Passage Performance of Technical Pool-Type Fishways for Potamodromous Cyprinids: Novel Experiences in Semiarid Environments
Abstract
:1. Introduction
2. Materials and Methods
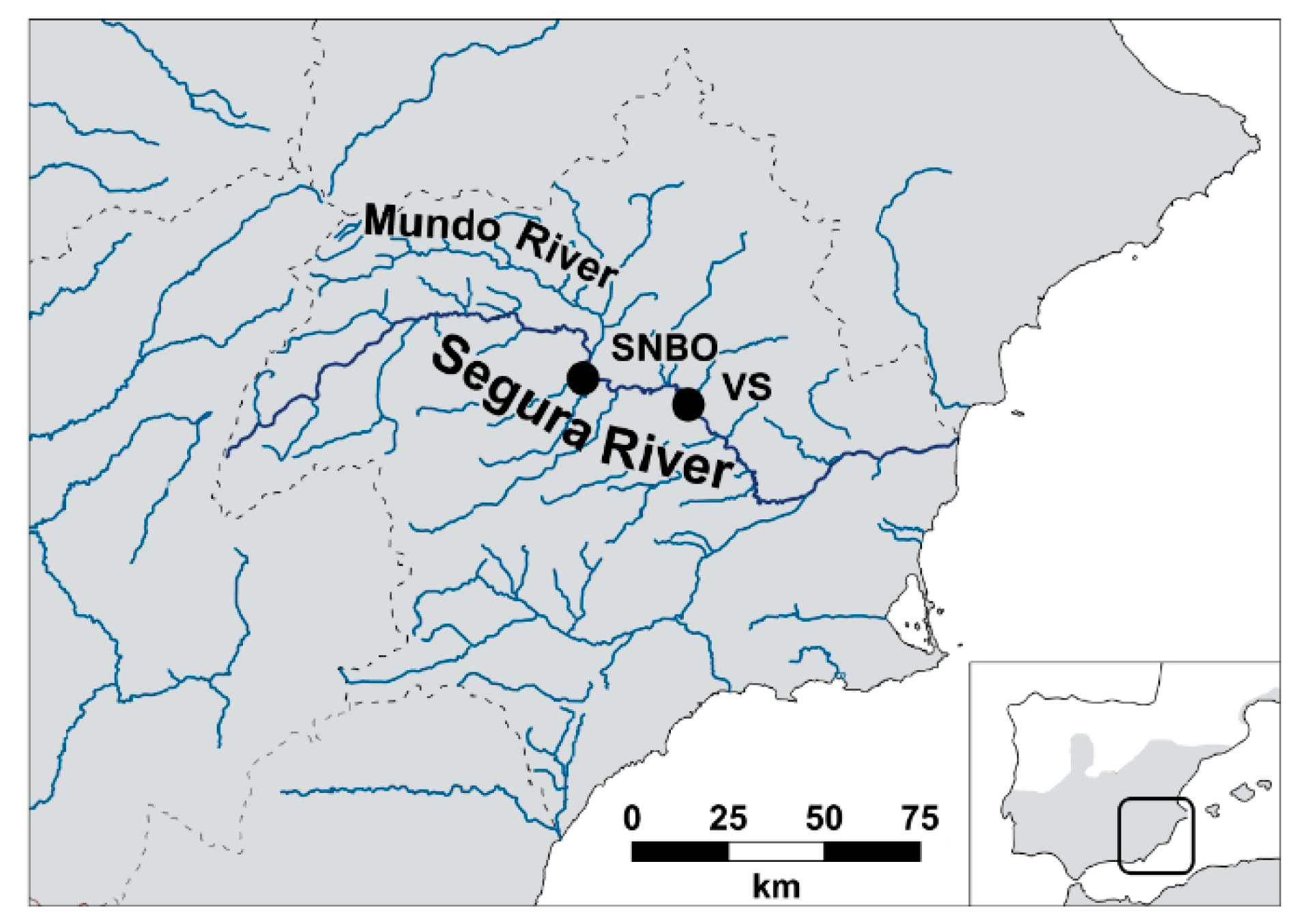
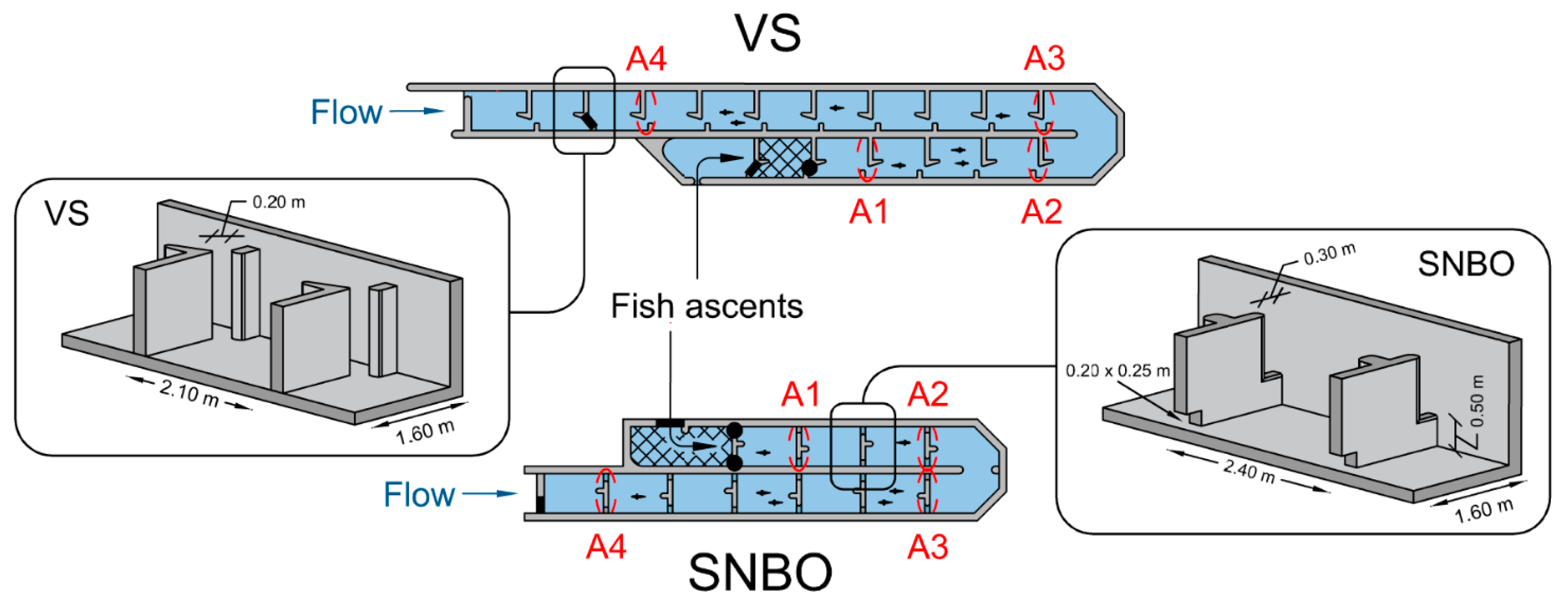
2.1. Study Area and Experimental Sites
2.2. Fish Capture, Tagging, and Handling
2.3. Trials
2.4. Data Analysis
2.4.1. Ascent Analysis
2.4.2. Motivation Analysis
3. Results
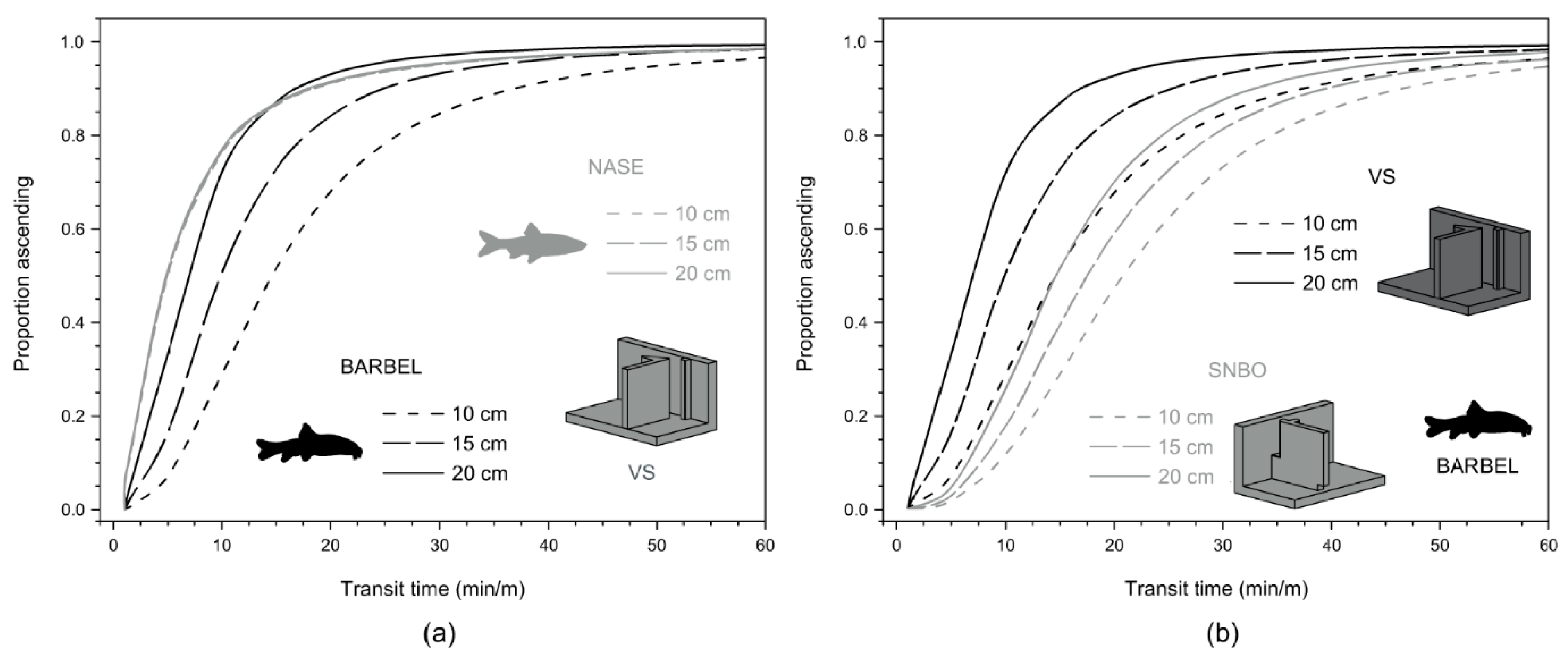
3.1. Ascent Analysis
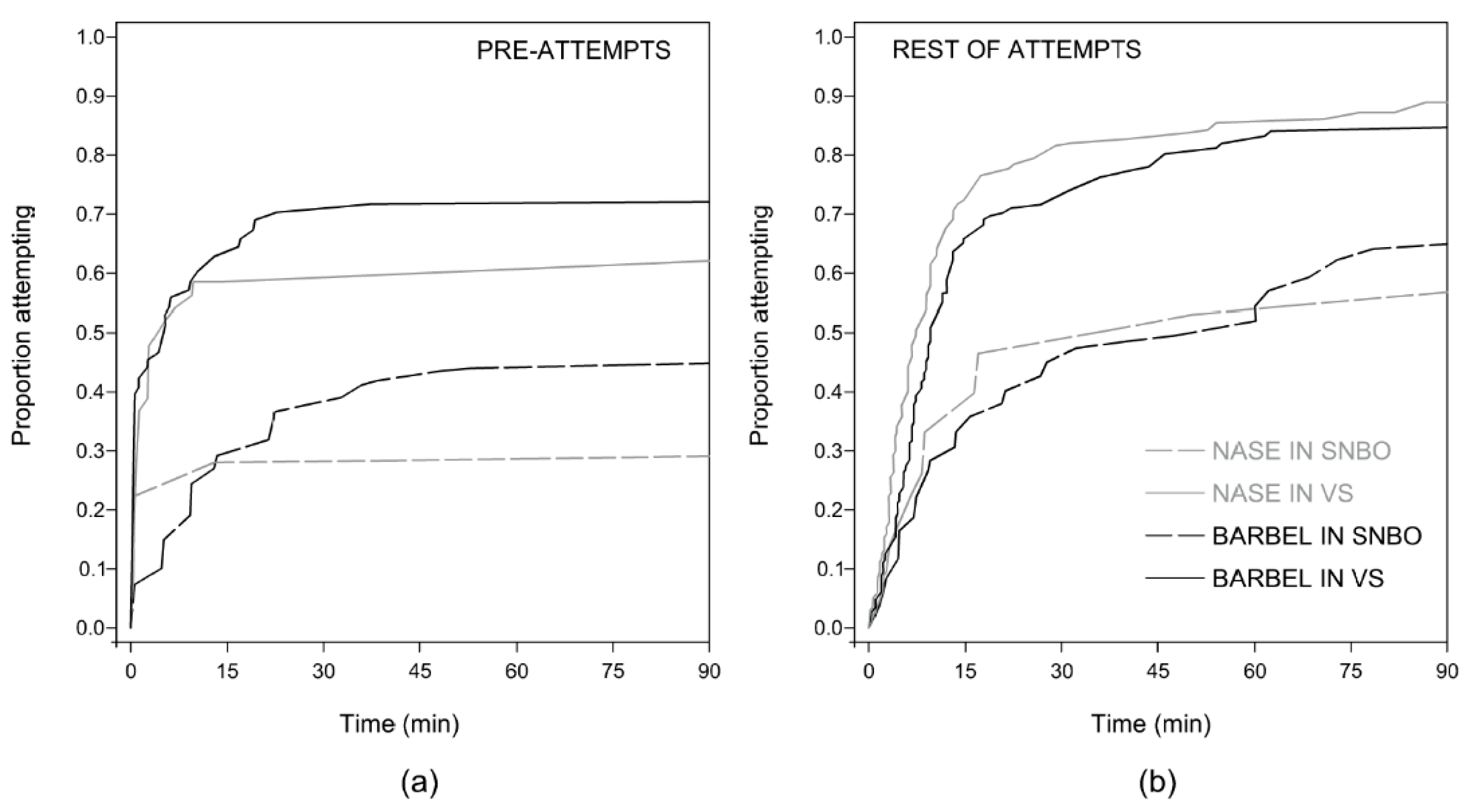
3.2. Motivation Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gasith, A.; Resh, V.H. Streams in Mediterranean climate regions: Abiotic influences and biotic responses to predictable seasonal events. Annu. Rev. Ecol. Syst. 1999, 30, 51–81. [Google Scholar] [CrossRef]
- Seager, R.; Osborn, T.J.; Kushnir, Y.; Simpson, I.R.; Nakamura, J.; Liu, H. Climate variability and change of mediterranean-type climates. J. Clim. 2019, 32, 2887–2915. [Google Scholar] [CrossRef]
- Grill, G.; Lehner, B.; Lumsdon, A.E.; MacDonald, G.K.; Zarfl, C.; Reidy Liermann, C. An index-based framework for assessing patterns and trends in river fragmentation and flow regulation by global dams at multiple scales. Environ. Res. Lett. 2015, 10, 015001. [Google Scholar] [CrossRef]
- Smith, K.G.; Barrios, V.; Darwall, W.R.T.; Numa, C. The Status and Distribution of Freshwater Biodiversity in the Eastern Mediterranean; IUCN: Gran, Switzerland, 2014; ISBN 9782831716992. [Google Scholar]
- Lucas, M.C.; Baras, E.; Thom, T.J.; Duncan, A.; Slavík, O. Migration of Freshwater Fishes; Blackwell Science: Oxford, UK, 2001; ISBN 0632057548. [Google Scholar]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef]
- Coad, B. Endemicity in the freshwater fishes of Iran. Iran. J. Anim. Biosyst. 2006, 1, 1–13. [Google Scholar]
- Maceda-Veiga, A. Towards the conservation of freshwater fish: Iberian Rivers as an example of threats and management practices. Rev. Fish Biol. Fish. 2013, 23, 1–22. [Google Scholar] [CrossRef]
- Hermoso, V.; Clavero, M. Threatening processes and conservation management of endemic freshwater fish in the Mediterranean basin: A review. Mar. Freshw. Res. 2011, 62, 244–254. [Google Scholar] [CrossRef]
- Vörösmarty, C.J.; Green, P.; Salisbury, J.; Lammers, R.B. Global Water Resources: Vulnerability from Climate Change and Population Growth. Science 2000, 289, 284–289. [Google Scholar] [CrossRef]
- Solomon, S.; Qin, D.; Manning, M.; Marquis, M.; Averty, K.; Tignor, M.M.B.; Miller, H.L.; Chen, Z. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Clavero, M.; Blanco-Garrido, F.; Prenda, J. Fish fauna in Iberian Mediterranean river basins: Biodiversity, introduced species and damming impacts. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, 575–585. [Google Scholar] [CrossRef]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland; Berlin, Germany, 2007; ISBN 978-2-8399-0298-4. [Google Scholar]
- Jouladeh-Roudbar, A.; Vatandoust, S.; Eagderi, S.; Jafari-Kenari, S.; Mousavi-Sabet, H. Freshwater fishes of Iran; an updated checklist. Aquac. Aquar. Conserv. Legis. 2015, 8, 855–909. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Available online: www.fishbase.org (accessed on 26 September 2019).
- Doadrio, I. Atlas y Libro Rojo de los Peces Continentales de España; Dirección General de Conservación de la Naturaleza; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2002; ISBN 84-8014-313-4.
- Collares-Pereira, M.; Martins, M.; Pires, A.; Geraldes, A.; Coelho, M. Feeding behaviour of Barbus bocagei assessed under a spatio-temporal approach. Folia Zool. 1996, 45, 65–76. [Google Scholar]
- Doadrio, I.; Perea, S.; Garzón-Heydt, P.; González, J.L. Ictiofauna Continental Española: Bases Para su Seguimiento; Ministerio de Medio Ambiente y Medio Rural y Marino: Madrid, Spain, 2011; ISBN 978-84-491-1158-7.
- Torralva, M.M.; Oliva-Paterna, F.J. First record of Chondrostoma polylepis Steindachner, 1865 (Ostariophysi, Cyprinidae) in the basin of the Segura River, SE of Spain. Limnetica 1997, 13, 1–3. [Google Scholar]
- Encina, L.; Rodriguez, A.; Granado-Lorencio, C. The Iberian ichthyofauna: Ecological contributions. Limnetica 2006, 25, 349–368. [Google Scholar]
- Pringle, C. What is hydrologic connectivity and why is it ecologically important? Hydrol. Process. 2003, 17, 2685–2689. [Google Scholar] [CrossRef]
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The River Continuum Concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Clay, C.H. Design of Fishways and Other Fish Facilities, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1994; ISBN 1566701112. [Google Scholar]
- Food and Agriculture Organization of the United Nations; Deutscher Verband für Wasserwirtschaft und Kulturbau e. V. Fish Passes: Design, Dimensions and Monitoring; FAO: Rome, Italy, 2002; ISBN 9251048940. [Google Scholar]
- Lynch, H.A. Report to the Government of Iraq on Fishways at Dams on the Tigris and Euphrates Rivers; Report No 526; FAO: Rome, Italy, 1956. [Google Scholar]
- Elvira, B.; Nicola, G.G.; Almodóvar, A. Impacto de las Obras Hidráulicas en la Ictiofauna: Dispositivos de Paso Para Peces en las Presas de España; Organismo Autónomo Parques Nacionales: Madrid, Spain, 1998; ISBN 8480142545.
- Verep, B.; Küçükali, S.; Turan, D.; Alp, A. A Critical Analysis of Existing Fish A Critical Analysis of Existing Fish Pass Structures at Small Hydropower Plants in Turkey. In Proceedings of the International Conference on Engineering and Ecohydrology for Fish Passage, Amherst, MA, USA, 22 June 2016; p. 53. [Google Scholar]
- Pervin, A. Fish passages from past to future in Turkey. In Proceedings of the International Conference on Engineering and Ecohydrology for Fish Passage, Corvallis, OR, USA, 19 June 2017; p. 49. [Google Scholar]
- Comoglio, C.; (Politecnico di Torino, Turin, Italy). Personal communication, 2019.
- Mimeche, F.; (Université de M´Sila, M´Sila, Algeria). Personal communication, 2019.
- Koutrakis, E.T.; (Fisheries Research Institute, National Agricultural Research Foundation, Kavala, Greece). Personal communication, 2019.
- Larinier, M. Pool fishways, pre-barrages and natural bypass channels. Bull. Français Pêche Piscic. 2002, 364, 54–82. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; García-Vega, A.; Sanz-Ronda, F.J.; Martínez de Azagra Paredes, A. Villemonte’s approach: Validation of a general method for modeling uniform and non-uniform performance in stepped fishways. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 11. [Google Scholar] [CrossRef]
- Silva, A.T.; Ferreira, M.T.; Santos, J.M.; Pinheiro, A.N.; Melo, J.F.; Bochechas, J.H. Development of a pool-type fishway facility for Iberian cyprinids. In Proceedings of the International Symposium on Ecohydraulics, Madrid, Spain, 12 September 2004; pp. 924–947. [Google Scholar]
- Noonan, M.J.; Grant, J.W.A.; Jackson, C.D. A quantitative assessment of fish passage efficiency. Fish Fish. 2012, 13, 450–464. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Bravo-Córdoba, F.J.; Fuentes-Pérez, J.F.; Castro-Santos, T. Ascent ability of brown trout, Salmo trutta, and two Iberian cyprinids− Iberian barbel, Luciobarbus bocagei, and northern straight-mouth nase, Pseudochondrostoma duriense− in a vertical slot fishway. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 10. [Google Scholar] [CrossRef]
- Bravo-Córdoba, F.J.; Sanz-Ronda, F.J.; Ruiz-Legazpi, J.; Fernandes Celestino, L.; Makrakis, S. Fishway with two entrance branches: Understanding its performance for potamodromous Mediterranean barbels. Fish. Manag. Ecol. 2018, 25, 12–21. [Google Scholar] [CrossRef]
- Bravo-Córdoba, F.J.; Sanz-Ronda, F.J.; Ruiz-Legazpi, J.; Valbuena-Castro, J.; Makrakis, S. Vertical slot versus submerged notch with bottom orifice: Looking for the best technical fishway type for Mediterranean barbels. Ecol. Eng. 2018, 122, 120–125. [Google Scholar] [CrossRef]
- Branco, P.; Amaral, S.D.; Ferreira, M.T.; Santos, J.M. Do small barriers affect the movement of freshwater fish by increasing residency? Sci. Total Environ. 2017, 581–582, 486–494. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Ronda, F.J.; Fuentes-Pérez, J.F.; García-Vega, A. Escala de artesas en el azud Soto Damián en el río Segura (Abarán, Murcia) LIFE12 ENV/ES/1140 RIVERLINK (Applied Ecohydraulics Group, Palencia, Spain). Available online: https://www.chsegura.es/chs/cuenca/segurariverlink/riverlink/ (accessed on 28 September 2017).
- Sanz-Ronda, F.J.; Fuentes-Pérez, J.F.; García-Vega, A. Escala de artesas en el azud Elevación Zona 1 Postrasvase en el río Segura (Calasparra, Murcia) LIFE12 ENV/ES/1140 RIVERLINK (Applied Ecohydraulics Group, Palencia, Spain). Available online: https://www.chsegura.es/chs/cuenca/segurariverlink/riverlink/ (accessed on 28 September 2017).
- James, R.S.; Johnston, I.A. Scaling of muscle performance during escape responses in the fish Myoxocephalus scorpius L. J. Exp. Biol. 1998, 201, 913–923. [Google Scholar] [PubMed]
- Plaut, I. Does pregnancy affect swimming performance of female Mosquitofish, Gambusia affinis? Funct. Ecol. 2002, 16, 290–295. [Google Scholar] [CrossRef]
- Clough, S.; Lee-Elliot, I.; Turnpenny, A.; Holden, S.; Hinks, C. Swimming Speeds in Fish: Phase 2. Technical Report W2-049/TR1; Environment Agency: Bristol, UK, 2004. [Google Scholar]
- Pedersen, L.F.; Koed, A.; Malte, H. Swimming performance of wild and F1-hatchery-reared Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) smolts. Ecol. Freshw. Fish 2008, 17, 425–431. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Cotel, A.; Webb, P. Fishway Evaluations for Better Bioengineering: An Integrative Approach. In Challenges for Diadromous Fishes in a Dynamic Global Environment, Proceedings of the American Fisheries Society Symposium, Halifax, NS, Canada, 18 June 2007; Haro, A., Smith, K.G., Rulifson, R.A., Moffitt, C.M., Klauda, R.J., Dadswell, M.J., Cunjak, R.A., Cooper, J.E., Beal, K.L., Avery, T.S., Eds.; American Fisheries Society: Bethesda, MD, USA, 2009; Volume 69, pp. 557–575. [Google Scholar]
- Castro-Santos, T.; Haro, A. Fish guidance and passage at barriers. In An Eco-Ethological Perspective; Domeneci, P., Kapoor, B.G., Eds.; Science Publishers: Enfield, NH, USA, 2010; pp. 62–89. [Google Scholar]
- Cooke, S.J.; Hinch, S.G. Improving the reliability of fishway attraction and passage efficiency estimates to inform fishway engineering, science, and practice. Ecol. Eng. 2013, 58, 123–132. [Google Scholar] [CrossRef]
- Romão, F.; Branco, P.; Quaresma, A.L.; Amaral, S.D.; Pinheiro, A.N. Effectiveness of a multi-slot vertical slot fishway versus a standard vertical slot fishway for potamodromous cyprinids. Hydrobiologia 2018, 816, 153–163. [Google Scholar] [CrossRef]
- Grindlay, A.L.; Zamorano, M.; Rodríguez, M.I.; Molero, E.; Urrea, M.A. Implementation of the European Water Framework Directive: Integration of hydrological and regional planning at the Segura River Basin, southeast Spain. Land Use Policy 2011, 28, 242–256. [Google Scholar] [CrossRef]
- Illies, J.; Botoseanu, L. Problèmes et méthodes de la classification et de la-zonation écologique des eaux courantes, considérées surtout-du point de vue faunistique. Mitt. Int. Ver. fuer Theor. Amgewandte Limnol. 1963, 12, 1–57. [Google Scholar] [CrossRef]
- Rosgen, D.L.; Silvey, H.L. Applied River Morphology; Wildland Hydrology Books: Fort Collins, CO, USA, 1996. [Google Scholar]
- Oliva-Paterna, F.J.; Verdiell-Cubedo, D.; Ruiz-Navarro, A.; Torralva, M. La ictiofauna continental de la Cuenca del río Segura (SE Península Ibérica): Décadas después de Mas (1986). An. Biol. 2014, 6, 37–45. [Google Scholar]
- Oliva-Paterna, F.J.; Lafuente, E.; Olivo del Amo, R.; Sanz-Ronda, F.J.; Sánchez-Balibrea, J.; Diaz-García, R.; Torralva, M. LIFE+ Segura-Riverlink: A green infrastructure approach to restore the longitudinal connectivity. Fish. Mediterr. Environ. 2016, 7, 1–3. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Van der Vinne, G.; Katopodis, C. Hydraulics of vertical slot fishways. J. Hydraul. Eng. 1986, 112, 909–927. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Vono, V. Posthandling survival and PIT tag retention by alewives- A comparison of gastric and surgical implants. N. Am. J. Fish. Manag. 2013, 33, 790–794. [Google Scholar] [CrossRef]
- Ostrand, K.G.; Zydlewski, G.B.; Gale, W.L.; Zydlewski, J.D. Long term retention, survival, growth, and physiological indicators of salmonids marked with passive integrated transponder tags. In Advances in Fish Tagging and Marking Technology; McKenzie, J., Parsons, B., Seitz, A., Kopf, R.K., Mesa, M.G., Phelps, Q., Eds.; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 135–145. [Google Scholar]
- Cooke, S.J.; Midwood, J.D.; Thiem, J.D.; Klimley, P.; Lucas, M.C.; Thorstad, E.B.; Eiler, J.; Holbrook, C.; Ebner, B.C. Tracking animals in freshwater with electronic tags: Past, present and future. Anim. Biotelemetry 2013, 1, 5. [Google Scholar] [CrossRef]
- Enders, E.C.; Castro-Santos, T.; Lacey, R.W.J. The effects of horizontally and vertically oriented baffles on flow structure and ascent performance of upstream-migrating fish. J. Ecohydraulics 2017, 2, 38–52. [Google Scholar] [CrossRef]
- Goerig, E.; Castro-Santos, T. Is motivation important to brook trout passage through culverts? Can. J. Fish. Aquat. Sci. 2017, 74, 885–893. [Google Scholar] [CrossRef]
- Allison, P.D. Survival Analysis Using SAS: A Practical Guide; SAS Institute: Cary, NC, USA, 2010; ISBN 978-1-59994-640-5. [Google Scholar]
- Akaike, H. A new look at the statistical model Identification. IEEE Trans. Automat. Contr. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Ferreira, M.T.; Almeida, P.R. Fish assemblages in non-regulated and regulated rivers from permanent and temporary Iberian systems. River Res. Appl. 2013, 29, 1042–1058. [Google Scholar] [CrossRef]
- Grimardias, D. Evaluation de l´efficacité des dispositifs de franchissement des barrages via la méthode télémetrique (module 2). In Proceedings of the Programme INTERREG IV A France-Suisse, Bonneville, France, 17 June 2015. [Google Scholar]
- Assumpção, L.; Makrakis, M.C.; Makrakis, S.; Wagner, R.L.; Da Silva, P.S.; de Lima, A.F.; Kashiwaqui, E.A.L. The use of morphometric analysis to predict the swimming efficiency of two Neotropical long-distance migratory species in fish passage. Neotrop. Ichthyol. 2012, 10, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Videler, J.J.; Wardle, C.S. Fish swimming stride by stride: Speed limits and endurance. Rev. Fish Biol. Fish. 1991, 1, 23–40. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Ruiz-Legazpi, J.; Bravo-Córdoba, F.J.; Makrakis, S.; Castro-Santos, T. Sprinting performance of two Iberian fish: Luciobarbus bocagei and Pseudochondrostoma duriense in an open channel flume. Ecol. Eng. 2015, 83, 61–70. [Google Scholar] [CrossRef]
- Ruiz-Legazpi, J.; Sanz-Ronda, F.J.; Bravo-Córdoba, F.J.; Fuentes-Pérez, J.F.; Castro-Santos, T. Influencia de factores ambientales y biométricos en la capacidad de nado del barbo ibérico (Luciobarbus bocagei Steindachner, 1864), un ciprínido potamódromo endémico de la Península Ibérica. Limnetica 2018, 37, 251–265. [Google Scholar]
- Oliva-Paterna, F.J.; Sánchez-Pérez, A.; Zamora-Marín, J.M.; Zamora-López, A.; Amat-Trigo, F.; Guillén-Beltrán, A.; Bravo-Córdoba, F.J.; Sanz-Ronda, F.J.; Lafuente, E.; Torralva, M. Use of fish passes in a highly regulated Mediterranean river: Final insights and lesson learned in the LIFE+ Segura-Riverlink. In Proceedings of the VII Iberian Congress on Ichthyology, Faro, Algarve, Portugal, 13 June 2018; p. 17. [Google Scholar]
- Alexandre, C.M.; Quintella, B.R.; Ferreira, A.F.; Romão, F.A.; Almeida, P.R. Swimming performance and ecomorphology of the Iberian barbel Luciobarbus bocagei (Steindachner, 1864) on permanent and temporary rivers. Ecol. Freshw. Fish 2014, 23, 244–258. [Google Scholar] [CrossRef]
- Santos, J.M.; Silva, A.; Katopodis, C.; Pinheiro, P.; Pinheiro, A.; Bochechas, J.; Ferreira, M.T. Ecohydraulics of pool-type fishways: Getting past the barriers. Ecol. Eng. 2012, 48, 38–50. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; Sanz-Ronda, F.J.; Martínez de Azagra, A.; García-Vega, A. Non-uniform hydraulic behavior of pool-weir fishways: A tool to optimize its design and performance. Ecol. Eng. 2016, 86, 5–12. [Google Scholar] [CrossRef]
- Larinier, M.; Travade, F.; Porcher, J.P. Fishways: Biological basis, design criteria and monitoring. Bull. Français Pêche Piscic. 2002, 364, 208. [Google Scholar]
| Variables | VS | SNBO |
|---|---|---|
| Pool dimension (length × width) | 2.10 m × 1.60 m | 2.40 m × 1.60 m |
| Slope | 6.52% | 7.31% |
| Number of pools between A1 and A4 | 11 | 8 |
| Width of the slot/notch 1 | 0.23 m (0.20–0.23) | 0.32 m (0.31–0.36) |
| Height of the notch sill 1 | NA | 0.49 m (0.45–0.52) |
| Bottom orifice size (length × width) | NA | 0.20 m × 0.25 m |
| Drop between pools 1 | 0.15 m (0.14–0.19) | 0.19 m (0.13–0.24) |
| Mean water depth 1 | 0.91 m | 0.99 m |
| Flow discharge 2 | 0.29 m3/s | 0.31 m3/s |
| Volumetric Energy Dissipation | 118 W/m3 (110–149) | 148 W/m3 (102–181) |
| Water velocity at the slot/notch 3 | 1.38 m/s (1.10–1.47) | 1.24 m/s (0.80–1.36) |
| Water velocity at the orifice 3 | NA | 1.72 m/s (1.42–1.93) |
| Fish Group | Species | N | Length (cm) | Weight (g) | K | |||
|---|---|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | Mean ± SD | Range | |||
| VS1 | Barbel | 36 | 20.0 ± 4.2 | 11.2–28.2 | 133 ± 72 | 21–298 | 1.51 ± 0.17 | 1.24–2.18 |
| Nase | 23 | 15.6 ± 1.7 | 13.5–20.0 | 44 ± 18 | 26–92 | 1.11 ± 0.11 | 0.95–1.34 | |
| VS2 | Barbel | 29 | 19.9.0 ± 3.3 | 15.6–27.9 | 127 ± 66 | 56–322 | 1.49 ± 0.10 | 1.23–1.73 |
| Nase | 21 | 15.0 ± 1.6 | 12.8–19.6 | 40 ± 17 | 25–99 | 1.14 ± 0.12 | 0.96–1.42 | |
| SNBO1 | Barbel 1 | 21 | 17.9 ± 8.9 | 11.5–43.7 | 166 ± 344 | 24–1326 | 1.55 ± 0.14 | 1.26–1.83 |
| Nase 1 | 10 | 14.8 ± 2.8 | 11.6–19.6 | 39 ± 24 | 19–85 | 1.12 ± 0.08 | 0.98–1.22 | |
| SNBO2 | Barbel 1 | 18 | 15.3 ± 2.2 | 11.8–20.0 | 56± 27 | 28–130 | 1.49 ± 0.11 | 1.32–1.77 |
| Nase 1 | 7 | 14.0 ± 3.3 | 11.0–20.5 | 35 ± 29 | 16–99 | 1.13 ± 0.18 | 0.92–1.45 | |
| Metrics | VS (N = 110) | SNBO (N = 56) | ||
|---|---|---|---|---|
| Barbel | Nase | Barbel | Nase | |
| Attempt percentage | (58/66) 87.9% | (39/44) 88.6% | (30/39) 76.9% | (8/17) 47.1% |
| Median number of attempts | 3 (1–16) | 5 (1–12) | 2 (1–6) | 3 (1–6) |
| Ascent success | (55/58) 94.9% | (38/39) 94.8% | (24/30) 80.0% | (7/8) 87.5% |
| Median transit time 1 | (54) 12.5 min | (37) 8.0 min | (19) 26.3 min | (6) 9.3 min |
| Median transit time per meter of height | 6.9 min/m (1.3–274.5) | 4.4 min/m (1.2–342.7) | 16.6 min/m (4.6–405.3) | 5.2 min/m (2.1–34.4) |
| Barbel | VS | SNBO | ||||
| Parameters | Coefficient | SE | p | Coefficient | SE | p |
| Intercept (μ) | 3.4601 | 0.5425 | <0.001 | 3.3816 | 0.3209 | <0.001 |
| Length (β) | −0.0784 | 0.0268 | 0.0034 | −0.0350 | 0.0155 | 0.0239 |
| Shape (σ) | 0.4236 | 0.0487 | 0.3657 | 0.0725 | ||
| Nase | VS | SNBO | ||||
| Parameters | Coefficient | SE | p-value | Coefficient | SE | p-value |
| Intercept (μ) | 1.6243 | 1.7664 | 0.3578 | 5.4887 | 1.6238 | <0.001 |
| Length (β) | −0.0028 | 0.1161 | 0.9809 | −0.2365 | 0.0979 | 0.0157 |
| Shape (σ) | 0.6001 | 0.0882 | 0.3033 | 0.1063 | ||
| Parameters | Pre-Attempt Rate | Rest of Attempts Rate | ||||
|---|---|---|---|---|---|---|
| β ± SE | p-Value | HR | β ± SE | p-Value | HR | |
| Fishway | −0.951 ± 0.189 | <0.001 | 0.386 | −0.599 ± 0.149 | <0.001 | 0.549 |
| Species | 0.595 | 0.124 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanz-Ronda, F.J.; Bravo-Córdoba, F.J.; Sánchez-Pérez, A.; García-Vega, A.; Valbuena-Castro, J.; Fernandes-Celestino, L.; Torralva, M.; Oliva-Paterna, F.J. Passage Performance of Technical Pool-Type Fishways for Potamodromous Cyprinids: Novel Experiences in Semiarid Environments. Water 2019, 11, 2362. https://doi.org/10.3390/w11112362
Sanz-Ronda FJ, Bravo-Córdoba FJ, Sánchez-Pérez A, García-Vega A, Valbuena-Castro J, Fernandes-Celestino L, Torralva M, Oliva-Paterna FJ. Passage Performance of Technical Pool-Type Fishways for Potamodromous Cyprinids: Novel Experiences in Semiarid Environments. Water. 2019; 11(11):2362. https://doi.org/10.3390/w11112362
Chicago/Turabian StyleSanz-Ronda, Francisco Javier, Francisco Javier Bravo-Córdoba, Ana Sánchez-Pérez, Ana García-Vega, Jorge Valbuena-Castro, Leandro Fernandes-Celestino, Mar Torralva, and Francisco José Oliva-Paterna. 2019. "Passage Performance of Technical Pool-Type Fishways for Potamodromous Cyprinids: Novel Experiences in Semiarid Environments" Water 11, no. 11: 2362. https://doi.org/10.3390/w11112362





