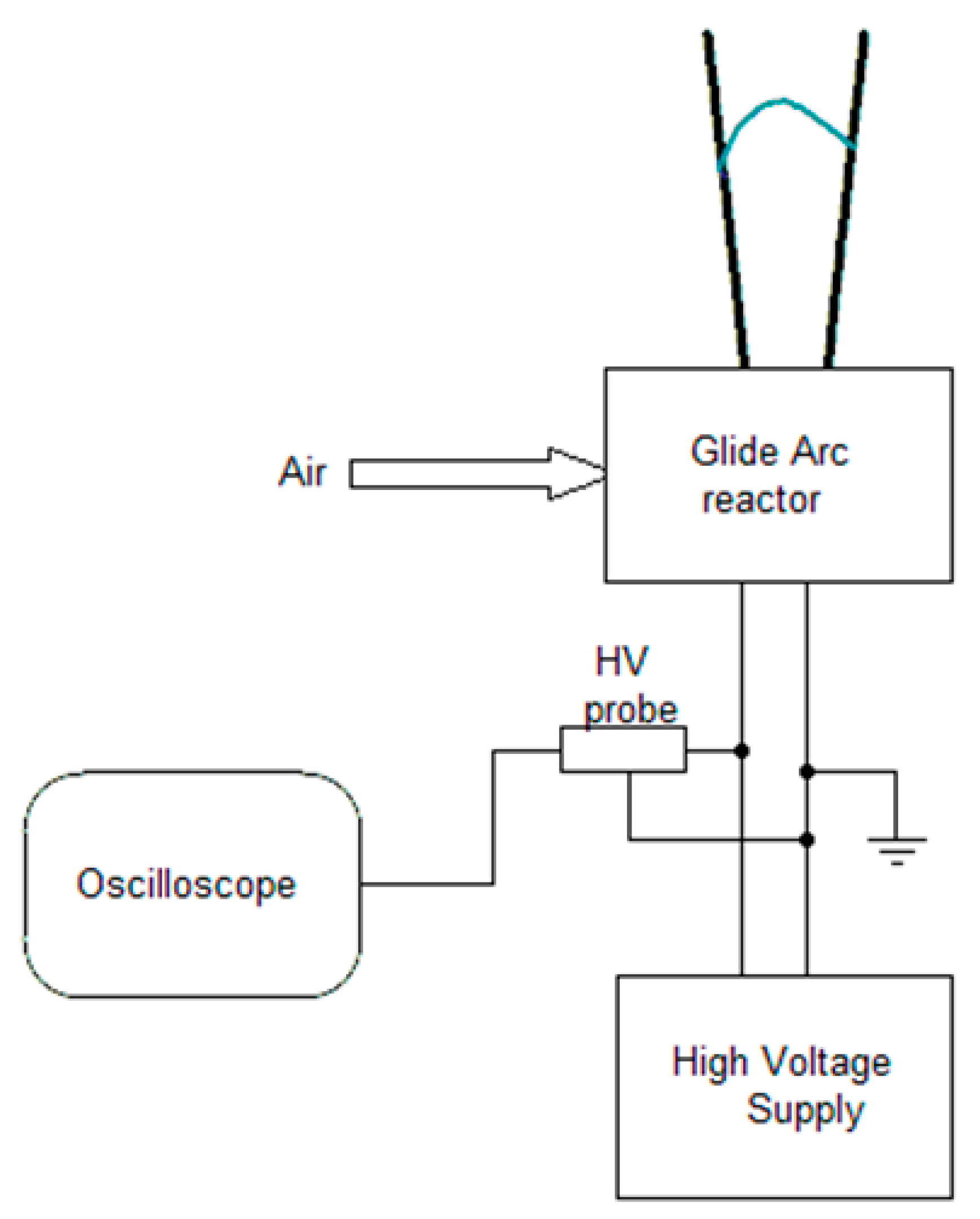
2.1. Non-Equilibrium Plasma Treatment
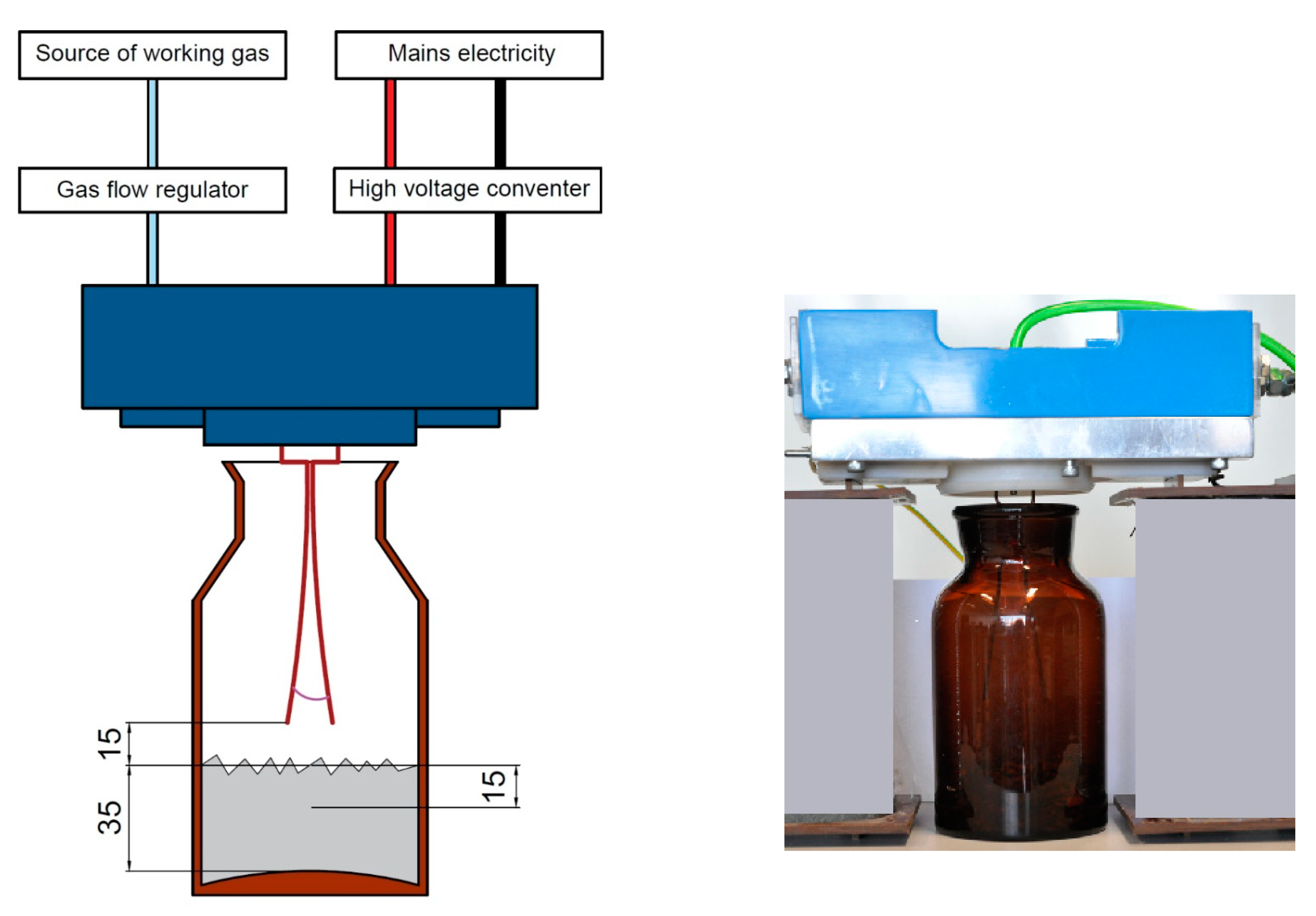
2.2. Tests Involving the Use of Low-Temperature Plasma on Municipal Waste Samples
3. Microbiological Analyzes
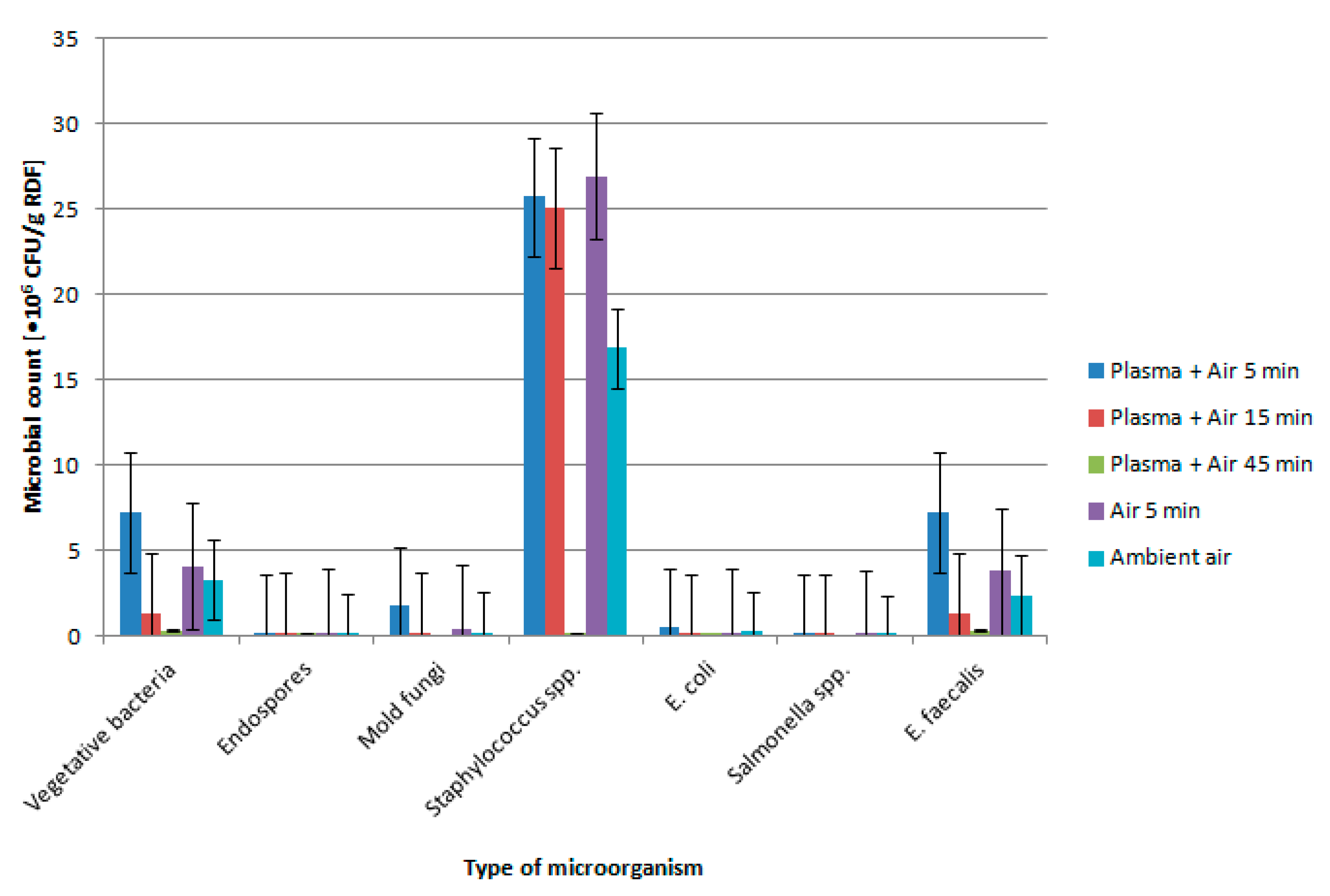
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brisset, J.-L.; Pawłat, J. Chemical effects of air plasma species on aqueous solutes in direct and delayed exposure modes: Discharge, post-discharge and plasma activated water. Plasma Chem. Plasma Process. 2016, 36, 355–381. [Google Scholar] [CrossRef]
- Hensel, K.; Kučerová, K.; Tarabová, B.; Janda, M.; Machala, Z.; Sano, K.; Mihai, C.T.; Gorgan, L.D.; Jijie, R.; Pohoata, V.; et al. Effects of air transient spark discharge and helium plasma jet on water, bacteria, cells and biomolecules. Biointerphases 2015, 10, 029515. [Google Scholar] [CrossRef]
- Janda, M.; Martišovitš, V.; Hensel, K.; Machala, Z. Generation of antimicrobial NOx by atmospheric air transient spark discharge. Plasma Chem. Plasma Proc. 2016. [Google Scholar] [CrossRef]
- Kovalová, Z.; Tarabová, K.; Hensel, K.; Machala, Z. Decontamination of Streptococci biofilms and Bacillus cereus spores on plastic surfaces with DC and pulsed corona discharges. Eur. Phys. J. Appl. Phys. 2013, 61, 24306. [Google Scholar] [CrossRef] [Green Version]
- Tak, G.; Gallagher, M.; Gangoli, S.; Gutsol, A.; Fridman, A. Use of Non-Thermal Atmospheric Pressure Plasma for Air Cleaning and Sterilization. In Proceedings of the 32nd IEEE International Conference on Plasma Science, Monterey, CA, USA, 20–23 June 2005. [Google Scholar]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.; Graham, W.G.; Graves, D.B. Plasma–liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar]
- Niedźwiedź, I.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The State of Research on Antimicrobial Activity of Cold Plasma. Pol. J. Microbiol. 2019, 68, 153–164. [Google Scholar]
- Pawlat, J.; Mizuno, A.; Yamabe, C.; Pollo, I. Absorption and Decomposition of CH3CHO in the Cylindrical Foaming System. J. Adv. Oxid. Technol. 2004, 7, 59–64. [Google Scholar]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Mizeraczyk, J.; Dors, M.; Jasiński, M.; Hrycak, B.; Czylkowski, D. Atmospheric pressure low-power microwave microplasma source for deactivation of microorganisms. Eur. Phys. J. Appl. Phys. 2013, 61, 24309. [Google Scholar] [CrossRef]
- Pawłat, J. Atmospheric pressure plasma jet for decontamination purposes. Eur. Phys. J. Appl. Phys. 2013, 61, 24323. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Balasubramanian, M.; Ayan, H.; Fridman, A.; Gutsol, A.; Brooks, A. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Krumpolec, R.; Zahoranova, A.; Černák, M.; Kováčik, D. Chemical and physical evaluation of hydrophobic pp-HMDSO layers deposited by plasma polymerization at atmospheric pressure. Chem. Listy 2012, 106, 1450–1454. [Google Scholar]
- Terebun, P.; Kwiatkowski, M.; Starek, A.; Reuter, S.; Mok, Y.-S.; Pawłat, J. Impact of Short Time Atmospheric Plasma Treatment on Onion Seeds. Plasma Chem. Plasma Process. 2021. [Google Scholar] [CrossRef]
- Sahni, M.; Finney, W.C.; Locke, B.R. Degradation of aqueous phase polychlorinated biphenyls (PCB) using pulsed corona discharges. J. Adv. Oxid. Technol. 2005, 8, 105–111. [Google Scholar] [CrossRef]
- Maïté Audemar, M.; Vallcorba, O.; Peral, I.; Thomann, J.; Przekora, A.; Pawlat, J.; Canal, C.; Ginalska, G.; Kwiatkowski, M.; Duday, D.; et al. Catalytic enrichment of plasma with hydroxyl radicals in the aqueous phase at room temperature. Catal. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Nishime, T.M.C.; Wannicke, N.; Horn, S.; Weltmann, K.-D.; Brust, H. A Coaxial Dielectric Barrier Discharge Reactor for Treatment of Winter Wheat Seeds. Appl. Sci. 2020, 10, 7133. [Google Scholar] [CrossRef]
- Pawłat, J.; Starek, A.; Sujak, A.; Terebun, P.; Kwiatkowski, M.; Budzeń, M.; Andrejko, D. Effects of atmospheric pressure plasma jet operating with DBD on Lavatera thuringiaca L. seeds’ germination. PLoS ONE 2018, 13, e0194349. [Google Scholar] [CrossRef] [Green Version]
- Krishna, S.; Ceriani, E.; Marotta, E.; Giardina, A.; Špatenka, P.; Paradisi, C. Products and mechanism of verapamil removal in water by air non-thermal plasma treatment. Chem. Eng. J. 2016, 292, 35–41. [Google Scholar] [CrossRef]
- Pawlat, J.; Ihara, S. Removal of color caused by various chemical compounds using electrical discharges in a foaming column. Plasma Process. Polym. 2007, 4, 753–759. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen-and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Krčma, F.; Blahová, L.; Fojtíková, P.; Graham, W.G.; Grossmannová, H.; Hlochová, L.; Horák, J.; Janová, D.; Kelsey, C.P. Application of low temperature plasmas for restoration/conservation of archaeological objects. J. Phys. Conf. Ser. 2014, 565, 1. [Google Scholar] [CrossRef] [Green Version]
- Škoro, N.; Puač, N.; Lazović, S.; Cvelbar, U.; Kokkoris, G.; Gogolides, E. Characterization and global modelling of low-pressure hydrogen-based RF plasmas suitable for surface cleaning processes. J. Phys. D 2013, 46, 475206. [Google Scholar] [CrossRef]
- Prysiazhnyi, V.; Zaporojchenko, V.; Kersten, H.; Černák, M. Influence of humidity on atmospheric pressure air plasma treatment of aluminium surfaces. Appl. Surf. Sci. 2012, 258, 5467–5471. [Google Scholar] [CrossRef]
- Kolacinski, Z.; Szymanski, L.; Raniszewski, G. LTE plasma reactors for materials conversion. Eur. Phys. J. Appl. Phys. 2013, 61, 24314. [Google Scholar] [CrossRef]
- Favia, P. Plasma deposited coatings for biomedical materials and devices: Fluorocarbon and PEO-like coatings. Surf. Coat. Technol. 2012, 211, 50–56. [Google Scholar]
- Dvořáková, H.; Čech, J.; Černák, M.; Sťahel, P. Plasma surface activation of high density polyethylene at atmospheric pressure. Composites 2015, 2, 3. [Google Scholar]
- Pawlat, J.; Terebun, P.; Kwiatkowski, M.; Diatczyk, J. RF atmospheric plasma jet surface treatment of paper. J. Phys. D 2016, 49, 374001. [Google Scholar] [CrossRef]
- Pawlat, J.; Kwiatkowski, M.; Terebun, P.; Murakami, T. RF-powered atmospheric-pressure plasma jet in surface treatment of high-impact polystyrene. IEEE Trans. Plasma Sci. 2016, 44, 314–320. [Google Scholar] [CrossRef]
- Janča, J.; Czernichowski, A. Wool treatment in the gas flow from gliding discharge plasma at atmospheric pressure. Surf. Coat. Technol. 1998, 98, 1112–1115. [Google Scholar] [CrossRef]
- Pawłat, J.; Terebun, P.; Kwiatkowski, M.; Tarabová, B.; Kovaľová, Z.; Kučerová, K.; Machala, Z.; Janda, M.; Hensel, K. Evaluation of oxidative species in gaseous and liquid phase generated by mini-gliding arc discharge. Plasma Chem. Plasma Process. 2019, 39, 627–642. [Google Scholar]
- Przekora, A.; Audemar, M.; Pawlat, J.; Canal, C.; Thomann, J.; Labay, C.; Wojcik, M.; Kwiatkowski, M.; Terebun, P.; Ginalska, G.; et al. Positive Effect of Cold Atmospheric Nitrogen Plasma on the Behavior of Mesenchymal Stem Cells Cultured on a Bone Scaffold Containing Iron Oxide-Loaded Silica Nanoparticles Catalyst. Int. J. Mol. Sci. 2020, 21, 4738. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Jamroz, P.; Nowak, P. Sterylizacja za pomocą niskotemperaturowej plazmy, generowanej w warunkach ciśnienia atmosferycznego. Postępy Mikrobiol. 2015, 54, 195–200. [Google Scholar]
- Laskowska, M.; Boguslawska-Was, E.; Kowal, P.; Holub, M.; Dabrowski, W. Skuteczność wykorzystania niskotemperaturowej plazmy w mikrobiologii i medycynie. Postępy Mikrobiologii 2016, 55, 172–181. [Google Scholar]
- Hana, L.; Patila, S.; Boehma, D.; Milosavljevića, V.; Cullena, P.J.; Bourkea, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma sterilization. Methods and mechanisms. Pure Appl. Chem. 2002, 74, 349. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Boehm, D.; Amias, E.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Atmospheric cold plasma interactions with modified atmosphere packaging inducer gases for safe food preservation. Innov. Food Sci. Emerg. Technol. 2016, 38, 384–392. [Google Scholar] [CrossRef] [Green Version]
- Min, S.C.; Roh, S.H.; Niemira, B.A.; Sites, J.E.; Boyd, G.; Lacombe, A. Dielectric barrier discharge atmospheric cold plasma inhibits O157:H7, and Tulane virus in Romaine lettuce. Int. J. Food. Microbiol. 2016, 237, 114–120. [Google Scholar] [CrossRef]
- Lai, A.C.K.; Cheung, A.C.T.; Wong, M.M.L.; Li, W.S. Evaluation of cold plasma inactivation efficacy against different airborne bacteria in ventilation duct flow. Build. Environ. 2016, 98, 39–46. [Google Scholar] [CrossRef]
- Hertwig, C.; Leslie, A.; Meneses, N.; Reineke, K.; Rauh, C.; Schlüter, O. Inactivation of Enteritidis PT30 on the surface of unpeeled almonds by cold plasma. Innov. Food Sci. Emerg. Technol. 2017, 44, 242–248. [Google Scholar] [CrossRef]
- Nasir, N.M.; Lee, B.K.; Yap, S.S.; Thong, K.L.; Yap, S.L. Cold plasma inactivation of chronic wound bacteria. Arch. Biochem. Biophys. 2016, 605, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Stepczyńska, M. Surface Modification by Low Temperature Plasma: Sterilization of Biodegradable Materials. Plasma Process. Polym. 2016, 13, 1080–1088. [Google Scholar] [CrossRef]
- Wielgosiński, G. Przegląd technologii termicznego przekształcania odpadów. Nowa Energ. 2001, 1, 55. [Google Scholar]
- Pawlat, J.; Inaba, T. Study on atmospheric pressure plasma reactor for biphenyl decomposition. IEEJ Trans. Power Energy 2003, 123, 844–851. [Google Scholar] [CrossRef] [Green Version]
- Pawłat, J.; Diatczyk, J.; Stryczewska, H.D. Low-temperature plasma for exhaust gas purification from paint shop—A case study. Przegląd Elektrotechniczny 2011, 87, 245–248. [Google Scholar]
- Gomez, E.; Rani, D.A.; Cheeseman, C.R.; Deegan, D.; Wise, M.; Boccaccini, A.R. Thermal plasma technology for the treatment of wastes: A critical review. J. Hazard. Mater. 2009, 161, 614–626. [Google Scholar] [CrossRef]
- Winnicki, T.; Tuźnik, P. Bezemisyjne technologie przetwarzania stałych odpadów komunalnych—Najkrótsza droga spełnienia trudnych wymogów unijnych. Czas. Inżynierii Lądowej Sr. Archit. 2013, 60, 223–237. [Google Scholar]
- Czernichowski, A.; Lesueur, H. Plasma Applications to Waste Treatment; First Annual INEL: Idaho Falls, ID, USA, 1991. [Google Scholar]
- Urashima, K.; Chang, J. Removal of volatile organic compounds from air streams and industrial flue gases by non-thermal plasma technology. IEEE Trans. Ind. Appl. 2000, 7, 602–614. [Google Scholar] [CrossRef]
- Oda, T. Non-thermal plasma processing for environmental protection: Decomposition of dilute VOCs in air. J. Electrostat. 2003, 57, 293–311. [Google Scholar] [CrossRef]
- Van Durme, J.; Dewulf, J.; Sysmans, W.; Leys, C.; Van Langenhove, H. Abatement and degradation pathways of toluene in indoor air by positive corona discharge. Chemosphere 2007, 68, 1821–1829. [Google Scholar] [CrossRef]
- Kim, H.; Prieto, G.; Takashima, K.; Katsura, S.; Mizuno, A. Performance evaluation of discharge plasma process for gaseous pollutant removal. J. Electrostat. 2002, 55, 25–41. [Google Scholar] [CrossRef]
- Kučerová, K.; Machala, Z.; Hensel, K. Transient Spark Discharge Generated in Various N2/O2 Gas Mixtures: Reactive Species in the Gas and Water and Their Antibacterial Effects. Plasma Chem. Plasma Process. 2020, 40, 749–773. [Google Scholar] [CrossRef]
- Liao, X.; Xiang, Q.; Liu, D.; Chen, S.; Ye, X.; Ding, T. Lethal and Sublethal Effect of a Dielectric Barrier Discharge Atmospheric Cold Plasma on Staphylococcus aureus. J. Food Prot. 2017, 80, 928–932. [Google Scholar] [CrossRef] [PubMed]
- Jacofsky, M.C.; Watson, G.A. Method and Apparatus for Cold Plasma Food Contact Surface Sanitation. U.S. Patent 9,295,280 B2, 29 March 2016. [Google Scholar]
- Dasan, B.; Onal-Ulusoy, B.; Pawlat, J.; Diatczyk, J.; Sen, Y.; Mutlu, M. A New and Simple Approach for Decontamination of Food Contact Surfaces with Gliding Arc Discharge Atmospheric Non-Thermal Plasma. Food Bioproc Tech. 2017, 10, 650–661. [Google Scholar] [CrossRef]
- Starek, A.; Pawłat, J.; Chudzik, B.; Kwiatkowski, M.; Terebun, P.; Sagan, A.; Andrejko, D. Evaluation of selected microbial and physicochemical parameters of fresh tomato juice after cold atmospheric pressure plasma treatment during refrigerated storage. Sci. Rep. 2019, 9, 8407. [Google Scholar] [CrossRef] [Green Version]
- Zemanek, J.; Wozniak, A.; Malinowski, M. The role and place of solid waste transfer station in the waste management system. Infrastruct. Ecol. Rural Areas Pan 2011, 11, 5–13. [Google Scholar]
- Liu, X.; Lendormi, T.; Lanoisellé, J. A Review of Hygienization Technology of Biowastes for Anaerobic Digestion: Effect on Pathogen Inactivation and Methane Production. Chem. Eng. Trans. 2018, 70, 529–534. [Google Scholar]
- Wolny-Koładka, K.; Malinowski, M.; Zukowski, W. Impact of Calcium Oxide on Hygienization and Self-Heating Prevention of Biologically Contaminated Polymer Materials. Materials 2020, 13, 4012. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Zukowski, W. Mixed Municipal Solid Waste Hygienisation for Refuse-Derived Fuel Production by Ozonation in the Novel Configuration Using Fluidized Bed and Horizontal Reactor. Waste Biomass Valor 2019, 10, 575–583. [Google Scholar] [CrossRef]
- Notes and Memoranda: Dr. Koch’s New Method of Pure Cultivation of Bacteria. J. Cell Sci. 1881, 2–21, 650–654.
- Koch, R. Sechster Bericht der deutschen wissenschaftlichen Commission zur Erforschung der Cholera. Dtsch. Med. Wochenscrift 1884, 10, 191–192. [Google Scholar]
- Basaran, P.; Basaran-Akgul, N.; Oksuz, L. Elimination of Aspergillus parasiticus from nut surface with low pressure cold plasma (LPCP) treatment. Food Microbiol. 2008, 25, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Ragni, L.; Berardinelli, A.; Vannini, L.; Montanari, C.; Sirri, F.; Guerzoni, M.E.; Guarnieri, A. Non-thermal atmospheric gas plasma device for surface decontamination of shell eggs. J. Food Eng. 2010, 100, 125–132. [Google Scholar] [CrossRef]
- Sato, T.; Fujioka, K.; Ramasamy, R.; Urayama, T.; Fujii, S. Sterilization efficacy of a coaxial microwave plasma flow at atmospheric pressure. IEEE Trans. Ind. Appl. 2006, 42, 399–404. [Google Scholar] [CrossRef]
- Miao, H.; Yun, G. The Effect of Air Plasma on Sterilization of Escherichia coli in Dielectric Barrier Discharge. Plasma Sci. Tech. 2012, 14, 735. [Google Scholar]
- Xiaohu, L.; Feng, H.; Ying, G.; Jing, Z.; Jianjun, S. Sterilization of Staphylococcus Aureus by an Atmospheric Non-Thermal Plasma Jet. Plasma Sci. Tech. 2013, 15, 439. [Google Scholar]
- Fernández, A.; Noriega, E.; Thompson, A. Inactivation of Salmonella enterica serovar Typhimurium on fresh produce by cold atmospheric gas plasma technology. Food Microbiol. 2013, 33, 24–29. [Google Scholar] [CrossRef]
- Ermolaeva, S.A.; Varfolomeev, A.F.; Chernukha, M.Y.; Yurov, D.S.; Vasiliev, M.M.; Kaminskaya, A.A.; Moisenovich, M.M.; Romanova, J.M.; Murashev, A.N. Bactericidal effects of non-thermal argon plasma in vitro, in biofilms and in the animal model of infected wounds. J. Med. Microbiol. 2011, 60, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Daeschlein, G.; Napp, M.; von Podewils, S.; Lutze, S.; Emmert, S.; Lange, A.; Klare, I.; Haase, H.; Gümbel, D. In Vitro Susceptibility of Multidrug Resistant Skin and Wound Pathogens against Low Temperature Atmospheric Pressure Plasma Jet (APPJ) and Dielectric Barrier Discharge Plasma (DBD). Plasma Process Polym. 2014, 11, 175–183. [Google Scholar] [CrossRef]
- Wolny-Koładka, K.; Malinowski, M.; Sikora, A.; Szymonik, K.; Pelczar, G.; Wawrzyniak-Turek, K. Effect of the Intensive Aerobic Biostabilization Phase on Selected Microbiological and Physicochemical Parameters of Wastes. Infrastrukt. Ekol. Teren. Wiej. 2016, 4, 1099–1115. [Google Scholar]
- Sung, S.-J.; Huh, J.-B.; Yun, M.-J.; Chang, B.M.W.; Jeong, C.-M.; Jeon, Y.-C. Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments. J. Adv. Prosthodont. 2013, 5, 2–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Apparent power drawn from power grid | 52 VA |
| RMS (Root-Mean-Squared) voltage (primary windings) | 230 V |
| RMS (Root-Mean-Squared) voltage (secondary windings) | 612 V |
| Max. voltage (secondary windings) | 3760 V |
| Frequency (primary and secondary windings of the transformer) | 50 Hz |
| No. | Treatment | Time [min] | Sample (15 g) | Temperature [°C] |
|---|---|---|---|---|
| 1. | Plasma + Air | 5 | I | 40.5 |
| 2. | Plasma + Air | 5 | II | 40.7 |
| 3. | Plasma + Air | 5 | III | 42.0 |
| 4. | Plasma + Air | 15 | I | 61.7 |
| 5. | Plasma + Air | 15 | II | 55.0 |
| 6. | Plasma + Air | 15 | III | 56.4 |
| 7. | Plasma + Air | 45 | I | 64.3 |
| 8. | Plasma + Air | 45 | II | 71.4 |
| 9. | Plasma + Air | 45 | III | 69.1 |
| 10. | Air | 5 | Control I | 22 |
| 11. | Air | 5 | Control I | 21 |
| 12. | Air | 5 | Control I | 21 |
| 13. | Ambient air | 45 | Control II | 71.5 |
| 14. | Ambient air | 45 | Control II | 71.5 |
| 15. | Ambient air | 45 | Control II | 71.5 |
| Time [min] | Av. Temperature [°C] |
|---|---|
| 5 | 41.1 |
| 15 | 57.7 |
| 45 | 68.3 |
| Air Flow Rate [dm3/h] | iBrid MX6 [ppm] | Eco Sensors A-21ZX [ppm] | Time [s] | Mode | ||
|---|---|---|---|---|---|---|
| NO2 | NO | CO | O3 | |||
| 480 | 0 | 11 ± 2 | 10 ± 1 | 0.10 ± 0.06 | 10 | With the sample |
| 480 | 0.5 ± 0.1 | 28 ± 3 | 19 ± 2 | 0.16 ± 0.04 | 120 | |
| 480 | 0.5 ± 0.1 | 35 ± 5 | 19 ± 5 | 0.20 ± 0.06 | 300 | |
| 480 | 0 | 12 ± 2 | 0 | 0.16 ± 0.04 | 10 | Without the sample |
| 480 | 0.4 ± 0.1 | 31 ± 4 | 2 ± 1 | 0.24 ± 0.02 | 120 | |
| 480 | 0.6 ± 0.1 | 39 ± 6 | 5 ± 2 | 0.31 ± 0.04 | 300 | |
| Sample | Plasma Treatment Time [min] | Vegetative Bacteria | Endospores | Mold Fungi | Staphylococcus spp. | E. coli | Salmonella spp. | E. faecalis | pH |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 5 | 4.12 ± 0.01 ab | 0.11 ± 0.01 ab | 1.39 ± 0.04 ab | 24.82 ± 0.06 ab | 0.54 ± 0.01 ab | 0.07 ± 0.01 ab | 4.11 ± 0.01 ab | 8.03 |
| 2. | 5 | 11.19 ± 0.03 ab | 0.19 ± 0.02 ab | 2.84 ± 0.01 ab | 27.92 ± 0.07 ab | 0.49 ± 0.01 ab | 0.02 ± 0.01 ab | 11.18 ± 0.01 ab | 7.71 |
| 3. | 5 | 6.28 ± 0.01 ab | 0.08 ± 0.01 a | 0.95 ± 0.05 ab | 24.38 ± 0.07 ab | 0.41 ± 0.01 ab | 0.01 ± 0.001 a | 6.29 ± 0.02 ab | 7.74 |
| 4. | 15 | 2.21 ± 0.03 a | 0.13 ± 0.03 a | 0.41 ± 0.01 a | 23.14 ± 0.05 a | 0.04 ± 0.01 a | 0.01 ± 0.001 a | 2.22 ± 0.02 a | 7.47 |
| 5. | 15 | 0.93 ± 0.01 a | 0.16 ± 0.03 a | 0.11 ± 0.02 a | 27.11 ± 0.04 ab | 0.05 ± 0.01 a | 0 ± 0 a | 0.94 ± 0.01 a | 7.41 |
| 6. | 15 | 0.61 ± 0.01 a | 0.07 ± 0.01 a | 0.09 ± 0.02 a | 24.87 ± 0.08 a | 0.08 ± 0.01 a | 0 ± 0 a | 0.61 ± 0.01 a | 7.29 |
| 7. | 45 | 0.49 ± 0.01 ab | 0.12 ± 0.01 ab | 0 ± 0 a | 0.09 ± 0.01 a | 0.01 ± 0.01 a | 0 ± 0 a | 0.49 ± 0.01 ab | 7.27 |
| 8. | 45 | 0.14 ± 0.01 ab | 0.03 ± 0.01 a | 0 ± 0 a | 0.12 ± 0.01 ab | 0 ± 0 a | 0 ± 0 a | 0.14 ± 0.01 a | 6.98 |
| 9. | 45 | 0.22 ± 0.01 ab | 0.02 ± 0.01 ab | 0 ± 0 a | 0.07 ± 0.01 a | 0 ± 0 a | 0 ± 0 a | 0.22 ± 0.01 a | 7.33 |
| 10. | Control I | 4.04 ± 0.02 a | 0.13 ± 0.02 a | 0.38 ± 0.02 a | 27.64 ± 0.09 ab | 0.18 ± 0.01 a | 0.03 ± 0.01 a | 4.04 ± 0.01 a | 7.31 |
| 11. | Control I | 3.82 ± 0.02 a | 0.15 ± 0.02 a | 0.41 ± 0.02 a | 25.53 ± 0.09 ab | 0.23 ± 0.01 a | 0.05 ± 0.01 a | 3.24 ± 0.01 a | 7.32 |
| 12. | Control I | 4.24 ± 0.02 a | 0.23 ± 0.02 a | 0.38 ± 0.02 a | 27.64 ± 0.09 ab | 0.19 ± 0.01 a | 0.07 ± 0.01 a | 3.94 ± 0.01 a | 7.34 |
| 13. | Control II | 3.14 ± 0.02 a | 0.13 ± 0.02 a | 0.17 ± 0.02 a | 18.84 ± 0.09 a | 0.21 ± 0.01 a | 0.06 ± 0.01 a | 2.14 ± 0.01 a | 7.84 |
| 14. | Control II | 3.58 ± 0.02 a | 0.18 ± 0.02 a | 0.21 ± 0.02 a | 15.23 ± 0.09 a | 0.33 ± 0.01 a | 0.07 ± 0.01 a | 2.34 ± 0.01 a | 7.54 |
| 15. | Control II | 3.08 ± 0.02 a | 0.15 ± 0.02 a | 0.25 ± 0.02 a | 16.48 ± 0.09 a | 0.34 ± 0.01 a | 0.04 ± 0.01 a | 2.58 ± 0.01 a | 7.61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawłat, J.; Terebun, P.; Kwiatkowski, M.; Wolny-Koładka, K. Possibility of Humid Municipal Wastes Hygienisation Using Gliding Arc Plasma Reactor. Water 2021, 13, 194. https://doi.org/10.3390/w13020194
Pawłat J, Terebun P, Kwiatkowski M, Wolny-Koładka K. Possibility of Humid Municipal Wastes Hygienisation Using Gliding Arc Plasma Reactor. Water. 2021; 13(2):194. https://doi.org/10.3390/w13020194
Chicago/Turabian StylePawłat, Joanna, Piotr Terebun, Michał Kwiatkowski, and Katarzyna Wolny-Koładka. 2021. "Possibility of Humid Municipal Wastes Hygienisation Using Gliding Arc Plasma Reactor" Water 13, no. 2: 194. https://doi.org/10.3390/w13020194





