Quantitative Lasting Effects of Drought Stress at a Growth Stage on Soybean Evapotranspiration and Aboveground BIOMASS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Crop Management
2.3. Experimental Design
2.4. Measurements
2.4.1. Pot Weight
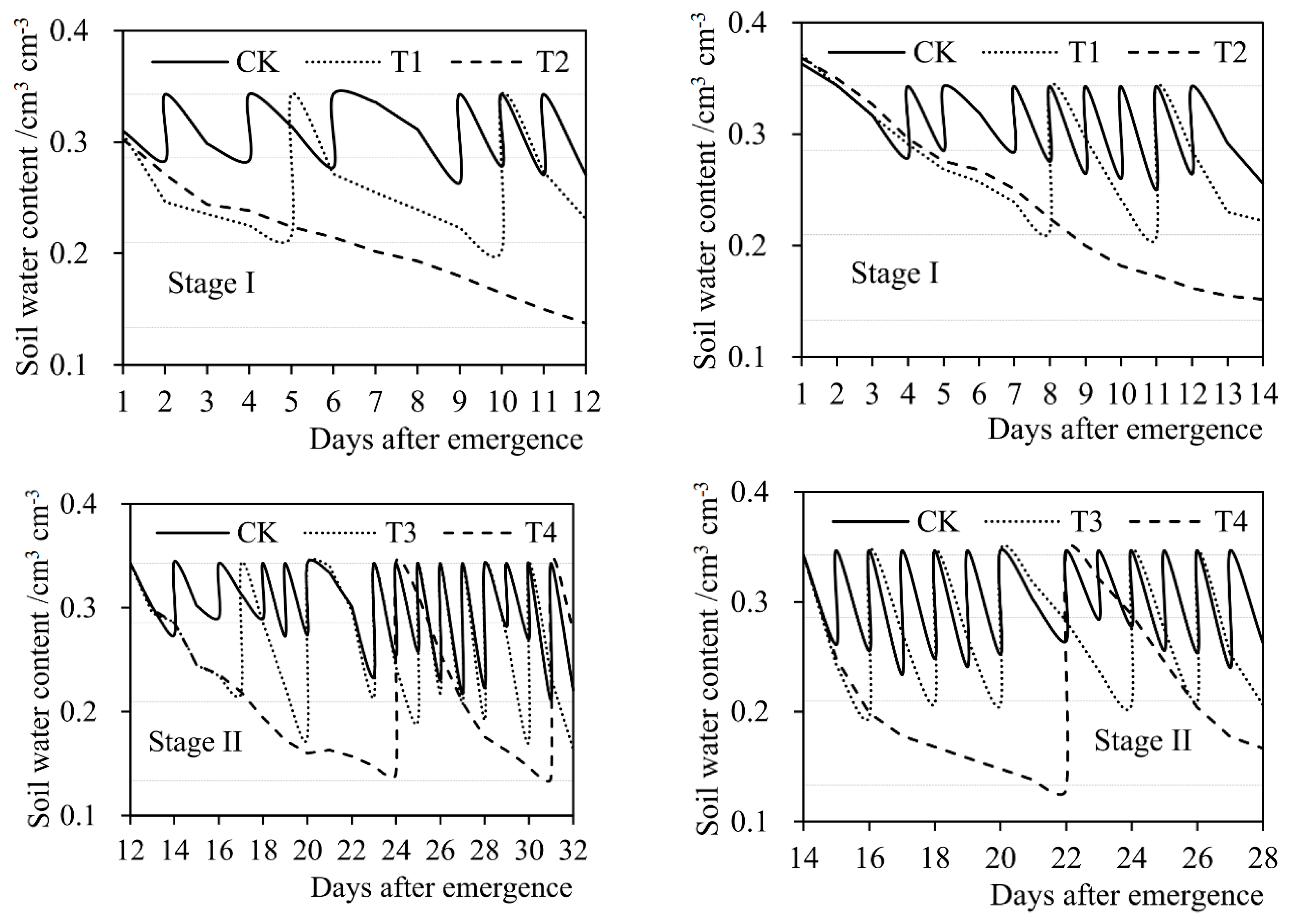
2.4.2. Soil Water Content
2.4.3. Irrigation Amount
2.4.4. Evapotranspiration
2.4.5. Aboveground Biomass
3. Results and Discussion
3.1. Current Influences of Drought Stress on Evapotranspiration
3.2. After-Effects of Drought Stress on Evapotranspiration
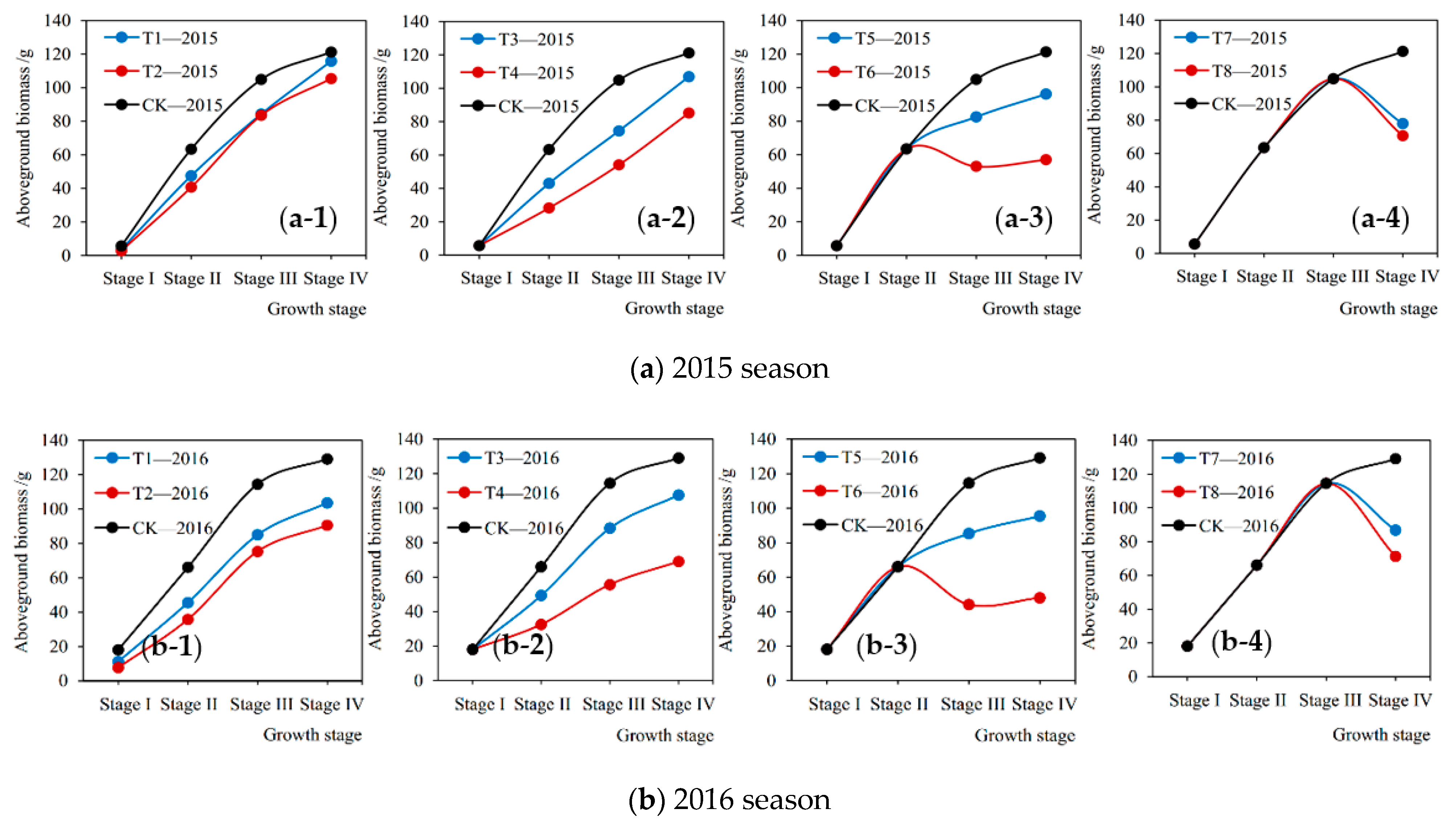
3.3. Current Influences of Drought Stress on Aboveground Biomass
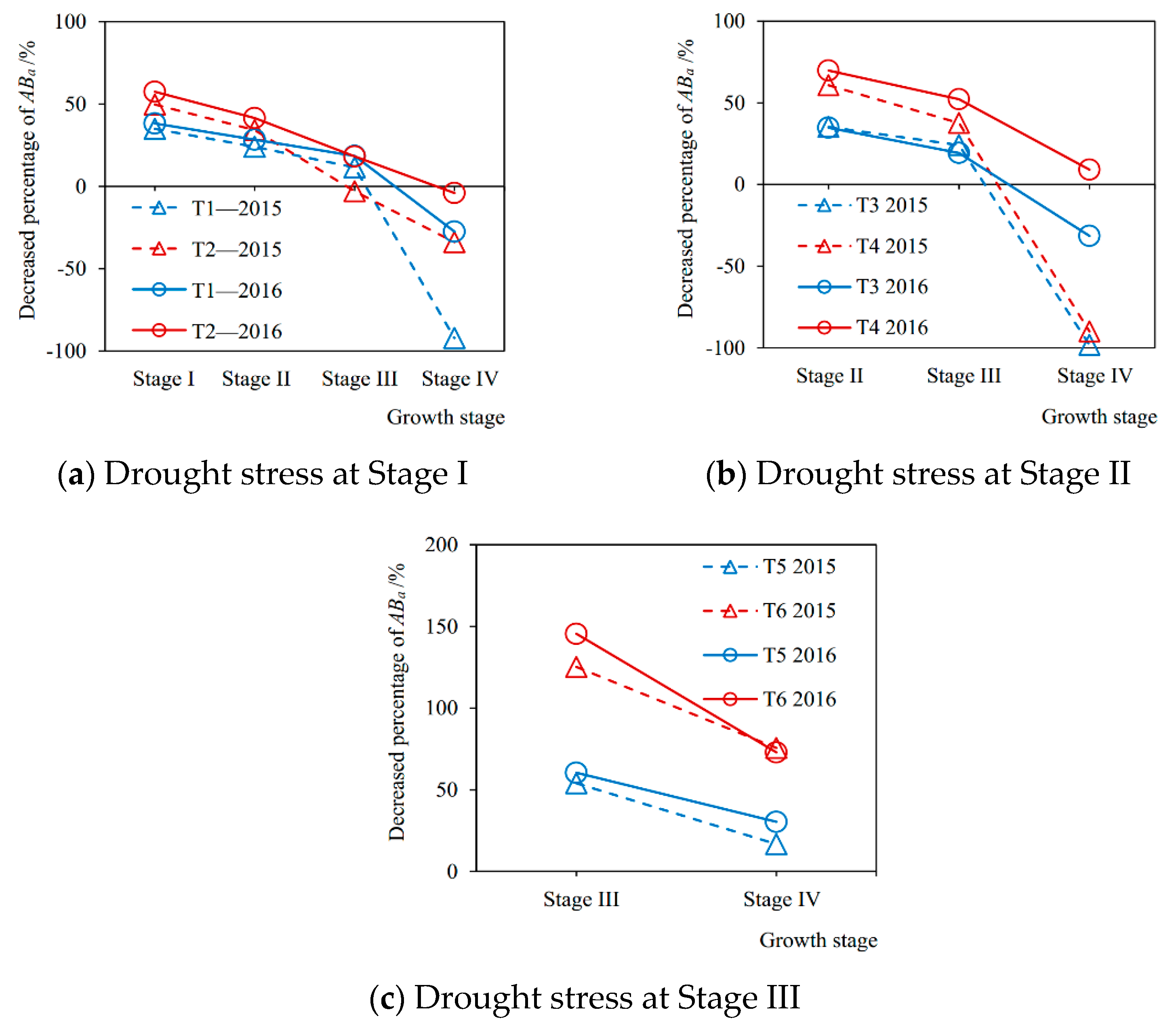
3.4. After-Effects of Drought Stress on Aboveground Biomass
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Sun, F.X.; Mejia, A.; Zeng, P.; Che, Y. Projecting meteorological, hydrological and agricultural droughts for the Yangtze River basin. Sci. Total Environ. 2019, 696, 134076. [Google Scholar] [CrossRef]
- Sánchez, N.; González-Zamora, Á.; Martínez-Fernández, J.; Piles, M.; Pablos, M. Integrated remote sensing approach to global agricultural drought monitoring. Agric. For. Meteorol. 2018, 259, 141–153. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Y.N.; Zhang, J.Q.; Guo, E.L.; Wang, R.; Li, D.J. Dynamic drought risk assessment for maize based on crop simulation model and multi-source drought indices. J. Clean. Prod. 2019, 233, 100–114. [Google Scholar] [CrossRef]
- Dai, A.G.; Trenberth, K.E.; Qian, T.T. A global dataset of Palmer Drought Severity Index for 1870–2002: Relationship with soil moisture and effects of surface warming. J. Hydrometeorol. 2004, 5, 1117–1130. [Google Scholar] [CrossRef]
- Fontaine, M.M.; Steinemann, A.C. Assessing vulnerability to natural hazards: Impact-based method and application to drought in Washington State. Nat. Hazards Rev. 2009, 10, 11–18. [Google Scholar] [CrossRef]
- Mishra, A.K.; Singh, V.P. Drought modeling—A review. J. Hydrol. 2011, 403, 157–175. [Google Scholar] [CrossRef]
- Khakwani, A.A.; Dennett, M.D.; Khan, N.U.; Munir, M.; Baloch, M.J.; Latif, A.; Gul, S. Stomatal and chlorophyll limitations of wheat cultivars subjected to water stress at booting and anthesis stages. Pak. J. Bot. 2013, 45, 1925–1932. [Google Scholar]
- Goldhamer, D.A.; Fereres, E. Irrigation scheduling protocols using continuously recorded trunk diameter measurements. Irrig. Sci. 2001, 20, 115–125. [Google Scholar] [CrossRef]
- Gallardo, M.; Thompson, R.B.; Valdez, L.C.; Fernández, M.D. Use of stem diameter variations to detect plant water stress in tomato. Irrig. Sci. 2006, 24, 241–255. [Google Scholar] [CrossRef]
- Meeks, C.D.; Snider, J.L.; Babb-Hartman, M.E.; Barnes, T. Evaluating the mechanisms of photosynthetic inhibition under growth-limiting, early-season water deficit stress in cotton. Crop Sci. 2019, 59, 1–11. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.Q.; Jin, J.L.; Jiang, S.M.; Ning, S.W.; Liu, L. Quantitative response of soybean development and yield to drought stress during different growth stages in the Huaibei Plain, China. Agronomy 2018, 8, 97. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.J.; Wang, L.; Li, J.; Zhu, A.X. An EPIC model-based wheat drought risk assessment using new climate scenarios in China. Clim. Chang. 2018, 147, 1–15. [Google Scholar] [CrossRef]
- Chen, Y.X.; Yu, J. A study on model of crop response to water in consideration of lag effect with limited water deficit. J. Hydraul. Eng. 1998, 4, 70–74. [Google Scholar]
- Liu, X.Y.; Luo, Y.P. Simulation of after-effect of water stress on growth of winter wheat. Trans. Chin. Soc. Agric. Eng. 2003, 19, 28–32. [Google Scholar]
- Cui, Y.; Jiang, S.M.; Feng, P.; Jin, J.L.; Yuan, H.W. Winter wheat evapotranspiration estimation under drought stress during several growth stages in Huaibei Plain, China. Water 2018, 10, 1208. [Google Scholar] [CrossRef] [Green Version]
- Alghory, A.; Yazar, A. Evaluation of crop water stress index and leaf water potential for deficit irrigation management of sprinkler-irrigated wheat. Irrig. Sci. 2019, 37, 61–77. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S.; Shimizu, H. Plant responses to drought and rewatering. Plant Signal. Behav. 2010, 5, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Boyer, J.S. Differing sensitivity of photosynthesis to low leaf water potentials in corn and soybean. Plant Physiol. 1970, 46, 236–239. [Google Scholar] [CrossRef] [Green Version]
- Desotgiu, R.; Pollastrini, M.; Cascio, C.; Gerosa, G.; Marzuoli, R.; Bussotti, F. Chlorophyll a fluorescence analysis along a vertical gradient of the crown in a poplar (Oxford clone) subjected to ozone and water stress. Tree Physiol. 2012, 32, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica 2016, 54, 65–73. [Google Scholar] [CrossRef]
- Guo, X.P.; Liu, Z.P.; Wang, Q.M.; Guo, F.; Yuan, J.; Chen, Z.P. Study on photosynthetic compensatory effects of PEG osmotic stress and rewatering on maize. J. Hohai Univ. 2007, 35, 286–290. [Google Scholar]
- Zegada-Lizarazu, W.; Monti, A. Photosynthetic response of sweet sorghum to drought and re-watering at different growth stages. Physiol. Plant. 2013, 149, 56–66. [Google Scholar] [CrossRef]
- Steduto, P.; Hsiao, T.C.; Fereres, E. On the conservative behavior of biomass water productivity. Irrig. Sci. 2007, 25, 189–207. [Google Scholar] [CrossRef] [Green Version]
- Board, J.E.; Modali, H. Dry matter accumulation predictors for optimal yield in soybean. Crop Sci. 2004, 45, 1790–1799. [Google Scholar] [CrossRef]
- Wu, Z.T.; Yu, L.; Du, Z.Q.; Zhang, H.; Fan, X.H.; Lei, T.J. Recent changes in the drought of China from 1960 to 2014. Int. J. Climatol. 2020, 40, 3281–3296. [Google Scholar] [CrossRef]
- Ministry of Water Resources, Office of State Flood Control and Drought Relief Headquarters. China Floods and Droughts Bulletin; Water and Power Press: Beijing, China, 2014.
- Wei, Z.; Paredes, P.; Liu, Y.; Chi, W.W.; Pereira, L.S. Modelling transpiration, soil evaporation and yield prediction of soybean in North China Plain. Agric. Water Manag. 2015, 147, 43–53. [Google Scholar] [CrossRef]
- Wei, Y.Q.; Jin, J.L.; Jiang, S.M.; Ning, S.W.; Cui, Y.; Zhou, Y.L. Simulated assessment of summer maize drought loss sensitivity in Huaibei Plain, China. Agronomy 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.L.; Ning, S.W.; Cui, Y.; Jin, J.L.; Zhou, Y.L.; Wu, C.G. Quantitative assessment and diagnosis for regional agricultural drought resilience based on set pair analysis and connection entropy. Entropy 2019, 21, 373. [Google Scholar] [CrossRef] [Green Version]
- Tsubo, M.; Walker, S. A model of radiation interception and use by a maize-bean intercrop canopy. Agric. For. Meteorol. 2002, 110, 203–215. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, S.M.; Jin, J.L.; Feng, P.; Ning, S.W. Decision-making of irrigation scheme for soybeans in the Huaibei Plain based on grey entropy weight and grey relation–projection pursuit. Entropy 2019, 21, 877. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Jiang, S.M.; Jin, J.L.; Ning, S.W.; Feng, P. Quantitative assessment of soybean drought loss sensitivity at different growth stages based on S-shaped damage curve. Agric. Water Manag. 2019, 213, 821–832. [Google Scholar] [CrossRef]
- Desclaux, D.; Huynh, T.; Roumet, P. Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci. 2000, 40, 716–722. [Google Scholar] [CrossRef]
- Dogan, E.; Kirnak, H.; Copur, O. Deficit irrigations during soybean reproductive stages and GROPGRO-soybean simulations under semi-arid climatic conditions. Field Crop. Res. 2007, 103, 154–159. [Google Scholar] [CrossRef]
- Sincik, M.; Candogan, B.N.; Demirtas, C.; Büyükcangaz, H.; Yazgan, S.; Göksoy, A.T. Deficit irrigation of soya bean [Glycine max (L.) Merr.] in a sub-humid climate. J. Agron. Crop Sci. 2008, 194, 200–205. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L. Effects of soil water deficit on yield and quality of processing tomato under a Mediterranean climate. Agric. Water Manag. 2010, 97, 131–138. [Google Scholar] [CrossRef]
- Chen, J.L.; Kang, S.Z.; Du, T.S.; Qiu, R.J.; Guo, P.; Chen, R.Q. Quantitative response of greenhouse tomato yield and quality to water deficit at different growth stages. Agric. Water Manag. 2013, 129, 152–162. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Liu, F.; Jensen, C.R. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa (Chenopodium quinoa Willd.). Sci. Hortic. 2009, 122, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.H.; Li, B.; Zhang, X.S.; Wang, R.Q. Effects of water stress on water use efficiency and water balance components of Hippophae rhamnoides and Caragana intermedia in the soil-plant-atmosphere continuum. Agrofor. Syst. 2010, 80, 423–435. [Google Scholar] [CrossRef]
- Čereković, N.; Pagter, M.; Kristensen, H.L.; Brennan, R.; Petersen, K.K. Effects of deficit irrigation during flower initiation of two blackcurrant (Ribes nigrum L.) cultivars. Sci. Hortic. 2014, 168, 193–201. [Google Scholar]
- Nosalewicz, A.; Siecińska, J.; Kondracka, K.; Nosalewicz, M. The functioning of Festuca arundinacea and Lolium perenne under drought is improved to a different extend by the previous exposure to water deficit. Environ. Exp. Bot. 2018, 156, 271–278. [Google Scholar] [CrossRef]
- Kaiser, W.M. Effects of water deficit on photosynthetic capacity. Physiol. Plant. 1987, 71, 142–149. [Google Scholar] [CrossRef]
- Igbadun, H.E.; Salim, B.A.; Tarimo, A.K.P.R.; Mahoo, H.F. Effects of deficit irrigation scheduling on yields and soil water balance of irrigated maize. Irrig. Sci. 2008, 27, 11–23. [Google Scholar] [CrossRef]
- Anda, A.; Soós, G.; Menyhárt, L.; Kucserka, T.; Simon, B. Yield features of two soybean varieties under different water supplies and field conditions. Field Crop. Res. 2020, 245, 107673. [Google Scholar] [CrossRef]
- Foroud, N.; Mündel, H.H.; Saindon, G.; Entz, T. Effect of level and timing of moisture stress on soybean plant development and yield components. Irrig. Sci. 1993, 13, 149–155. [Google Scholar] [CrossRef]
- Jha, P.K.; Kumar, S.N.; Ines, A.V.M. Responses of soybean to water stress and supplemental irrigation in upper Indo-Gangetic plain: Field experiment and modeling approach. Field Crop. Res. 2018, 219, 76–86. [Google Scholar] [CrossRef]
- Xu, S.Q.; Song, J.; Wu, Y. Discussion of soybean water demand regulation and sprinkling irrigation pattern. Water Sav. Irrig. 2003, 3, 23–25. [Google Scholar]
- Egli, D.B.; Bruening, W.P. Water stress, photosynthesis, seed sucrose levels and seed growth in soybean. J. Agric. Sci. 2004, 142, 1–8. [Google Scholar] [CrossRef]
- Oya, T.; Nepomuceno, A.L.; Neumaier, N.; Farias, J.R.B.; Tobita, S.; Ito, O. Drought tolerance characteristics of Brazilian soybean cultivars—Evaluation and characterization of drought tolerance of various Brazilian soybean cultivars in the field. Plant Prod. Sci. 2004, 7, 129–137. [Google Scholar] [CrossRef] [Green Version]
- Karam, F.; Masaad, R.; Thérèse, S.; Mounzer, O.; Rouphael, Y. Evapotranspiration and seed yield of field grown soybean under deficit irrigation conditions. Agric. Water Manag. 2005, 75, 226–244. [Google Scholar] [CrossRef]
- Siahpoosh, M.R.; Dehghanian, E.; Kamgar, A. Drought tolerance evaluation of bread wheat genotypes using water use efficiency, evapotranspiration efficiency, and drought susceptibility index. Crop Sci. 2011, 51, 1198–1204. [Google Scholar] [CrossRef]
- Lecoeur, J.; Wery, J.; Turc, O.; Tardieu, F. Expansion of pea leaves subjected to short water deficit: Cell number and cell size are sensitive to stress at different periods of leaf development. J. Exp. Bot. 1995, 46, 1093–1101. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.G.; Xu, M.; Zhang, J.X. The effect of water deficit and waterlogging on the yield components of cotton. Crop Sci. 2018, 58, 1751–1761. [Google Scholar] [CrossRef]
- Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.R.; Henry, W.B.; Reddy, K.R. Water deficit effects on soybean root morphology and early-season vigor. Agronomy 2019, 9, 836. [Google Scholar] [CrossRef] [Green Version]
- Van Heerden, P.D.R.; Krüger, G.H.J. Separately and simultaneously induced dark chilling and drought stress effects on photosynthesis, proline accumulation and antioxidant metabolism in soybean. J. Plant Physiol. 2002, 159, 1077–1086. [Google Scholar] [CrossRef]
- Liu, F.L.; Jensen, C.R.; Andersen, M.N. Pod set related to photosynthetic rate and endogenous ABA in soybeans subjected to different water regimes and exogenous ABA and BA at early reproductive stages. Ann. Bot. 2004, 94, 405–411. [Google Scholar] [CrossRef] [Green Version]
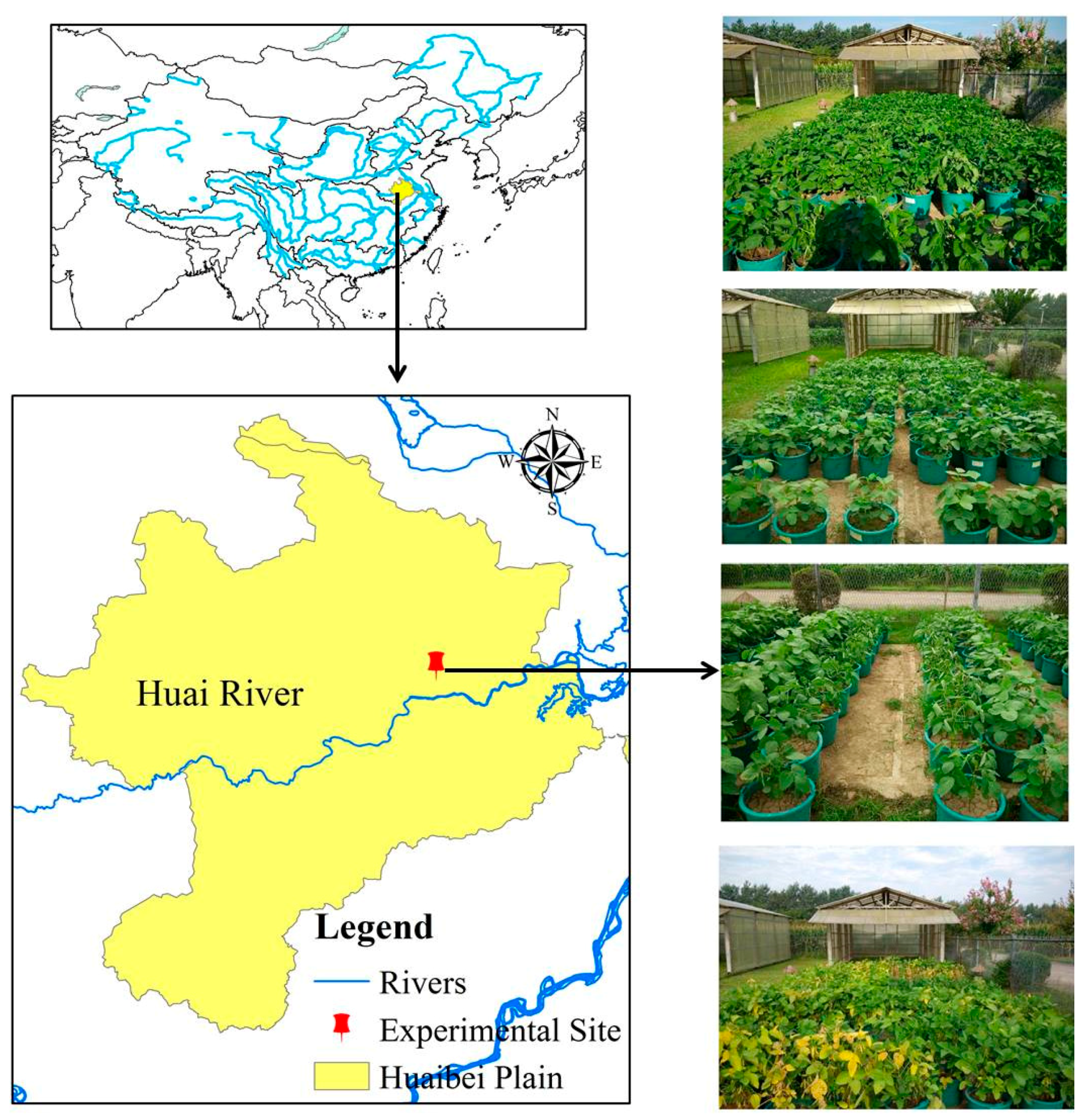
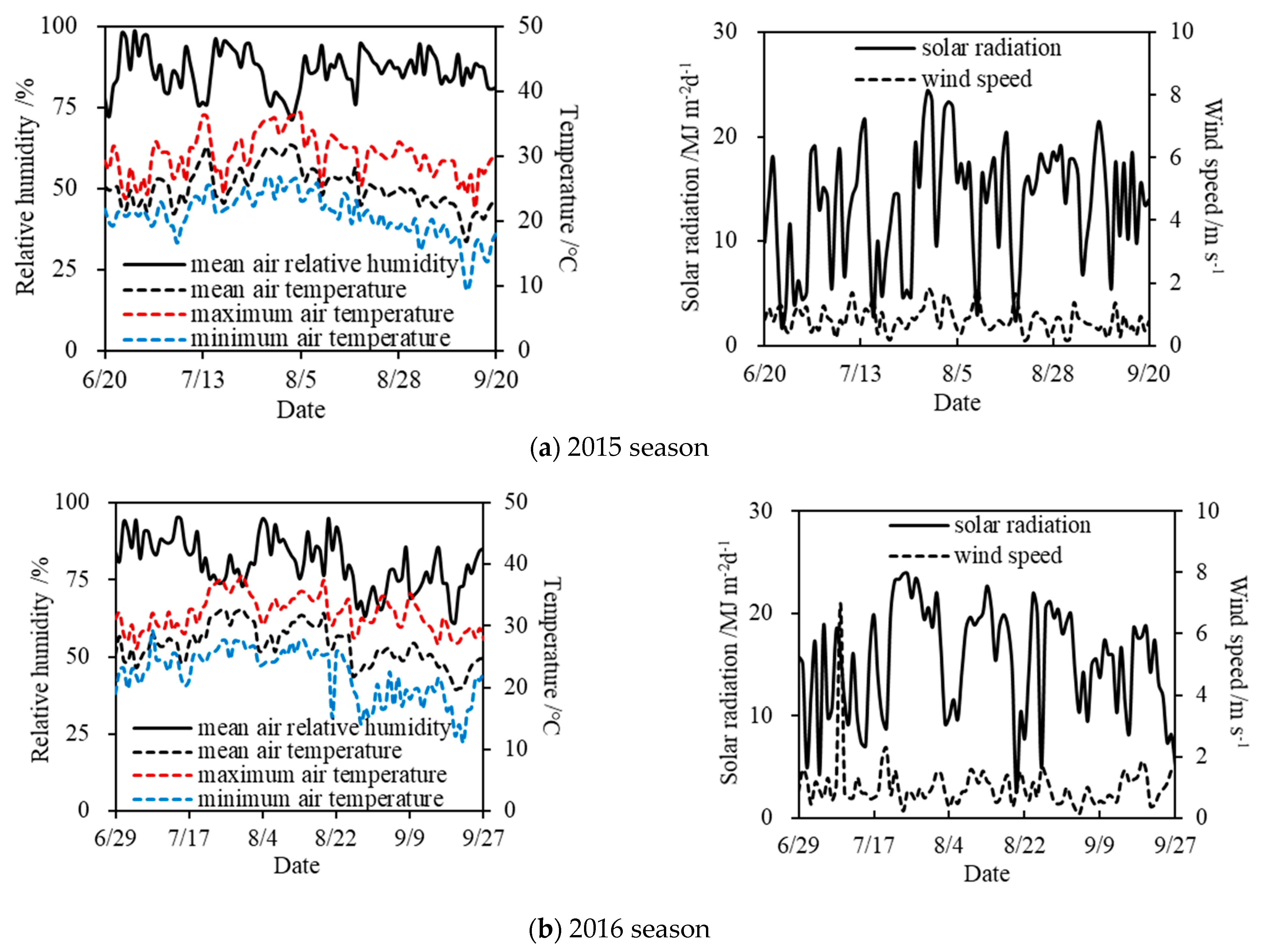


| Soil Characteristics | Values |
|---|---|
| Sand (%) | 3.45 |
| Silt (%) | 70.52 |
| Clay (%) | 26.03 |
| pH (in water solution) | 7.5 |
| Organic matter (%) | 0.85 |
| Bulk density (g/cm3) | 1.36 |
| Field capacity at −0.03 MPa (cm3/cm3) | 0.38 |
| Wilting point at −1.5 MPa (cm3/cm3) | 0.12 |
| Description of Growth Stage | 2015 Season | 2016 Season |
|---|---|---|
| germination stage, from sowing to seed germination | 20 June to 3 July, 14 days | 29 June to 14 July, 16 days |
| seedling stage (Stage I), from seed germination to plants with four fully expanded leaves | 4 July to 14 July, 11 days | 15 July to 27 July, 13 days |
| branching stage (Stage II), from plants with four fully expanded leaves to first flower appearance | 15 July to 3 August, 20 days | 28 July to 10 August, 14 days |
| flowering-podding stage (Stage III), from first flower appearance to the beginning of pod filling | 4 August to 20 August, 17 days | 11 August to 31 August, 21 days |
| seed filling stage (Stage IV), from the beginning of pod filling to plant maturation | 21 August to 20 September, 31 days | 1 September to 27 September, 27 days |
| Cropping Season | Treatment | Seedling Stage (Stage I) | Branching Stage (Stage II) | Flowering-Podding Stage (Stage III) | Seed Filling Stage (Stage IV) |
|---|---|---|---|---|---|
| 2015 and 2016 | T1 | 55% | 75% | 75% | 75% |
| T2 | 35% | 75% | 75% | 75% | |
| T3 | 75% | 55% | 75% | 75% | |
| T4 | 75% | 35% | 75% | 75% | |
| T5 | 75% | 75% | 55% | 75% | |
| T6 | 75% | 75% | 35% | 75% | |
| T7 | 75% | 75% | 75% | 55% | |
| T8 | 75% | 75% | 75% | 35% | |
| CK | 75% | 75% | 75% | 75% |
| Cropping Season | Treatment | Seedling Stage (Stage I) | Branching Stage (Stage II) | Flowering-Podding Stage (Stage III) | Seed Filling Stage (Stage IV) | ||||
|---|---|---|---|---|---|---|---|---|---|
| IA (mm) | IT | IA (mm) | IT | IA (mm) | IT | IA (mm) | IT | ||
| 2015 | T1 | 44.20 | 2 | 209.61 | 11 | 217.84 | 13 | 430.16 | 26 |
| T2 | 0 | 0 | 240.83 | 13 | 245.35 | 15 | 446.85 | 26 | |
| T3 | 69.87 | 6 | 157.17 | 9 | 225.26 | 11 | 436.33 | 26 | |
| T4 | 65.46 | 5 | 63.40 | 3 | 185.23 | 9 | 424.80 | 28 | |
| T5 | 63.77 | 6 | 235.32 | 14 | 157.61 | 8 | 385.67 | 26 | |
| T6 | 66.78 | 6 | 239.88 | 15 | 84.49 | 3 | 206.45 | 13 | |
| T7 | 60.69 | 6 | 231.94 | 14 | 248.40 | 15 | 281.63 | 12 | |
| T8 | 65.90 | 6 | 246.05 | 15 | 248.40 | 14 | 46.00 | 2 | |
| CK | 71.93 | 6 | 261.84 | 15 | 277.54 | 15 | 466.82 | 27 | |
| 2016 | T1 | 40.96 | 2 | 153.07 | 11 | 295.90 | 17 | 338.00 | 25 |
| T2 | 0 | 0 | 124.94 | 10 | 272.37 | 17 | 326.77 | 25 | |
| T3 | 92.65 | 8 | 135.62 | 6 | 282.53 | 17 | 330.72 | 24 | |
| T4 | 89.24 | 8 | 50.92 | 2 | 239.35 | 16 | 312.94 | 23 | |
| T5 | 82.38 | 7 | 171.86 | 11 | 205.82 | 9 | 291.43 | 23 | |
| T6 | 84.89 | 8 | 171.31 | 12 | 107.73 | 4 | 183.62 | 16 | |
| T7 | 95.02 | 8 | 164.62 | 12 | 300.91 | 17 | 176.30 | 11 | |
| T8 | 87.86 | 8 | 165.21 | 12 | 320.79 | 18 | 64.95 | 4 | |
| CK | 94.09 | 8 | 177.11 | 13 | 332.72 | 18 | 340.86 | 26 | |
| Cropping Season | Treatment | Seedling Stage (Stage I) | Branching Stage (Stage II) | Flowering-Podding Stage (Stage III) | Seed Filling Stage (Stage IV) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ET (mm) | ETd (mm d−1) | ET (mm) | ETd (mm d−1) | ET (mm) | ETd (mm d−1) | ET (mm) | ETd (mm d−1) | ||
| 2015 | T1 | 50.37 | 4.58 | 233.82 | 11.69 | 237.72 | 13.98 | 434.81 | 14.03 |
| T2 | 30.06 | 2.73 | 234.79 | 11.74 | 248.50 | 14.62 | 454.86 | 14.67 | |
| T3 | 77.67 | 7.06 | 178.73 | 8.94 | 226.23 | 13.31 | 435.79 | 14.06 | |
| T4 | 70.36 | 6.40 | 82.54 | 4.13 | 196.69 | 11.57 | 435.30 | 14.04 | |
| T5 | 69.54 | 6.32 | 253.31 | 12.67 | 166.39 | 9.79 | 385.67 | 12.44 | |
| T6 | 67.43 | 6.13 | 252.50 | 12.63 | 114.23 | 6.72 | 205.78 | 6.64 | |
| T7 | 69.54 | 6.32 | 241.45 | 12.07 | 255.10 | 15.01 | 285.65 | 9.21 | |
| T8 | 73.61 | 6.69 | 257.70 | 12.89 | 251.53 | 14.80 | 71.82 | 2.32 | |
| CK Significance | 78.16 *** | 7.11 *** | 274.44 *** | 13.72 *** | 273.95 *** | 16.11 *** | 461.43 *** | 14.88 *** | |
| 2016 | T1 | 68.31 | 5.69 | 147.08 | 10.51 | 302.84 | 14.42 | 330.48 | 12.24 |
| T2 | 40.43 | 3.37 | 134.86 | 9.63 | 277.97 | 13.24 | 314.86 | 11.66 | |
| T3 | 115.50 | 9.62 | 127.67 | 9.12 | 286.67 | 13.65 | 312.32 | 11.57 | |
| T4 | 116.11 | 9.68 | 67.71 | 4.84 | 225.55 | 10.74 | 337.92 | 12.52 | |
| T5 | 113.10 | 9.43 | 157.26 | 11.23 | 207.85 | 9.90 | 277.02 | 10.26 | |
| T6 | 110.09 | 9.17 | 166.53 | 11.89 | 103.59 | 4.93 | 169.84 | 6.29 | |
| T7 | 109.47 | 9.12 | 165.68 | 11.83 | 322.55 | 15.36 | 200.89 | 7.44 | |
| T8 | 106.31 | 8.86 | 177.20 | 12.66 | 330.74 | 15.75 | 73.02 | 2.70 | |
| CK Significance | 111.55 *** | 9.30 *** | 176.66 *** | 12.62 *** | 344.74 *** | 16.42 *** | 359.03 *** | 13.30 *** | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Ning, S.; Jin, J.; Jiang, S.; Zhou, Y.; Wu, C. Quantitative Lasting Effects of Drought Stress at a Growth Stage on Soybean Evapotranspiration and Aboveground BIOMASS. Water 2021, 13, 18. https://doi.org/10.3390/w13010018
Cui Y, Ning S, Jin J, Jiang S, Zhou Y, Wu C. Quantitative Lasting Effects of Drought Stress at a Growth Stage on Soybean Evapotranspiration and Aboveground BIOMASS. Water. 2021; 13(1):18. https://doi.org/10.3390/w13010018
Chicago/Turabian StyleCui, Yi, Shaowei Ning, Juliang Jin, Shangming Jiang, Yuliang Zhou, and Chengguo Wu. 2021. "Quantitative Lasting Effects of Drought Stress at a Growth Stage on Soybean Evapotranspiration and Aboveground BIOMASS" Water 13, no. 1: 18. https://doi.org/10.3390/w13010018





