In Situ Monitoring of a Eutrophicated Pond Revealed Complex Dynamics of Nitrogen and Phosphorus Triggered by Decomposition of Floating-Leaved Macrophytes
Abstract
:1. Introduction
2. Materials and Methods
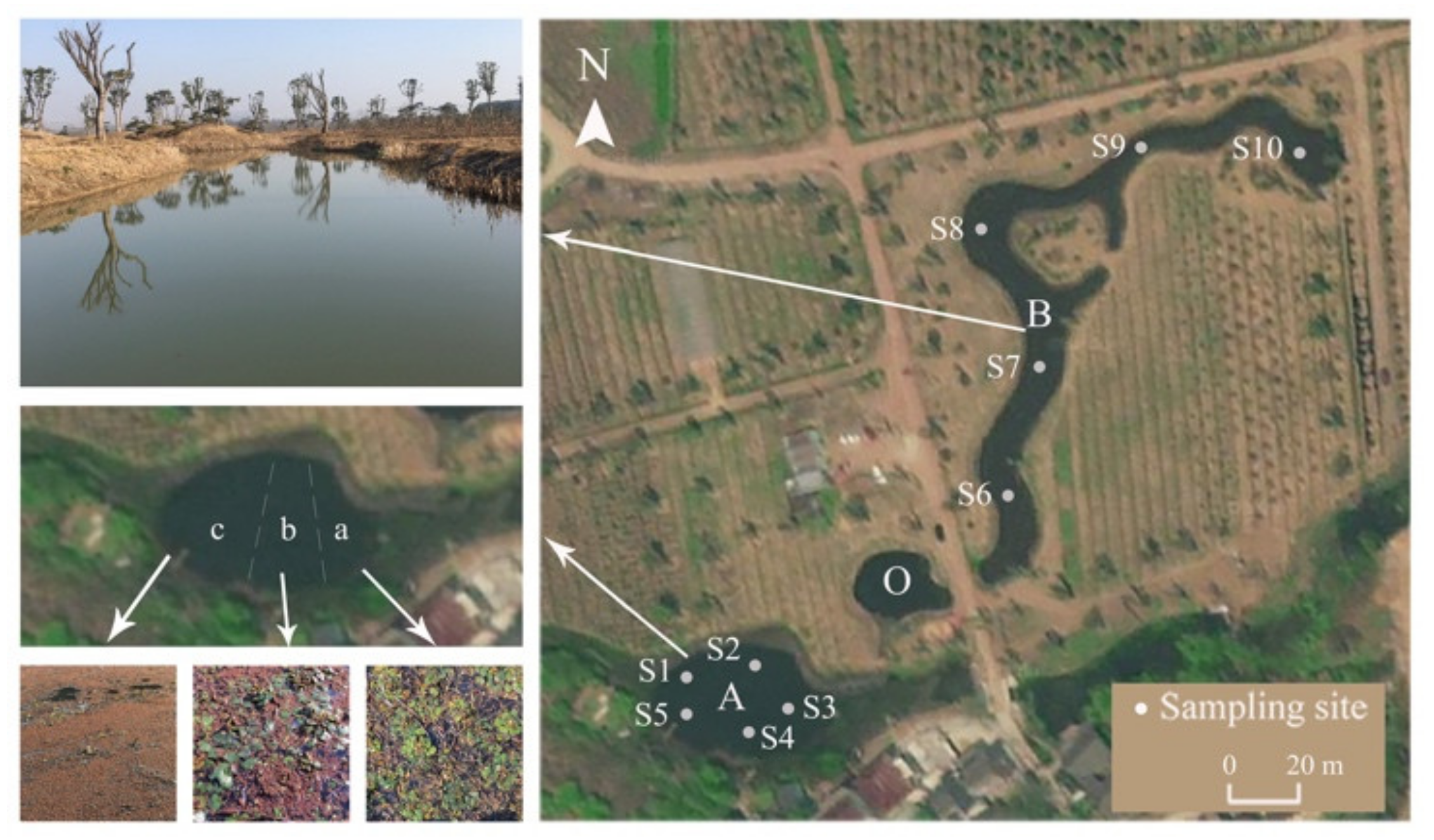
2.1. Study Area
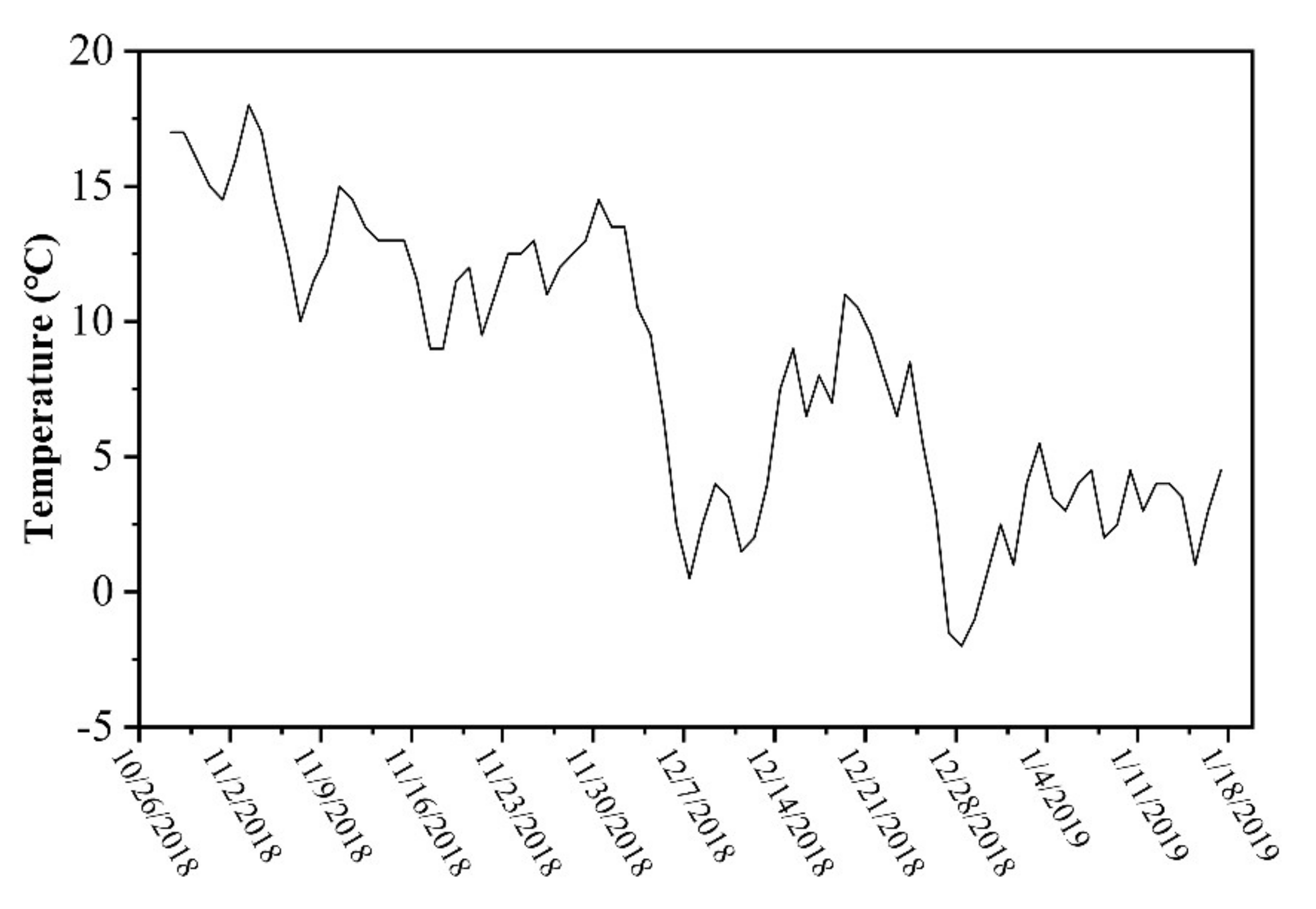
2.2. Sampling and Analyses
2.2.1. Plant Coverage and Biomass
2.2.2. Water Quality Parameters
2.2.3. Sediment Parameters
2.3. Statistical Analysis
3. Results
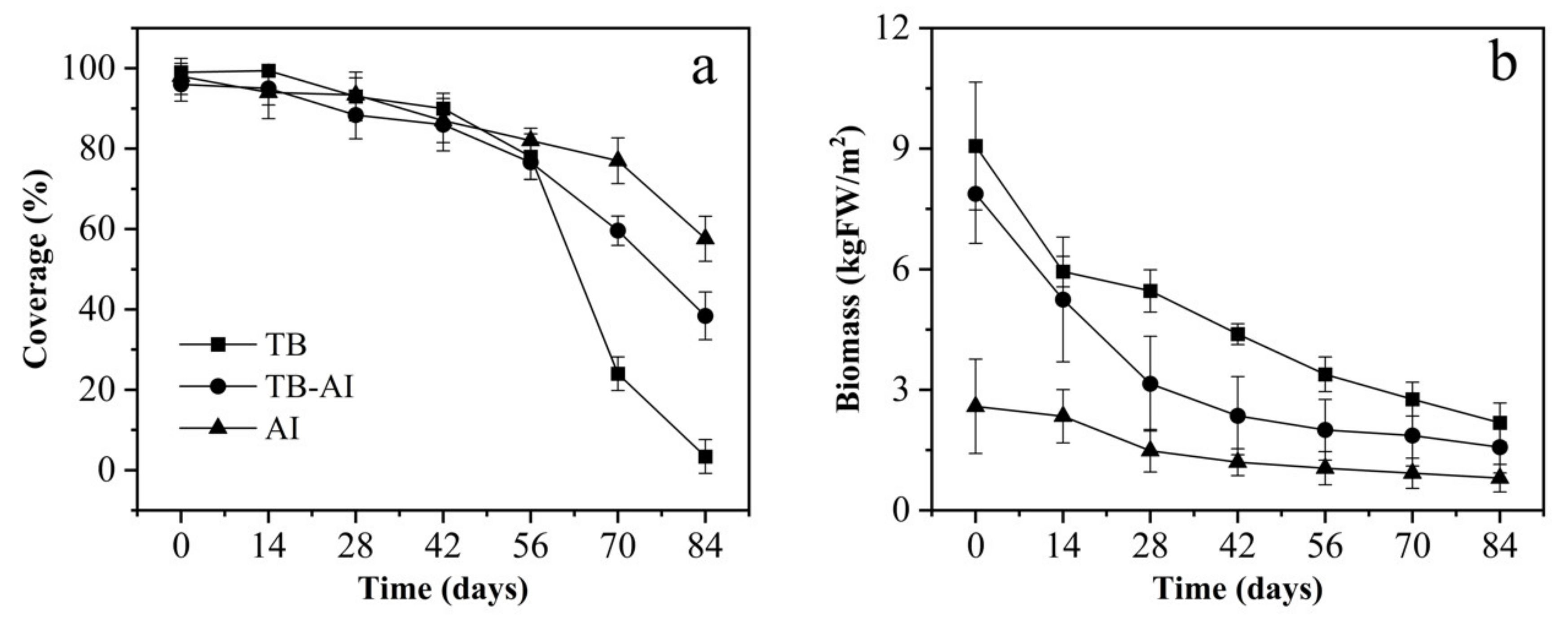
3.1. Coverage and Biomass of Pond A
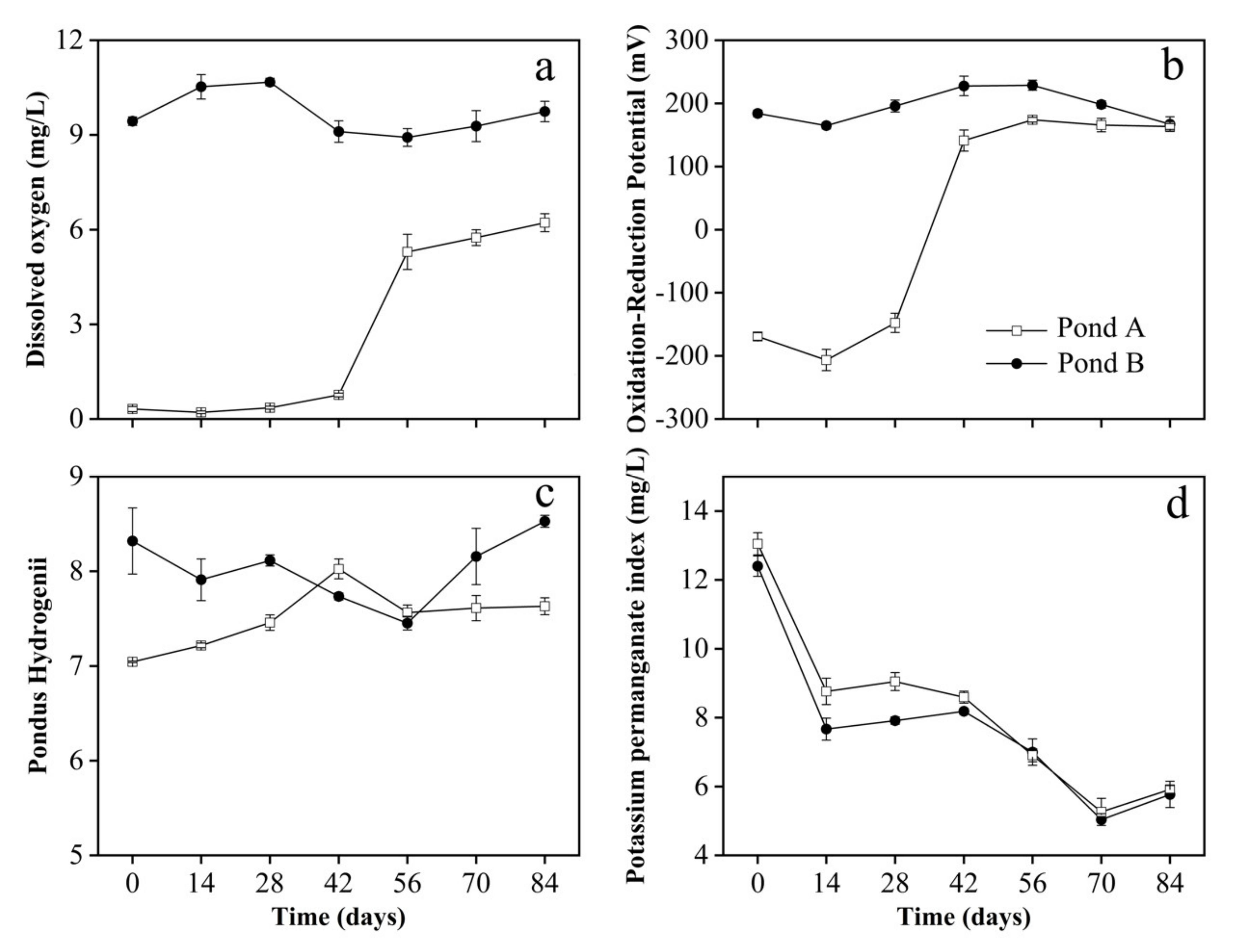
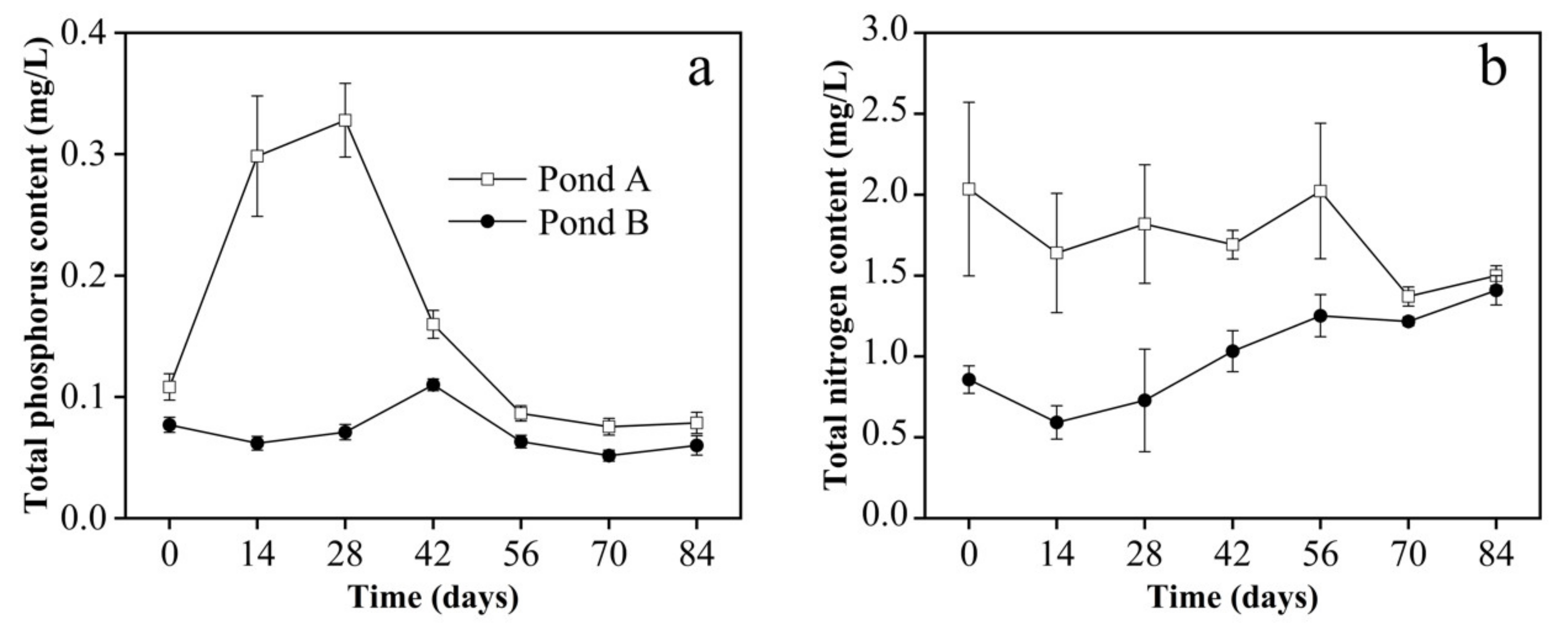
3.2. Water Parameters
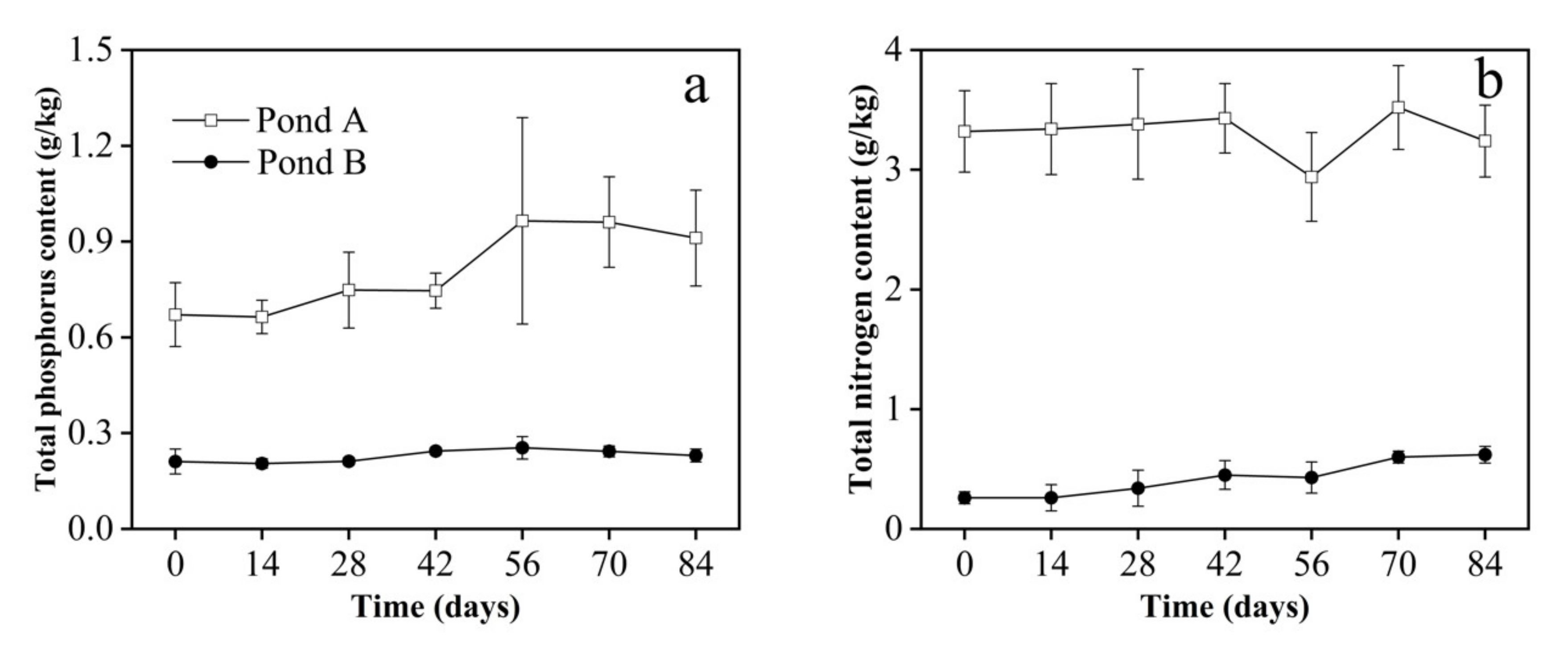
3.3. Sediment Parameters
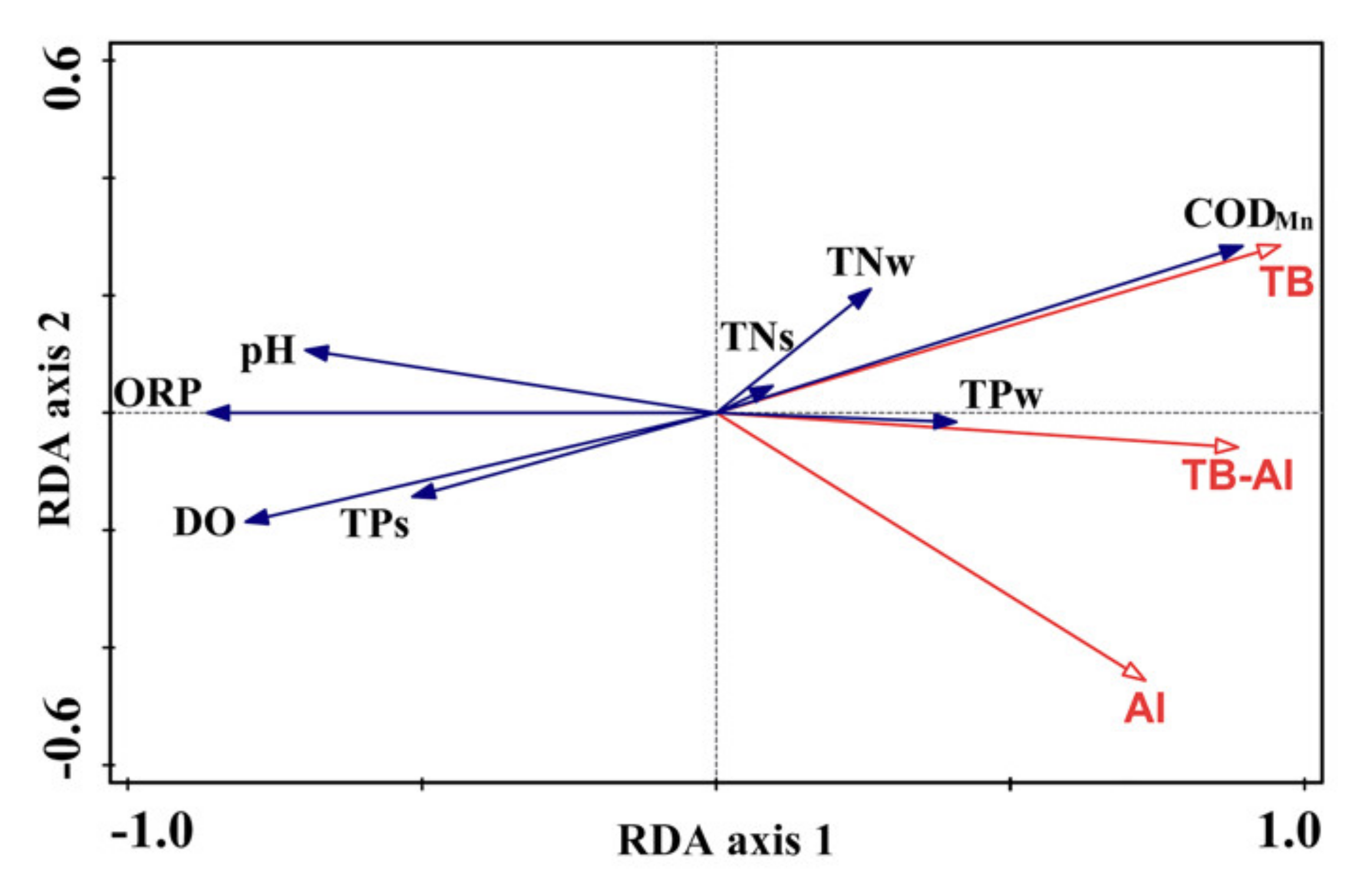
3.4. Correlations between Floating-Leaved Macrophytes and Environmental Parameters
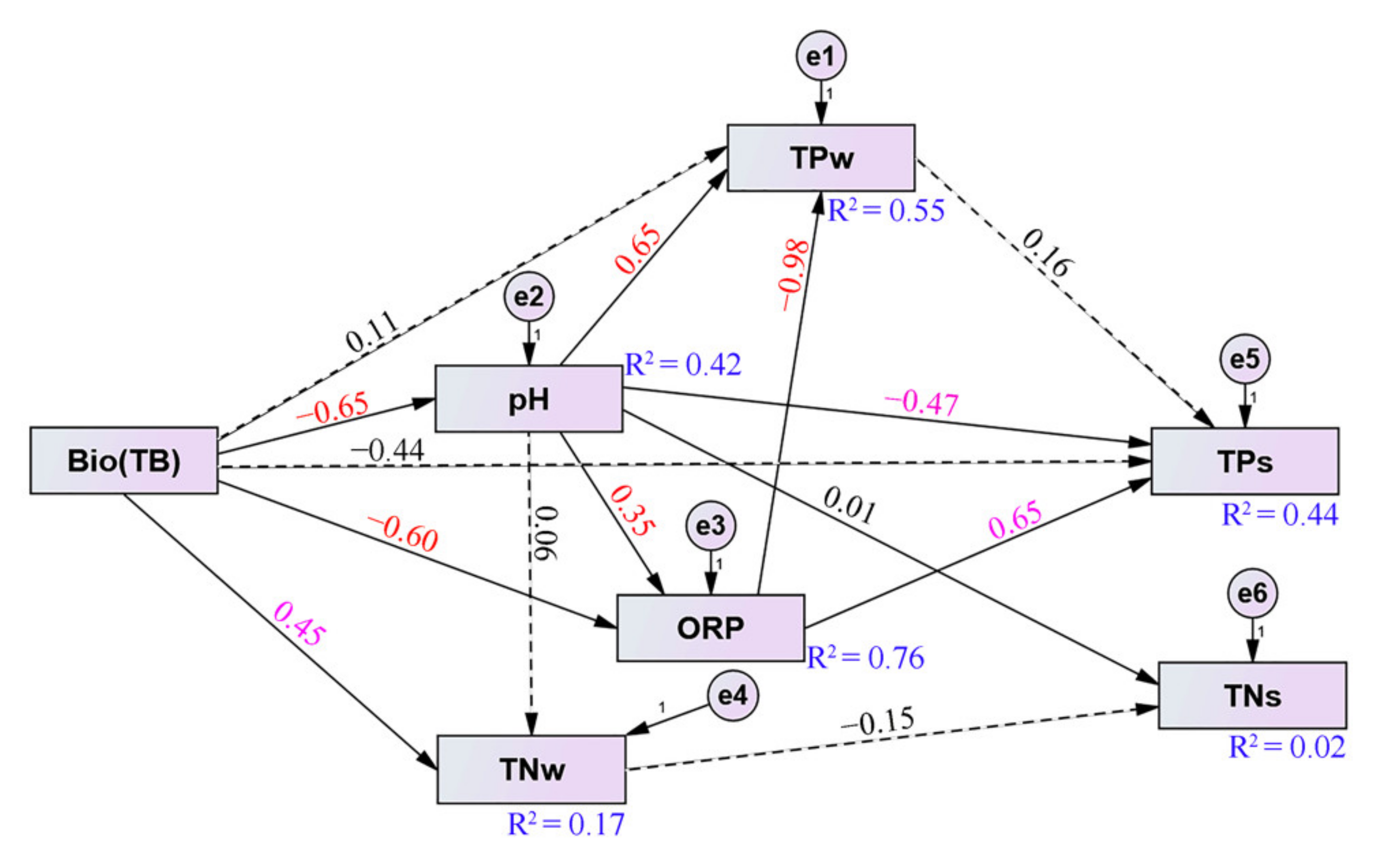
3.5. Structural Equation Model
4. Discussion
4.1. Decomposition Process of Floating-Leaved Macrophytes
4.2. Effect of Floating-Leaved Plant Decomposition on Nitrogen and Phosphorus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brix, H.; Schierup, H.H. The use of aquatic macrophytes in water-pollution control. Ambio 1989, 18, 100–107. [Google Scholar]
- Dhote, S.; Dixit, S. Water quality improvement through macrophytes—A review. Environ. Monit. Assess. 2009, 152, 149–153. [Google Scholar] [CrossRef]
- Wu, S.Q.; He, S.B.; Zhou, W.L.; Gu, J.Y.; Huang, J.C.; Gao, L.; Zhang, X. Decomposition characteristics of three different kinds of aquatic macrophytes and their potential application as carbon resource in constructed wetland. Environ. Pollut. 2017, 231, 1122–1133. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Qin, B.Q.; Gao, G.; Zhu, G.W.; Zhang, Y.L.; Song, Y.Z.; Tang, X.M.; Xu, H.; Deng, J.M. Lake eutrophication and its ecosystem response. Chin. Sci. Bull. 2013, 58, 961–970. [Google Scholar] [CrossRef] [Green Version]
- Ping, Y.M.; Pan, X.; Cui, L.J.; Li, W.; Lei, Y.R.; Zhou, J.; Wei, J.M. Effects of plant growth form and water substrates on the decomposition of submerged litter: Evidence of constructed wetland plants in a greenhouse experiment. Water 2017, 9, 827. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.L.; Cheng, H.F.; Zhan, M.H.; Wang, B.Y. Research progress of aquatic macrophytes in Taihu Lake. Wetl. Sci. 2019, 17, 9–15. [Google Scholar]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef]
- Xiao, L.; Zhu, B.; Kumwimba, M.N.; Jiang, S. Plant soaking decomposition as well as nitrogen and phosphorous release in the water-level fluctuation zone of the Three Gorges Reservoir. Sci. Total Environ. 2017, 592, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Y.; Zhang, M.Y.; Cui, L.J.; Pan, X.; Liu, W.W.; Li, W.; Lei, Y.R. Winter Decomposition of emergent macrophytes affects water quality under ice in a temperate shallow lake. Water 2020, 12, 2640. [Google Scholar] [CrossRef]
- Li, X.; Cui, B.; Yang, Q.; Lan, Y.; Wang, T.; Han, Z. Effects of plant species on macrophyte decomposition under three nutrient conditions in a eutrophic shallow lake, North China. Ecol. Modell. 2013, 252, 121–128. [Google Scholar] [CrossRef]
- Tanner, C.C.; Clayton, J.S.; Upsdell, M.P. Effect of loading rate and planting on treatment of dairy farm wastewaters in constructed wetlands—II. removal of nitrogen and phosphorus. Water Res. 1995, 29, 27–34. [Google Scholar] [CrossRef]
- Qiang, R.R.; Wang, G.X.; Zhang, L.M.; Zhu, M.; Xia, M.F. The continuous effects of decaying Eichhornia crassipes on water quality. Environ. Monit. China 2005, 21, 24–27. [Google Scholar]
- Mutshekwa, T.; Cuthbert, R.N.; Wasserman, R.J.; Murungweni, F.M.; Dalu, T. Nutrient release dynamics associated with native and invasive leaf litter decomposition: A mesocosm experiment. Water 2020, 12, 2350. [Google Scholar] [CrossRef]
- Bocock, K.L.; Gilbert, O.J.W. The disappearance of leaf litter under different woodland conditions. Plant Soil 1957, 9, 179–185. [Google Scholar] [CrossRef]
- Ferreira, V.; Elosegi, A.; Tiegs, S.D.; von Schiller, D.; Young, R. Organic matter decomposition and ecosystem metabolism as tools to assess the functional integrity of streams and rivers-a systematic review. Water 2020, 12, 3523. [Google Scholar] [CrossRef]
- Wu, S.Q.; He, S.B.; Huang, J.C.; Gu, J.Y.; Zhou, W.L.; Gao, L. Decomposition of emergent aquatic plant (Cattail) litter under different conditions and the influence on water quality. Water Air Soil Pollut. 2017, 228, 70. [Google Scholar] [CrossRef]
- Pu, G.Z.; Du, J.J.; Ma, X.; Lv, Y.N.; Jia, Y.Y.; Jia, X.Q.; Tian, X.J. Contribution of ambient atmospheric exposure to Typha angustifolia litter decomposition in aquatic environment. Ecol. Eng. 2014, 67, 144–149. [Google Scholar] [CrossRef]
- Peng, Y.; Yang, W.Q.; Yue, K.; Tan, B.; Huang, C.P.; Xu, Z.F.; Ni, X.Y.; Zhang, L.; Wu, F.Z. Temporal dynamics of phosphorus during aquatic and terrestrial litter decomposition in an alpine forest. Sci. Total Environ. 2018, 642, 832–841. [Google Scholar] [CrossRef]
- Woodward, G.; Gessner, M.O.; Giller, P.S.; Gulis, V.; Hladyz, S.; Lecerf, A.; Malmqvist, B.; McKie, B.G.; Tiegs, S.D.; Cariss, H.; et al. Continental-scale effects of nutrient pollution on stream ecosystem functioning. Science 2012, 336, 1438–1440. [Google Scholar] [CrossRef] [Green Version]
- Knorr, M.; Frey, S.D.; Curtis, P.S. Nitrogen additions and litter decomposition: A meta-analysis. Ecology 2005, 86, 3252–3257. [Google Scholar] [CrossRef]
- Bao, S.D. Soil and Agricultural Chemistry Analysis, 3rd ed.; China Agriculture Press: Beijing, China, 2007; pp. 268–270. [Google Scholar]
- Wu, J.Z.; Ge, Y.; Wang, X.Y. UV absorptio photometric determination of total nitrogen in plant after K2S2O8 oxidation. Phys. Test. Chem. Anal. Part B 2000, 36, 166–167. [Google Scholar]
- Wu, H.T.; Lu, X.G.; Yang, Q.; Jiang, M.; Tong, S.Z. The early-stage litter decomposition and its influencing factors in the wetland of the Sanjiang Plain, China. Acta Ecol. Sin. 2007, 27, 4027–4035. [Google Scholar]
- Overbeek, C.C.; van der Geest, H.G.; van Loon, E.E.; Klink, A.D.; van Heeringen, S.; Harpenslager, S.F.; Admiraal, W. Decomposition of aquatic pioneer vegetation in newly constructed wetlands. Ecol. Eng. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Carvalho, C.; Hepp, L.U.; Palma-Silva, C.; Albertoni, E.F. Decomposition of macrophytes in a shallow subtropical lake. Limnologica 2015, 53, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Wang, D. Effects of micro-, meio- and macroinvertebrates associated with burial on the decomposition of an aquatic macrophyte (Vallisneria natans) in a eutrophic shallow lake in China. Mar. Freshw. Res. 2019, 70, 554–562. [Google Scholar] [CrossRef]
- Passerini, M.D.; Cunha-Santino, M.B.; Bianchini, I. Oxygen availability and temperature as driving forces for decomposition of aquatic macrophytes. Aquat. Bot. 2016, 130, 1–10. [Google Scholar] [CrossRef]
- Christophoridis, C.; Fytianos, K. Conditions affecting the release of phosphorus from surface lake sediments. J. Environ. Qual. 2006, 35, 1181–1192. [Google Scholar] [CrossRef]
- Enriquez, S.; Duarte, C.M.; Sandjensen, K. Patterns in decomposition rates among photosynthetic organisms: The importance of detritus C:N:P content. Oecologia 1993, 94, 457–471. [Google Scholar] [CrossRef]
- Chimney, M.J.; Pietro, K.C. Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecol. Eng. 2006, 27, 301–321. [Google Scholar] [CrossRef]
- Mbaka, J.G.; Mwaniki, M.W. A critical review of the effect of water storage reservoirs on organic matter decomposition in rivers. Environ. Rev. 2017, 25, 193–198. [Google Scholar] [CrossRef]
- Hang, Q.; Wang, H.; Chu, Z.; Ye, B.; Li, C.; Hou, Z. Application of plant carbon source for denitrification by constructed wetland and bioreactor: Review of recent development. Environ. Sci. Pollut. Res. 2016, 23, 8260–8274. [Google Scholar] [CrossRef] [PubMed]
- Attermeyer, K.; Premke, K.; Hornick, T.; Hilt, S.; Grossart, H.-P. Ecosystem-level studies of terrestrial carbon reveal contrasting bacterial metabolism in different aquatic habitats. Ecology 2013, 94, 2754–2766. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.J.; Tian, W.L.; Guan, X.D.; Chen, X. The characteristics of the composition of various phosphorus forms in lake surface sediments in Wuhan city. J. Ecol. Rural Environ. 2020, 36, 1540–1548. [Google Scholar]
- Xiang, J.; Xu, L.P.; Li, H.P.; Li, J.; Tang, J.S. Research on and application of oxidation-reduction potential. Earth Environ. 2014, 42, 430–436. [Google Scholar]
- Parsons, C.T.; Rezanezhad, F.; O’Connell, D.W.; Van Cappellen, P. Sediment phosphorus speciation and mobility under dynamic redox conditions. Biogeosciences 2017, 14, 3585–3602. [Google Scholar] [CrossRef] [Green Version]
- Petticrew, E.L.; Arocena, J.M. Evaluation of iron-phosphate as a source of internal lake phosphorus loadings. Sci. Total Environ. 2001, 266, 87–93. [Google Scholar] [CrossRef]
- Ding, S.M.; Wang, Y.; Wang, D.; Li, Y.Y.; Gong, M.D.; Zhang, C.S. In situ, high-resolution evidence for iron-coupled mobilization of phosphorus in sediments. Sci. Rep. 2016, 6, 24341. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Feng, M.Q.; Hao, X.Y. Study on effect of overlying water environment on the nitrogen and phosphorus release of sediment. Environ. Pollut. Control 2020, 42, 7–11. [Google Scholar]
- Jin, X.C.; Wang, S.R.; Pang, Y.; Wu, F.C. Phosphorus fractions and the effect of pH on the phosphorus release of the sediments from different trophic areas in Taihu Lake, China. Environ. Pollut. 2006, 139, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Z.S.; Zhang, Y.L.; Dai, Z.G.; He, F.; Wu, Z.B. Effects of varying environmental conditions on release of sediment phosphorus in West Lake, Hang Zhou, China. Acta Hydrobiol. Sin. 2017, 41, 1354–1361. [Google Scholar]
- Chen, J.; Xu, H.; Zhan, X.; Zhu, G.W.; Qin, B.Q.; Zhang, Y.L. Mechanisms and research methods of phosphorus migration and transformation across sediment-water interface. J. Lake Sci. 2019, 31, 907–918. [Google Scholar]
- Zeng, J.; Yang, L.Y.; Xiao, L.; Yin, D.Q.; Qin, B.Q. Biogeochemical cycling of nitrogen in lakes and the role of microorganisms in conversion of nitrogen compounds. J. Lake Sci. 2007, 19, 382–389. [Google Scholar]
- Luo, P.; Tong, X.; Liu, F.; Huang, M.; Xu, J.; Xiao, R.L.; Wu, J.S. Nutrients release and greenhouse gas emission during decomposition of Myriophyllum aquaticum in a sediment-water system. Environ. Pollut. 2020, 260, 114015. [Google Scholar] [CrossRef]
| Parameters | TN/(g/kgDW) | TP/(g/kgDW) | TOC/(g/kgDW) | TOC/TN | TOC/TP |
|---|---|---|---|---|---|
| AI | 22.12 ± 1.74 b | 0.75 ± 0.08 b | 265.2 ± 36.14 a | 12.09 ± 2.08 a | 357.03 ± 68.25 a |
| TB | 34.25 ± 1.92 a | 2.13 ± 0.28 a | 240.9 ± 34.21 a | 7.06 ± 1.15 b | 114.89 ± 23.99 b |
| Parameters | Bio | DO | ORP | TPW | TPS | TNW | TNS | pH | CODMn |
|---|---|---|---|---|---|---|---|---|---|
| Bio | 1 | ||||||||
| DO | −0.937 ** | 1 | |||||||
| ORP | −0.777 ** | 0.833 ** | 1 | ||||||
| TPW | 0.726 ** | −0.796 ** | −0.710 ** | 1 | |||||
| TPS | −0.694 ** | 0.756 ** | 0.603 ** | −0.609 ** | 1 | ||||
| TNW | 0.386 * | −0.376 * | −0.131 | 0.289 | −0.490 ** | 1 | |||
| TNS | 0.078 | −0.080 | −0.153 | 0.073 | −0.134 | −0.055 | 1 | ||
| pH | −0.778 ** | 0.741 ** | 0.593 ** | −0.395 * | 0.527 ** | −0.262 | 0.063 | 1 | |
| CODMn | 0.895 ** | −0.838 ** | −0.770 ** | −0.717 ** | −0.662 ** | 0.559 ** | 0.042 | −0.662 ** | 1 |
| Fitting Index | χ2/df | GFI | NFI | CFI | IFI | RMSEA |
|---|---|---|---|---|---|---|
| Standard value | <3 | >0.9 | >0.9 | >0.9 | >0.9 | <0.08 |
| Modified fitting value | 1.165 | 0.940 | 0.936 | 0.989 | 0.990 | 0.070 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, C.; Li, J.; Zhang, C.; Pan, F.; Zhou, C. In Situ Monitoring of a Eutrophicated Pond Revealed Complex Dynamics of Nitrogen and Phosphorus Triggered by Decomposition of Floating-Leaved Macrophytes. Water 2021, 13, 1751. https://doi.org/10.3390/w13131751
Yi C, Li J, Zhang C, Pan F, Zhou C. In Situ Monitoring of a Eutrophicated Pond Revealed Complex Dynamics of Nitrogen and Phosphorus Triggered by Decomposition of Floating-Leaved Macrophytes. Water. 2021; 13(13):1751. https://doi.org/10.3390/w13131751
Chicago/Turabian StyleYi, Cuiyu, Jiafeng Li, Chenrong Zhang, Fan Pan, and Changfang Zhou. 2021. "In Situ Monitoring of a Eutrophicated Pond Revealed Complex Dynamics of Nitrogen and Phosphorus Triggered by Decomposition of Floating-Leaved Macrophytes" Water 13, no. 13: 1751. https://doi.org/10.3390/w13131751






