Gas Pressure Dynamics in Small and Mid-Size Lakes
Abstract
:1. Introduction
2. Environmental Gases and Methods
2.1. Solubility of Gases
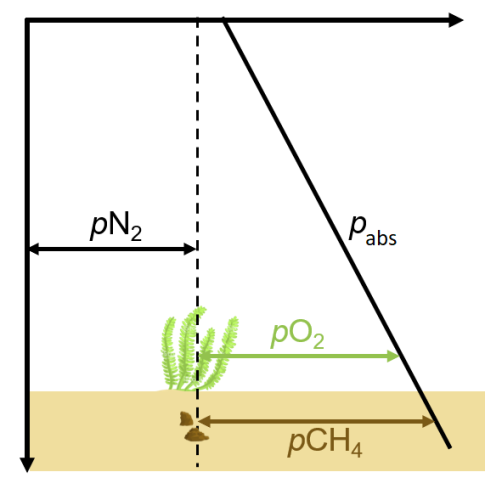
2.2. Gas Pressure, Saturation, Total Gas Pressure
2.3. Relevant Gases
2.4. Simulations
3. Measurements
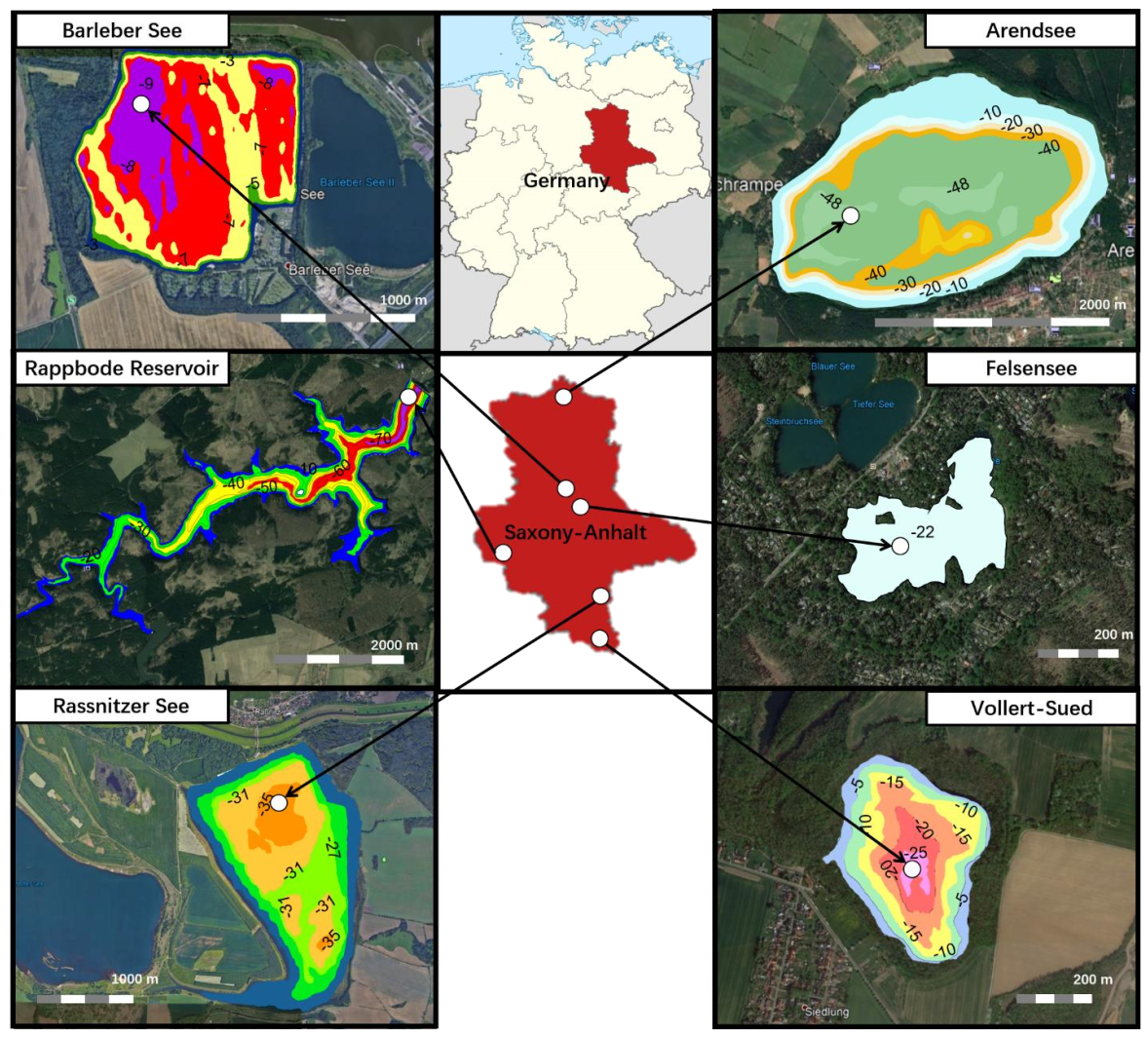
3.1. Investigated Lakes
- Rassnitzer See formed in the abandoned lignite mine Merseburg Ost 1b in the 1990s. Fresh and salty groundwater filled the void, resulting in a salinity-stratified water body, which does not overturn completely in winter. The final water level was reached by introducing freshwater from the nearby river Weisse Elster in 2002 [42,43,44,45].
- Barleber See is the residual of a gravel pit (gravel excavations took place at the beginning of the 1930s). As the local open air swimming facility of the city of Magdeburg, it is intensively used for recreation. Increasing nutrient concentrations led to heavy algal and cyanobacteria blooms and a restoration by alum treatment in 1986 [46,47]. A further use as recreational area required a second chemical treatment of the waters with poly-aluminum chloride from 9th July to 15th October 2019. Inflow and outflow exclusively happen through exchange with groundwater [48].
- Felsensee is a small lake, which formed in a former quarry. After stone production ceased, the quarry filled with groundwater. The water level has reached about 22 m. Higher conductivity groundwater inflows have turned the lake meromictic [49].
- Lake Vollert-Sued is a flooded opencast lignite mine. The pit was (until 1969) used to dispose of wastewater from lignite processing. There is no surficial inflow or outflow but exchange with groundwater balancing the evaporation deficit and causing groundwater contamination in the near vicinity of the lake [50,51]. Hence it is heavily affected through its history as a dumping site. The water has been treated in 1999 to reduce the unpleasant smell and the impact on animals in the area [52,53]. The lake has since been meromictic.
3.2. Equipment
- Arendsee: CTD profiles 2017: YSI 6600 V2, 2019: EXO2 from YSI, USA; optical oxygen sensor; CO2 and gas pressure measurement in a gas volume behind a permeable membrane; CO2 detection by IR spectrometry) CONTROS HydroC® CO2 from Kongsberg Maritime, Germany;
- Rappbode Reservoir: CTM90 from Sea & Sun Technology, Germany; optical oxygen sensor;
- Rassnitzer See: CTM90 from Sea & Sun Technology, Germany; optical oxygen sensor;
- Barleber See: CTM90 from Sea & Sun Technology, Germany; optical oxygen sensor;
- Felsensee: Ocean Seven 316 from Idronaut, Italy; amperometric oxygen sensor;
- Vollert Sued: CTD + O2: Ocean Seven 316 from Idronaut, Italy; amperometric oxygen sensor; gas pressure: (TDG-sensor pressure measurement in a gas filled permeable silicon tube) Hydrolab, USA; CH4, CO2 and N2: samples in GC thermal conductivity detector (see [53]).
4. Results
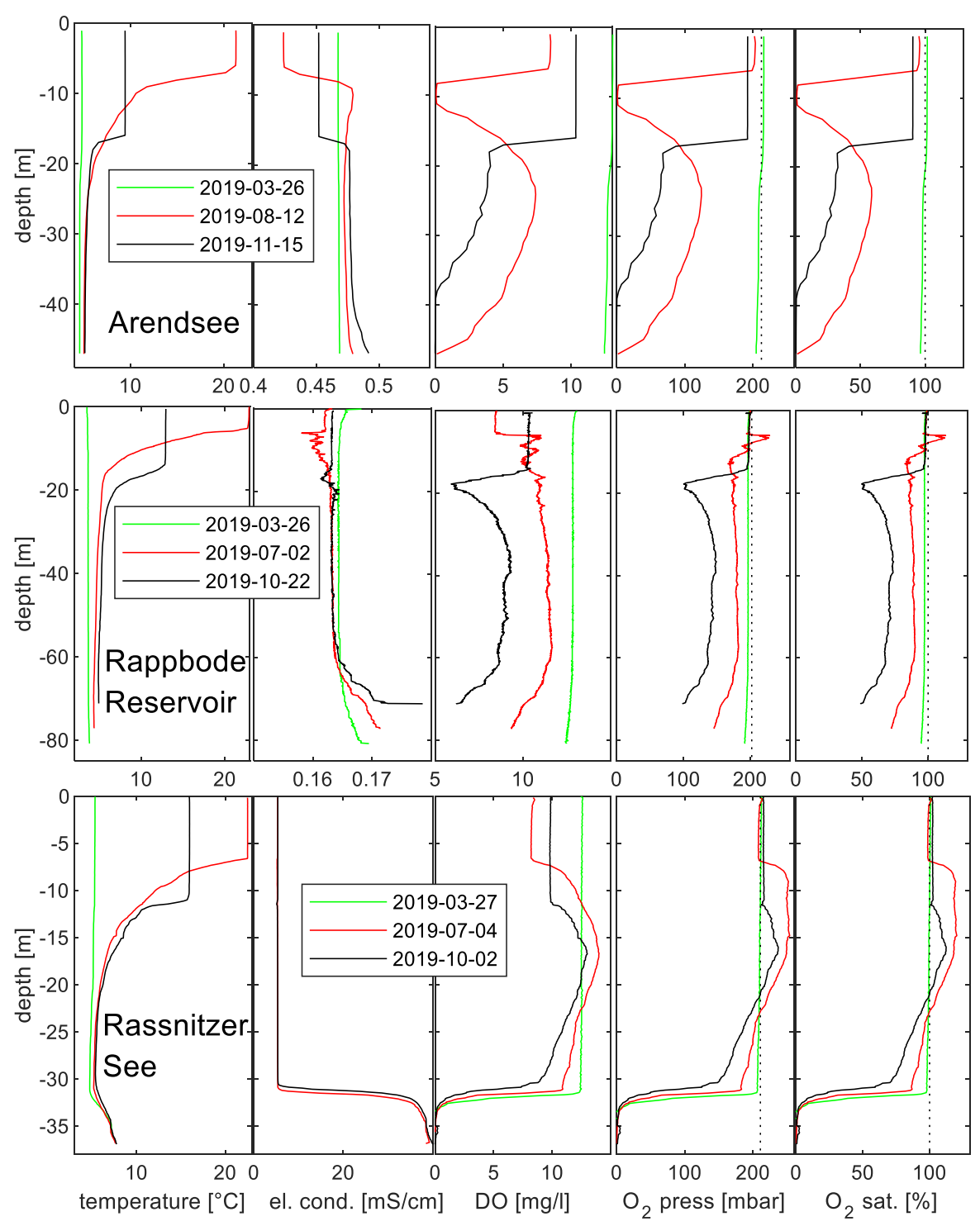
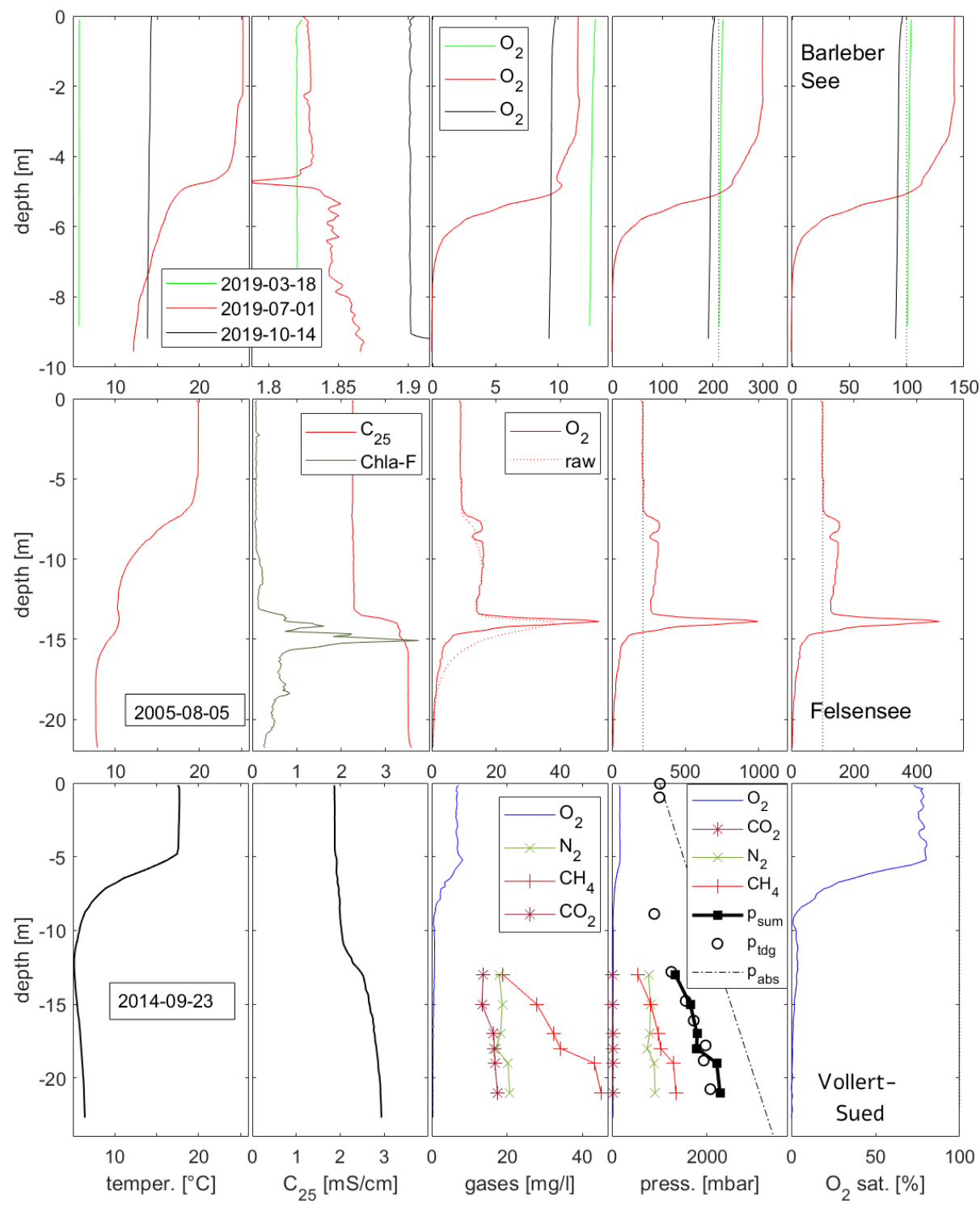
4.1. General Picture of Circulation and Atmospheric Recharge
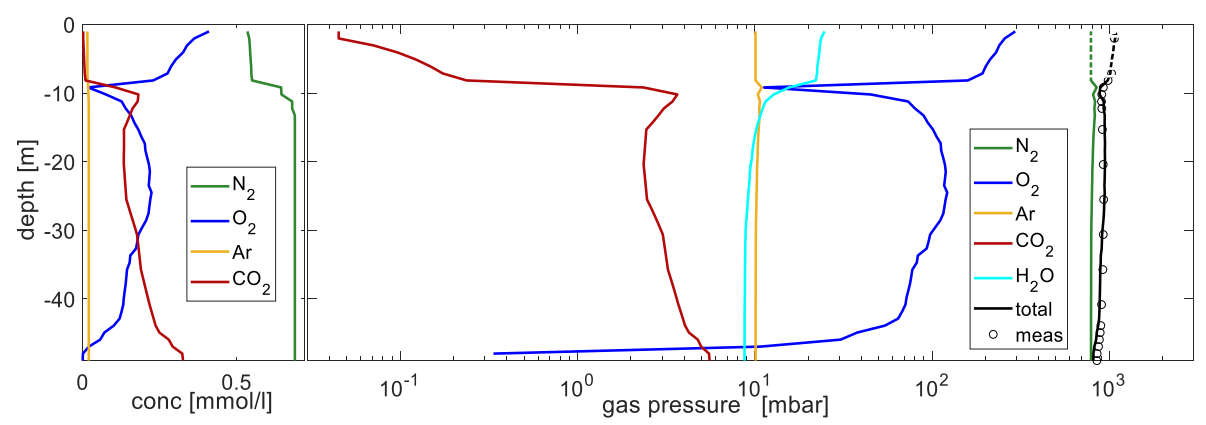
4.2. Complementing Gas Concentrations for Gas Pressure
4.3. Elevated Gas Pressure Observations
5. Discussion
5.1. Ebullition
5.2. Other Mechanisms for Raising Gas Pressure and Releasing Gas Bubbles
5.3. Accumulation of Gases in Monimolimnia
5.4. Final Remarks
- 1.
- Nitrogen always contributes to gas pressure decisively and must be considered;
- 2.
- Oxygen from the atmosphere and from photosynthesis can contribute decisively to the gas pressure;
- 3.
- Methane (mainly from biodegradation or volcanic sources) or
- 4.
- Carbon dioxide (from external sources such as volcanic vents and geochemical reactions) can become an important or even the leading contribution to gas pressure.
- 5.
- Vapor pressure from water;
- 6.
- Argon from atmospheric sources.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.; Enrich-Prast, A. Freshwater Methane Emissions Offset the Continental Carbon Sink. Science 2011, 331, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, J.J.; Prairie, Y.T.; Caraco, N.F.; McDowell, W.H.; Tranvik, L.J.; Striegl, R.G.; Duarte, C.M.; Kortelainen, P.; Downing, J.A.; Middelburg, J.J.; et al. Plumbing the global carbon cycle: Integrating inland waters into the terrestrial carbon budget. Ecosystems 2007, 10, 171–184. [Google Scholar]
- Myhre, G.; Shindell, D.; Bréon, F.; Collins, W.; Fuglestvedt, J.; Huang, J. Anthropogenic and natural radiative forcing. In Climate Change the Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin., D., Plattner, G., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Turner, A.J.; Frankenberg, C.; Wennberg, P.O.; Jacob, D.J. Ambiguity in the causes for decadal trends in atmospheric methane and hydroxyl. Proc. Nat. Acad. Sci. USA 2017, 114, 5367–5372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, P.S.; Catalán, N.; Von Schiller, D.; Grossart, H.-P.; Koschorreck, M.; Obrador, B.; Frassl, M.A.; Karakaya, N.; Barros, N.; Howitt, J.A.; et al. Global CO2 emissions from dry inland waters share common drivers across ecosystems. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: Cambridge, MA, USA, 2001. [Google Scholar]
- Higgins, S.N.; Paterson, M.J.; Hecky, R.E.; Schindler, D.W.; Venkiteswaran, J.J.; Findlay, D.L. Biological Nitrogen Fixation Prevents the Response of a Eutrophic Lake to Reduced Loading of Nitrogen: Evidence from a 46-Year Whole-Lake Experiment. Ecosystems 2018, 21, 1088–1100. [Google Scholar] [CrossRef]
- Miyake, Y. The Possibility and the Allowable Limit of Formation of Air Bubbles in the Sea. Pap. Meteorol. Geophys. 1951, 2, 95–101. [Google Scholar] [CrossRef]
- Koschorreck, M.; Hentschel, I.; Boehrer, B. Oxygen Ebullition from Lakes. Geophys. Res. Lett. 2017, 44, 9372–9378. [Google Scholar] [CrossRef]
- Langenegger, T.; Vachon, D.; Donis, D.; McGinnis, D.F. What the bubble knows: Lake methane dynamics revealed by sediment gas bubble composition. Limnol. Oceanogr. 2019, 64, 1526–1544. [Google Scholar] [CrossRef] [Green Version]
- McGinnis, D.F.; Greinert, J.; Artemov, Y.; Beaubien, S.E.; Wüest, A. Fate of rising methane bubbles in stratified waters: How much methane reaches the atmosphere? J. Geophys. Res. Space Phys. 2006, 111. [Google Scholar] [CrossRef] [Green Version]
- Halbwachs, M.; Sabroux, J.-C.; Grangeon, J.; Kayser, G.; Tochon-Danguy, J.-C.; Felix, A.; Beard, J.-C.; Villevieille, A.; Vitter, G.; Richon, P.; et al. Degassing the “killer lakes” Nyos and Monoun, Cameroon. Eos Transacti. Am. Geophys. 2004, 85, 281–285. [Google Scholar] [CrossRef] [Green Version]
- Sigurdsson, H.; Devine, J.; Tchua, F.; Presser, F.; Pringle, M.; Evans, W. Origin of the lethal gas burst from Lake Monoun, Cameroun. J. Volcanol. Geotherm. Res. 1987, 31, 1–16. [Google Scholar] [CrossRef]
- Kling, G.; Clark, M.A.; Wagner, G.N.; Compton, H.R.; Humphrey, A.M.; Devine, J.D.; Evans, W.C.; Lockwood, J.; Tuttle, M.L.; Koenigsberg, E.J. The 1986 Lake Nyos Gas Disaster in Cameroon, West Africa. Science 1987, 236, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Freeth, S.J.; Kling, G.; Kusakabe, M.; Maley, J.; Tchoua, F.M.; Tietze, K.; Freeth, G.W.K.S.J. Conclusions from Lake Nyos disaster. Nature 1990, 348, 201. [Google Scholar] [CrossRef]
- Wüest, A.; Jarc, L.; Bürgmann, H.; Pasche, N.; Schmid, M. Methane Formation and Future Extraction in Lake Kivu. In Lake Kivu; Springer: Berlin/Heidelberg, Germany, 2012; pp. 165–180. [Google Scholar]
- Lorke, A.; Tietze, K.; Halbwachs, M.; Wüest, A. Response of Lake Kivu stratification to lava inflow and climate warming. Limnol. Oceanogr. 2004, 49, 778–783. [Google Scholar] [CrossRef] [Green Version]
- Boehrer, B.; Yusta, I.; Magin, K.; Sanchez-España, J. Quantifying, assessing and removing the extreme gas load from meromictic Guadiana pit lake, Southwest Spain. Sci. Total Environ. 2016, 563–564, 468–477. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmosph. Chem. Phys. Discuss. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Roedel, W. Physik Unserer Umwelt: Die Atmosphäre; Springer: Heidelberg, Germany, 1992. [Google Scholar]
- Worch, E. Hydrochemistry. Basic Concepts and Exercises; De Gruyter: Berlin, Germany, 2015; (De Gruyter Graduate). [Google Scholar]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The Global Methane Budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- Weiss, R. The solubility of nitrogen, oxygen and argon in water and seawater. Deep Sea Res. Oceanogr. Abstr. 1970, 17, 721–735. [Google Scholar] [CrossRef]
- Jenkins, W.; Lott, D.; Cahill, K. A determination of atmospheric helium, neon, argon, krypton, and xenon solubility concentrations in water and seawater. Mar. Chem. 2019, 211, 94–107. [Google Scholar] [CrossRef]
- Wiesenburg, D.A.; Guinasso, N.L. Equilibrium solubilities of methane, carbon monoxide and hydrogen in water and seawater. J. Chem. Eng. Data 1979, 24, 357–360. [Google Scholar] [CrossRef]
- Weiss, R. Carbon dioxide in water and seawater: The solubility of a non-ideal gas. Mar. Chem. 1974, 2, 203–215. [Google Scholar] [CrossRef]
- Dietz, S.; Lessmann, D.; Boehrer, B. Contribution of Solutes to Density Stratification in a Meromictic Lake (Waldsee/Germany). Mine Water Environ. 2012, 31, 129–137. [Google Scholar] [CrossRef]
- Bärenbold, F.; Boehrer, B.; Grilli, R.; Mugisha, A.; von Tümpling, W.; Umutoni, A.; Schmid, M. Updated dissolved gas concentrations in Lake Kivu from an intercomparison project. PLoS ONE 2020, 15, e0237836. [Google Scholar]
- Kipfer, R.; Aeschbach-Hertig, W.; Peeters, F.; Stute, M. Noble Gases in Lakes and Ground Waters. Rev. Miner. Geochem. 2002, 47, 615–700. [Google Scholar] [CrossRef]
- Christenson, B.; Tassi, F. Gases in Volcanic Lake Environments. In Advances in Volcanology; Springer: Berlin/Heidelberg, Germany, 2015; pp. 125–153. [Google Scholar]
- Worch, E. Wasser und Wasserinhaltsstoffe. Eine Einführung in die Hydrochemie. Wiesbaden, s.l.; Vieweg+Teubner Verlag: Berlin, Germany, 1997. [Google Scholar]
- Boehrer, B.; von Tuempling, W.; Mugisha, A.; Rogemont, C.; Umutoni, A. Reliable reference for the methane concentrations in Lake Kivu at the beginning of industrial exploitation. Hydrol. Earth Syst. Sci. 2019, 23, 4707–4716. [Google Scholar] [CrossRef] [Green Version]
- Alduchov, O.A.; Eskridge, R.E. Improved Magnus Form Approximation of Saturation Vapor Pressure. J. Appl. Meteorolog. 1996, 4, 601–609. [Google Scholar] [CrossRef]
- Wagner, W.; Pruss, A. International Equations for the Saturation Properties of Ordinary Water Substance. Revised According to the International Temperature Scale of Addendum to 1990. J. Phys. Chem. Ref. Data 1993, 16, 783–787. [Google Scholar] [CrossRef] [Green Version]
- Huang, J. A Simple Accurate Formula for Calculating Saturation Vapor Pressure of Water and Ice. J. Appl. Meteorol. Clim. 2018, 57, 1265–1272. [Google Scholar] [CrossRef]
- Hartmann, O.; Schönberg, G. Geologische Entwicklungsgeschichte und Untersuchungsergebnisse am Arendsee. Nachr. Arb. Unterwasserarchäologie 2009, 15, 58–64. [Google Scholar]
- Meinikmann, K.; Lewandowski, J.; Nützmann, G. Lacustrine groundwater discharge: Combined determination of volumes and spatial patterns. J. Hydrol. 2013, 502, 202–211. [Google Scholar] [CrossRef]
- Hannappel, S.; Köpp, C.; Rejman-Rasinska, E. Aufklärung der Ursachen zur Phosphorbelastung des oberflächennahen Grundwassers im hydraulischen Zustrom zum Arendsee in der Altmark. Hydrol. Wasserbewirtsch. 2020, 62, 25–38. [Google Scholar]
- Rinke, K.; Kuehn, B.; Bocaniov, S.; Wendt-Potthoff, K.; Büttner, O.; Tittel, J.; Schultze, M.; Herzsprung, P.; Rönicke, H.; Rink, K.; et al. Reservoirs as sentinels of catchments: The Rappbode Reservoir Observatory (Harz Mountains, Germany). Environ. Earth Sci. 2013, 69, 523–536. [Google Scholar] [CrossRef]
- Pöhlein, F.; Schultze, M.; Donner, J.; Dietze, M.; Wilhayn, S. Reaktionen eines zweistufigen Talsperrensystems auf Veränderungen des Stoffeintrags am Beispiel des Bodeswerks. Forum Hydrol. Wasserbewirtsch. 2004, 34, 137–144. [Google Scholar]
- Wentzky, V.C.; Tittel, J.; Jäger, C.G.; Rinke, K. Mechanisms preventing a decrease in phytoplankton biomass after phosphorus reductions in a German drinking water reservoir-results from more than 50 years of observation. Freshw. Biol. 2018, 63, 1063–1076. [Google Scholar] [CrossRef]
- Boehrer, B.; Heidenreich, H.; Schimmele, M.; Schultze, M. Numerical prognosis for salinity profiles of future lakes in the opencast mine Merseburg-Ost. Int. J. Salt Lake Res. 1998, 7, 235–260. [Google Scholar] [CrossRef]
- Heidenreich, H.; Boehrer, B.; Kater, R.; Hennig, G. Gekoppelte Modellierung geohydraulischer und limnophysikalischer Vorgänge in Tagebaurestseen und ihrer Umgebung. Grundwasser 1999, 4, 49–54. [Google Scholar] [CrossRef]
- Trettin, R.; Gläßer, W.; Lerche, I.; Seelig, U.; Treutler, H.-C. Flooding of lignite mines: Isotope variations and processes in a system influenced by saline groundwater. Isot. Environ. Health Stud. 2006, 42, 159–179. [Google Scholar] [CrossRef]
- Boehrer, B.; Kiwel, U.; Rahn, K.; Schultze, M. Chemocline erosion and its conservation by freshwater introduction to meromictic salt lakes. Limnology 2014, 44, 81–89. [Google Scholar] [CrossRef]
- Kong, X.; Seewald, M.; Dadi, T.; Friese, K.; Mi, C.; Boehrer, B.; Schultze, M.; Rinke, K.; Shatwell, T. Unravelling winter diatom blooms in temperate lakes using high frequency data and ecological modeling. Water Res. 2021, 190, 116681. [Google Scholar] [CrossRef]
- Rönicke, H.; Frassl, M.A.; Rinke, K.; Tittel, J.; Beyer, M.; Kormann, B.; Gohr, F.; Schultze, M. Suppression of bloom-forming colonial cyanobacteria by phosphate precipitation: A 30 years case study in Lake Barleber (Germany). Ecol. Eng. 2021, 162, 106171. [Google Scholar] [CrossRef]
- Hannappel, S.; Strom, A. Methode zur Ermittlung des Phosphoreintrags über das Grundwasser in den Barleber See bei Magdeburg. Korresp. Wasserwirtsch. 2020, 13, 24–30. [Google Scholar]
- Spott, D. Zum Chemismus der künstlichen Seen des Steinbruchgebiets zwischen Plötzky, Gommern und Pretzien. Nat. Nat. Heim. Bez. Halle Magdebg. 1967, 4, 43–53. [Google Scholar]
- Eccarius, B. Groundwater Monitoring and Isotope Investigation of Contaminated Wastewater from an Open Pit Mining Lake. Environ. Geosci. 1998, 5, 156–161. [Google Scholar] [CrossRef]
- Eccarius, B.; Christoph, G.; Ebhardt, G.; Glaser, W. Grundwassermodellierung zur Gefährdungsabschätzung eines phenolverseuchten Tagebaurestsees. Grundwasser 2001, 6, 61–70. [Google Scholar] [CrossRef]
- Stottmeister, U.; Kuschk, P.; Wiessner, A. Full-scale bioremediation and long-term monitoring of a phenolic wastewater disposal lake. Pure Appl. Chem. 2010, 82, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Horn, C.; Metzler, P.; Ullrich, K.; Koschorreck, M.; Boehrer, B. Methane storage and ebullition in monimolimnetic waters of polluted mine pit lake Vollert-Sued, Germany. Sci. Total Environ. 2017, 584–585, 1–10. [Google Scholar] [CrossRef]
- Wentzky, V.C.; Frassl, M.A.; Rinke, K.; Boehrer, B. Metalimnetic oxygen minimum and the presence of Planktothrix rubescens in a low-nutrient drinking water reservoir. Water Res. 2019, 148, 208–218. [Google Scholar] [CrossRef]
- Mi, C.; Shatwell, T.; Ma, J.; Wentzky, V.C.; Boehrer, B.; Xu, Y.; Rinke, K. The formation of a metalimnetic oxygen minimum exemplifies how ecosystem dynamics shape biogeochemical processes: A modelling study. Water Res. 2020, 175, 115701. [Google Scholar] [CrossRef]
- Boehrer, B.; Schultze, M. Stratification of lakes. Rev. Geophys. 2008, 46. [Google Scholar] [CrossRef] [Green Version]
- Kreling, J.; Bravidor, J.; Engelhardt, C.; Hupfer, M.; Koschorreck, M.; Lorke, A. The importance of physical transport and oxygen consumption for the development of a metalimnetic oxygen minimum in a lake. Limnol. Oceanogr. 2016, 62, 348–363. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, W.; Lorke, A.; Woodhouse, J.; Grossart, H.-P. A Fast-Response Automated Gas Equilibrator (FaRAGE) for continuous in situ measurement of CH4 and CO2 dissolved in water. Hydrol. Earth Syst. Sci. 2002, 24, 3871–3880. [Google Scholar] [CrossRef]
- Aeschbach-Hertig, W.; Peeters, F.; Beyerle, U.; Kipfer, R. Interpretation of dissolved atmospheric noble gases in natural waters. Water Resour. Res. 1999, 35, 2779–2792. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.; Tietze, K.; Halbwachs, M.; Lorke, A.; McGinnis, D.F.; Wüest, A. How hazardous is the gas accumulation in Lake Kivu? Arguments for a risk assesment in light of the Nyiragongo volcano eruption of 2002. Acta Vulcanol. 2004, 14, 115–122. [Google Scholar]
- Avagyan, A.; Sahakyan, L.; Meliksetian, K.; Karakhanyan, A.; Lavrushin, V.; Atalyan, T.; Hovakimyan, H.; Avagyan, S.; Tozalakyan, P.; Shalaeva, E.; et al. New evidences of Holocene tectonic and volcanic activity of the western part of Lake Sevan (Armenia). Geol. Q. 2020, 64, 288–303. [Google Scholar] [CrossRef]
- Bastviken, D. Methan. In Encyclopedia of Inland Waters; Elsevier: Oxford, UK, 2009; pp. 783–805. [Google Scholar]
- Ramsey, W.L. Bubble growth from dissolved oxygen near the sea surface. Limnol. Oceanogr. 1962, 7, 1–7. [Google Scholar] [CrossRef]
- D’Aoust, B.G. Technical Note: Total Dissolved Gas Pressure (TDGP) Sensing in the Laboratory. Dissolut. Technol. 2007, 14, 38–41. [Google Scholar] [CrossRef]
- Long, M.H.; Sutherland, K.; Wankel, S.D.; Burdige, D.J.; Zimmerman, R.C. Ebullition of oxygen from seagrasses under supersaturated conditions. Limnol. Oceanogr. 2020, 65, 314–324. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, O.; Colmer, T.D.; Esand-Jensen, K. Underwater Photosynthesis of Submerged Plants—Recent Advances and Methods. Front. Plant. Sci. 2013, 4, 140. [Google Scholar] [CrossRef] [Green Version]
- Mendoza-Lera, C.; Federlein, L.L.; Knie, M.; Mutz, M. The algal lift: Buoyancy-mediated sediment transport. Water Resourc. Res. 2016, 52, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Boudreau, B.P. The physics of bubbles in surficial, soft, cohesive sediments. Mar. Pet. Geol. 2012, 38, 1–18. [Google Scholar] [CrossRef]
- Schmid, M.; Ostrovsky, I.; McGinnis, D.F. Role of gas ebullition in the methane budget of a deep subtropical lake: What can we learn from process-based modeling? Limnol. Oceanogr. 2017, 62, 2674–2698. [Google Scholar] [CrossRef] [Green Version]
- Reeburgh, W.S. Observations of gases in chesapeake bay sediments. Limnol. Oceanogr. 1969, 14, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Brennwald, M.; Kipfer, R.; Imboden, D. Release of gas bubbles from lake sediment traced by noble gas isotopes in the sediment pore water. Earth Planet. Sci. Lett. 2005, 235, 31–44. [Google Scholar] [CrossRef]
- Maeck, A.; Hofmann, H.; Lorke, A. Pumping methane out of aquatic sediments—Ebullition forcing mechanisms in an impounded river. Biogeosciences 2014, 11, 2925–2938. [Google Scholar] [CrossRef] [Green Version]
- Joyce, J.; Jewell, P.W. Physical controls on methane ebullition from reservoirs and lakes. Environ. Eng. Geosci. 2003, 9, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Chanton, J.P.; Martens, C.S.; Kelley, C.A. Gas transport from methane-saturated, tidal freshwater and wetland sediments. Limnol. Oceanogr. 1989, 34, 807–819. [Google Scholar] [CrossRef]
- Varadharajan, C.; Hemond, H.F. Time-series analysis of high-resolution ebullition fluxes from a stratified, freshwater lake. J. Geophys. Res. Space Phys. 2012, 117. [Google Scholar] [CrossRef]
- Gächter, R.; Wehrli, B. Ten years of artificial mixing and oxygenation: No effect on the internal phosphorus loading of two eutrophic lakes. Environ. Sci. Technol. 1998, 32, 3659–3665. [Google Scholar] [CrossRef]
- Bürgi, H.; Stadelmann, P. Change of phytoplankton composition and biodiversity in Lake Sempach before and during restoration. Hydrobiologia 2002, 469, 33–48. [Google Scholar] [CrossRef]
- Cooke, G.D.; Welch, E.B.; Peterson, S.A.; Nicols, S.L. Restoration and Management of Lakes and Reservoirs, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Boehrer, B.; Von Rohden, C.; Schultze, M. Physical Features of Meromictic Lakes: Stratification and Circulation. In Mediterranean-Type Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 15–34. [Google Scholar]
- Schultze, M.; Boehrer, B.; Wendt-Potthoff, K.; Katsev, S.; Brown, E.T. Chemical Setting and Biogeochemical Reactions in Meromictic Lakes. In Mediterranean-Type Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 35–59. [Google Scholar]
- Schmid, M.; Halbwachs, M.; Wehrli, B.; Wüest, A. Weak mixing in Lake Kivu: New insights indicate increasing risk of uncontrolled gas eruption. Geochem. Geophys. Geosyst. 2005, 6, Q07009. [Google Scholar] [CrossRef]
- Caracausi, A.; Nuccio, P.M.; Favara, R.; Nicolosi, M.; Paternoster, M. Gas hazard assessment at the Monticchio crater lakes of Mt. Vulture, a volcano in Southern Italy. Terra Nova 2009, 21, 83–87. [Google Scholar] [CrossRef]
- Tietze, K.; Geyh, M.; Müller, H.; Schröder, L.; Stahl, W.; Wehner, H. The genesis of the methane in Lake Kivu (Central Africa). Acta Diabetol. 1980, 69, 452–472. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Boehrer, B.; Yusta, I. Extreme carbon dioxide concentrations in acidic pit lakes provoked by water/rock interaction. Environ. Sci. Technol. 2014, 48, 4273–4281. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Falagán, C.; Ayala, D.; Wendt-Potthoff, K. Adaptation of Coccomyxa sp. to extremely low light conditions causes deep chlorophyll and oxygen maxima in acidic pit lakes. Microorganisms 2020, 8, 1218. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-España, J.; Yusta, I.; Boehrer, B. Degassing Pit Lakes: Technical Issues and Lessons Learnt from the HERCO2 Project in the Guadiana Open Pit (Herrerías Mine, SW Spain). Mine Water Environ. 2020, 39, 517–534. [Google Scholar] [CrossRef]
- Strøm, K.; Str, K. A Lake with Trapped Sea-Water? Nat. Cell Biol. 1957, 180, 982–983. [Google Scholar] [CrossRef]
- Williams, P.M.; Mathews, W.H.; Pickard, G.L. A Lake in British Columbia containing Old Sea-Water. Nat. Cell Biol. 1961, 191, 830–832. [Google Scholar] [CrossRef]
- Sanderson, B.; Perry, K.; Pedersen, T. Vertical diffusion in meromictic Powell Lake, British Columbia. J. Geophys. Res. Space Phys. 1986, 91, 7647. [Google Scholar] [CrossRef]
- Gulati, R.D.; Zadereev, E.S.; Degermendzhi, A.G. Ecology of meromictic lakes. In Ecological Studies 228; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Zadereev, E.S.; Gulati, R.D.; Camacho, A. Biological and Ecological Features, Trophic Structure and Energy Flow in Meromictic Lakes. In Mediterranean-Type Ecosystems; Springer: Berlin/Heidelberg, Germany, 2017; pp. 61–86. [Google Scholar]
- Oikomonou, A.; Filker, S.; Breiner, H.-W.; Stoeck, T. Protistan diversity in a permanently stratified meromictic lake (Lake Alatsee, SW Germany). Environ. Microbiol. 2015, 17, 2144–2157. [Google Scholar] [CrossRef]
- Nitzsche, H.M. Chemical and Isotope Investigations in Dissolved Gases of a Meromictic Residual Lake. Isotop. Environ. Health Stud. 1999, 35, 63–73. [Google Scholar] [CrossRef]
- Maiss, M.; Ilmberger, J.; Münnich, K.O. Vertical mixing in Überlingersee (Lake Constance) traced by SF 6 and heat. Aquat. Sci. 1994, 56, 329–346. [Google Scholar] [CrossRef]
- Von Rohden, C.; Ilmberger, J.; Boehrer, B. Assessing groundwater coupling and vertical exchange in a meromictic mining lake with an SF6-tracer experiment. J. Hydrol. 2009, 372, 102–108. [Google Scholar] [CrossRef]
- Aeschbach-Hertig, W.; Kipfer, R.; Hofer, M.; Imboden, D.; Wieler, R.; Signer, P. Quantification of gas fluxes from the subcontinental mantle: The example of Laacher See, a maar lake in Germany. Geochim. Cosmochim. Acta 1996, 60, 31–41. [Google Scholar] [CrossRef]
- Bärenbold, F.; Schmid, M.; Brennwald, M.S.; Kipfer, R. Missing atmospheric noble gases in a large, tropical lake: The case of Lake Kivu, East-Africa. Chem. Geol. 2020, 532, 119374. [Google Scholar] [CrossRef]
- Baulch, H.M.; Dillon, P.J.; Maranger, R.; Schiff, S.L. Diffusive and ebullitive transport of methane and nitrous oxide from streams: Are bubble-mediated fluxes important? J. Geophys. Res. Space Phys. 2011, 116. [Google Scholar] [CrossRef]
- Bräuer, K.; Geissler, W.H.; Kämpf, H.; Niedermann, S.; Rman, N. Helium and carbon isotope signatures of gas exhala-tions in the westernmost part of the Pannonian Basin (SE Austria/NE Slovenia): Evidence for active lithospheric mantle de-gassing. Chem. Geol. 2016, 422, 60–70. [Google Scholar] [CrossRef]
- Burton, M.R.; Sawyer, G.M.; Granieri, D. Deep Carbon Emissions from Volcanoes. Rev. Miner. Geochem. 2013, 75, 323–354. [Google Scholar] [CrossRef] [Green Version]
- Chambers, L.; Gooddy, D.; Binley, A. Use and application of CFC-11, CFC-12, CFC-113 and SF6 as environmental tracers of groundwater residence time: A review. Geosci. Front. 2019, 10, 1643–1652. [Google Scholar] [CrossRef]
- Emerson, S.; Quay, P.D.; Stump, C.; Wilbur, D.; Schudlich, R. Chemical tracers of productivity and respiration in the subtropical Pacific Ocean. J. Geophys. Res. Space Phys. 1995, 100, 15873. [Google Scholar] [CrossRef]
- Etiope, G.; Klusman, R. Microseepage in drylands: Flux and implications in the global atmospheric source/sink budget of methane. Glob. Planet. Chang. 2010, 72, 265–274. [Google Scholar] [CrossRef]
- Etiope, G.; Lollar, B.S. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Etiope, G.; Ciotoli, G.; Schwietzke, S.; Schoell, M. Gridded maps of geological methane emissions and their isotopic signature. Earth Syst. Sci. Data 2019, 11, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Frondini, F.; Cardellini, C.; Caliro, S.; Beddini, G.; Rosiello, A.; Chiodini, G. Measuring and interpreting CO2 fluxes at regional scale: The case of the Appennines, Italy. J. Geol. Soc. 2019, 176, 408–416. [Google Scholar] [CrossRef] [Green Version]
- Giggenbach, W.E.; Sano, Y.; Schmincke, H.U. CO2-rich gases from Lakes Nyos and Monoun, Cameroon; Laacher See, Germany; Dieng, Indonesia, and Mt. Gambier, Australia—Variations on a common theme. J. Volcanol. Geotherm. Res. 1991, 45, 311–323. [Google Scholar] [CrossRef]
- Guidotti, T.L. Hydrogen Sulphide. Occup. Med. 1996, 46, 367–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamersley, M.R.; Turk, K.; Leinweber, A.; Gruber, N.; Zehr, J.; Gunderson, T.; Capone, D. Nitrogen fixation within the water column associated with two hypoxic basins in the Southern California Bight. Aquat. Microb. Ecol. 2011, 63, 193–205. [Google Scholar] [CrossRef] [Green Version]
- Hamersley, M.R.; Woebken, D.; Boehrer, B.; Schultze, M.; Lavik, G.; Kuypers, M.M. Water column anammox and denitrification in a temperate permanently stratified lake (Lake Rassnitzer, Germany). Syst. Appl. Microbiol. 2009, 32, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.A.; Zafu, A.; Mather, T.A.; Pyle, D.M.; Barry, P. Spatially Variable CO2 Degassing in the Main Ethiopian Rift: Implications for Magma Storage, Volatile Transport, and Rift-Related Emissions. Geochem. Geophys. Geosystems 2017, 18, 3714–3737. [Google Scholar] [CrossRef]
- Kämpf, H.; Bräuer, K.; Schumann, J.; Hahne, K.; Strauch, G. CO2 discharge in an active, non-volcanic continental rift area (Czech Republic): Characterisation (δ13C, 3He/4He) and quantification of diffuse and vent CO2 emissions. Chem. Geol. 2013, 339, 71–83. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Manning, C.E. Reevaluating carbon fluxes in subduction zones, what goes down, mostly comes up. Proc. Natl. Acad. Sci. USA 2015, 112, E3997–E4006. [Google Scholar] [CrossRef] [Green Version]
- Kerrick, D.M. Present and past nonanthropogenic CO2 degassing from the solid earth. Rev. Geophys. 2001, 39, 565–585. [Google Scholar] [CrossRef]
- Koutsoyiannis, D. Clausius–Clapeyron equation and saturation vapour pressure: Simple theory reconciled with practice. Eur. J. Phys. 2012, 33, 295–305. [Google Scholar] [CrossRef]
- Lee, S.; Kang, N.; Park, M.; Hwang, J.Y.; Yun, S.H.; Jeong, H.Y. A review on volcanic gas compositions related to volcanic activities and non-volcanological effects. Geosci. J. 2018, 22, 183–197. [Google Scholar] [CrossRef]
- Lewis, A.E. Review of metal sulphide precipitation. Hydrometallurgy 2010, 104, 222–234. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Stahl, D.A.; Clark, D.P. Brock Mikrobiologie, 13th ed.; Pearson: Munich, Germany, 2013. [Google Scholar]
- Macpherson, G. CO2 distribution in groundwater and the impact of groundwater extraction on the global C cycle. Chem. Geol. 2009, 264, 328–336. [Google Scholar] [CrossRef]
- Moreira, S.; Schultze, M.; Rahn, K.; Boehrer, B. A practical approach to lake water density from electrical conductivity and temperature. Hydrol. Earth Syst. Sci. 2016, 20, 2975–2986. [Google Scholar] [CrossRef] [Green Version]
- Van de Graaf, A.A.; Mulder, A.; de Bruijn, P.; Jetten, M.S.; Robertson, L.A.; Kuenen, J.G. Anaerobic ammonium oxidation discovered in a denitri-fying fluidized bed reactor. FEMS Microbiol. Ecol. 1995, 16, 177–183. [Google Scholar] [CrossRef]
- Poissant, L.; Constant, P.; Pilote, M.; Canário, J.; O’Driscoll, N.; Ridal, J.; Lean, D. The ebullition of hydrogen, carbon monoxide, methane, carbon dioxide and total gaseous mercury from the Cornwall Area of Concern. Sci. Total Environ. 2007, 381, 256–262. [Google Scholar] [CrossRef]
- Press, F.; Siever, R.; Jordan, T.H.; Grontzinger, J. Understanding Earth, 4th ed.; W. H. Freeman & Co.: New York, NY, USA, 2003. [Google Scholar]
- Raymond, P.A.; Hartmann, J.; Lauerwald, R.; Sobek, S.; McDonald, C.; Hoover, M.; Butman, D.; Striegl, R.; Mayorga, E.; Humborg, C.; et al. Global carbon dioxide emissions from inland waters. Nature 2013, 503, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Ross, K.A.; Gashugi, E.; Gafasi, A.; Wüest, A.; Schmid, M. Characterisation of the Subaquatic Groundwater Discharge That Maintains the Permanent Stratification within Lake Kivu; East Africa. PLoS ONE 2015, 10, e0121217. [Google Scholar] [CrossRef]
- Schink, B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol. Mol. Biol. Rev. 1997, 61, 262–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schink, B. Microbially driven redox reactions in anoxic environments: Pathways, energetics, and biochemical conse-quences. Eng. Life Sci. 2006, 6, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Sobolewski, A. Metal species indicate the potential of constructed wetlands for long-term treatment of metal mine drainage. Ecol. Eng. 1996, 6, 259–271. [Google Scholar] [CrossRef]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1996. [Google Scholar]
| Atmosphere | kH (25 °C) | kH (25 °C) | TE | kH (25 °C) | A1 | A2 | A3 | u | |
|---|---|---|---|---|---|---|---|---|---|
| [%] | [mol/m3/Pa] | [mol/L/bar] | [K] | [-] | Coefficients for Equation (4) | ||||
| N2 | 78.09 | 6.4 × 10−6 | 6.4 × 10−4 | 1300 | 0.016 | −59.6274 | 85.7661 | 24.3696 | 986.9/ 22391 |
| O2 | 20.95 | 1.3 × 10−5 | 1.3 × 10−3 | 1500 | 0.032 | −58.3877 | 85.8079 | 23.8439 | |
| Ar | 0.93 | 1.4 × 10−5 | 1.4 × 10−3 | 1400 | 0.035 | −55.6578 | 82.0262 | 22.5929 | |
| CH4 | 0.00019 | 1.4 × 10−5 | 1.4 × 10−3 | 1600 | 0.035 | −68.8862 | 101.4956 | 28.7314 | |
| CO2 | ~0.039 | 3.3 × 10−4 | 3.3 × 10−2 | 2400 | 0.82 | −58.0931 | 90.5069 | 22.2940 | 1/ 1.01325 |
| Lake Name | Surface Area [km2] | Volume [106 m3] | Max. Depth [m] | Inflow [106 m3/y] | Outflow [106 m3/y] | Residence Time [y] | Age in 2020 [y] | Origin |
|---|---|---|---|---|---|---|---|---|
| Arendsee | 5.1 | 149 | 49 | 6.03 | 2.65 | 56 | >10,000 | Dissolution of salt dome and subsidence |
| Rappbode Reservoir | 3.95 | 113 | 89 | 109.8 | 89.6 | 0.942 | 61 | Artificial dam |
| Rassnitzer See | 3.1 | 68 | 38 | 2.07 | 0.4 | 170 | 18 | Lignite surface mine |
| Barleber See | 1.03 | 6.9 | 9.8 | 1.18 | 0.53 | 13 | 88 | Gravel and sand excavations |
| Felsensee | 0.085 | Unknown (~1) | 22 | little GW flow | little GW flow | Unknown | >55 | Stone quarry |
| Vollert-Sued | 0.09 | 2 | 27 | 0.055 | 0.005 | 400 | 51 | Polluted opencast lignite mine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boehrer, B.; Jordan, S.; Leng, P.; Waldemer, C.; Schwenk, C.; Hupfer, M.; Schultze, M. Gas Pressure Dynamics in Small and Mid-Size Lakes. Water 2021, 13, 1824. https://doi.org/10.3390/w13131824
Boehrer B, Jordan S, Leng P, Waldemer C, Schwenk C, Hupfer M, Schultze M. Gas Pressure Dynamics in Small and Mid-Size Lakes. Water. 2021; 13(13):1824. https://doi.org/10.3390/w13131824
Chicago/Turabian StyleBoehrer, Bertram, Sylvia Jordan, Peifang Leng, Carolin Waldemer, Cornelis Schwenk, Michael Hupfer, and Martin Schultze. 2021. "Gas Pressure Dynamics in Small and Mid-Size Lakes" Water 13, no. 13: 1824. https://doi.org/10.3390/w13131824






