Mesocosm Experiments at a Tunnelling Construction Site for Assessing Re-Use of Spoil Material as a By-Product
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soils and Foaming Agents
2.2. Soil Mesocosm Set-Up at the Construction Site
2.3. Chemicals and SLES Analyses
2.4. Ecotoxicological Tests
2.4.1. Vibrio fischeri Acute Toxicity Test
2.4.2. Lepidium sativum Seed Germination and Seedling Growth Tests
2.4.3. Eisenia fetida Acute and Chronic Tests
2.4.4. Danio rerio Acute Toxicity Test
2.5. Toxicity Test Battery Integrated Index
2.6. Statistical Analysis
3. Results
3.1. Pre-Screening of the Four Foaming Agents with V. fischeri
3.2. Soil Mesocosm Experiment at the Construction Site
3.2.1. Organic Carbon and Microbial Abundance, WHC, Soil Moisture, pH, Temperature
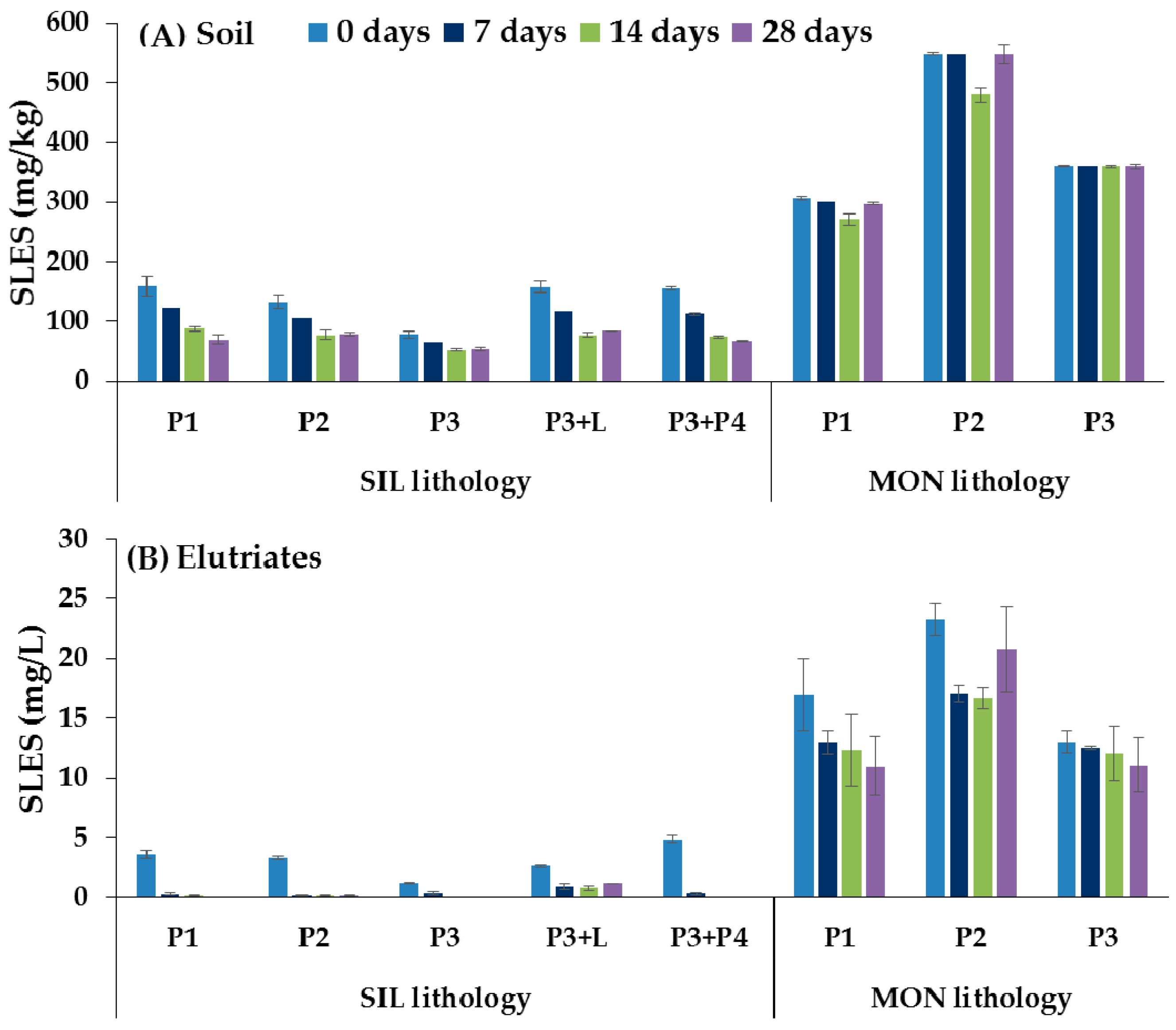
3.2.2. Analytical Determination of SLES in Soils and Elutriates
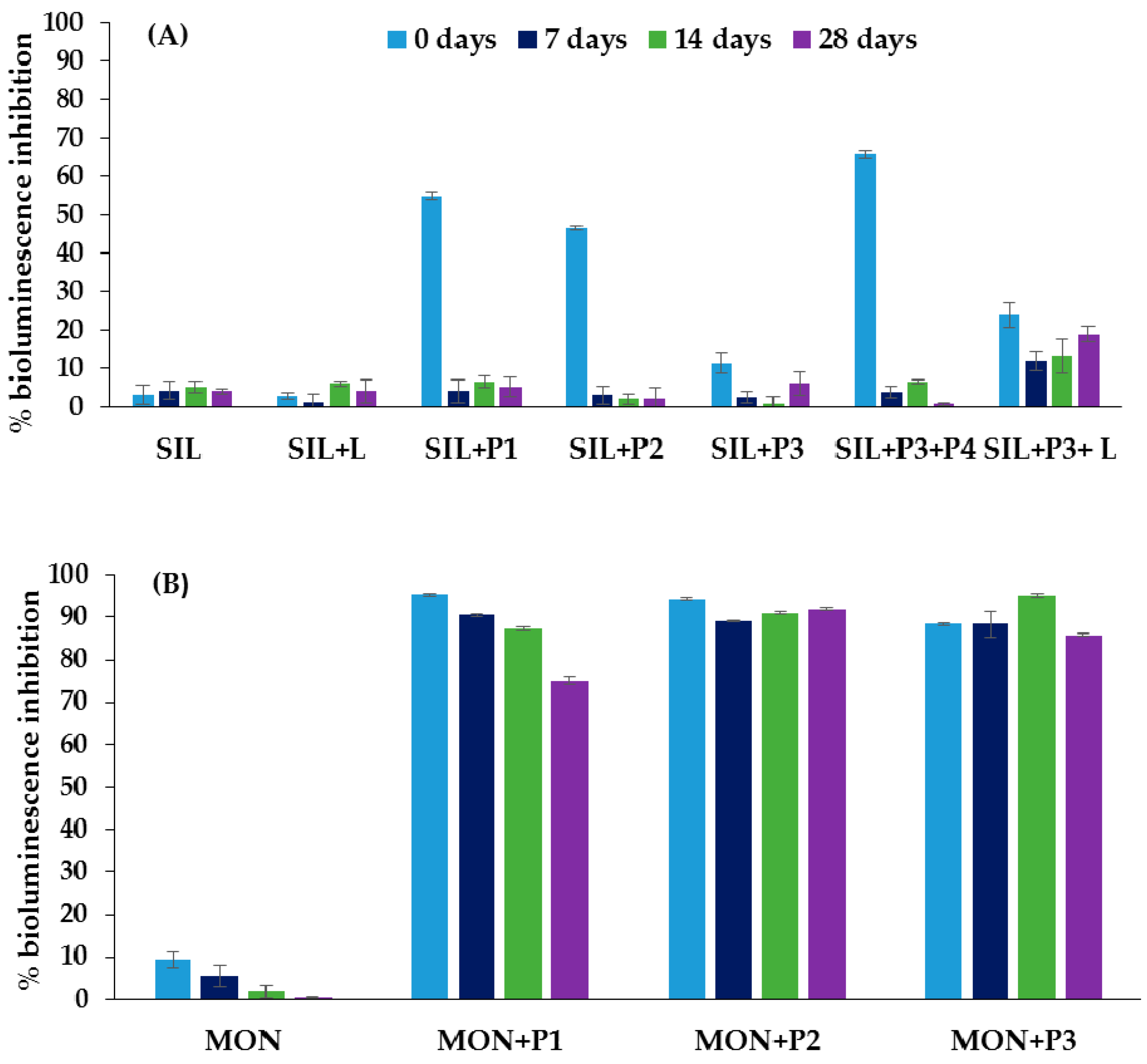
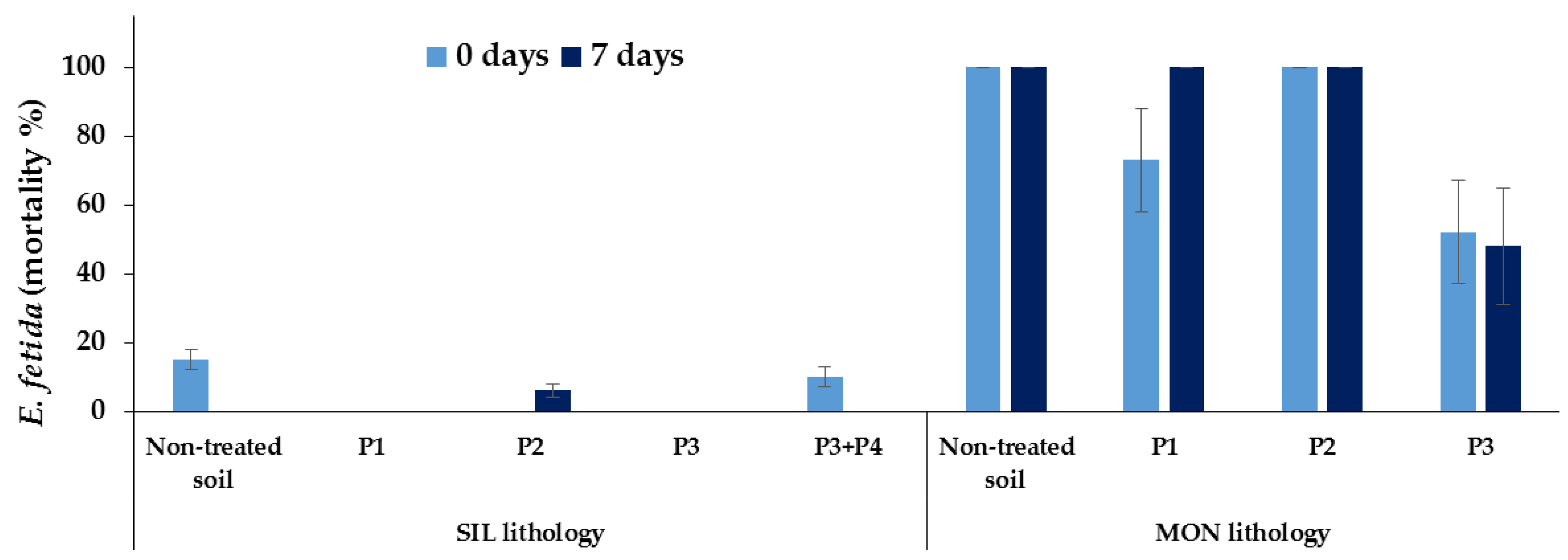
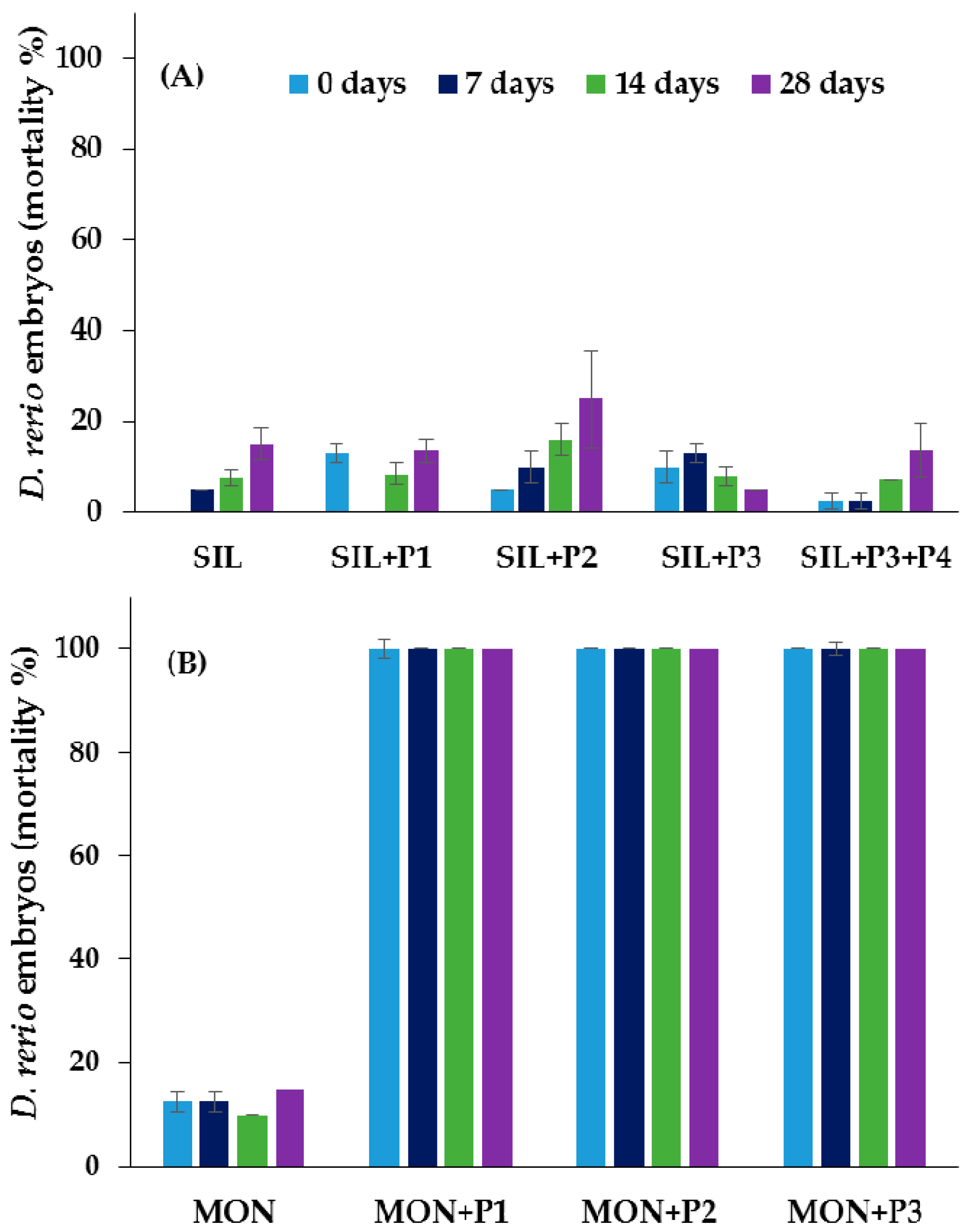
3.3. Ecotoxicological Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- EC. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. Closing the Loop—An EU Action Plan for the Circular Economy; COM(2015) 614 Final; European Commission: Brussels, Belgium, 2015; p. 21. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52015DC0614&from=EN (accessed on 10 November 2020).
- EC. Environment Action Programme to 2020. European Commission. Available online: https://ec.europa.eu/environment/action-programme (accessed on 18 June 2019).
- EC. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. A New Circular Economy Action Plan for a Cleaner and More Competitive Europe; COM(2020) 98 Final; European Commission: Brussels, Belgium, 2020; p. 20. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:52020DC0098&from=EN (accessed on 10 November 2020).
- Entacher, M.; Resch, D.; Reichel, P.; Galler, R. Recycling of tunnel spoil—Laws affecting waste from mining and tunnelling/Wiederverwertung von Tunnelausbruchmaterial—Abfallrecht im Berg- und Tunnelbau. Geomech. Tunn. 2011, 4, 692–701. [Google Scholar] [CrossRef]
- EU. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives (Text with EEA relevance). Off. J. Eur. Union 2008, 51, 312. [Google Scholar]
- Oggeri, C.; Fenoglio, T.M. Muck classification: Raw material or waste in tunnelling operation. Rev. Min. 2014, 20, 16–25. [Google Scholar]
- Oggeri, C.; Fenoglio, T.M.; Vinai, R. Tunnel spoil classification and applicability of lime addition in weak formations for muck reuse. Tunn. Undergr. Space Technol. 2014, 44, 97–107. [Google Scholar] [CrossRef]
- Oggeri, C.; Fenoglio, T.M.; Vinai, R. Tunnelling Muck Classification: Definition and Application. In Proceedings of the World Tunnel Congress 2017—Surface Challenges—Underground Solutions, Bergen, Norway, 9–15 June 2017; pp. 1–10. [Google Scholar]
- Magnusson, S.; Lundberg, K.; Svedberg, B.; Knutsson, S. Sustainable management of excavated soil and rock in urban areas—A literature review. J. Clean. Prod. 2015, 93, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Rahimzadeh, A.; Tang, W.; Sher, W.; Davis, P. Management of Excavated Material in Infrastructure Construction—A Critical Review of Literature. In Proceedings of the International Conference on Architecture and Civil Engineering, Sydney, Australia, 10–12 January 2018; pp. 1–7. [Google Scholar]
- Voit, K.; Kuschel, E. Rock material recycling in tunnel engineering. Appl. Sci. 2020, 10, 2722. [Google Scholar] [CrossRef] [Green Version]
- Bellopede, R.; Marini, P. Aggregates from tunnel muck treatments. Properties and uses. Physicochem. Probl. Miner. Process. 2011, 40, 259–266. [Google Scholar]
- Thames Tideway Tunnel. Excavated Materials Options Assessment (EMOA). 2003; p. 408. Available online: https://infrastructure.planninginspectorate.gov.uk/wp-content/ipc/uploads/projects/WW010001/WW010001-003342-9.10.03_Excavated_Materials_Options_Assessment_(EMOA)%20_Annex_D9_to_D16.pdf (accessed on 13 January 2020).
- D.P.R. 120/2017. Regulation on the simplified discipline of the excavated soil and rock management, pursuant to article 8 of the decree-law 12 September 2014, n. 133 (Decreto del Presidente della Repubblica 13 giugno 2017, n. 120. Regolamento recante la disciplina semplificata della gestione delle terre e rocce da scavo, ai sensi dell’articolo 8 del decreto-legge 12 settembre 2014, n. 133, convertito, con modificazioni, dalla legge 11 novembre 2014, n. 164. In Italian). Gazz. Uff. Repubb. Ital. Ser. Gen. 2017, 183, 1–40. Available online: https://www.gazzettaufficiale.it/eli/gu/2017/08/07/183/sg/pdf (accessed on 13 January 2020).
- D.M. 161/2012. Decree of the Ministry of the Environment and the Protection of the Territory and the Sea of 10 August 2012, n. 161 Regulation regulating the use of excavated soil and rocks (Regolamento recante la disciplina dell’utilizzazione delle terre e rocce da scavo. In Italian). Gazz. Uff. Repubb. Ital. Ser. Gen. 2012, 221, 1–34. Available online: http://www.arpa.fvg.it/export/sites/default/tema/rifiuti/allegati/DM_161-12.pdf (accessed on 13 January 2020).
- Mariani, L.; Grenni, P.; Barra Caracciolo, A.; Donati, E.; Rauseo, J.; Rolando, L.; Patrolecco, L. Toxic response of the bacterium Vibrio fischeri to sodium lauryl ether sulphate residues in excavated soils. Ecotoxicology 2020, 29, 815–824. [Google Scholar] [CrossRef]
- Grenni, P.; Barra Caracciolo, A.; Patrolecco, L.; Ademollo, N.; Rauseo, J.; Saccà, M.; Mingazzini, M.; Palumbo, M.; Galli, E.; Muzzini, V.; et al. A bioassay battery for the ecotoxicity assessment of soils conditioned with two different commercial foaming products. Ecotoxicol. Environ. Saf. 2018, 148, 1067–1077. [Google Scholar] [CrossRef]
- Peila, D. Soil conditioning for EPB shield tunnelling. KSCE J. Civ. Eng. 2014, 18, 831–836. [Google Scholar] [CrossRef]
- Peila, D.; Martinelli, D.; Todaro, C.; Luciani, A. Soil conditioning in EPB shield tunnelling—An overview of laboratory tests. Géoméch. Tunnelbau 2019, 12, 491–498. [Google Scholar] [CrossRef]
- Vinai, R.; Oggeri, C.; Peila, D. Soil conditioning of sand for EPB applications: A laboratory research. Tunn. Undergr. Space Technol. 2008, 23, 308–317. [Google Scholar] [CrossRef]
- Peila, D.; Picchio, A.; Martinelli, D.; Negro, E.D. Laboratory tests on soil conditioning of clayey soil. Acta Geotech. 2016, 11, 1061–1074. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, B.K.; Lee, K.H.; Lee, I.M. Soil conditioning of weathered granite soil used for EPB shield TBM: A laboratory scale study. KSCE J. Civ. Eng. 2019, 23, 1829–1838. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Ademollo, N.; Cardoni, M.; Di Giulio, A.; Grenni, P.; Pescatore, T.; Rauseo, J.; Patrolecco, L. Assessment of biodegradation of the anionic surfactant sodium lauryl ether sulphate used in two foaming agents for mechanized tunnelling excavation. J. Hazard. Mater. 2019, 365, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Padulosi, S.; Martelli, F.; Sciotti, A.; Putzu, D.F.D.; Filippone, M.; Mininni, G.; Martelli, A.; Sciotti, A.; Putzu, D.F.D.; Filippone, M.; et al. Environmental risk assessment of conditioned soil: Some Italian case studies. In Tunnels and Underground Cities. Engineering and Innovation Meet Archaeology, Architecture and Art: Proceedings of the WTC 2019 ITA-AITES World Tunnel Congress (WTC, Naples, 3–9 May 2019); Peila, D., Viggiani, G., Celestino, T., Eds.; CRC Press: London, UK, 2019; pp. 505–514. [Google Scholar]
- Finizio, A.; Patrolecco, L.; Grenni, P.; Galli, E.; Muzzini, V.; Rauseo, J.; Rizzi, C.; Barra Caracciolo, A. Environmental risk assessment of the anionic surfactant sodium lauryl ether sulphate in site-specific conditions arising from mechanized tunnelling. J. Hazard. Mater. 2020, 383, 121116. [Google Scholar] [CrossRef]
- Rolando, L.; Grenni, P.; Rauseo, J.; Pescatore, T.; Patrolecco, L.; Garbini, G.L.; Visca, A.; Barra Caracciolo, A. Isolation and characterization in a soil conditioned with foaming agents of a bacterial consortium able to degrade sodium lauryl ether sulfate. Front. Microbiol. 2020, 11, 1542. [Google Scholar] [CrossRef]
- Bakırel, T.; Keleş, O.; Karatas, S.; Özcan, M.; Türkmen, G.; Candan, A.; Bakırel, T.; Candan, A. Effect of linear alkylbenzene sulphonate (LAS) on non-specific defence mechanisms in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 2005, 71, 175–181. [Google Scholar] [CrossRef]
- Barra Caracciolo, A.; Cardoni, M.; Pescatore, T.; Patrolecco, L. Characteristics and environmental fate of the anionic surfactant sodium lauryl ether sulphate (SLES) used as the main component in foaming agents for mechanized tunnelling. Environ. Pollut. 2017, 226, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Jardak, K.; Drogui, P.; Daghrir, R. Surfactants in aquatic and terrestrial environment: Occurrence, behavior, and treatment processes. Environ. Sci. Pollut. Res. 2016, 23, 3195–3216. [Google Scholar] [CrossRef]
- Lechuga, M.; Fernández-Serrano, M.; Jurado, E.; Núñez-Olea, J.; Rios, F. Acute toxicity of anionic and non-ionic surfactants to aquatic organisms. Ecotoxicol. Environ. Saf. 2016, 125, 1–8. [Google Scholar] [CrossRef]
- Liwarska-Bizukojc, E.; Miksch, K.; Malachowska-Jutsz, A.; Kalka, J. Acute toxicity and genotoxicity of five selected anionic and nonionic surfactants. Chemosphere 2005, 58, 1249–1253. [Google Scholar] [CrossRef] [PubMed]
- ECHA. Alcohols, C12-14, Ethoxylated, Sulphates, Sodium Salts. European Chemicals Agency. Available online: https://echa.europa.eu/it/registration-dossier/-/registered-dossier/15887/6/1 (accessed on 13 January 2020).
- Budach, C.; Thewes, M. Application ranges of EPB shields in coarse ground based on laboratory research. Tunn. Undergr. Space Technol. 2015, 50, 296–304. [Google Scholar] [CrossRef]
- Peila, D.; Oggeri, C.; Vinai, R. Screw conveyor device for laboratory tests on conditioned soil for EPB tunneling operations. J. Geotech. Geoenviron. Eng. 2007, 133, 1622–1625. [Google Scholar] [CrossRef]
- UNI EN 12457-2:2004. Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 l/kg for Materials with Particle Size Below 4 mm (Without or With Size Reduction). 2004, p. 30. Available online: http://store.uni.com/catalogo/index.php/uni-en-12457-2-2004.html (accessed on 10 January 2020).
- AWWA 5540 C. Anionic Surfactants as MBAS. In Standard Methods for the Examination of Water and Wastewater; Rice, E.W., Baird, R.B., Eaton, A.D., Eds.; American Water Works Association/American Public Works Association/Water Environment Federation; American Public Health Association: Washington, DC, USA, 2012; pp. 53–55. [Google Scholar]
- Thompson, M.; Ellison, S.L.R.; Wood, R. Harmonized guidelines for single-laboratory validation of methods of analysis (IUPAC Technical Report). Pure Appl. Chem. 2002, 74, 835–855. [Google Scholar] [CrossRef]
- Firouzei, Y.; Grenni, P.; Barra Caracciolo, A.; Patrolecco, L.; Todaro, C.; Martinelli, D.; Carigi, A.; Hajipour, G.; Hassanpour, J.; Peila, D. The most common laboratory procedures for the evaluation of EPB TBMs excavated material ecotoxicity in Italy: A review. GEAM Geoing. Ambient. Min. 2020, 2, 44–56. [Google Scholar]
- UNI EN ISO 11348-3:2019. Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio Fischeri (Luminescent Bacteria Test)—Part 3: Method Using Freeze-Dried Bacteria. 2019, p. 44. Available online: http://store.uni.com/catalogo/uni-en-iso-11348-3-2019 (accessed on 10 January 2020).
- Persoone, G.; Marsalek, B.; Blinova, I.; Törökne, A.; Zarina, D.; Manusadzianas, L.; Nalecz-Jawecki, G.; Tofan, L.; Stepanova, N.; Tothova, L.; et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters. Environ. Toxicol. 2003, 18, 395–402. [Google Scholar] [CrossRef]
- Environment Canada. Guidance Document on Statistical Methods for Environmental Toxicity Tests; EPS 1/RM/46; Environmental Protection Series; Environmental Technology Centre, Environment Canada: Ottawa, ON, Canada, 2007; p. 283. Available online: http://publications.gc.ca/site/eng/278313/publication.html (accessed on 10 January 2020).
- US EPA. Ecological Effects Test Guidelines. OPPTS 850.4200, Seed Germination/Root Elongation Toxicity Test. Publication No. 96–154; US-EPA: Washington, DC, USA, 1996; p. 283.
- APAT. Proposal of a Technical Guidance on Analysis Methods for Soil and Contaminated Sites. Use of Ecotoxicological and Biological Indicators (Proposta di Guida Tecnica su Metodi di Analisi per il Suolo e i Siti Contaminati. Utilizzo di Indicatori Biologici ed ecotossicologici; APAT, Agenzia per la Protezione dell’Ambiente e per i servizi Tecnici: Rome, Italy, 2004; p. 160. Available online: https://www.isprambiente.gov.it/files/biodiversita/APAT_Guida_tecnica_indicatori_2004.pdf (accessed on 15 October 2019). (In Italian)
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef]
- Galli, E.; Muzzini, V.G.; Finizio, A.; Fumagalli, P.; Grenni, P.; Barra Caracciolo, A.; Rauseo, J.; Patrolecco, L. Ecotoxicity of foaming agent conditioned soils tested on two terrestrial organisms. Environ. Eng. Manag. J. 2019, 18, 1703–1710. [Google Scholar] [CrossRef]
- OECD. Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia Andrei); OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publisher: Paris, France, 2016; p. 21. [Google Scholar]
- OECD. Test No. 207: Earthworm, Acute Toxicity Tests; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 1984; p. 9. [Google Scholar]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013; p. 22. [Google Scholar]
- Wernersson, A.-S.; Carere, M.; Maggi, C.; Tusil, P.; Soldan, P.; James, A.; Sanchez, W.; Dulio, V.; Broeg, K.; Reifferscheid, G.; et al. The European technical report on aquatic effect-based monitoring tools under the water framework directive. Environ. Sci. Eur. 2015, 27, 7. [Google Scholar] [CrossRef] [Green Version]
- Cristiano, W.; Lacchetti, I.; Mancini, L.; Corti, M.; Di Domenico, K.; Di Paolo, C.; Hollert, H.; Carere, M. Promoting zebrafish embryo tool to identify the effects of chemicals in the context of Water Framework Directive monitoring and assessment. Microchem. J. 2019, 149, 104035. [Google Scholar] [CrossRef]
- Lammer, E.; Carr, G.J.; Wendler, K.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- Sobanska, M.A.; Scholz, S.; Nyman, A.-M.; Cesnaitis, R.; Alonso, S.G.; Klüver, N.; Kühne, R.; Tyle, H.; De Knecht, J.; Dang, Z.; et al. Applicability of the fish embryo acute toxicity (FET) test (OECD 236) in the regulatory context of Registration, Evaluation, Authorisation, and Restriction of Chemicals (REACH). Environ. Toxicol. Chem. 2018, 37, 657–670. [Google Scholar] [CrossRef]
- Joint Research Centre. EURL ECVAM RECOMMENDATION on the Zebrafish Embryo Acute Toxicity Test Method (ZFET) for Acute Aquatic Toxicity Testing; European Commission, Joint Research Centre, Institute for Health and Consumer Protection: Ispra, Italy, 2014; p. 37. Available online: https://publications.jrc.ec.europa.eu/repository/bitstream/JRC91098/eur%2026710_eurl%20ecvam%20zfet%20recommendation__online.pdf (accessed on 11 January 2019).
- Busquet, F.; Strecker, R.; Rawlings, J.M.; Belanger, S.E.; Braunbeck, T.; Carr, G.J.; Cenijn, P.H.; Fochtman, P.; Gourmelon, A.; Hübler, N.; et al. OECD validation study to assess intra- and inter-laboratory reproducibility of the zebrafish embryo toxicity test for acute aquatic toxicity testing. Regul. Toxicol. Pharmacol. 2014, 69, 496–511. [Google Scholar] [CrossRef]
- ISPRA. Ecotoxicological Bioassay Batteries for Sediments and Inland Waters (Batterie di Saggi Ecotossicologici per Sedimenti e Acque interne); ISPRA, Istituto Superiore per la Protezione e la Ricerca Ambientale, Manuali e linee guida 88/2013: Rome, Italy, 2013; p. 79. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/manuali-e-linee-guida/batterie-di-saggi-ecotossicologici-per-sedimenti-e-acque-interne (accessed on 15 July 2020).
- Manzo, S.; Schiavo, S.; Aleksi, P.; Tabaku, A. Application of a toxicity test battery integrated index for a first screening of the ecotoxicological threat posed by ports and harbors in the southern Adriatic Sea (Italy). Environ. Monit. Assess. 2014, 186, 7127–7139. [Google Scholar] [CrossRef]
- Hartwell, S.I. Demonstration of a toxicological risk ranking method to correlate measures of ambient toxicity and fish community diversity. Environ. Toxicol. Chem. 1997, 16, 361–371. [Google Scholar] [CrossRef]
- OECD. Test No. 208: Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2006; p. 21. [Google Scholar]
- UNI EN ISO 11268-2:2015. Soil Quality—Effects of Pollutants on Earthworms—Part 2: Determination of Effects on Reproduction of Eisenia Fetida/Eisenia Andrei; 2015; p. 20. Available online: http://store.uni.com/catalogo/uni-en-iso-11268-2-2015 (accessed on 10 January 2020).
- Patrolecco, L.; Pescatore, T.; Mariani, L.; Rolando, L.; Grenni, P.; Finizio, A.; Spataro, F.; Rauseo, J.; Ademollo, N.; Muzzini, V.G.; et al. Environmental fate and effects of foaming agents containing sodium lauryl ether sulphate in soil debris from mechanized tunneling. Water 2020, 12, 2074. [Google Scholar] [CrossRef]
- Giannakis, I.; Emmanouil, C.; Mitrakas, M.; Manakou, V.; Kungolos, A. Chemical and ecotoxicological assessment of sludge-based biosolids used for corn field fertilization. Environ. Sci. Pollut. Res. 2020, 1–13. [Google Scholar] [CrossRef]
- Malara, A.; Oleszczuk, P. Application of a battery of biotests for the determination of leachate toxicity to bacteria and invertebrates from sewage sludge-amended soil. Environ. Sci. Pollut. Res. 2012, 20, 3435–3446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abreu-Junior, C.H.; de Lima Brossi, M.J.; Monteiro, R.T.; Silveira Cardoso, P.H.; da Silva Mandu, T.; Nogueira, T.H.R.; Ganga, A.; Filzmoser, P.; Oliveira, F.C.; Pittol Firme, L.; et al. Effects of sewage sludge application on unfertile tropical soils evaluated by multiple approaches: A field experiment in a commercial Eucalyptus plantation. Sci. Total Environ. 2019, 655, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Barra Caracciolo, A.; Patrolecco, L. Site-specific protocols for evaluating environmental compatibility of spoil materials produced by EPB-TBMs. In Tunnels and Underground Cities: Engineering and Innovation meet Archaeology, Architecture and Art; Peila, D., Viggiani, G., Celestino, T., Eds.; CRC Press: London, UK, 2019; pp. 360–366. [Google Scholar]
- Garbarino, E.; Orveillon, G.; Saveyn, H.G.M.; Barthe, P.; Eder, P. Best Available Techniques (BAT) Reference Document for the Management of Waste from Extractive Industries, in Accordance with Directive 2006/21/EC; European Commission, Joint Research Centre, Directorate B—Growth and Innovation Circular Economy and Industrial Leadership; EUR 28963 EN; Publications Office of the European Union: Luxembourg, 2018; p. 72. Available online: https://op.europa.eu/it/publication-detail/-/publication/74b27c3c-0289-11e9-adde-01aa75ed71a1 (accessed on 10 January 2020). [CrossRef]

| Product | Density (g/cm3) | Main Components, CAS# and EC# | Component % | TR (L/m3) | |
|---|---|---|---|---|---|
| SIL | MON | ||||
| P1 | ~1 | Sodium lauryl ether sulphate (SLES) 9004-82-4; 618-398-5 | 10–30 | 0.53 | 1.46 |
| P2 | 1.35–1.45 | Alcohols, C12-14, ethoxylated, sulphates, sodium salts (SLES) 68891-38-3; 500-234-8 | 10–50 | 0.35 | 1.2 |
| P3 | 1.04 | (a) Alcohols, C12-14, ethoxylated, sulphates, sodium salts (SLES) 68891-38-3; 500-234-8 | 10–20 | 0.59 | 1.46 |
| (b) 1,2-benzisotiazol-3(2H)-one 2634-33-5; 220-120-9 | 0.005–0.01 | 0.44 * | |||
| P4 ** | 1.04 | Alcohols, C12-14, ethoxylated, sulphates, sodium salts (SLES) 68891-38-3; 500-234-8 | 25–30 | 0.165 | - |
| Soil Lithology | Foaming Agent | Lime | Mesocosm Acronym |
|---|---|---|---|
| SIL | P1 | - | SIL + P1 |
| P2 | - | SIL + P2 | |
| P3 | - | SIL + P3 | |
| P3 and P4 | - | SIL + P3 + P4 | |
| P3 | Yes | SIL + P3 + L | |
| - | - | SIL | |
| - | Yes | SIL + L | |
| MON | P1 | - | MON + P1 |
| P2 | - | MON + P2 | |
| P3 | - | MON + P3 | |
| - | - | MON |
| Product | EC20 (mg/L) | s.e. | EC50 (mg/L) | s.e. |
|---|---|---|---|---|
| P1 | 2.21 | 0.29 | 6.97 | 0.87 |
| P2 | 2.23 | 0.27 | 6.88 | 0.89 |
| P3 | 5.75 | 0.45 | 17.86 | 1.48 |
| P4 | 1.96 | 0.28 | 6.10 | 0.89 |
| P3 + P4 | 4.11 | 0.00 | 12.76 | 0.00 |
| 0 days | 7 days | 14 days | 28 days | |||||
|---|---|---|---|---|---|---|---|---|
| GI (%) | s.e. | GI (%) | s.e. | GI (%) | s.e. | GI (%) | s.e. | |
| SIL | 131.4 | 13.1 | 96.2 | 24.4 | 130.0 | 8.9 | 181.6 | 10.1 |
| SIL + L | 97.3 | 7.6 | 93.7 | 15.8 | 126.8 | 3.2 | 140.8 | 19.6 |
| SIL + P1 | 55.4 | 5.3 | 89.8 | 11.6 | 105.4 | 11.5 | 146.5 | 35.0 |
| SIL + P2 | 122.2 | 10.9 | 113.0 | 10.1 | 101.1 | 20.3 | 151 | 22.1 |
| SIL + P3 | 117.1 | 32.2 | 136.7 | 4.6 | 120.4 | 3.2 | 168.9 | 8.5 |
| SIL + P3 + P4 | 118.6 | 5.0 | 116.5 | 24.3 | 135.6 | 22.2 | 165.8 | 13.9 |
| SIL + P3 + L | 96.3 | 9.9 | 112.5 | 7.8 | 88.2 | 12.6 | 147.0 | 3.4 |
| MON | 83.3 | 14.2 | 92.5 | 23.4 | 117.6 | 3.0 | 155.6 | 12.3 |
| MON + P1 | 108.8 | 31.5 | 137.7 | 8.9 | 109.9 | 29.1 | 157.4 | 10.7 |
| MON + P2 | 109.3 | 14.2 | 106.9 | 17.0 | 106.1 | 16.2 | 155.2 | 11.4 |
| MON + P3 | 125.7 | 4.3 | 132.6 | 13.7 | 118.5 | 2.5 | 174.2 | 6.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barra Caracciolo, A.; Grenni, P.; Mariani, L.; Rauseo, J.; Di Lenola, M.; Muzzini, V.G.; Donati, E.; Lacchetti, I.; Gucci, P.M.B.; Finizio, A.; et al. Mesocosm Experiments at a Tunnelling Construction Site for Assessing Re-Use of Spoil Material as a By-Product. Water 2021, 13, 161. https://doi.org/10.3390/w13020161
Barra Caracciolo A, Grenni P, Mariani L, Rauseo J, Di Lenola M, Muzzini VG, Donati E, Lacchetti I, Gucci PMB, Finizio A, et al. Mesocosm Experiments at a Tunnelling Construction Site for Assessing Re-Use of Spoil Material as a By-Product. Water. 2021; 13(2):161. https://doi.org/10.3390/w13020161
Chicago/Turabian StyleBarra Caracciolo, Anna, Paola Grenni, Livia Mariani, Jasmin Rauseo, Martina Di Lenola, Valerio Giorgio Muzzini, Enrica Donati, Ines Lacchetti, Paola Margherita Bianca Gucci, Antonio Finizio, and et al. 2021. "Mesocosm Experiments at a Tunnelling Construction Site for Assessing Re-Use of Spoil Material as a By-Product" Water 13, no. 2: 161. https://doi.org/10.3390/w13020161








