Impact of Green Roofs and Vertical Greenery Systems on Surface Runoff Quality
Abstract
:1. Introduction
Literature on Green Roofs and Vertical Greening Systems
- Providing an overview of GR and VGS systems, the materials used, and the potential pollutants associated with these materials (Section 2);
- Assessing the impact of greenery systems on the quality of urban runoff and identifying environmentally relevant pollutants in their runoff (Section 3);
- Identifying mitigation strategies for the circulation of these pollutants in urban areas (Section 6).
2. Green Building Systems
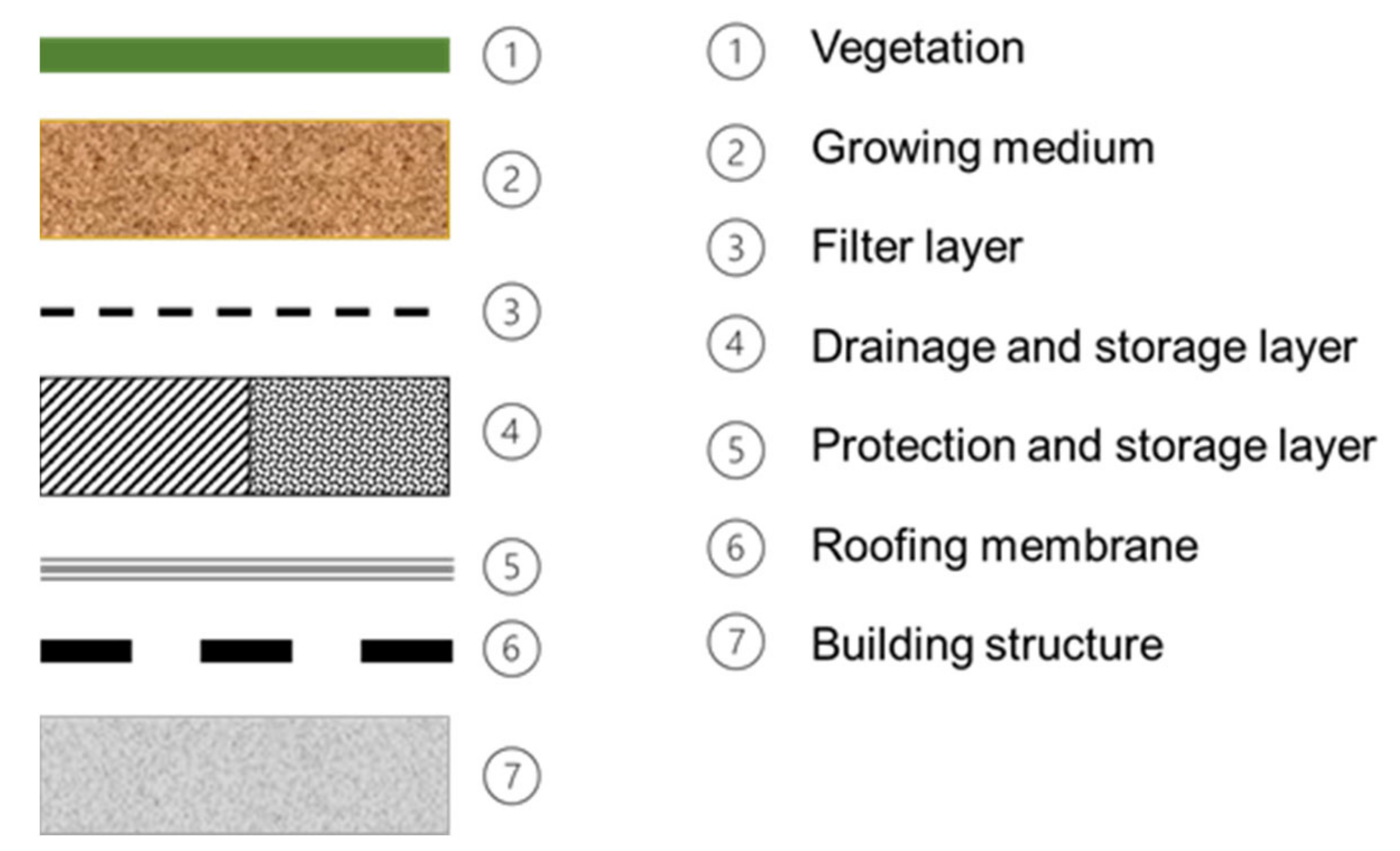
2.1. Green Roofs
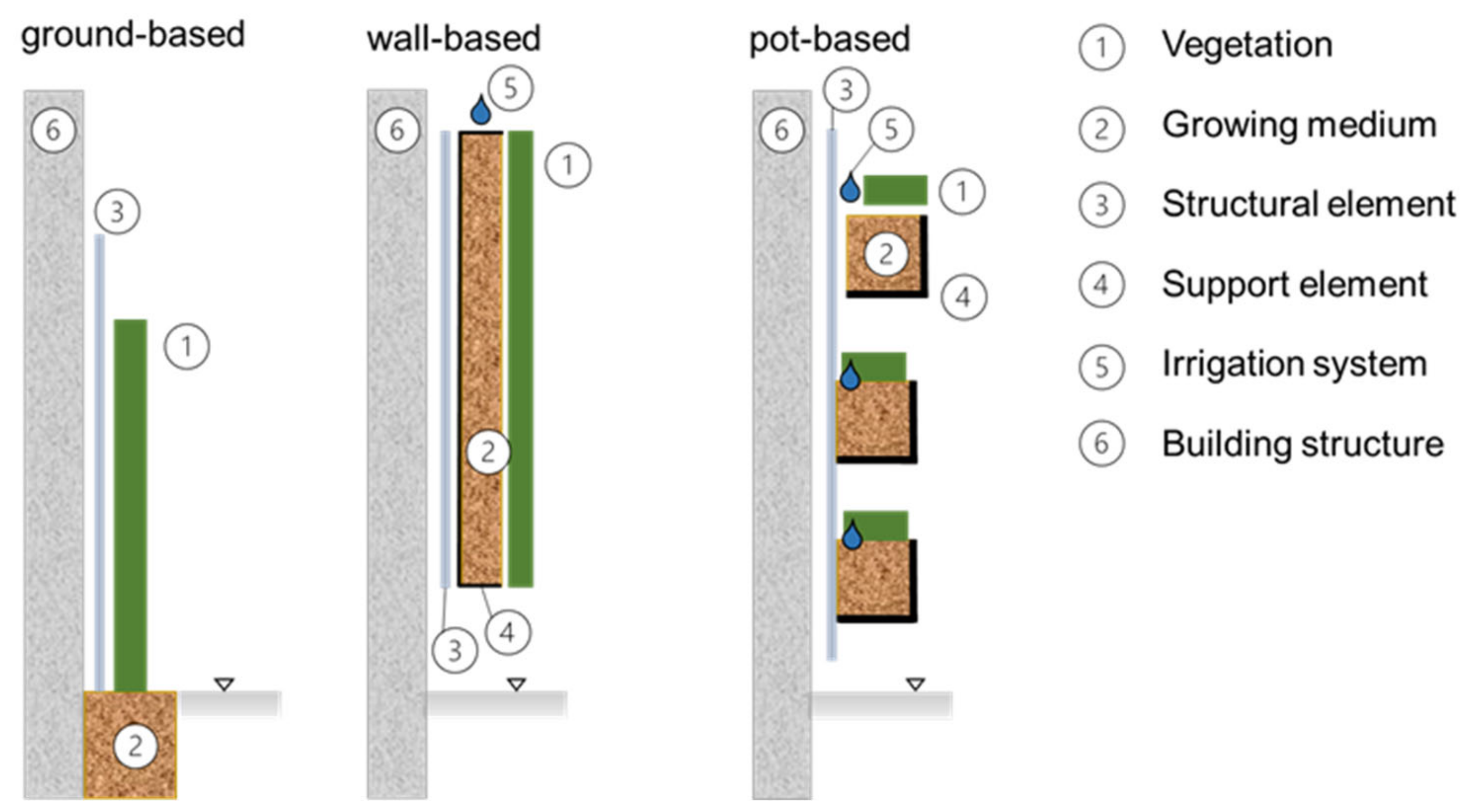
2.2. Vertical Greenery Systems (VGSs)
3. Green Buildings and Runoff Quality
- Metals, which are released from metallic building constructions (mostly aluminium and steel);
- Additives, which represent chemicals used for a certain purpose in GRs and/or VGSs. These can be substances applied during the construction of the green element for various purposes (biocides, herbicides, and flame retardants) and others used to improve certain properties of construction materials (plasticizers, vulcanization accelerators, etc.); and
- Nutrients, which are mainly used as fertilizers in GRs and VGSs.
3.1. Metals
3.2. Additives
3.2.1. Biocides/Herbicides
3.2.2. Plasticizers
3.2.3. Flame Retardants
3.3. Nutrients
4. Pollutants’ Environmental Fate
5. Environmental Risk Potential
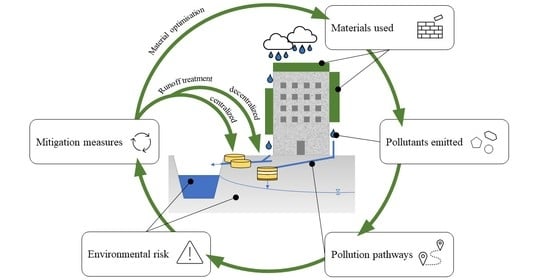
6. Mitigation Strategies
- Mitigation at the source, through:
- ◦
- optimization of construction practices of green elements and/or materials used; and
- ◦
- use of decentralized treatments; and
- Conventional mitigation—centralized treatment in stormwater TPs and WWTPs
6.1. Mitigation at the Source—Optimization of Construction Practices and Materials Used
6.2. Mitigation at the Source—Decentralized Treatment Options
6.3. Mitigation at the End-of-Pipe—Conventional Centralized Treatment Options
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lavell, A.; Oppenheimer, M.; Diop, C.; Hess, J.; Lempert, R.; Li, J.; Muir-Wood, R.; Myeong, S.; Moser, S.; Takeuchi, K.; et al. Climate Change: New Dimensions in Disaster Risk, Exposure, Vulnerability, and Resilience: A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC). In Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation: Special Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2012; pp. 25–64. [Google Scholar] [CrossRef]
- Oke, T.R. The energetic basis of the urban heat island. Q. J. R. Meteorol. Soc. 1982, 108, 1–24. [Google Scholar] [CrossRef]
- Vicedo-Cabrera, A.M.; Scovronick, N.; Sera, F.; Royé, D.; Schneider, R.; Tobias, A.; Astrom, C.; Guo, Y.; Honda, Y.; Hondula, D.M.; et al. The burden of heat-related mortality attributable to recent human-induced climate change. Nat. Clim. Chang. 2021, 11, 492–500. [Google Scholar] [CrossRef]
- Pearlmutter, D.; Pucher, B.; Calheiros, C.S.C.; Hoffmann, K.A.; Aicher, A.; Pinho, P.; Stracqualursi, A.; Korolova, A.; Pobric, A.; Galvão, A.; et al. Closing Water Cycles in the Built Environment through Nature-Based Solutions: The Contribution of Vertical Greening Systems and Green Roofs. Water. 2021, 13, 2165. [Google Scholar] [CrossRef]
- Fletcher, T.D.; Shuster, W.; Hunt, W.F.; Ashley, R.; Butler, D.; Arthur, S.; Trowsdale, S.; Barraud, S.; Semadeni-Davies, A.; Bertrand-Krajewski, J.-L.; et al. SUDS, LID, BMPs, WSUD and more—The evolution and application of terminology surrounding urban drainage. Urban. Water J. 2015, 12, 525–542. [Google Scholar] [CrossRef]
- Yin, D.; Chen, Y.; Jia, H.; Wang, Q.; Chen, Z.; Xu, C.; Li, Q.; Wang, W.; Yang, Y.; Fu, G.; et al. Sponge city practice in China: A review of construction, assessment, operational and maintenance. J. Clean. Prod. 2021, 280, 124963. [Google Scholar] [CrossRef]
- Simperler, L.; Ertl, T.; Matzinger, A. Spatial Compatibility of Implementing Nature-Based Solutions for Reducing Urban Heat Islands and Stormwater Pollution. Sustainability. 2020, 12, 5967. [Google Scholar] [CrossRef]
- Langergraber, G.; Pucher, B.; Simperler, L.; Kisser, J.; Katsou, E.; Buehler, D.; Mateo, M.C.G.; Atanasova, N. Implementing nature-based solutions for creating a resourceful circular city. Blue-Green Syst. 2020, 2, 173–185. [Google Scholar] [CrossRef]
- Atanasova, N.; Castellar, J.A.; Pineda-Martos, R.; Nika, C.E.; Katsou, E.; Istenič, D.; Pucher, B.; Andreucci, M.B.; Langergraber, G. Nature-Based Solutions and Circularity in Cities. Circ. Econ. Sustain. 2021, 1, 319–332. [Google Scholar] [CrossRef]
- Gräf, M.; Immitzer, M.; Hietz, P.; Stangl, R. Water-Stressed Plants Do Not Cool: Leaf Surface Temperature of Living Wall Plants under Drought Stress. Sustainability. 2021, 13, 3910. [Google Scholar] [CrossRef]
- Allabashi, R.; Haile, T.M.; Fuerhacker, M.; Pitha, U.; Scharf, B.; Stach, W.; Ziegenbalg, F.; Heidinger, S.; Ertl, T. Simultaneous removal of heavy metals from synthetic storm water using sustainable urban drainage systems. Urban. Water J. 2019, 16, 444–450. [Google Scholar] [CrossRef]
- Blecken, G.-T.; Marsalek, J.; Viklander, M. Laboratory Study of Stormwater Biofiltration in Low Temperatures: Total and Dissolved Metal Removals and Fates. Water Air Soil Pollut. 2011, 219, 303–317. [Google Scholar] [CrossRef]
- Hatt, B.E.; Fletcher, T.D.; Deletic, A. Hydraulic and pollutant removal performance of stormwater filters under variable wetting and drying regimes. Water Sci. Technol. 2007, 56, 11–19. [Google Scholar] [CrossRef]
- Hatt, B.E.; Steinel, A.; Deletic, A.; Fletcher, T.D. Retention of heavy metals by stormwater filtration systems: Breakthrough analysis. Water Sci. Technol. 2011, 64, 1913–1919. [Google Scholar] [CrossRef]
- Alsup, S.; Ebbs, S.; Battaglia, L.; Retzlaff, W. Green Roof Systems as Sources or Sinks Influencing Heavy Metal Concentrations in Run. off. J. Environ. Eng. 2013. [Google Scholar] [CrossRef]
- Paijens, C.; Bressy, A.; Frère, B.; Moilleron, R. Biocide emissions from building materials during wet weather: Identification of substances, mechanism of release and transfer to the aquatic environment. Environ. Sci. Pollut. Res. Int. 2020, 27, 3768–3791. [Google Scholar] [CrossRef]
- Alsup, S.; Ebbs, S.; Retzlaff, W. The exchangeability and leachability of metals from select green roof growth substrates. Urban. Ecosyst. 2010, 13, 91–111. [Google Scholar] [CrossRef]
- Pearlmutter, D.; Theochari, D.; Nehls, T.; Pinho, P.; Piro, P.; Korolova, A.; Papaefthimiou, S.; Mateo, M.C.G.; Calheiros, C.; Zluwa, I.; et al. Enhancing the circular economy with nature-based solutions in the built urban environment: Green building materials, systems and sites. Blue-Green Syst. 2020, 2, 46–72. [Google Scholar] [CrossRef] [Green Version]
- Castellar, J.A.C.; Popartan, L.A.; Pueyo-Ros, J.; Atanasova, N.; Langergraber, G.; Säumel, I.; Corominas, L.; Comas, J.; Acuña, V. Nature-based solutions in the urban context: Terminology, classification and scoring for urban challenges and ecosystem services. Sci. Total Environ. 2021, 779, 146237. [Google Scholar] [CrossRef]
- Langergraber, G.; Castellar, J.A.; Pucher, B.; Baganz, G.F.; Milosevic, D.; Andreucci, M.B.; Kearney, K.; Pineda-Martos, R.; Atanasova, N. A Framework for Addressing Circularity Challenges in Cities with Nature-based Solutions. Water. 2021, 17, 2355. [Google Scholar] [CrossRef]
- Özyavuz, M.; Karakaya, B.; Ertin, D.G. The Effects of Green Roofs on Urban Ecosystems. Conference: Green Age Symp. 2015, 1–9. Available online: https://www.researchgate.net/publication/315792406_The_Effects_of_Green_Roofs_on_Urban_Ecosystems (accessed on 1 September 2021).
- Weiler, S.; Scholz-Barth, K. Green Roof Systems: A Guide to the Planning Design and Construction of Landscapes over Structure; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 92–93. [Google Scholar]
- Grant, G.; Gedge, D. Living roofs and walls from policy to practice: 10 years of urban greening in London and beyond. In Report of the European Federation of Green Roof and Green Wall Associations (EFB) and Livingroofs. org on Behalf of the Greater London Authority; Blanche Cameron of The Bartlett, University College London (UCL): London, UK, 2019. [Google Scholar]
- Manso, M.; Castro-Gomes, J. Green wall systems: A review of their characteristics. Renew. Sustain. Energy Rev. 2015, 41, 863–871. [Google Scholar] [CrossRef]
- Ottelé, M.; Perini, K.; Fraaij, A.; Haas, E.M.; Raiteri, R. Comparative life cycle analysis for green façades and living wall systems. Energy Build. 2011, 43, 3419–3429. [Google Scholar] [CrossRef]
- Cortês, A.; Almeida, J.; de Brito, J.; Tadeu, A. Water retention and drainage capability of expanded cork agglomerate boards intended for application in green vertical systems. Constr. Build. Mater. 2019, 224, 439–446. [Google Scholar] [CrossRef]
- Oquendo-Di Cosola, V.; Olivieri, F.; Ruiz-García, L.; Bacenetti, J. An environmental Life Cycle Assessment of Living Wall Systems. J. Environ. Manag. 2020, 254, 109743. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, M.; Rahimi, M.; Sen, S.; Mackenzie, N.; Imanbayev, Y. Living wall systems: Evaluating life-cycle energy, water and carbon impacts. Urban Ecosyst. 2015, 18, 1–11. [Google Scholar] [CrossRef]
- Pucher, B.; ZluwA, I. Entwicklung Eines Multifunktionalen Living-Wall-Systems zur Reinigung und Nutzung von Grauwasser; Wasser und Abfall: Hennef, Germany, 2020. [Google Scholar] [CrossRef]
- Boano, F.; Caruso, A.; Costamagna, E.; Ridolfi, L.; Fiore, S.; Demichelis, F.; Galvão, A.; Pisoeiro, J.; Rizzo, A.; Masi, F. A review of nature-based solutions for greywater treatment: Applications, hydraulic design, and environmental benefits. Sci. Total Environ. 2020, 711, 134731. [Google Scholar] [CrossRef]
- Burkhardt, M.; Rohr, M.; Heisterkamp, I.; Gartiser, S. niederschlahswasser von kunststoffdachbahnen_auslaugung von stoffen und deren ökotoxizität für aquatische organismen. Korresp. Wasserwirtsch. 2020, 13, 418–424. [Google Scholar]
- Bandow, N.; Gartiser, S.; Ilvonen, O.; Schoknecht, U. Evaluation of the impact of construction products on the environment by leaching of possibly hazardous substances. Environ. Sci. Eur. 2018, 30, 14. [Google Scholar] [CrossRef] [Green Version]
- Rasul, M.G.; Arutla, L. Environmental impact assessment of green roofs using life cycle assessment. Energy Rep. 2020, 6, 503–508. [Google Scholar] [CrossRef]
- Gromaire, M.C.; Bretaudeau, K.L.; Bret, C.M.; Caupos, E.; Seidl, M. Organic Micropollutants in Roof Runoff—A Study of the Emission/Retention Potential of Green Roofs. In Proceedings of the 13th International Conference on Urban Drainage, Kuching, Sarawak, 7–12 September 2014; p. 2516832. [Google Scholar]
- Winters, N.L.; Graunke, K. Roofing Materials Assessment: Investigation of Toxic Chemicals in Roof Runoff; Washington State Department of Ecology Olympia: Olympia, WA, USA, 2014. [Google Scholar]
- Gromaire, M.C.; Van De Voorde, A.; Lorgeoux, C.; Chebbo, G. Benzalkonium runoff from roofs treated with biocide products - In situ pilot-scale study. Water Res. 2015, 81, 279–287. [Google Scholar] [CrossRef]
- EFRA. Frequently Asked Questions on Flame Retardants: How do They Work? EFRA: Brussels, Belgium, 2011. [Google Scholar]
- Ghisari, M.; Bonefeld-Jorgensen, E.C. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol. Lett. 2009, 189, 67–77. [Google Scholar] [CrossRef]
- Togerö, Å. Leaching of Hazardous Substances from Additives and Admixtures in Concrete. Environ. Eng. Sci. 2006, 23, 102–117. [Google Scholar] [CrossRef]
- Müller, A.; Österlund, H.; Nordqvist, K.; Marsalek, J.; Viklander, M. Building surface materials as sources of micropollutants in building runoff: A pilot study. Sci. Total Environ. 2019, 680, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Gerzhova, N.; Blanchet, P.; Dagenais, C.; Ménard, S.; Côté, J. Flammability Characteristics of Green Roofs. Buildings. 2020, 10, 126. [Google Scholar] [CrossRef]
- Capolupo, M.; Sørensen, L.; Jayasena, K.D.R.; Booth, A.M.; Fabbri, E. Chemical composition and ecotoxicity of plastic and car tire rubber leachates to aquatic organisms. Water Res. 2020, 169, 115270. [Google Scholar] [CrossRef]
- Morau, D.; Rabarison, T.; Rakotondramiarana, H. Life Cycle Analysis of Green Roof Implemented in a Global South Low-income Country. Br. J. Environ. Clim. Chang. 2017, 7, 43–55. [Google Scholar] [CrossRef] [Green Version]
- Kotsiris, G.; Androutsopoulos, A.; Polychroni, E.; Souliotis, M.; Kavga, A. Carbon footprint of green roof installation on school buildings in Greek Mediterranean climatic region. Int. J. Sustain. Energy. 2019, 38, 866–883. [Google Scholar] [CrossRef]
- Bianchini, F.; Hewage, K. How “green” are the green roofs? Lifecycle analysis of green roof materials. Build Environ. 2012, 48, 57–65. [Google Scholar] [CrossRef]
- Manso, M.; Castro-Gomes, J.; Paulo, B.; Bentes, I.; Teixeira, C.A. Life cycle analysis of a new modular greening system. Sci. Total Environ. 2018, 627, 1146–1153. [Google Scholar] [CrossRef]
- Koura, J.; Manneh, R.; Belarbi, R.; El Khoury, V.; El Bachawati, M. Comparative cradle to grave environmental life cycle assessment of traditional and extensive vegetative roofs: An application for the Lebanese context. Int. J. Life Cycle Assess. 2020, 25, 423–442. [Google Scholar] [CrossRef]
- Rider, C.V.; Janardhan, K.S.; Rao, D.; Morrison, J.P.; McPherson, C.A.; Harry, G.J. Evaluation of N-butylbenzenesulfonamide (NBBS) neurotoxicity in Sprague-Dawley male rats following 27-day oral exposure. Neurotoxicology. 2012, 33, 1528–1535. [Google Scholar] [CrossRef] [Green Version]
- Chenani, S.B.; Lehvävirta, S.; Häkkinen, T. Life cycle assessment of layers of green roofs. J. Clean. Prod. 2015, 90, 153–162. [Google Scholar] [CrossRef]
- Maqbool, A.; Ahmas, B.S. Leaching of styrene and other aromatic compounds in drinking water from PS bottles. J. Environ. Sci. 2007, 19, 421–426. [Google Scholar]
- Karczmarczyk, A.; Baryła, A.; Fronczyk, J.; Bus, A.; Mosiej, J. Phosphorus and Metals Leaching from Green Roof Substrates and Aggregates Used in Their Composition. Minerals. 2020, 10, 112. [Google Scholar] [CrossRef] [Green Version]
- Alsup, S.E.; Ebbs, S.D.; Battaglia, L.L.; Retzlaff, W.A. Heavy metals in leachate from simulated green roof systems. Ecol. Eng. 2011, 37, 1709–1717. [Google Scholar] [CrossRef]
- El Bachawati, M.; Manneh, R.; Belarbi, R.; Dandres, T.; Nassab, C.; El Zakhem, H. Cradle-to-gate Life Cycle Assessment of traditional gravel ballasted, white reflective, and vegetative roofs: A Lebanese case study: Cradle-to-gate Life Cycle Assessment of traditional gravel ballasted, white reflective, and vegetative roofs: A Lebanese case study. J. Clean. Prod. 2016, 137, 10. [Google Scholar]
- Burkhardt, M.; Kupper, T.; Hean, S.; Haag, R.; Schmid, P.; Kohler, M.; Boller, M. Biocides used in building materials and their leaching behavior to sewer systems. Water Sci. Technol. 2007, 56, 63–67. [Google Scholar] [CrossRef]
- Berndtsson, J.C.; Bengtsson, L.; Jinno, K. Runoff water quality from intensive and extensive vegetated roofs. Ecol. Eng. 2009, 35, 369–380. [Google Scholar] [CrossRef]
- Speak, A.F.; Rothwell, J.J.; Lindley, S.J.; Smith, C.L. Metal and nutrient dynamics on an aged intensive green roof. Environ. Pollut. 2014, 184, 33–43. [Google Scholar] [CrossRef]
- Hashemi, S.S.; Mahmud, H.B.; Ashraf, M.A. Performance of green roofs with respect to water quality and reduction of energy consumption in tropics: A review. Renew. Sustain. Energy Rev. 2015, 52, 669–679. [Google Scholar] [CrossRef]
- Li, Y.; Babcock, R.W. Green roofs against pollution and climate change. A review. Agron. Sustain. Dev. 2014, 34, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Qin, J.; Hu, Y. Are green roofs a source or sink of runoff pollutants? Ecol. Eng. 2017, 107, 65–70. [Google Scholar] [CrossRef]
- Sainte, P. Contribution des Matériaux de Couverture à la Contamination Métallique des Eaux de Ruissellement. Ph.D. Thesis, Université Paris-Est, Paris, France, 2009. [Google Scholar]
- Berndtsson, J.C.; Emilsson, T.; Bengtsson, L. The influence of extensive vegetated roofs on runoff water quality. Sci. Total Environ. 2006, 355, 48–63. [Google Scholar] [CrossRef]
- Berndtsson, J.C. Green roof performance towards management of runoff water quantity and quality: A review. Ecol. Eng. 2010, 36, 351–360. [Google Scholar] [CrossRef]
- Clark, S.E.; Lalor, M.M.; Pitt, R.; Field, R. Contribution of commonly used building materials to wet weather pollution. Proc. Water Environ. Fed. 2003, 12, 804–830. [Google Scholar] [CrossRef]
- Gromaire, M.C.; GARNAUD, S.; Saad, M.; Chebbo, G. Contribution of Different Sources to THE Pollution of Wet Weather Flows in Combined Sewers. Water Res. 2001, 35, 521–533. [Google Scholar] [CrossRef]
- Robert-Sainte, P.; Gromaire, M.C.; de Gouvello, B.; Saad, M.; Chebbo, G. Annual metallic flows in roof runoff from different materials: Test-bed scale in Paris conurbation. Environ. Sci. Technol. 2009, 43, 5612–5618. [Google Scholar] [CrossRef]
- Clark, S.E.; Steele, K.A.; Spicher, J.; Siu, C.Y.; Lalor, M.M.; Pitt, R.; Kirby, J.T. Roofing Materials’ Contributions to Storm-Water Runoff Pollution. J. Irrig. Drain Eng. 2008, 134, 638–645. [Google Scholar] [CrossRef] [Green Version]
- McIntyre, J.K.; Winters, N.; Rozmyn, L.; Haskins, T.; Stark, J.D. Metals leaching from common residential and commercial roofing materials across four years of weathering and implications for environmental loading. Environ. Pollut. 2019, 255, 113262. [Google Scholar] [CrossRef]
- Winters, N.; Granuke, K.; McCall, M. Roofing Materials Assessment: Investigation of Five Metals in Runoff from Roofing Materials. Water Environ. Res. 2015, 87, 835–848. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Joshi, U.M. Can green roof act as a sink for contaminants? A methodological study to evaluate runoff quality from green roofs. Environ. Pollut. 2014, 194, 121–129. [Google Scholar] [CrossRef]
- USEPA. In National Recommended Water Quality Criteria–Correction; EPA 822-Z-99-001; US Environmental Protection Agency, Office of Water, S.W: Washington, DC, USA, 1999.
- EC. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off. J. Eur. Union. L 226/1-16. 2013. [Google Scholar]
- Giacomello, E. Green Roofs, Facades, and Vegetative Systems. Chapter 3: Preliminary considerations: Safety Aspects in the Standards, 1st ed.; Butterworth-Heinemann_Elsevier: Amsterdam, The Netherlands, 2021; pp. 7–14. [Google Scholar] [CrossRef]
- Elgizawy, E.M. The Effect of Green Facades in Landscape Ecology. Procedia. Environ. Sci. 2016, 34, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Bollmann, U.E.; Fernández-Calviño, D.; Brandt, K.K.; Storgaard, M.S.; Sanderson, H.; Bester, K. Biocide Runoff from Building Facades: Degradation Kinetics in Soil. Environ. Sci. Technol. 2017, 51, 3694–3702. [Google Scholar] [CrossRef] [PubMed]
- Bollmann, U.E.; Tang, C.; Eriksson, E.; Jönsson, K.; Vollertsen, J.; Bester, K. Biocides in urban wastewater treatment plant influent at dry and wet weather: Concentrations, mass flows and possible sources. Water Res. 2014, 60, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Burkhardt, M.; Zuleeg, S.; Vonbank, R.; Bester, K.; Carmeliet, J.; Boller, M.; Wangler, T. Leaching of biocides from façades under natural weather conditions. Environ. Sci. Technol. 2012, 46, 5497–5503. [Google Scholar] [CrossRef]
- Wangler, T.P.; Zuleeg, S.; Vonbank, R.; Bester, K.; Boller, M.; Carmeliet, J.; Burkhardt, M. Laboratory scale studies of biocide leaching from façade coatings. Build. Env. 2012, 54, 168–173. [Google Scholar] [CrossRef]
- Schoknecht, U.; Gruycheva, J.; Mathies, H.; Bergmann, H.; Burkhardt, M. Leaching of biocides used in façade coatings under laboratory test conditions. Environ. Sci. Technol. 2009, 43, 9321–9328. [Google Scholar] [CrossRef]
- European Parliament and Council. REGULATION (EU) No 528/2012 concerning the making available on the market and use of biocidal products. Off. J. Eur. Union. 2012, 1–122. Available online: http://data.europa.eu/eli/reg/2012/528/oj (accessed on 1 September 2021).
- Paulus, W. Directory of Microbicides for the Protection of Materials: A Handbook; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Jungnickel, C.; Stock, F.; Brandsch, T.; Ranke, J. Risk assessment of biocides in roof paint. Part 1: Experimental determination and modelling of biocide leaching from roof paint. Environ. Sci. Pollut. Res. Int. 2008, 15, 258–265. [Google Scholar] [CrossRef]
- Burkhardt, M.; Zuleeg, S.; Eugster, J.; Boller, M.; Hean, S.; Haag, R.; Schmid, P.; Kohler, M. Mecoprop in Bitumenbahnen: Auswaschung von Mecoprop aus Bitumenbahnen und Vorkommen im Regenkanal. Eawag: Das Wasserforschungs-Institut des ETH-Bereichs Abteilung Siedlungswasserwirtschaft 2009. [Google Scholar] [CrossRef]
- Bucheli, T.D.; Müller, S.R.; Voegelin, A.; Schwarzenbach, R.P. Bituminous Roof Sealing Membranes as Major Sources of the Herbicide (R, S )-Mecoprop in Roof Runoff Waters: Potential Contamination of Groundwater and Surface Waters. Environ. Sci. Technol. 1998, 32, 3465–3471. [Google Scholar] [CrossRef]
- Pillonel, C.; Hitzfeld, B.; Burkhardt, M. Information über Mecoprop in Bitumen-Dachbahnen. In Eawag: Das Wasserforschungs-Institut des ETH-Bereichs Abteilung Siedlungswasserwirtschaft; Eawag: Dübendorf, Switzerland, 2009; pp. 1–4. [Google Scholar]
- Gerecke, A.C.; Scharer, M.; Singer, H.P.; Muller, S.R.; Schwarzenbach, R.P.; Sagesser, M.; Ochsenbein, U.; Popow, G. Sources of pesticides in surface waters in Switzerland: Pesticide load through waste water treatment plants—Current situation and reduction potential. Chemosphere. 2002, 48, 307–315. [Google Scholar] [CrossRef]
- Lamprea, K.; Bressy, A.; Mirande-Bret, C.; Caupos, E.; Gromaire, M.C. Alkylphenol and bisphenol A contamination of urban runoff: An evaluation of the emission potentials of various construction materials and automotive supplies. Environ. Sci. Pollut. Res. Int. 2018, 25, 21887–21900. [Google Scholar] [CrossRef]
- Björklund, K. Substance flow analyses of phthalates and nonylphenols in stormwater. Water Sci. Technol. 2010, 62, 1154–1160. [Google Scholar] [CrossRef]
- Bressy, A.; Gromaire, M.-C.; Lorgeoux, C.; Chebbo, G. Alkylphenols in atmospheric depositions and urban runoff. Water Sci. Technol. 2011, 63, 671–679. [Google Scholar] [CrossRef]
- Drozd, W. Problems and benefits of using green roofs in Poland. IOP Conf. Ser. Earth Environ. Sci. 2019, 214, 12076. [Google Scholar] [CrossRef]
- Bayen, S.; Obbard, J.P.; Thomas, G.O. Chlorinated paraffins: A review of analysis and environmental occurrence. Environ. Int. 2006, 32, 915–929. [Google Scholar] [CrossRef]
- Estill, C.F.; Slone, J.; Mayer, A.; Chen, I.C.; La Guardia, M.J. Worker exposure to flame retardants in manufacturing, construction and service industries. Environ. Int. 2020, 135, 105349. [Google Scholar] [CrossRef]
- Cristale, J.; Hurtado, A.; Gómez-Canela, C.; Lacorte, S. Occurrence and sources of brominated and organophosphorus flame retardants in dust from different indoor environments in Barcelona, Spain. Environ. Res. 2016, 149, 66–76. [Google Scholar] [CrossRef] [Green Version]
- Fohner, J.M. Nutrient Dynamics in Stormwater Runoff from Green Roofs with Varying Substrate. Ph.D. Thesis, University of Arkansas, Fayetteville, AR, USA, 2012. [Google Scholar]
- Buffam, I.; Mitchell, M.E. Chapter 5: Nutrient Cycling in Green Roof Ecosystems. In Green Roof Ecosystems; Springer International Publishing: Cham, Switzerland, 2015; Volume 223, pp. 107–137. [Google Scholar] [CrossRef]
- Aitkenhead-Peterson, J.A.; Dvorak, B.D.; Volder, A.; Stanley, N.C. Chemistry of growth medium and leachate from green roof systems in south-central Texas. Urban Ecosyst. 2011, 14, 17–33. [Google Scholar] [CrossRef]
- Gregoire, B.G.; Clausen, J.C. Effect of a modular extensive green roof on stormwater runoff and water quality. Ecol. Eng. 2011, 37, 963–969. [Google Scholar] [CrossRef]
- Fahimah, N.; Oginawati, K. Fate and spatial distribution of Pb, Cd, Cu and Zn in the water column and in the surface sediment of Indonesian Estuary (Citarum River Estuary). In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2020. [Google Scholar]
- National Research Council. 4, Toxicity and Related Data on Selected Cadmium Compounds: Toxicologic Assessment of the Army’s Zinc Cadmium Sulfide Dispersion Tests. 1997. Available online: https://www.ncbi.nlm.nih.gov/books/NBK233504/ (accessed on 17 September 2021).
- Masindi, V.; Muedi, K. Environmental Contamination by Heavy Metals. Heavy Metals. 2018, 10, 115–132. [Google Scholar]
- NORMAN Network. NORMAN Substance Database. Available online: https://www.norman-network.com/nds/factsheets/ (accessed on 7 December 2020).
- ECHA. Information on Chemicals. Available online: https://echa.europa.eu/de/information-on-chemicals (accessed on 1 September 2020).
- Cuppett, J.D.; Duncan, S.E.; Dietrich, A.M. Evaluation of Copper Speciation and Water Quality Factors That Affect Aqueous Copper Tasting Response. Chem. Senses. 2006, 31, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadin, H.; Ashizawa, A.; Stevens, Y. Toxicological Profile for Lead. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US). Table 4-2, Physical and Chemical Properties of Lead and Compounds. Available online: https://www.ncbi.nlm.nih.gov/books/NBK158769/table/T15/ (accessed on 12 April 2021).
- IARC. Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 49.) NICKEL AND NICKEL COMPOUNDS: Chromium, Nickel and Welding. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519249/ (accessed on 22 April 2021).
- ChemSpider. Search and Share Chemistry. Available online: http://www.chemspider.com/ (accessed on 7 December 2020).
- National Pollutant Inventory. Zinc and Compunds. Available online: http://www.npi.gov.au/resource/zinc-and-compounds#:~:text=Zinc%20is%20insoluble%20in%20water,oxygen%20to%20give%20zinc%20oxide (accessed on 9 September 2020).
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Zinc. 2005. Atlanta, Georgia, US. Available online: https://www.atsdr.cdc.gov/ToxProfiles/tp60.pdf (accessed on 4 May 2021).
- University of Hertfordshire. PPDB: Pesticide Properties DataBase. Available online: https://sitem.herts.ac.uk/aeru/ppdb/en/atoz.htm (accessed on 15 October 2020).
- PubChem. Explore Chemistry. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 9 December 2020).
- US EPA. CompTox: Chemicals Dashboard. Available online: https://comptox.epa.gov/dashboard (accessed on 9 December 2020).
- SciFinder. Substance Identifier. Available online: https://scifinder.cas.org/scifinder/view/scifinder/scifinderExplore.jsf (accessed on 15 December 2020).
- EU. European Union Risk Assessment Report—Bisphenol A. European Communities: Luxemburg, 2008. [Google Scholar]
- Chen, C.; Guo, W.; Ngo, H.H. Pesticides in stormwater runoff—A mini review. Front. Environ. Sci. Eng. 2019, 13. [Google Scholar] [CrossRef]
- Launay, M.A. Organic Micropollutants in Urban Wastewater Systems during Dry and Wet Weather: Occurrence, Spatio-temporal Distribution and Emissions to Surface Waters. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2017. [Google Scholar]
- Margot, J. Micropollutant Removal from Municipal Wastewater from Conventional Treatments to Advanced Biological Processes. Swiss Federal Institute of Technology Lausanne: Lausanne, Switzerland, 2015. [Google Scholar]
- Deffontis, S.; Breton, A.; Vialle, C.; Montréjaud-Vignoles, M.; Vignoles, C.; Sablayrolles, C. Impact of dry weather discharges on annual pollution from a separate storm sewer in Toulouse, France. Sci. Total Environ. 2013, 452-453, 394–403. [Google Scholar] [CrossRef] [Green Version]
- Rippy, M.A.; Deletic, A.; Black, J.; Aryal, R.; Lampard, J.-L.; Tang, J.Y.-M.; McCarthy, D.; Kolotelo, P.; Sidhu, J.; Gernjak, W. Pesticide occurrence and spatio-temporal variability in urban run-off across Australia. Water Res. 2017, 115, 245–255. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Pedersen, T.; Fischer, M.; White, R.; Young, T.M. Herbicide runoff along highways. 1. Field observations. Environ. Sci. Technol. 2004, 38, 3263–3271. [Google Scholar] [CrossRef] [PubMed]
- Vialle, C.; Sablayrolles, C.; Silvestre, J.; Monier, L.; Jacob, S.; Huau, M.-C.; Montrejaud-Vignoles, M. Pesticides in roof runoff: Study of a rural site and a suburban site. J. Environ. Manage. 2013, 120, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Ramírez, P.; García-Fernández, A.J. Mecoprop. Encycl. Toxicol. 2014, 3, 176–179. [Google Scholar] [CrossRef]
- Wang, S.; He, Q.; Ai, H.; Wang, Z.; Zhang, Q. Pollutant concentrations and pollution loads in stormwater runoff from different land uses in Chongqing. J. Environ. Sci. 2013, 25, 502–510. [Google Scholar] [CrossRef]
- Nickel, J.P.; Fuchs, S. Micropollutant emissions from combined sewer overflows. Water Sci. Technol. 2019, 80, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Bressy, A.; Gromaire, M.-C.; Lorgeoux, C.; Saad, M.; Leroy, F.; Chebbo, G. Towards the determination of an optimal scale for stormwater quality management: Micropollutants in a small residential catchment. Water Res. 2012, 46, 6799–6810. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, S.; Moilleron, R.; Chebbo, G. Screening of priority pollutants in urban stormwater: Innovative methodology. Water Pollut. IX. 2008, 111, 235–244. [Google Scholar]
- Zgheib, S.; Moilleron, R.; Chebbo, G. Priority pollutants in urban stormwater: Part 1—Case of separate storm sewers. Water Res. 2012, 46, 6683–6692. [Google Scholar] [CrossRef]
- Launay, M.A.; Dittmer, U.; Steinmetz, H. Organic micropollutants discharged by combined sewer overflows—Characterisation of pollutant sources and stormwater-related processes. Water Res. 2016, 104, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Hollender, J.; Rothardt, J.; Radny, D.; Loos, M.; Epting, J.; Huggenberger, P.; Borer, P.; Singer, H. Comprehensive micropollutant screening using LC-HRMS/MS at three riverbank filtration sites to assess natural attenuation and potential implications for human health. Water. Res. X. 2018, 1, 100007. [Google Scholar] [CrossRef]
- Schaffer, M.; Licha, T. A guideline for the identification of environmentally relevant, ionizable organic molecule species. Chemosphere. 2014, 103, 12–25. [Google Scholar] [CrossRef]
- ECHA. Guidance on Information Requirements and Chemical Safety Assessment: Chapter R.11: PBT/vPvB Assessment; ED-01-17-294-EN-N; European Chemicals Agency: Helsinki, Finland, 2017. [Google Scholar]
- Von der Ohe, P.C.; Dulio, V. NORMAN Prioritisation Framework for Emerging Substances; Norman Association: Verneuil-en-Halatt, France, 2013; ISBN 978-2-9545254-0-2. [Google Scholar]
- Wicke, D.; Matzinger, A. Relevanz Organischer Spurenstoffe im Regenwasserabfluss Berlins—Ogre; Kompetenzzentrum Wasser Berlin GmbH: Berlin, Germany, 2015. [Google Scholar]
- Clara, M.; Gruber, G.; Humer, F.; Hofer, T.; Kretschmer, F.; Ertl, T.; Scheffknecht, C.; Weiß, S. Spurenstoffemissionen aus Siedlungsgebieten und von Verkehrsflächen. In Stuttgarter Berichte zur Siedlungswasserwirtschaft: Spurenstoffe im Regen-und Mischwasserabfluss; SCHTURM: Wien, Austria, 2014; Available online: https://www.bmnt.gv.at/service/publikationen/wasser/Spurenstoffemissionen-aus-Siedlungsgebieten-und-von-Verkehrsflaechen.html (accessed on 7 December 2020).
- Gasperi, J.; Sebastian, C.; Ruban, V.; Delamain, M.; Percot, S.; Wiest, L.; Mirande, C.; Caupos, E.; Demare, D.; Kessoo, M.D.K.; et al. Micropollutants in urban stormwater: Occurrence, concentrations, and atmospheric contributions for a wide range of contaminants in three French catchments. Environ. Sci. Pollut. Res. 2014, 21, 5267–5281. [Google Scholar] [CrossRef] [Green Version]
- Birch, H.; Mikkelsen, P.S.; Jensen, J.K.; Lützhøft, H.-C.H. Micropollutants in stormwater runoff and combined sewer overflow in the Copenhagen area, Denmark. Water Sci. Technol. 2011, 64, 485–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clara, M.; Gruber, G.; Hohenblum, P.; Hofer, T.; Kittlaus, S.; Lenz, K.; Liebmann, B.; Liedermann, M.; Maier, R.; Mallow, O.; et al. TEMPEST: Erfassung von Emissionen Ausgewählter Spurenstoffe aus Kanalsystemen, Handlungsoptionen zu Deren Minderung und Opimierung einer Alternativen Nachweismethode für Kunststoffpartikel in Wasserproben; Institute of Urban Water Management and Landscape Water Engineering: Wien, Austria, 2020. [Google Scholar]
- EEA. Simulated Land Average Maximum Number of Consecutive Dry Days for Different European Regions (1860-2100). Available online: https://www.eea.europa.eu/data-and-maps/figures/simulated-land-average-maximum-number-of-consecutive-dry-days-for-different-european-regions-1860-2100 (accessed on 17 June 2021).
- Council of the European Communities. Directive 2000/60/Ec of the European Parliament and of The Council establishing a Framework for Community Action in the Field of Water Policy. Off. J. Eur. Communities. 2000, 327, 1–72. [Google Scholar]
- More, S.J.; Bampidis, V.; Benford, D.; Bennekou, S.H.; Bragard, C.; Halldorsson, T.I.; Hernández-Jerez, A.F.; Koutsoumanis, K.; Naegeli, H.; Schlatter, J.R.; et al. Guidance on harmonised methodologies for human health, animal health and ecological risk assessment of combined exposure to multiple chemicals. EFSA J. 2019, 17, e05634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zessner, M.; Kroiss, H.; Gabriel, O. Präzisierung von Qualitätszielen im Falle einer Anwendung bei der Einleitung aus Punktquellen im Auftrag von Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft; Institut für Wassergüte und Abfallwirtschaft, Technische Universität Wien: Vienna, Austria, 2004. [Google Scholar]
- ECHA. Guidance for the Identification of Endocrine Disruptors. Available online: https://echa.europa.eu/hot-topics/endocrine-disruptors (accessed on 30 August 2021).
- Toshovski, S.; Kaiser, M.; Fuchs, S.; Sacher, F.; Thoma, A.; Kümmel, V.; Lambert, B. Prioritäre Stoffe in Kommunalen Kläranlagen: Ein Deutschlandweit Harmonisiertes Vorgehen No. 173, Dessau-Roßlau. 2020. Available online: https://www.umweltbundesamt.de/publikationen/prioritaere-stoffe-in-kommunalen-klaeranlagen (accessed on 12 October 2020).
- Boller, M. Nachhaltige Regenwasserentsorgung auf dem Weg in die Praxis. EAWAG News. 2003, 57, 25–28. [Google Scholar]
- Göbel, P.; Dierkes, C.; Coldewey, W. Storm water runoff concentration matrix for urban areas. J. Contam. Hydrol. 2007, 91, 26–42. [Google Scholar] [CrossRef]
- Hürlimann, J. Auswirkungen von Straßenabwasser aus Oberflächengewässer. GWA. 2011, 91, 793–801. [Google Scholar]
- Fuerhacker, M.; Haile, T.M.; Monai, B.; Mentler, A. Performance of a filtration system equipped with filter media for parking lot runoff treatment. Desalination. 2011, 275, 118–125. [Google Scholar] [CrossRef]
- Fürhacker, M.; Haile, T.B.; Schärfinger, B.; Kammerer, G.; Allabashi, R.; Magnat, S.; Lins, A. Entwicklung von Methoden zur Prüfung der Eignung von Substraten für die Oberflächenwasserbehandlung von Dach- und Verkehrsflächen; Grant agreement GZ B100121; Bundesministerium für Land- und Forstwirtschaf: Vienna, Austria, 2013. [Google Scholar]
- Zgheib, S.; Moilleron, R.; Saad, M.; Chebbo, G. Partition of pollution between dissolved and particulate phases: What about emerging substances in urban stormwater catchments? Water Res. 2011, 45, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Kasting, U. Reinigungsleistung von Zentralen Anlagen zur Behandlung von Abflüssen Stark Befahrener Straßen; Technische Universität Kaiserslautern: Kaiserslautern, Germany, 2002. [Google Scholar]
- Burkhardt, M.; Zuleeg, S.; Vonbank, R.; Schmid, P.; Hean, S.; Lamani, X.; Bester, K.; Boller, M. Leaching of additives from construction materials to urban storm water runoff. Water Sci. Technol. 2011, 63, 1974–1982. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Vollertsen, J.; Carmeliet, J.; Bester, K. Dynamics of biocide emissions from buildings in a suburban stormwater catchment – Concentrations, mass loads and emission processes. Water Res. 2014, 56, 66–76. [Google Scholar] [CrossRef]
- Bollmann, U.E.; Minelgaite, G.; Schlüsener, M.; Ternes, T.A.; Vollertsen, J.; Bester, K. Photodegradation of octylisothiazolinone and semi-field emissions from facade coatings. Sci. Rep. 2017, 7, 41501. [Google Scholar] [CrossRef]
- Gasperi, J.; Garnaud, S.; Rocher, V.; Moilleron, R. Priority pollutants in wastewater and combined sewer overflow. Sci. Total Env. 2008, 407, 263–272. [Google Scholar] [CrossRef]
- Fuchs, S.; Snezhina, T.; Kaiser, M.; Sacher, F.; Thoma, A. Belastung der Umwelt mit Bioziden Realistischer Erfassen - Schwerpunkt Einträge über Kläranlagen; UBA-Texte 169/2020, Dessau-Roßlau. 2020. Available online: https://www.umweltbundesamt.de/sites/default/files/medien/479/publikationen/texte_169-2020_belastung_der_umwelt_mit_bioziden_realistischer_erfassen_-_schwerpunkt_eintraege_ueber_klaeranlagen.pdf (accessed on 9 February 2021).
- Bollmann, U.E.; Minelgaite, G.; Schlüsener, M.; Ternes, T.; Vollertsen, J.; Bester, K. Leaching of Terbutryn and Its Photodegradation Products from Artificial Walls under Natural Weather Conditions. Environ. Sci. Technol. 2016, 50, 4289–4295. [Google Scholar] [CrossRef] [PubMed]
- Couderc, M.; Poirier, L.; Zalouk-Vergnoux, A.; Kamari, A.; Blanchet-Letrouvé, I.; Marchand, P.; Vénisseau, A.; Veyrand, B.; Mouneyrac, C.; Le Bizec, B. Occurrence of POPs and other persistent organic contaminants in the European eel (Anguilla anguilla) from the Loire estuary, France. Sci. Total Environ. 2015, 505, 199–215. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L.; et al. Urban Stormwater: An Overlooked Pathway of Extensive Mixed Contaminants to Surface and Groundwaters in the United States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cladière, M.; Gasperi, J.; Lorgeoux, C.; Tassin, B. Discharges of endocrine disrupting chemicals by combined sewer overflows into receiving water: Case-study of the Paris conurbation. In Proceedings of the 11th Edition of the World Wide Workshop for Young Environmental Scientists (WWW-YES-2011)—Urban Waters: Resource or Risks? Arcueil, France, 6–10 June 2011. [Google Scholar]
- Boyd, G.R.; Grimm, D.A.; Palmeri, J.M.; Zhang, S. Pharmaceuticals and personal care products (PPCPs) and endocrine disrupting chemicals (EDCs) in stormwater canals and Bayou St. John in New Orleans, Louisiana, USA. Sci. Total Environ. 2004, 333, 137–148. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Khan, E.; Chen, H.; Nguyen, V.H.; Li, Y.; Goh, S.G.; Nguyen, Q.B.; Saeidi, N.; Gin, K.N. Emerging contaminants in wastewater, stormwater runoff, and surface water: Application as chemical markers for diffuse sources. Sci. Total Environ. 2019, 676, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Barriuso, F.; Urbano, B. Green Roofs and Walls Design Intended to Mitigate Climate Change in Urban Areas across All Continents. Sustainability. 2021, 13, 2245. [Google Scholar] [CrossRef]
- Köhler, M.; Schmidt, M. Study of Extensive Green Roofs in Berlin: Part 3 Retention of Contaminatnts; Köhler and Schmidt: Waldrohrbach, Germany, 2003. [Google Scholar]
- Long, B.; Clark, S.E.; Baker, K.H.; Berghage, R. Green Roof Media Selection for the Minimization of Pollutant Loadings in Roof Runoff. Proc. Water Environ. Fed. 2006, 2006, 5528–5548. [Google Scholar] [CrossRef] [Green Version]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jane Clark, M.; Zheng, Y. Plant Nutrition Requirements for an Installed Sedum-Vegetated Green Roof Module System: Effects of Fertilizer Rate and Type on Plant Growth and Leachate Nutrient Content. HortScience 2013, 48, 1173–1180. [Google Scholar] [CrossRef]
- Flanagan, K.; Branchu, P.; Boudahmane, L.; Caupos, E.; Demare, D.; Deshayes, S.; Dubois, P.; Meffray, L.; Partibane, C.; Saad, M.; et al. Field performance of two biofiltration systems treating micropollutants from road runoff. Water Res. 2018, 145, 562–578. [Google Scholar] [CrossRef]
- Austrian Standards Institute. ÖNORM B 2506-3: Regenwasser-Sickeranlagen Für Abläufe von Dachflächen und Befestigten Flächen; Austrian Standards Institute: Vienna, Austria, 2018. [Google Scholar]
- Al-Anbari, R.H.; Wootton, K.P.; Durmanic, S.; Deletic, A.; Fletcher, T.D. Evaluation of media for the adsorption of stormwater pollutants. In Proceedings of the 11th International Conference on Urban Drainage, Edinburgh, Scotland, 31 August–5 September 2008. [Google Scholar]
- Cheng, Y.; Fassman-Beck, E. The effect of zeolite amendments on nitrogen leaching from extensive sedum green roofs. NOVATECH 2019, 1–4. Available online: http://www.novatech.graie.org/documents/auteurs/3D74-202CHE.pdf (accessed on 17 May 2021).
- Kuoppamäki, K.; Lehvävirta, S. Mitigating nutrient leaching from green roofs with biochar. Landsc. Urban Plan. 2016, 152, 39–48. [Google Scholar] [CrossRef]
- Qianqian, Z.; Liping, M.; Huiwei, W.; Long, W. Analysis of the effect of green roof substrate amended with biochar on water quality and quantity of rainfall runoff. Environ. Monit. Assess. 2019, 191, 304. [Google Scholar] [CrossRef] [PubMed]
- Spahr, S.; Teixidó, M.; Sedlak, D.L.; Luthy, R.G. Hydrophilic trace organic contaminants in urban stormwater: Occurrence, toxicological relevance, and the need to enhance green stormwater infrastructure. Environ. Sci. Water Res. Technol. 2020, 6, 15–44. [Google Scholar] [CrossRef] [Green Version]
- Bester, K.; Banzhaf, S.; Burkhardt, M.; Janzen, N.; Niederstrasser, B.; Scheytt, T. Activated soil filters for removal of biocides from contaminated run-off and waste-waters. Chemosphere. 2011, 85, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Braswell, A.S.; Anderson, A.R.; Hunt, W.F. Hydrologic and Water Quality Evaluation of a Permeable Pavement and Biofiltration Device in Series. Water. 2018, 10, 33. [Google Scholar] [CrossRef] [Green Version]
- Ashoori, N.; Teixido, M.; Spahr, S.; LeFevre, G.H.; Sedlak, D.L.; Luthy, R.G. Evaluation of pilot-scale biochar-amended woodchip bioreactors to remove nitrate, metals, and trace organic contaminants from urban stormwater runoff. Water Res. 2019, 154, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Jacobson, A. Environmental Fate of Isothiazolone Biocides. In CORROSION 99; OnePetro: San Antonio, TX, USA, 1999. [Google Scholar]
- Zhang, D.; Gersberg, R.M.; Ng, W.J.; Tan, S.K. Conventional and decentralized urban stormwater management: A comparison through case studies of Singapore and Berlin, Germany. Urban Water J. 2017, 14, 113–124. [Google Scholar] [CrossRef]
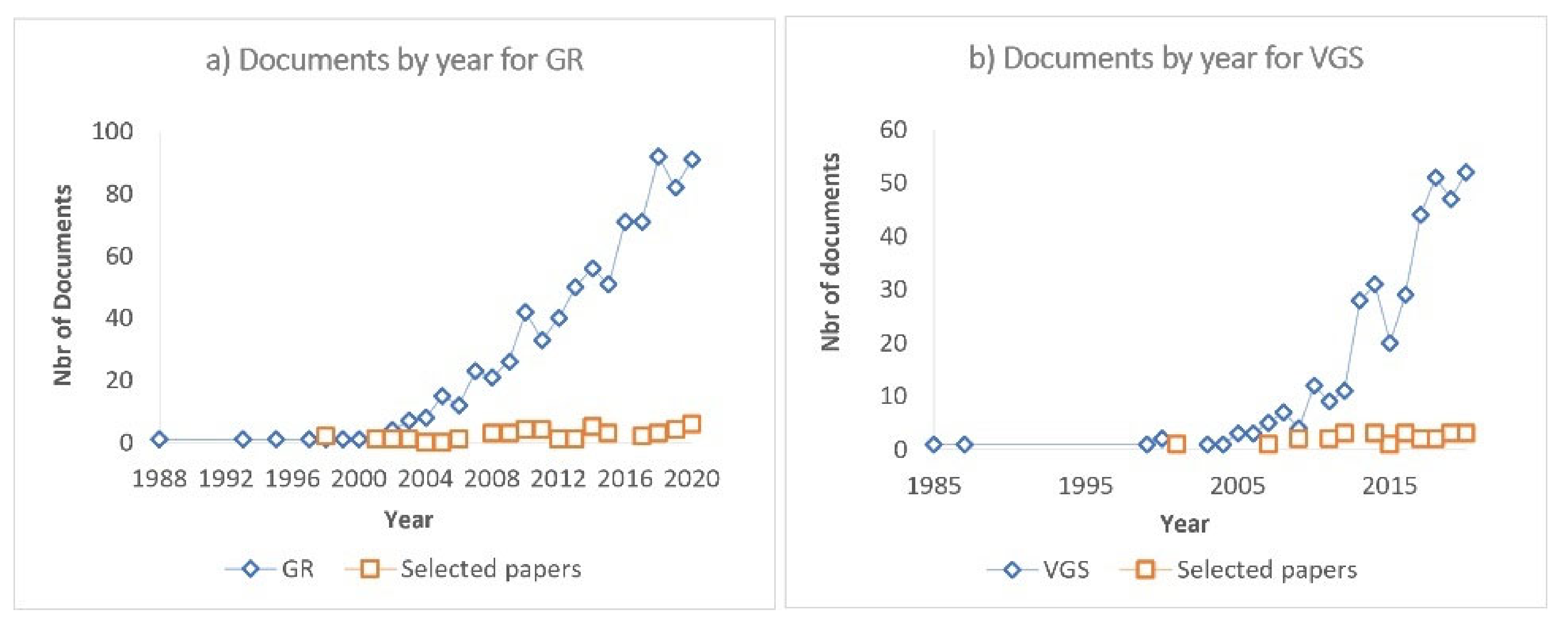
| General | Search strings for ABS-TITLE-KEY search |
|---|---|
| GR | (green AND roof* AND runoff*) |
| VGS | (green AND wall* OR living AND wall* OR green AND fa?ade*) |
| Focus on pollutants | |
| GR | AND (pollutants OR biocides OR metals OR plasticizers*) |
| VGS | AND (Leach* AND quality AND runoff AND (Pollutants OR chemicals OR biocides OR metals OR plasticizers*)) |
| Material | Full Name | Application | NBS Unit | Potential Pollutants | Literature |
|---|---|---|---|---|---|
| Plastic-based materials | |||||
| FPO/TPO | Flexible Polyolefins | Protection layer Root barrier | GR | Zn; Ba; Trimethylpropane; Metilox | [31,32] |
| PVC | Polyvinylchloride | Root barrier Support element | GR; VGS | Zn; Ba; Cu; Sr; Sb2O3; DINP; DIDP; Alkylphenols (NP2EO, OP2EO, 4-NP, NP1EC, NP1EO, OP); Phthalate (DIDP, DINP, DMP, DEP); BPA; HBCD; BBP; Brominated flame retardant (PBD, PBDE); acetophenone | [25,31,32,33,34,35,36,37,38,39,40,41,42] |
| PE, HDPE, LDPE | Polyethylene High-Density Polyethylene, Low-Density Polyethylene, | Drainage layer Storage layer Waterproof layer Protection layer Root barrier Support element Geotextile Irrigation system | GR; VGS | DEHP in HDPE; 4-NP in PE, several additives in geotextiles | [25,26,33,34,35,43,44,45,46,47] |
| PA | Polyamide | Geotextile | VGS | N-Butylbenzenesulfonamide (NBBS); several additives in geotextiles | [25,48] |
| PP | Polypropylene | Drainage layer Protection layer Filter layer Geotextile Support element | GR; VGS | 4-NP; acetophenone; phenoxyethanol; Pb; BPA; Cu; Zn; several additives in geotextiles | [25,27,34,42,44,45,47,49] |
| PS, HIPS, EPS | Polystyrene High-impact polystyrene, Expanded polystyrene | Drainage layer Insulation layer | GR | Styrene; acetophenone; BPA; Al | [42,43,44,47,49,50] |
| PES, PET | Polyester, Polyethylene Terephthalate | Protection layer Drainage layer Support element | GR; VGS | Co; Sb; Cu (in PET) | [26,28,42] |
| PIB | Polyisobutene | Roofing membrane | GR | Product provider | |
| Polymerbitumen, APP-Bitumen, SBS-Bitumen | Atactic polypropylene (APP), Styrene-butadiene-styrene (SBS) | Waterproof layer, Root barrier, Roofing membrane | GR | Mecoprop; MCPA (in Polymerbitumen and APP); 4-NP; OP; BPA; mecoprop; MCPA; DIDP (in SBS) | [31,34,36,44] |
| PC | Polycarbonate | Support element | VGS | BPA; N-Butylbenzenesulfonamide (NBBS) | [26,27,48] |
| EPDM | Ethylene-Propylene-Diene Rubber | Roofing membrane Waterproof layer | GR; VGS | Zn; Ba; Hexamethylendiamine; Aniline; Benzothiazole, Metilox; Di-N-octylphthalate | [31,32,35,46] |
| TPE | Thermoplastic Elastomers | Roofing membrane | GR | [31,32] | |
| Metal-based materials | |||||
| Steel (Zn-coated) | Additional element | GR | Zn | [51] | |
| Stainless steel | Additional element Support element | GR; VGS | NP | [25,40] | |
| Steel S235 | Support element | VGS | [25] | ||
| Aluminum | Additional element Structural element | GR; VGS | [26] | ||
| Galvanized iron | Structural element | VGS | Fe | [26] | |
| Mineral-based materials | |||||
| Expanded clay, clay | Growing medium | GR; VGS | Ni; Zn; Cu; Cd; Ni | [33,49,51,52] | |
| Crushed brick | Growing medium | GR | Ni; Zn; Cu | [49,51] | |
| Foam glass gravel | Growing medium | GR | Product provider | ||
| Expanded shale | Growing medium | GR | Product provider | ||
| Volcanic rock: lava, pozzolan, pumice | Growing medium | GR | Product provider; [47,49,53] | ||
| Sand | Growing medium | GR; VGS | Ni; Zn | [49,51] | |
| Perlite | Growing medium | GR | [53] | ||
| Rockwool, mineral wool | Storage layer | GR | [49] | ||
| Organic-based materials | |||||
| Compost, solid manure | Growing medium | GR; VGS | N; P | [43,47,49] | |
| Wood | Structural element Support element | GR | DMP; Propiconazole; Permethrin; IPBC, Bis(2-ethylhexyl) phthalate | [16,35,43,54] | |
| Fertilizer | Growing medium | GR | N; P; B; Cu; Fe; Mn; Mo; Zn | [47,53] | |
| Soil | Growing medium | GR; VGS | [26,43] | ||
| Organic material: Coconut fiber, peat moss, peat | Growing medium | GR; VGS | [26,47] | ||
| Viscose | Waterproof layer Support element | VGS | [26,27] | ||
| Physical–Chemical Properties | Literature | ||||||
|---|---|---|---|---|---|---|---|
| LogKOW | Sw (mg/L) | LogKoc | Clasification | Half-life Soil or Water/Sediment (Days) | |||
| Heavy Metals | |||||||
| 7440-43-9 | Cadmium (Cd) | 3.87 | Insoluble in water | 3.58 | mainly sediment-related | Non biodegradable | [97,98,99] |
| 7440-47-3 | Chromium (Cr) | 0.23 a | Insoluble in water | mainly sediment-related | Non biodegradable | [99,100,101] | |
| 7440-50-8 | Copper (Cu) | 3.84 | 1.3 | 3.55 | water and/or sediment related | Non biodegradable | [97,99,102] |
| 7439-92-1 | Lead (Pb) | 3.35 | Insoluble in water | 3.11 | mainly sediment-related | Non biodegradable | [97,99,103] |
| 7440-02-0 | Nickel (Ni) | −0.57 | Insoluble in water | 1.16 | mainly sediment-related | Non biodegradable | [99,100,104,105] |
| 7440-66-6 | Zinc (Zn) | 3.72 | Insoluble in water | 3.44 | mainly sediment-related | Non biodegradable | [97,99,106,107] |
| Biocides/Herbicides | |||||||
| 93-65-2 | Mecoprop | −0.19 | 471–250,000 | 1.67 | water-related | 44 b | [16,74,108] |
| 10605-21-7 | Carbendazim | 1.48 | 8 | 2.35 | water-related | 40 b | [100,101,108] |
| 55406-53-6 | Iodopropynyl butylcarbamate (IPBC) | 2.81 | 168 | 2.18 a | water-related | 1.05 b | [74,100,101] |
| 2634-33-5 | Benzisothiazolinone (BIT) | 0.7 | 1288 | 0.97 | water-related | 0.52 b | [74,101] |
| 26172-55-4 | Chloromethylisothiazolinone (CMIT) | 0.401 | 706,000–751,000 | 1.72 a | water-related | 4.76 a | [109,110] |
| 64359-81-5 | Dichloro-N-octylisothiazolinone (DCOIT) | 3.59–4.9 | 27 | 3.22 a | water and/or sediment-related | 4.8 b | [16,74,110] |
| 2682-20-4 | Methylisothiazolinone (MIT) | −0.486 | 489,000–1,000,000 | 1.74 a | water-related | 0.28 b | [74,101,110] |
| 26530-20-1 | Octhilinone (OIT) or Octylisothiazolinone | 2.61 | 500 | 2.85 a | water-related | 30 b | [101,110] |
| 330-54-1 | Diuron | 2.84–2.89 | 29 | 2.57 | water-related | >2500 b | [74,101] |
| 34123-59-6 | Isoproturon (IP) | 2.69 | 63.9 | 2 a | water-related | 100 b; 149 c | [74,100,108] |
| 52645-53-1 | Permethrin | 6.1 | 0.2 | 5 | sediment-related | 42 b; 40 c | [108] |
| 8001-54-5 | Benzalkonium chloride (BZC) | 1.69–3.42 | 100,000 | mainly water-related | [16] | ||
| 28159-98-0 | Cybutryne (or Irgarol 1051) | 3.95 | 7 | 3.2 | water and/or sediment-related | 3.18 a | [100,108] |
| 886-50-0 | Terbutryn | 3.56 | 24.11 | 2.85 | mainly water-related | 231 b; 60 c | [74,100,108] |
| 60207-90-1 | Propiconazole | 3.7 | 110 | 3.26 | water and/or sediment-related | 68.3 b; 561 c | [101,108] |
| 107534-96-3 | Tebuconazole | 3.7 | 36 | 2.63 | mainly water-related | 47.1 b; 365 c | [100,108] |
| Plasticizers | |||||||
| 156609-10-8 | Diethoxylates (NP2EO) (Nonylphenol diethoxylate) | - | - | - | - | - | |
| 1173020-69-3 | Diethoxylates (OP2EO) (Octylphenol diethoxylate) | 5.12 a | 50 a | 3.37 a | mainly sediment-related | 5 a | [110,111] |
| 84852-15-3 | Nonylphenol (NP) (4-Nonylphenol) | 5.4 | 5.7 | 4.04 | mainly sediment-related | - | [101] |
| 3115-49-9 | Nonylphenol acetic acid (NP1EC) ((4-nonylphenoxy)acetic acid) | 5.8 | 40 | 3.36 | mainly sediment-related | - | [101] |
| 104-35-8 | Nonylphenol mono-ethoxylate (NP1EO) | 5.6 a | 18 a | 4.51 a | mainly sediment-related | 4.45 b | [108,110,111] |
| 140-66-9 | Octylphenol (OP) (4-(1,1′,3,3′- tetramethylbutyl)-phenol)) | 5.18 a | 7; 62 a | 4.25 | mainly sediment-related | 50 c | [101,111] |
| 85-68-7 | Benzyl butylphthalate (BBP) | 4.84 | 2.69 | 3.72 | water and/or sediment-related | 3.35 a | [101,110] |
| 117-81-7 | Bis (2-ethylhexyl) phthalate (DEHP) | 7.5 | 0.086 | 4.94 | sediment-related | 300 b | [100,101] |
| 84-66-2 | Diethyl phthalate (DEP) | 2.2 | 932 | 2.34 | water-related | 5.14 a | [101,110] |
| 28553-12-0 | Di-isononyl phthalate (DINP) | 8.8–9.7 | 0.0006 | 5.46 | sediment-related | 51 b | [101] |
| 26761-40-0 | Di-isodecyl phthalate (DIDP) | 10.36 | 0.28 | - | sediment-related | - | [109] |
| 131-11-3 | Dimethyl phthalate (DMP) | 1.54 | 4000 | 1.59 | water-related | 3.81 a | [100,101,110] |
| 117-84-0 | Di-N-octylphthalate (DnOP) | 8.1 | 0.09 a | 4.38 | sediment-related | 5.75 a | [110,111] |
| 77-99-6 | 1,1,1-Trimethylpropane | −0.47 | 100,000,000 | 1.22 a | water-related | 5.34 a | [101,110] |
| 80-05-7 | Bisphenol A (BPA) | 3.4 | 300 | 3.15 a | water and/or sediment-related | 30 a | [100,101,112] |
| Flame retardant | |||||||
| 32534-81-9 | Polybrominated diphenyl ethers (PBDEs) | 6.85 | 0.0113 | - | sediment-related | - | [109] |
| 79-94-7 | Tetrabromobisphenol A (TBBPA) | 5.903 | 0.0351 | 5.62 | sediment-related | 6.4 b; 26 c | [101] |
| 25637-99-4 | Hexabromocyclododecane (HBCD) | 5.625 | 0.0656 | - | sediment-related | - | [101] |
| 1309-64-4 | Sb-Trioxide (Sb2O3) | 6.23 a | 0.37 | - | sediment-related | - | [101,110] |
| Group | logKow | Sw | logKoc | Category |
|---|---|---|---|---|
| 1 | ≤3 | >1 | <3 | water-related |
| 2 | <3–≤5 | >1 | <3 | mainly water-related |
| 3 | >5 | <1 | >3 | sediment-related |
| 4 | <3–≤5 | <1 | >3 | mainly sediment-related |
| 5 | <3–≤5 | >1 | >3 | water and/or sediment-related |
| 6 | <3–≤5 | <1 | <3 | water and/or sediment-related |
| Environmental Concentration in Untreated Stormwater Runoff (µg/L) | Literature | Environmental Concentrations in GR and VGS Runoff (µg/L) | Literature | EQS 1 (µg/L) | PNEC 2 Fresh-Water (µg/L) | MoE (Stormwater) | MoE (GR and VGS) | ||
|---|---|---|---|---|---|---|---|---|---|
| Heavy Metals | |||||||||
| 7440-43-9 | Cd | 0.019–30 | [131,132,134,135,141,142,143,144,145,146] | 0.05–0.1 | [17,40,52] | 0.45 (class 1) | 0.19 | 0.112–23.67 | 4.5–9 |
| 7440-47-3 | Cr | 0.25–94 | [125,131,132,134,135,141,143,144,146,147] | 0.9–8.1 | [40] | 3.4 | 6.5 | 0.036–13.6 | 0.42–3.78 |
| 7440-50-8 | Cu | 1.2–352 | [125,131,132,134,135,141,143,144,145,146,147,148] | 0.02–58 | [17,40,52,56,61] | 1.00–28 | 7.8 | 0.0028–0.83; 0.08–23.33 | 50–0.017; 0.48–1400 |
| 7439-92-1 | Pb | 0.95–300 | [125,131,132,134,135,141,142,143,144,145,146,147,148] | 0.0025–6 | [17,52,61] | 14 | 2.4 | 0.047–14.73 | 2.3–5600 |
| 7440-02-0 | Ni | 0.91–130 | [131,132,134,135,141,143,144,146] | 0.6–3.5 | [17,40,52] | 34 | 7.1 | 0.26–37.36 | 9.71–56.6 |
| 7440-66-6 | Zn | 4–2000 | [125,132,134,135,142,143,144,145,146,147,148] | 0.06–468 | [17,40,52,56,61] | 8.0–25 | 20.6 | 0.004–2; 0.012- 6.25 | 0.017–133; 0.06–417 |
| Biocides/Herbicides | |||||||||
| 93-65-2 | Mecoprop | 0.51–6.9 | [131] | 1–10 | [149,150] | 3.6 | 0.52–7.06 | 3.6–0.36 | |
| 10605-21-7 | Carbendazim | 0.13–1.5 | [131] | 40 | [149,150] | 0.34 | 0.23–2.6 | 0.0085 | |
| 55406-53-6 | PBC | 0.53 | |||||||
| 2634-33-5 | BIT | 0.09–1.6 | [131] | 4.03 | |||||
| 26172-55-4 | CMIT | ||||||||
| 64359-81-5 | DCOIT | ||||||||
| 2682-20-4 | MIT | 3.39 | |||||||
| 26530-20-1 | OIT | 20–14,000 | [151] | ||||||
| 330-54-1 | Diuron | 0.055–1.21 | [131,132,133,134,135] | 70 | [149] | 1.8 | 320 | 1.48–32.7 | 0.026 |
| 34123-59-6 | IP | 0.02–0.12 | [131,133,134,141,152] | 1 | 8.33–50 | ||||
| 52645-53-1 | Permethrin | 0.005–0.017 | [153] | ||||||
| 8001-54-5 | BZC | ||||||||
| 28159-98-0 | Cybutryne | 0.01–0.02 | [131] | 7000–12,000 | [76,149] | 0.016 | 0.8–1.6 | 0.00000133–0.00000228 | |
| 886-50-0 | Terbutryn | 0.05–0.36 | [131,135] | 360–1000 | [76,149,154] | 0.34 | 0.94–6.8 | 0.00034–0.00094 | |
| 60207-90-1 | Propiconazole | 0.04 | [16] | ||||||
| 107534-96-3 | Tebuconazole | 0.02–0.09 | [131] | ||||||
| Plasticizers | |||||||||
| 156609-10-8 | NP2EO | 0.085–0.4 | [34,86] | ||||||
| 1173020-69-3 | OP2EO | 0.005–0.04 | [34,86] | ||||||
| 84852-15-3 | 4-NP | 0.059–9.2 | [123,125,141] | 0.6–26 | [34,40,86,155] | 2 | 0.21–33.9 | 0.08–3.3 | |
| 3115-49-9 | NP1EC | 0.011–0.12 | [34,86] | ||||||
| 104-35-8 | NP1EO | 0.05–2.39 | [116,132,133,156,157] | 0.078–0.4 | [34,86] | ||||
| 140-66-9 | OP | 0.026–1 | [123,131,141,147] | 0.017–0.08 | [34,40,86] | 0.1 a | 0.1–3.85 | 1.25–5.88 | |
| 85-68-7 | BBP | 20,000 | |||||||
| 117-81-7 | DEHP | 0.2–14 | [131,132,135] | 1.3 a | 0.09–6.5 | ||||
| 84-66-2 | DEP | 200 | |||||||
| 28553-12-0 | DINP | 0.73–130 | [131,132] | 0.68–455 | [40,86] | ||||
| 26761-40-0 | DIDP | ||||||||
| 131-11-3 | DMP | 0.04–0.61 | [131] | 800 | 192 | 1300–20,000 | |||
| 117-84-0 | DnOP | ||||||||
| 77-99-6 | 1,1,1-Trimethylpropan | ||||||||
| 80-05-7 | BPA | 0.0117–10.402 | [131,132,133,158,159] | 0.017–0.57 | [34,86,155] | 1.5 | 18 | 0.144–128.2 | 2.63–88.2 |
| Flame retardant | |||||||||
| 32534-81-9 | PBDE | 0.000013–0.00029 | [135] | 0.14 | 483–10,700 | ||||
| 79-94-7 | TBBPA | 16 | |||||||
| 25637-99-4 | HBCD | 0.5 | 310 | ||||||
| 1309-64-4 | Sb2O3 | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hachoumi, I.; Pucher, B.; De Vito-Francesco, E.; Prenner, F.; Ertl, T.; Langergraber, G.; Fürhacker, M.; Allabashi, R. Impact of Green Roofs and Vertical Greenery Systems on Surface Runoff Quality. Water 2021, 13, 2609. https://doi.org/10.3390/w13192609
Hachoumi I, Pucher B, De Vito-Francesco E, Prenner F, Ertl T, Langergraber G, Fürhacker M, Allabashi R. Impact of Green Roofs and Vertical Greenery Systems on Surface Runoff Quality. Water. 2021; 13(19):2609. https://doi.org/10.3390/w13192609
Chicago/Turabian StyleHachoumi, Imane, Bernhard Pucher, Elisabetta De Vito-Francesco, Flora Prenner, Thomas Ertl, Guenter Langergraber, Maria Fürhacker, and Roza Allabashi. 2021. "Impact of Green Roofs and Vertical Greenery Systems on Surface Runoff Quality" Water 13, no. 19: 2609. https://doi.org/10.3390/w13192609