Spoiled for Choice during Cold Season? Habitat Use and Potential Impacts of the Invasive Silurus glanis L. in a Deep, Large, and Oligotrophic Lake (Lake Maggiore, North Italy)
Abstract
:1. Introduction
2. Materials and Methods
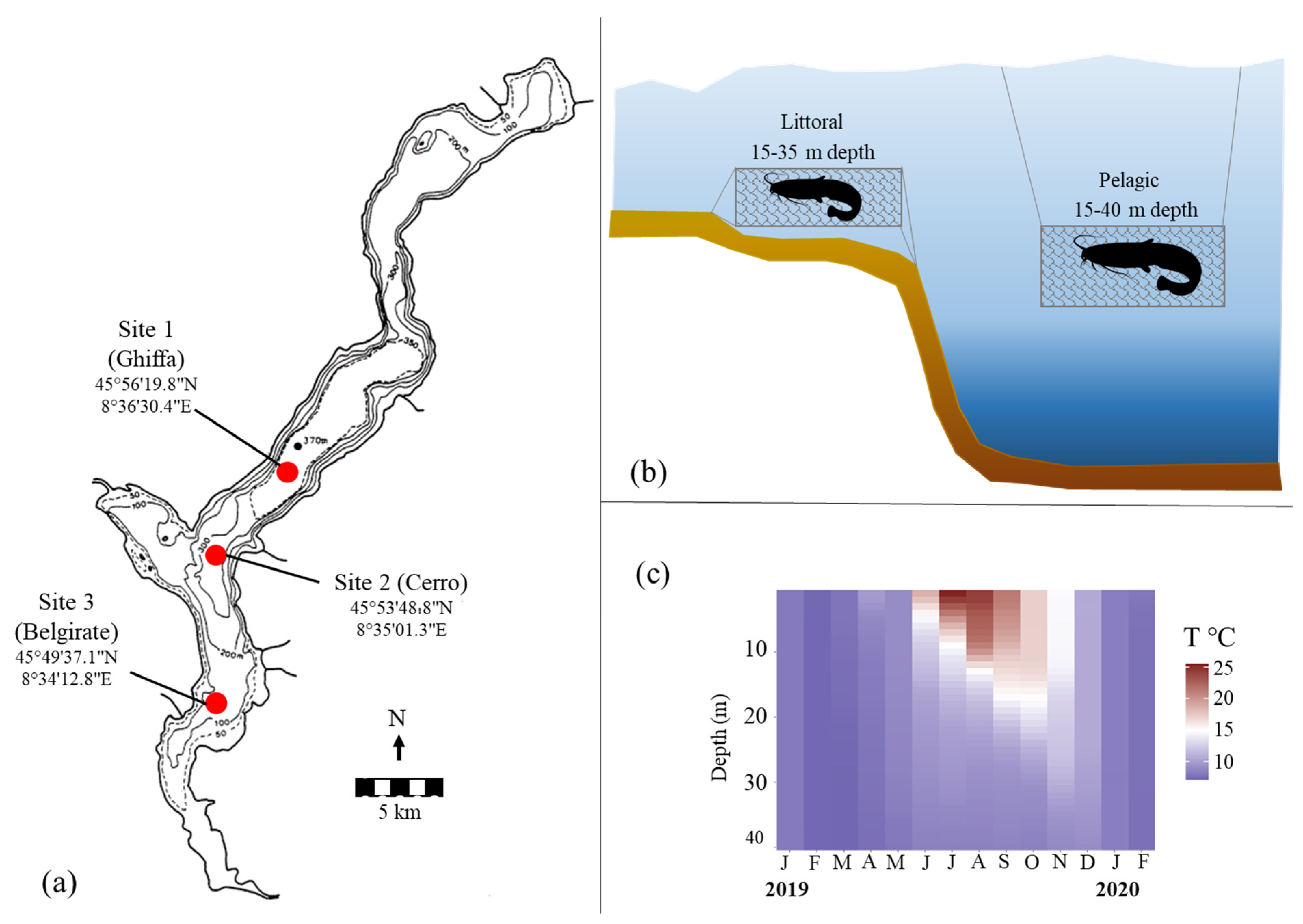
2.1. Sampling Locations and Fishing Gears
2.2. Stomach Content and Prey Detection
2.3. Data Analyses
2.4. Additional Information: L. Maggiore’s Fish Commercial Harvest
3. Results
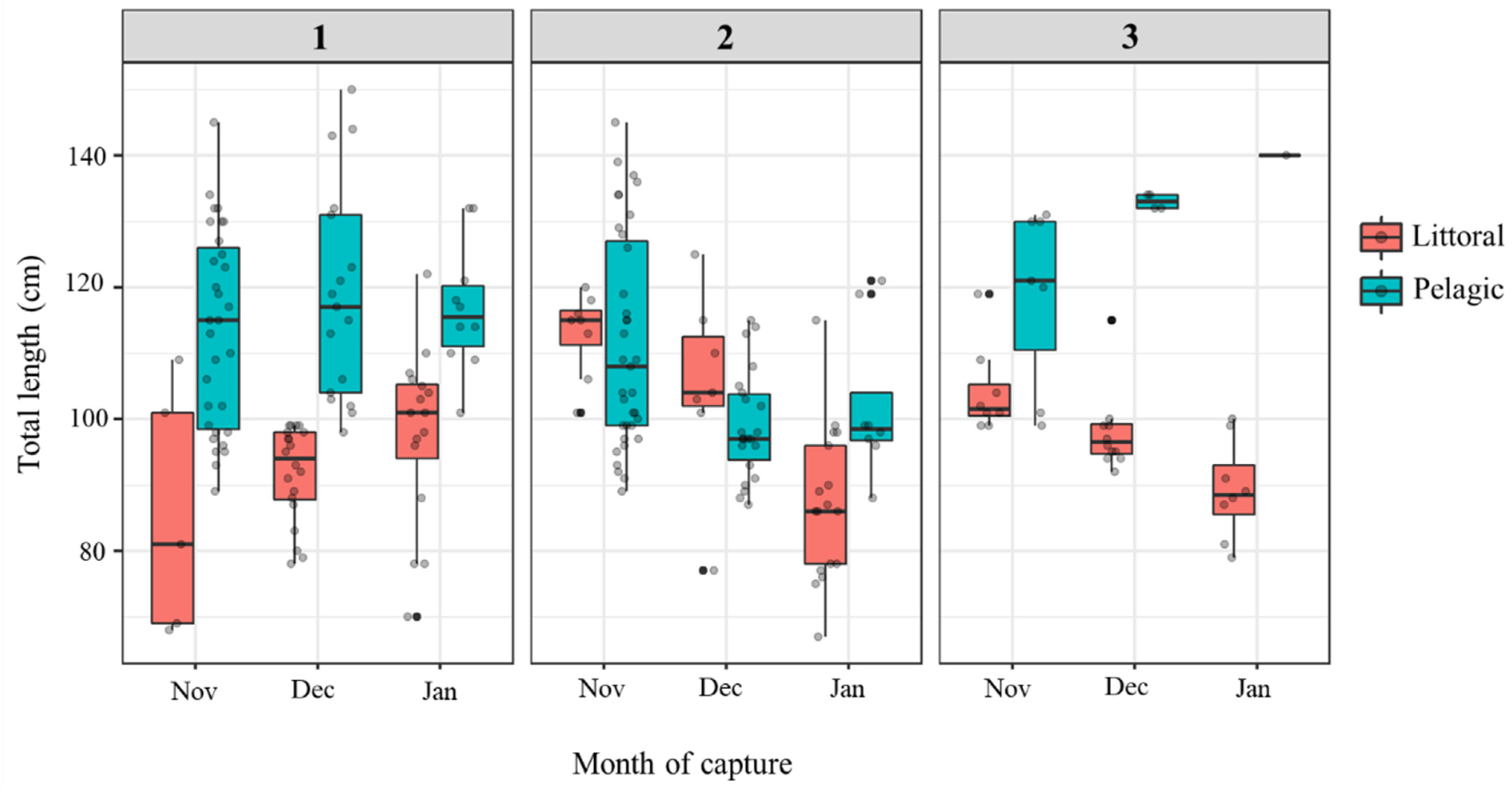
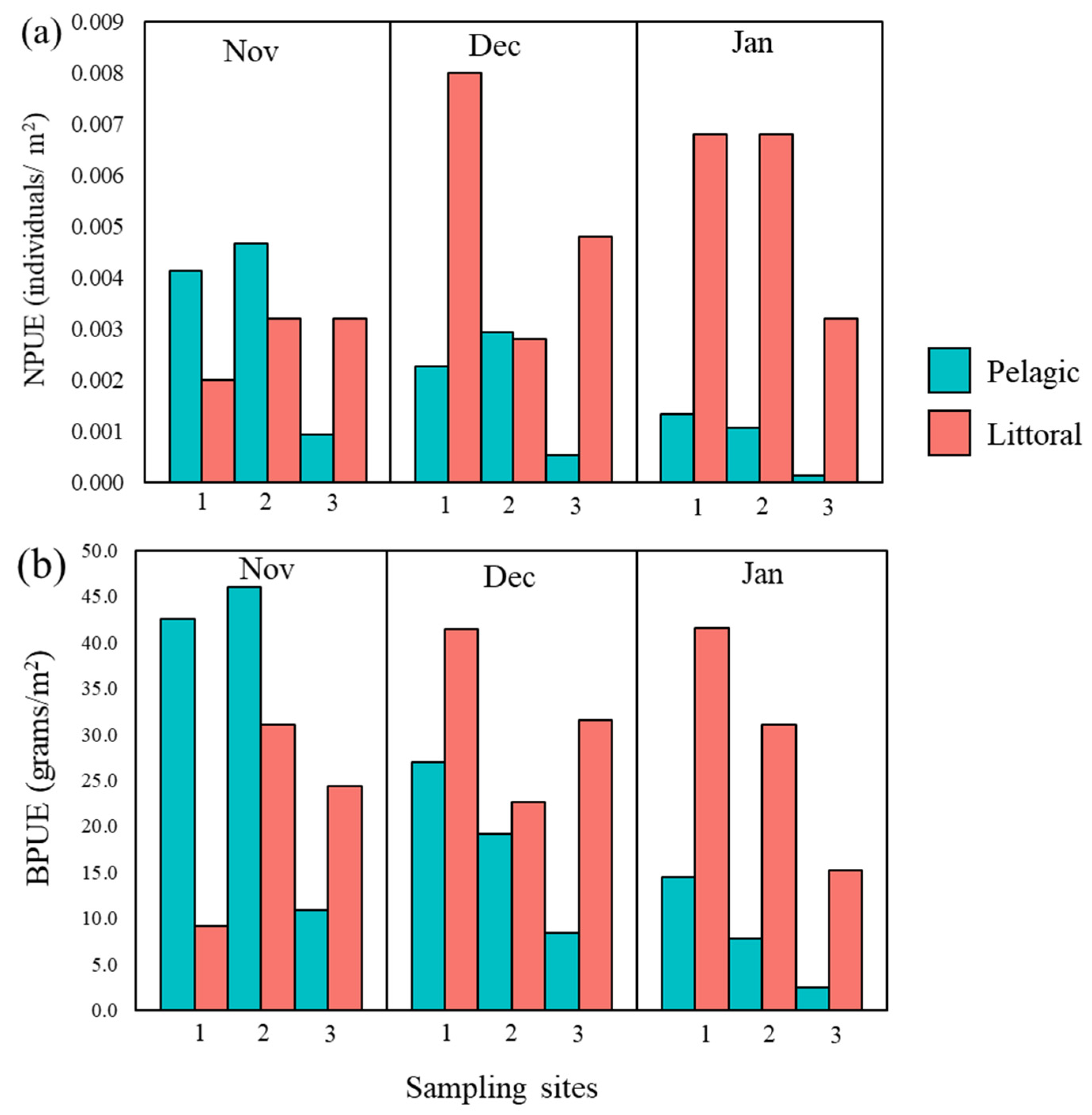
3.1. Catfish Harvest in Late Autumn/Early Winter 2019/2020 at Three Sampling Sites
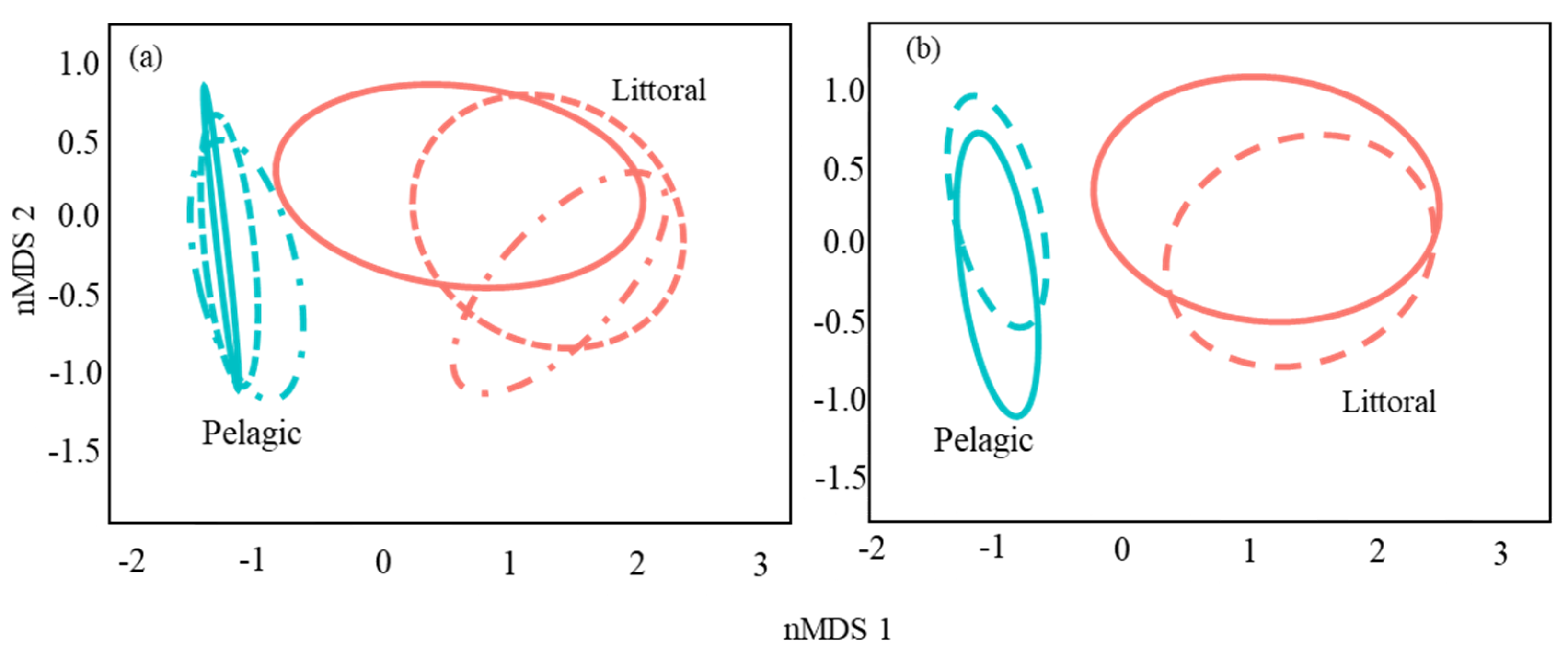
3.2. Catfish Diet Composition in Pelagic and Littoral Habitats
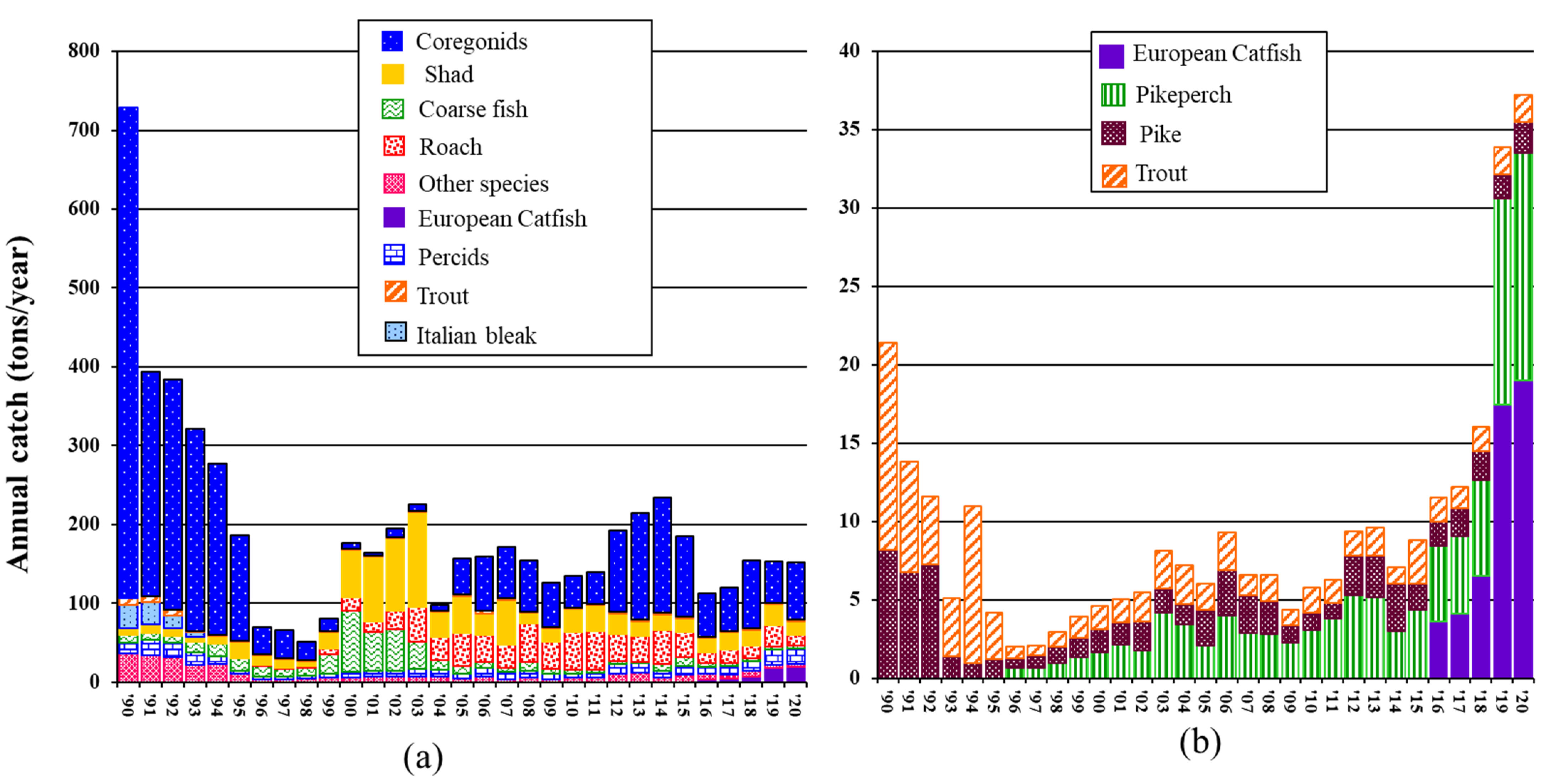
3.3. Trends in L. Maggiore’s Fishing Harvest during the 20 Years of Catfish Invasion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conser, C.; Seebacher, L.; Fujino, D.W.; Reichard, S.; DiTomaso, J.M. The development of a plant risk evaluation (PRE) tool for assessing the invasive potential of ornamental plants. PLoS ONE 2015, 10, e0121053. [Google Scholar] [CrossRef]
- Copp, G.H.; Vilizzi, L.; Tidbury, H.; Stebbing, P.D.; Tarkan, A.S.; Miossec, L.; Goulletquer, P. Development of a generic decision-support tool for identifying potentially invasive aquatic taxa: AS-ISK. Manag. Biol. Invasions 2016, 7, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Dick, J.T.; Laverty, C.; Lennon, J.J.; Barrios-O’Neill, D.; Mensink, P.J.; Robert Britton, J.; Médoc, V.; Boets, P.; Alexander, M.E.; Taylor, N.G.; et al. Invader Relative Impact Potential: A new metric to understand and predict the ecological impacts of existing, emerging and future invasive alien species. J. Appl. Ecol. 2017, 54, 1259–1267. [Google Scholar] [CrossRef]
- Matthews, J.; Beringen, R.; Creemers, R.; Hollander, H.D.; Kessel, N.V.; Kleef, H.V.; van de Koppel, S.; Lemaire, A.J.J.; Baudewijn, O.; Verbrugge, L.N.H.; et al. A new approach to horizon-scanning: Identifying potentially invasive alien species and their introduction pathways. Manag. Biol. Invasions 2017, 8, 37–52. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.R.; Gutmann Roberts, C.; Amat Trigo, F.; Nolan, E.T.; De Santis, V. Predicting the ecological impacts of an alien invader: Experimental approaches reveal the trophic consequences of competition. J. Anim. Ecol. 2019, 88, 1066–1078. [Google Scholar] [CrossRef]
- Vilizzi, L.; Copp, G.H.; Hill, J.E.; Adamovich, B.; Aislabie, L.; Akin, D.; Al-Faisal, A.J.; Almeida, D.; Azmai, M.A.; Bakiu, R.; et al. A global-scale screening of non-native aquatic organisms to identify potentially invasive species under current and future climate conditions. Sci. Total Environ. 2021, 788, 147868. [Google Scholar] [CrossRef] [PubMed]
- Copp, G.H.; Garthwaite, R.; Gozlan, R.E. Risk identification and assessment of non-native freshwater fishes: A summary of concepts and perspectives on protocols for the UK. J. Appl. Ichthyol. 2005, 21, 371–373. [Google Scholar] [CrossRef]
- Ricciardi, A.; Hoopes, M.F.; Marchetti, M.P.; Lockwood, J.L. Progress toward understanding the ecological impacts of nonnative species. Ecol. Monogr. 2013, 83, 263–282. [Google Scholar] [CrossRef] [Green Version]
- Boets, P.; Laverty, C.; Fukuda, S.; Verreycken, H.; Green, K.; Britton, R.J.; Caffrey, J.; Goethals, P.L.M.; Pegg, J.; Médoc, V.; et al. Intra-and intercontinental variation in the functional responses of a high impact alien invasive fish. Biol. Invasions 2019, 21, 1751–1762. [Google Scholar] [CrossRef]
- Grimm, J.; Dick, J.T.; Verreycken, H.; Jeschke, J.M.; Linzmaier, S.; Ricciardi, A. Context-dependent differences in the functional responses of conspecific native and non-native crayfishes. NeoBiota 2020, 54, 71–88. [Google Scholar] [CrossRef]
- Ricciardi, A.; Iacarella, J.C.; Aldridge, D.C.; Blackburn, T.M.; Carlton, J.T.; Catfors, J.A.; Dick, J.T.A.; Hulme, P.E.; Jeschke, J.M.; Liebhold, A.M.; et al. Four priority areas to advance invasion science in the face of rapid environmental change. Environ. Rev. 2021, 29, 119–141. [Google Scholar] [CrossRef]
- Cucherousset, J.; Horky, P.; Slavík, O.; Ovidio, M.; Arlinghaus, R.; Boulêtreau, S.; Britton, R.; García-Berthou, E.; Santoul, F. Ecology, behaviour, and management of the European catfish. Rev. Fish Biol. Fish. 2018, 28, 177–190. [Google Scholar] [CrossRef] [Green Version]
- Lyach, R.; Remr, J. Changes in recreational catfish Silurus glanis harvest rates between years 1986–2017 in Central Europe. J. Appl. Ichthyol. 2019, 35, 1094–1104. [Google Scholar] [CrossRef]
- Proteau, J.-P.; Hilge, V.; Linhart, O. État actuel et perspectives de la production aquacole des poissons-chats (Siluroidei) en Europe. Aquat. Living Res. 1996, 9, 229–235. [Google Scholar] [CrossRef]
- Copp, G.H.; Robert Britton, J.; Cucherousset, J.; García-Berthou, E.; Kirk, R.; Peeler, E.; Stakėnas, S. Voracious invader or benign feline? A review of the environmental biology of European catfish Silurus glanis in its native and introduced ranges. Fish Fish. 2009, 10, 252–282. [Google Scholar] [CrossRef]
- Carol, J.; Benejam, L.; Benito, J.; García-Berthou, E. Growth and diet of European catfish (Silurus glanis) in early and late invasion stages. Fundam. Appl. Limnol. 2009, 174, 317–328. [Google Scholar] [CrossRef]
- Castaldelli, G.; Pluchinotta, A.; Milardi, M.; Lanzoni, M.; Giari, L.; Rossi, R.; Fano, E.A. Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy: The decline of native fish species in the lower Po basin. Aquat. Conserv. 2013, 23, 405–417. [Google Scholar] [CrossRef]
- Vejřík, L.; Vejříková, I.; Kočvara, L.; Sajdlová, Z.; Hoang The, S.C.; Šmejkal, M.; Peterka, J.; Čech, M. Thirty-year-old paradigm about unpalatable perch egg strands disclaimed by the freshwater top-predator, the European Catfish (Silurus glanis). PLoS ONE 2017, 12, e0169000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillerault, N.; Bouletreau, S.; Iribar, A.; Valentini, A.; Santoul, F. Application of DNA metabarcoding on faeces to identify European catfish Silurus glanis diet: DNA metabarcoding of S. glanis faeces. J. Fish Biol. 2017, 90, 2214–2219. [Google Scholar] [CrossRef] [PubMed]
- Vejřík, L.; Vejříková, I.; Blabolil, P.; Eloranta, A.P.; Kočvara, L.; Peterka, J.; Sajdlová, Z.; Chung, S.H.T.; Šmejkal, M.; Kiljunen, M.; et al. European catfish (Silurus glanis) as a freshwater apex predator drives ecosystem via its diet adaptability. Sci. Rep. 2017, 7, 15970. [Google Scholar] [CrossRef] [PubMed]
- Syväranta, J.; Cucherousset, J.; Kopp, D.; Crivelli, A.; Céréghino, R.; Santoul, F. Dietary breadth and trophic position of introduced European catfish Silurus glanis in the River Tarn (Garonne River basin), southwest France. Aquat. Biol. 2010, 8, 137–144. [Google Scholar] [CrossRef]
- Reading, A.J.; Britton, J.R.; Davies, G.D.; Shinn, A.P.; Williams, C.F. Introduction and spread of non-native parasites with Silurus glanis L. (Teleostei: Siluridae) in UK fisheries. J. Helminthol. 2012, 86, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Rees, A. The Impact of Introduced European Catfish (Silurus glanis L.) in UK Waters: A Three Pond Study. Ph.D. Thesis, University of Hertfordshire, Hatfield, UK, May 2020. [Google Scholar]
- Cucherousset, J.; Boulêtreau, S.; Azémar, F.; Compin, A.; Guillaume, M.; Santoul, F. “Freshwater killer whales”: Beaching behavior of an alien fish to hunt land birds. PLoS ONE 2012, 7, e50840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haubrock, P.J.; Azzini, M.; Balzani, P.; Inghilesi, A.F.; Tricarico, E. When alien catfish meet—Resource overlap between the North American Ictalurus punctatus and immature European Silurus glanis in the Arno River (Italy). Ecol. Freshw. Fish 2020, 29, 4–17. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, M.; Gago, J.; Ribeiro, F. Diet of European Catfish in a newly invaded region. Fishes 2019, 4, 58. [Google Scholar] [CrossRef] [Green Version]
- Alp, A. Diet shift and prey selection of the native European catfish, Silurus glanis, in a Turkish reservoir. J. Limnol. Freshw. Fish. Res. 2017, 3, 15–23. [Google Scholar] [CrossRef]
- Manfredi, P. Cattura di un Silurus glanis nell’Adda Presso Lecco; Natura, Società Italiana Scienze Naturali e del Museo Civico di Storia Naturale, Acquario Civico Milano: Milano, Italy, 1957; pp. 28–30. [Google Scholar]
- Gandolfi, G.; Giannini, M. La Presenza del Silurus glanis nel Fiume Po (Osteichthyes, Siluridae); Natura, Società Italiana Scienze Naturali e del Museo Civico di Storia Naturale, Acquario Civico Milano: Milano, Italy, 1979; pp. 3–6. [Google Scholar]
- Cerri, J.; Ciappelli, A.; Lenuzza, A.; Zaccaroni, M.; Nocita, A. Recreational angling as a vector of freshwater invasions in Central Italy: Perceptions and prevalence of illegal fish restocking. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 38. [Google Scholar] [CrossRef]
- Specchi, M.; Pizzul, E. First observations of Silurus glanis (L. 1758) in the streams of the Isonzo basin (North-eastern Italy) (Osteichthyes, Siluridae). In Gortania; Atti del Museo Friulano di Storia Naturale di Udine: Udine, Italy, 1994; pp. 213–216. [Google Scholar]
- Capra, H.; Pella, H.; Ovidio, M. Individual movements, home ranges and habitat use by native rheophilic cyprinids and non-native catfish in a large regulated river. Fish. Manag. Ecol. 2018, 25, 136–149. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.R.; Cucherousset, J.; Davies, G.D.; Godard, M.J.; Copp, G.H. Non-native fishes and climate change: Predicting species responses to warming temperatures in a temperate region. Freshw. Biol. 2010, 55, 1130–1141. [Google Scholar] [CrossRef]
- Volta, P.; Jeppesen, E.; Sala, P.; Galafassi, S.; Foglini, C.; Puzzi, C.; Winfield, I.J. Fish assemblages in deep Italian subalpine lakes: History and present status with an emphasis on non-native species. Hydrobiologia 2018, 824, 255–270. [Google Scholar] [CrossRef]
- Salmaso, N.; Mosello, R. Limnological research in the deep southern subalpine lakes: Synthesis, directions and perspectives. Adv. Oceanogr. Limnol. 2010, 1, 29–66. [Google Scholar] [CrossRef]
- Grimaldi, E. Lago Maggiore: Effects of exploitation and introductions on the salmonid community. J. Fish. Res. Board Can. 1972, 29, 777–785. [Google Scholar] [CrossRef]
- De Bernardi, R.; Calderoni, A.; Mosello, R. Environmental problems in Italian lakes, and lakes Maggiore and Orta as successful examples of correct management leading to restoration. Int. Ver. Theor. Angew. Limnol. Verh. 1996, 26, 123–138. [Google Scholar] [CrossRef]
- Galassi, S.; Volta, P.; Calderoni, A.; Guzzella, L. Cycling pp’DDT and pp’DDE at a watershed scale: The case of Lago Maggiore (Italy). J. Limnol. 2006, 65, 100–106. [Google Scholar] [CrossRef]
- Volta, P.; Tremolada, P.; Neri, M.C.; Giussani, G.; Galassi, S. Age-dependent bioaccumulation of organochlorine compounds in fish and their selective biotransformation in top predators from Lake Maggiore (Italy). Water Air Soil Pollut. 2009, 197, 193–209. [Google Scholar] [CrossRef]
- Guzzella, L.M.; Novati, S.; Casatta, N.; Roscioli, C.; Valsecchi, L.; Binelli, A.; Parolini, M.; Solcà, N.; Bettinetti, R.; Manca, M.; et al. Spatial and temporal trends of target organic and inorganic micropollutants in Lake Maggiore and Lake Lugano (Italian-Swiss water bodies): Contamination in sediments and biota. Hydrobiologia 2018, 824, 271–290. [Google Scholar] [CrossRef]
- Mazzoni, M.; Buffo, A.; Cappelli, F.; Pascariello, S.; Polesello, S.; Valsecchi, S.; Volta, P.; Bettinetti, R. Perfluoroalkyl acids in fish of Italian deep lakes: Environmental and human risk assessment. Sci. Total Environ. 2019, 653, 351–358. [Google Scholar] [CrossRef]
- Galafassi, S.; Sighicelli, M.; Pusceddu, A.; Bettinetti, R.; Cau, A.; Temperini, M.E.; Gilliber, R.; Ortolani, M.; Pietrelli, L.; Zaupa, S.; et al. Microplastic pollution in perch (Perca fluviatilis, Linnaeus 1758) from Italian south-alpine lakes. Environ. Pollut. 2021, 288, 117782. [Google Scholar] [CrossRef]
- Douglas, M.R.; Brunner, P.C. Biodiversity of central alpine Coregonus (Salmoniformes): Impact of one-hundred years of management. Ecol. Appl. 2002, 12, 154–172. [Google Scholar] [CrossRef]
- Hudson, A.; Vonlanthen, P.; Lundsgaard-Hansen, B.; Denis, R.; Seehausen, O. Untersuchungen zur Verwandtschaft der Felchen aus dem Lago di Como, di Lugano und Maggiore; Rapporto Tecnico per il Dipartimento del Territorio; Servizio Cantonale della Caccia e Pesca: Bellinzona, Switzerland, 2008. [Google Scholar]
- Schindler, D.E.; Scheuerell, M.D. Habitat coupling in lake ecosystems. Oikos 2002, 98, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Volta, P.; Sala, P.; Campi, B.; Cerutti, I. Caratterizzazione tassonomica e funzionale della comunità ittica nel Lago Maggiore con particolare riferimento alle specie alloctone invasive di recente comparsa e alla sovrapposizione della nicchia trofica. In Ricerche Sull’Evoluzione del Lago Maggiore: Aspetti Limnologici; CIPAIS—Consiglio Nazionale delle Ricerche: Verbania, Italy, 2013; pp. 82–98. [Google Scholar]
- Jeppesen, E.; Mehner, T.; Winfield, I.J.; Kangur, K.; Sarvala, J.; Gerdeaux, D.; Rask, M.; Malmquist, H.J.; Holmgren, K.; Volta, P.; et al. Impacts of climate warming on lake fish assemblages: Evidence from 24 European long-term data series. Hydrobiologia 2012, 694, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Vejřík, L.; Vejříková, I.; Kočvara, L.; Blabolil, P.; Peterka, J.; Sajdlová, Z.; Jůza, T.; Šmejkal, M.; Kolařík, T.; Bartoň, D.; et al. The pros and cons of the invasive freshwater apex predator, European catfish Silurus glanis, and powerful angling technique for its population control. J. Environ. Manag. 2019, 241, 374–382. [Google Scholar] [CrossRef]
- Volta, P.; Jepsen, N. The recent invasion of Rutilus rutilus (L.) (Pisces: Cyprinidae) in a large South-Alpine Lake: Lago Maggiore. J. Limnol. 2008, 67, 163–170. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; La Porta, G.; Pedicillo, G.; Lorenzoni, M. Biology of Procambarus clarkii (Girard, 1852) in Lake Trasimeno. Bull. Français Pêche Piscic. 2006, 380–381, 1155–1168. [Google Scholar] [CrossRef] [Green Version]
- Endrizzi, S.; Bruno, M.C.; Maiolini, B. Distribution and biometry of native and alien crayfish in Trentino (Italian Alps). J. Limnol. 2013, 72, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Batistić, M.; Tutman, P.; Bojanić, D.; Skaramuca, B.; Kŏzul, V.; Glavić, N.; Bartulović, V. Diet and diel feeding activity of juvenile pompano (Trachinotus ovatus) (Teleostei: Carangidae) from the southern Adriatic, Croatia. J. Mar. Biol. Assoc. 2005, 85, 1533–1534. [Google Scholar] [CrossRef]
- Hureau, J.C. Biologie compare de quelques poisons antarctiques Nothotheniidae. Bulletin De L’institut Océanographique Monaco 1969, 68, 1–44. [Google Scholar]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef] [Green Version]
- De Santis, V.; Quadroni, S.; Britton, R.J.; Carosi, A.; Roberts, C.G.; Lorenzoni, M.; Crosa, G.; Zaccara, S. Biological and trophic consequences of genetic introgression between endemic and invasive Barbus fishes. Biol. Invasions 2021, 1–18. [Google Scholar] [CrossRef]
- Costello, M.J. Predator feeding strategy and prey importance: A new graphical analysis. J. Fish Biol. 1990, 36, 261–263. [Google Scholar] [CrossRef]
- Amundsen, P.A.; Gabler, H.M.; Staldvik, F.J. A new approach to graphical analysis of feeding strategy from stomach contents data—Modification of the Costello (1990) method. J. Fish Biol. 1996, 48, 607–614. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. 2019. Available online: https://CRAN.R-project.org/package=vegan (accessed on 10 August 2021).
- Slavík, O.; Horký, P.; Bartoš, L.; Kolářová, J.; Randák, T. Diurnal and seasonal behaviour of adult and juvenile European catfish as determined by radio-telemetry in the River Berounka, Czech Republic. J. Fish Biol. 2007, 71, 101–114. [Google Scholar] [CrossRef]
- Daněk, T.; Horký, P.; Kalous, L.; Filinger, K.; Břicháček, V.; Slavík, O. Seasonal changes in diel activity of juvenile European catfish Silurus glanis (Linnaeus, 1758) in Byšická Lake, Central Bohemia. J. Appl. Ichthyol. 2016, 32, 1093–1098. [Google Scholar] [CrossRef]
- Lenhardt, M.B.; Smederevac-Lalić, M.M.; Spasić, S.Z.; Honţ, Ş.; Paraschiv, M.; Iani, M.I.; Nikčević, M.V.; Klimley, P.A.; Suciu, R. Seasonal changes in depth position and temperature of European catfish (Silurus glanis) tracked by acoustic telemetry in the Danube River. Int. Rev. Hydrobiol. 2021, 106, 191–201. [Google Scholar] [CrossRef]
- Slavík, O.; Horký, P.; Maciak, M.; Wackermannová, M. Familiarity, prior residency, resource availability and body mass as predictors of the movement activity of the European catfish. J. Ethol. 2016, 34, 23–30. [Google Scholar] [CrossRef]
- Portt, C.B.; Coker, G.A.; Ming, D.L.; Randall, R.G. A Review of Fish Sampling Methods Commonly Used in Canadian Freshwater Habitats; Canadian Technical Report of Fisheries and Aquatic Sciences 2604; Fisheries and Oceans: Ottawa, ON, Canada, 2006.
- Haubrock, P.J. Seasonal variability in the diet of juvenile European catfish, Silurus glanis, in the Arno River (Italy) in Florence. Fish. Aquat. Life 2021, 29, 54–61. [Google Scholar] [CrossRef]
- Haertel-Borer, S.S.; Zak, D.; Eckmann, R.; Baade, U.; Hölker, F. Population density of the crayfish, Orconectes limosus, in relation to fish and macroinvertebrate densities in a small mesotrophic lake—Implications for the lake’s food web. Int. Rev. Hydrobiol. 2005, 90, 523–533. [Google Scholar] [CrossRef]
- Czarnecki, M.; Andrzejewski, W.; Mastyñski, J. The feeding selectivity of wels (Silurus glanis L.) In lake Góreckie. Archiv. Pol. Fish. 2003, 11, 141–147. [Google Scholar]
- Pohlmann, K.; Grasso, F.W.; Breithaupt, T. Tracking wakes: The nocturnal predatory strategy of piscivorous catfish. Proc. Natl. Acad. Sci. USA 2001, 98, 7371–7374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carol, J.; Zamora, L.; García-Berthou, E. Preliminary telemetry data on the movement patterns and habitat use of European catfish (Silurus glanis) in a reservoir of the River Ebro, Spain. Ecol. Freshw. Fish 2007, 16, 450–456. [Google Scholar] [CrossRef]
- Wheeler, A. The Fishes of the British Isles and North West Europe; Macmillan and Co.: London, UK, 1969. [Google Scholar]
- Lever, C. The Naturalised Animals of the British Isles; Hutchinson and Company, Limited: London, UK, 2007; 600p. [Google Scholar]
- Gorman, O.T.; Yule, D.L.; Stockwell, J.D. Habitat use by fishes of Lake Superior. II. Consequences of diel habitat use for habitat linkages and habitat coupling in nearshore and offshore waters. Aquat. Ecosyst. Health Manag. 2012, 15, 355–368. [Google Scholar] [CrossRef]
- Agnetta, D.; Badalamenti, F.; Colloca, F.; D’Anna, G.; Di Lorenzo, M.; Fiorentino, F.; Garofalo, G.; Gristina, M.; Labanchi, L.; Patti, B.; et al. Benthic-pelagic coupling mediates interactions in Mediterranean mixed fisheries: An ecosystem modeling approach. PLoS ONE 2019, 14, e0210659. [Google Scholar] [CrossRef] [Green Version]
- Guillerault, N.; Delmotte, S.; Boulêtreau, S.; Lauzeral, C.; Poulet, N.; Santoul, F. Does the non-native European catfish Silurus glanis threaten French river fish populations? Freshw. Biol. 2015, 60, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, J.R.; Alexander, M.E.; Dalu, T.; Ellender, B.L.; Kaiser, H.; Weyl, O.L. Using functional responses to quantify interaction effects among predators. Funct. Ecol. 2016, 30, 1988–1998. [Google Scholar] [CrossRef]
- Wysujack, K.; Mehner, T. Can feeding of European catfish prevent cyprinids from reaching a size refuge? Ecol. Freshw. Fish 2005, 14, 87–95. [Google Scholar] [CrossRef]
- Bettinetti, R.; Croce, V.; Galassi, S.; Volta, P. pp´DDT and pp´DDE accumulation in a food chain of Lake Maggiore (Northern Italy): Testing steady-state condition. Environ. Sci. Pollut. Res. Int. 2006, 13, 59–66. [Google Scholar] [CrossRef]
- Saidi, H.; Dresti, C.; Manca, D.; Ciampittiello, M. Climate projections in Lake Maggiore watershed using statistical downscaling model. Clim. Res. 2020, 81, 113–130. [Google Scholar] [CrossRef]
- Fenocchi, A.; Rogora, M.; Sibilla, S.; Ciampittiello, M.; Dresti, C. Forecasting the evolution in the mixing regime of a deep subalpine lake under climate change scenarios through numerical modelling (Lake Maggiore, Northern Italy/Southern Switzerland). Clim. Dyn. 2018, 51, 3521–3536. [Google Scholar] [CrossRef]
- Iacarella, J.C.; Dick, J.T.A.; Alexander, M.E.; Ricciardi, A. Ecological impacts of invasive alien species along temperature gradients: Testing the role of environmental matching. Ecol. Appl. 2015, 25, 706–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Denley, D.; Metaxas, A.; Fennel, K. Community composition influences the population growth and ecological impact of invasive species in response to climate change. Oecologia 2019, 189, 537–548. [Google Scholar]
- Volta, P.; Jeppesen, E.; Campi, B.; Sala, P.; Matthias, E.; Winfield, I.J. The population biology and life history traits of Eurasian ruffe [Gymnocephalus cernuus (L.), Pisces: Percidae] introduced into eutrophic and oligotrophic lakes in Northern Italy. J. Limnol. 2013, 72, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Muscalu, R.; Muscalu, C.; Nagy, M.; Bura, M.; Szelei, Z.T. Studies on wels catfish (Silurus glanis) development during cold season as an auxiliary species in sturgeon recirculated aquaculture systems. Aquac. Aquar. Conserv. Legis. 2010, 3, 362–366. [Google Scholar]
- Larson, E.R.; Graham, B.M.; Achury, R.; Coon, J.J.; Daniels, M.K.; Gambrell, D.K.; Jonasen, K.L.; King, G.D.; LaRacuente, N.; Perrin-Stowe, T.I.; et al. From eDNA to citizen science: Emerging tools for the early detection of invasive species. Front. Ecol. Environ. 2020, 18, 194–202. [Google Scholar] [CrossRef]
- Martelo, J.; da Costa, L.; Ribeiro, D.; Gago, J.; Magalhães, M.; Gante, H.; Alves, M.; Cheoo, G.; Gkenas, C.; Banha, F.; et al. Evaluating the range expansion of recreational non-native fishes in Portuguese freshwaters using scientific and citizen science data. BioInvasions Rec. 2021, 10, 378–389. [Google Scholar] [CrossRef]
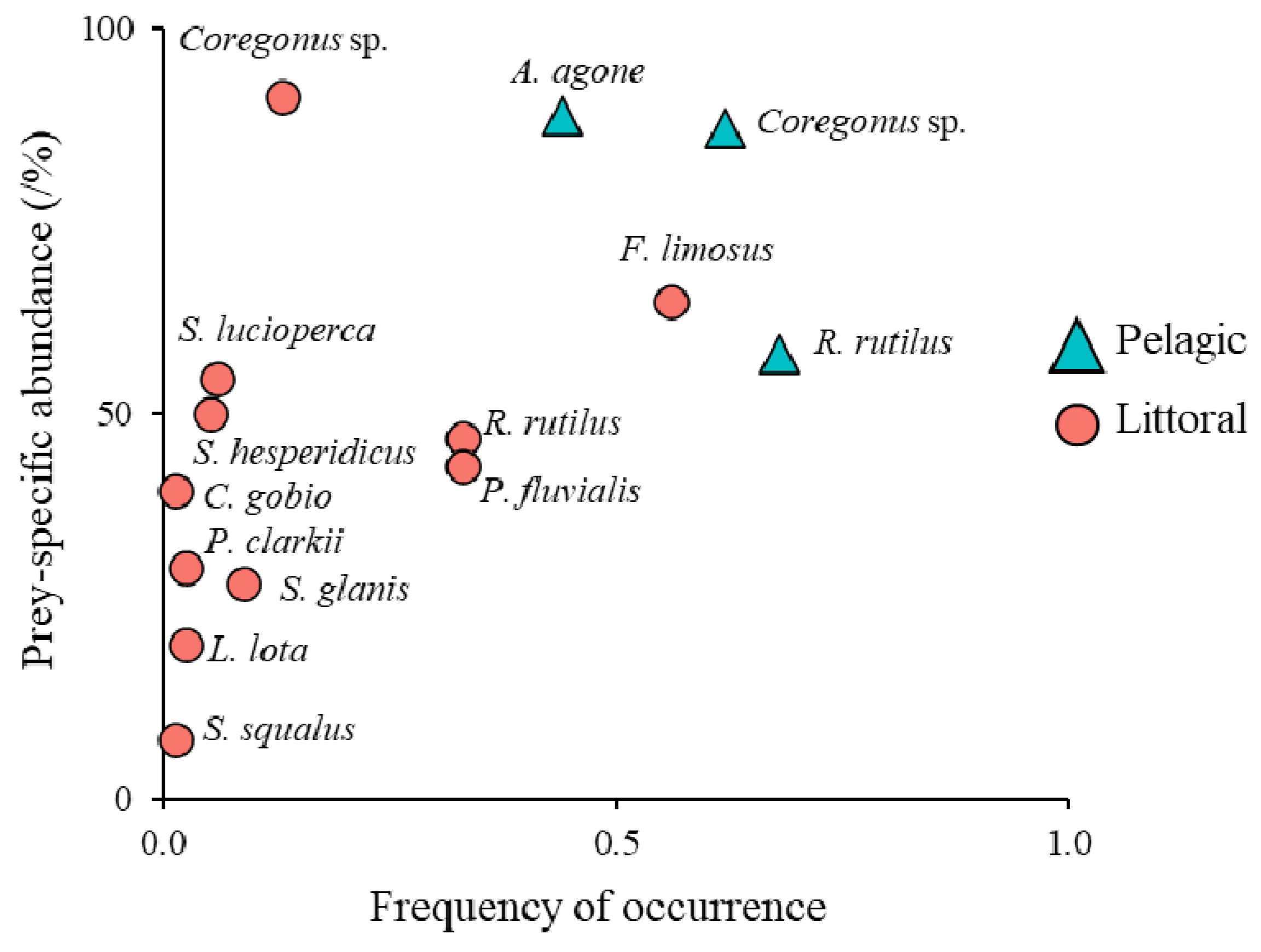
| Site | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| Habitat | Pelagic | Littoral | Pelagic | Littoral | Pelagic | Littoral |
| N | 58 | 41 | 65 | 32 | 12 | 28 |
| TL (cm) | 115.7 (89–150) | 93.4 (68–122) | 106.1 (87–145) | 97.5 (67–125) | 125.3 (99–140) | 97.8 (79–119) |
| W (g) | 10,861.4 (4619.8–22,940.4) | 5623.9 (2022.1–12,165.4) | 8421.3 (4308.5–20,672.8) | 6617.9 (1932.2–13,107.3) | 13,612.5 (6406.2–18,561.5) | 6346.1 (3204.2–11,270.1) |
| NPUE | 0.008 | 0.016 | 0.009 | 0.013 | 0.002 | 0.011 |
| BPUE | 113.9 | 95.9 | 73.0 | 120.2 | 21.8 | 177.0 |
| Habitat | Littoral | Pelagic | ||
|---|---|---|---|---|
| Size Class | Class I | Class II | Class I | Class II |
| Nstomach | 53 | 26 | 31 | 72 |
| VI (%) | 20.9 | 23.5 | 27.9 | 21.7 |
| Mean FI (%) ± 95% CI | 5.81 ± 1.55 | 5.56 ± 1.46 | 4.32 ± 0.94 | 3.53 ± 0.61 |
| Total Nprey | 220 | 86 | 79 | 196 |
| Mean Nprey | 4.15 ± 1.11 | 3.31 ± 0.99 | 2.55 ± 0.57 | 2.72 ± 0.40 |
| Total W (kg) | 11.8 | 15.3 | 7.62 | 28.6 |
| Mean W (g) ± 95% CI | 289 ± 78.9 | 454.7 ± 108.6 | 245.8 ± 57.1 | 397.3 ± 64 |
| Total | Pelagic | Littoral | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Status | N | TL (cm) | W (g) | Wtot (Kg) | FO% | N% | W% | FO% | N% | W% | FO% | N% | W% |
| Coregonus sp. | NN | 156 | 26.1 (15–45) | 169.5 (23.9–789.2) | 26.45 | 40.7 | 26.9 | 42.0 | 62.1 | 49.1 | 65.7 | 12.7 | 6.9 | 10.0 |
| Alosa agone | N | 125 | 21.9 (15–31) | 86 (26.2–242.7) | 10.75 | 25.3 | 21.5 | 17.1 | 44.7 | 45.5 | 30.2 | 0 | 0 | 0 |
| Rutilus rutilus | NN | 67 | 18 (13–31) | 79.9 (13–373.2) | 5.35 | 18.7 | 11.5 | 8.5 | 6.8 | 5.5 | 4.5 | 32.9 | 17.0 | 13.8 |
| Perca fluviatilis | N | 58 | 14.2 (8–24) | 38.7 (5.7–160.6) | 2.45 | 14.8 | 10.0 | 3.9 | 0 | 0 | 0 | 32.9 | 19.0 | 9.1 |
| Sander lucioperca | NN | 6 | 34.8 (25–45) | 428.5 (126.9–808.4) | 2.57 | 2.7 | 1.0 | 4.1 | 0 | 0 | 0 | 6.3 | 2.0 | 9.6 |
| Scardinius hesperidicus | N | 6 | 26.3 (23–31.4) | 296.3 (202–412) | 1.78 | 2.2 | 1.0 | 2.8 | 0 | 0 | 0 | 5.1 | 2.0 | 6.6 |
| Squalius squalus | N | 1 | 35 | 450 | 0.45 | 0.5 | 0.2 | 0.7 | 0 | 0 | 0 | 1.3 | 0.3 | 1.7 |
| Silurus glanis | NN | 7 | 48.1 (49–59) | 584.2 (396.6–786.7) | 5.11 | 3.8 | 1.2 | 8.1 | 0 | 0 | 0 | 8.9 | 2.3 | 19.0 |
| Cottus gobio | N | 2 | 10.5 (9–12) | 11.9 (7.6–16.1) | 0.02 | 0.5 | 0.3 | 0.0 | 0 | 0 | 0 | 1.3 | 0.7 | 0.1 |
| Lota lota | N | 2 | 28.5 (28–29) | 170.7 (161.2–180.2) | 0.34 | 1.1 | 0.3 | 0.5 | 0 | 0 | 0 | 2.5 | 0.7 | 1.3 |
| Faxonius limosus | NN | 148 | 8 (5–13) | 52 (25.5–93.5) | 7.7 | 24.2 | 25.5 | 12.2 | 0 | 0 | 0 | 55.7 | 48.4 | 28.6 |
| Procambarus clarkii | NN | 3 | 13.6 (13–14) | 60 (50–65) | 0.18 | 1.1 | 0.5 | 0.3 | 0 | 0 | 0 | 2.5 | 1.0 | 0.7 |
| Source | df | SS | MS | Pseudo F | R2 | p-Value |
|---|---|---|---|---|---|---|
| Habitat | 1 | 16.02 | 16.02 | 75.65 | 0.25 | 0.0001 |
| Size class | 1 | 0.62 | 0.62 | 2.94 | 0.01 | 0.025 |
| Site | 2 | 1.40 | 0.70 | 3.31 | 0.02 | 0.002 |
| Month | 2 | 3.77 | 1.89 | 8.91 | 0.06 | 0.0001 |
| Habitat × Month | 2 | 2.30 | 1.15 | 5.45 | 0.04 | 0.0001 |
| Size class × Month | 2 | 0.90 | 0.45 | 2.13 | 0.01 | 0.04 |
| Site × Month | 4 | 1.78 | 0.45 | 2.10 | 0.03 | 0.01 |
| Habitat × Size class | 1 | 1.29 | 1.29 | 6.10 | 0.02 | 0.0006 |
| Habitat × Site | 2 | 0.38 | 0.19 | 0.73 | 0.006 | 0.49 |
| Size class × Site | 2 | 1.91 | 0.96 | 4.51 | 0.03 | 0.0002 |
| Habitat × Size class × Site | 2 | 0.67 | 0.34 | 1.58 | 0.01 | 0.12 |
| Habitat × Size class × Month | 2 | 1.15 | 0.57 | 2.70 | 0.02 | 0.009 |
| Habitat × Site × Month | 2 | 0.34 | 0.17 | 0.81 | 0.005 | 0.58 |
| Size class × Site × Month | 1 | 0.10 | 0.10 | 0.46 | 0.001 | 0.79 |
| Residuals | 170 | 32.83 | 0.22 | 0.50 | ||
| Total | 181 | 65.48 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Santis, V.; Volta, P. Spoiled for Choice during Cold Season? Habitat Use and Potential Impacts of the Invasive Silurus glanis L. in a Deep, Large, and Oligotrophic Lake (Lake Maggiore, North Italy). Water 2021, 13, 2549. https://doi.org/10.3390/w13182549
De Santis V, Volta P. Spoiled for Choice during Cold Season? Habitat Use and Potential Impacts of the Invasive Silurus glanis L. in a Deep, Large, and Oligotrophic Lake (Lake Maggiore, North Italy). Water. 2021; 13(18):2549. https://doi.org/10.3390/w13182549
Chicago/Turabian StyleDe Santis, Vanessa, and Pietro Volta. 2021. "Spoiled for Choice during Cold Season? Habitat Use and Potential Impacts of the Invasive Silurus glanis L. in a Deep, Large, and Oligotrophic Lake (Lake Maggiore, North Italy)" Water 13, no. 18: 2549. https://doi.org/10.3390/w13182549







