The Impact of River Discharge and Water Temperature on Manganese Release from the Riverbed during Riverbank Filtration: A Case Study from Dresden, Germany
Abstract
:1. Introduction
2. Materials and Methods
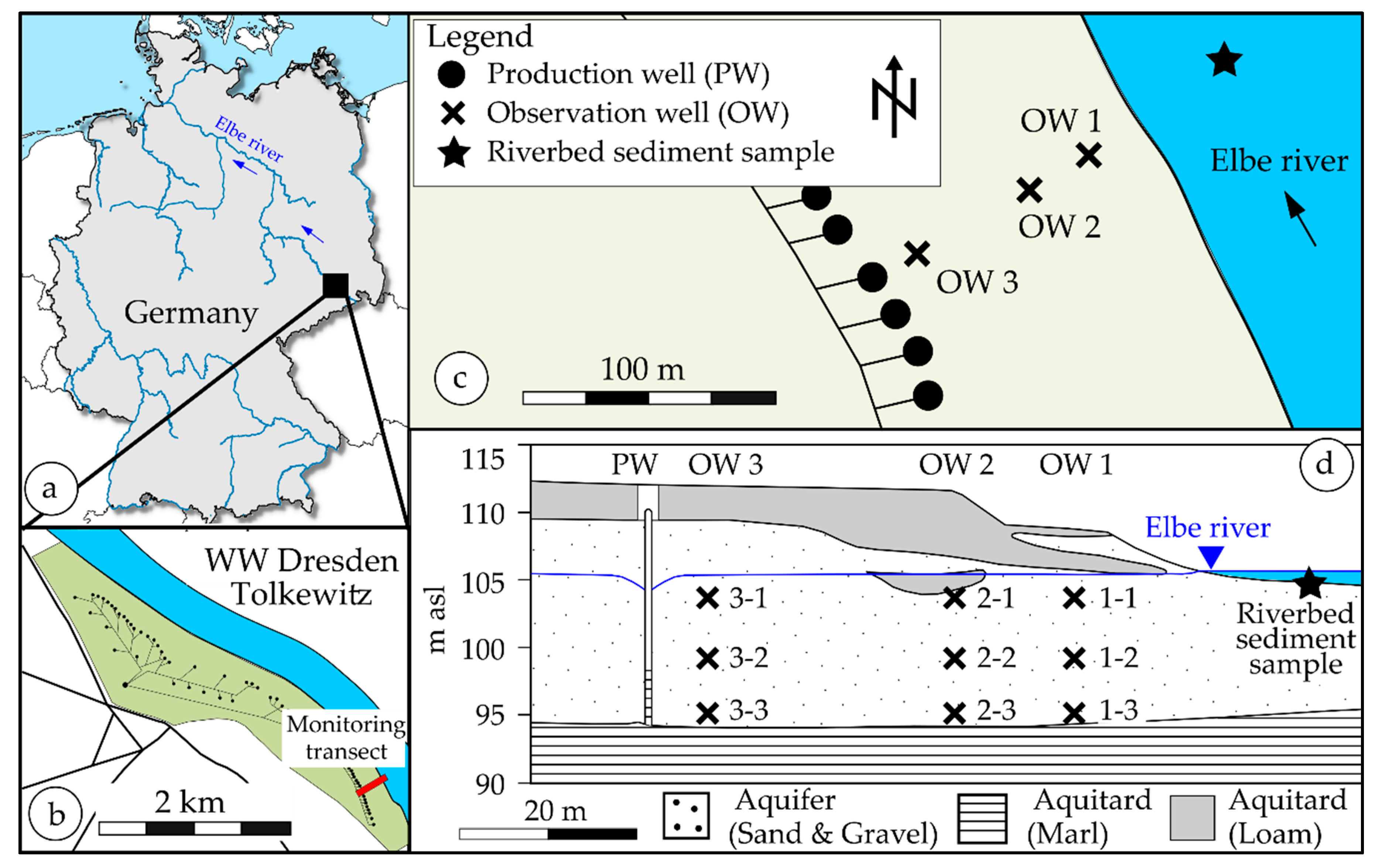
2.1. Description of the RBF Waterworks Dresden-Tolkewitz
2.2. Regular Monitoring in Dresden-Tolkewitz
2.3. Set-Up of the Column Experiments
2.4. Water Analysis
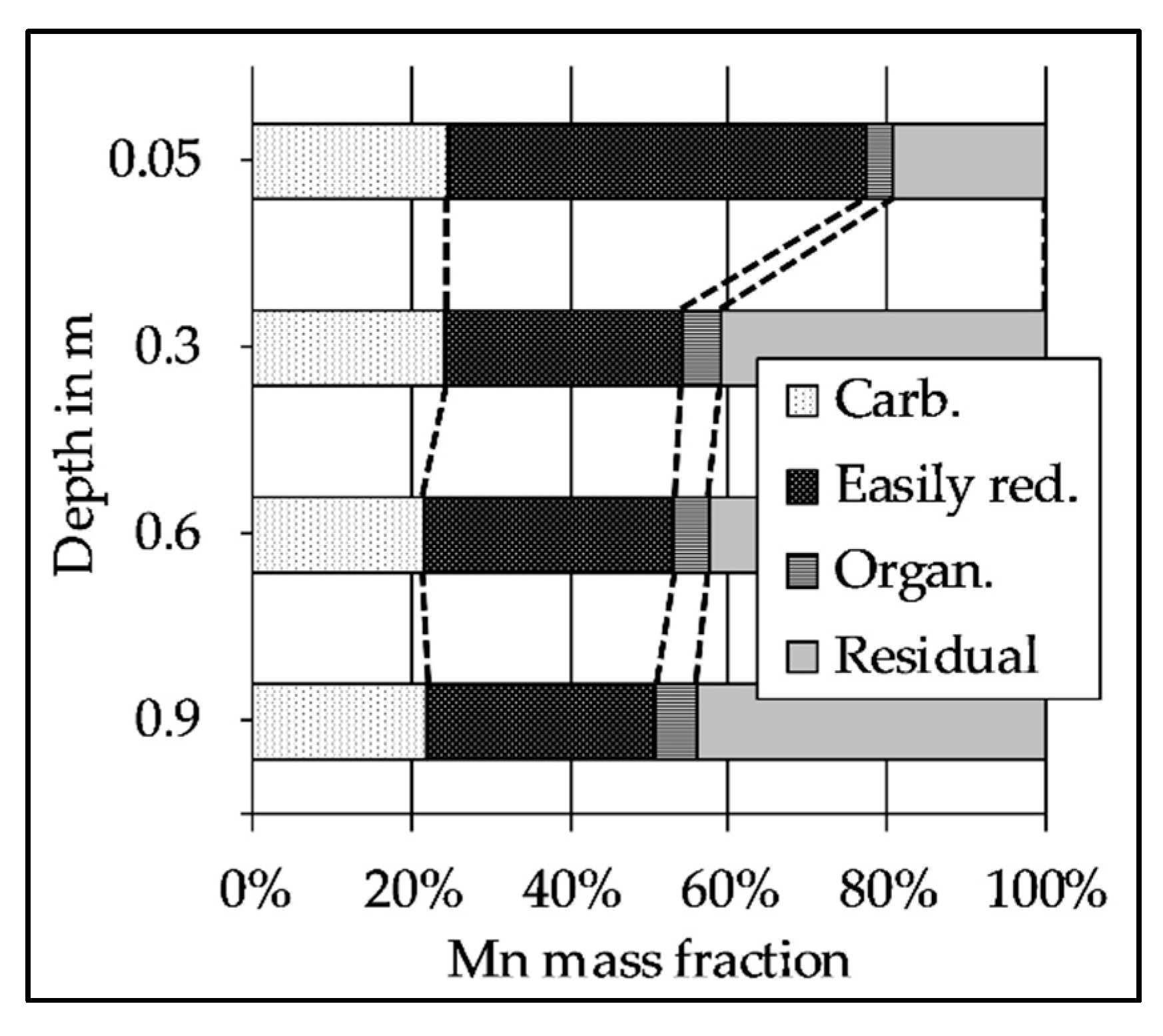
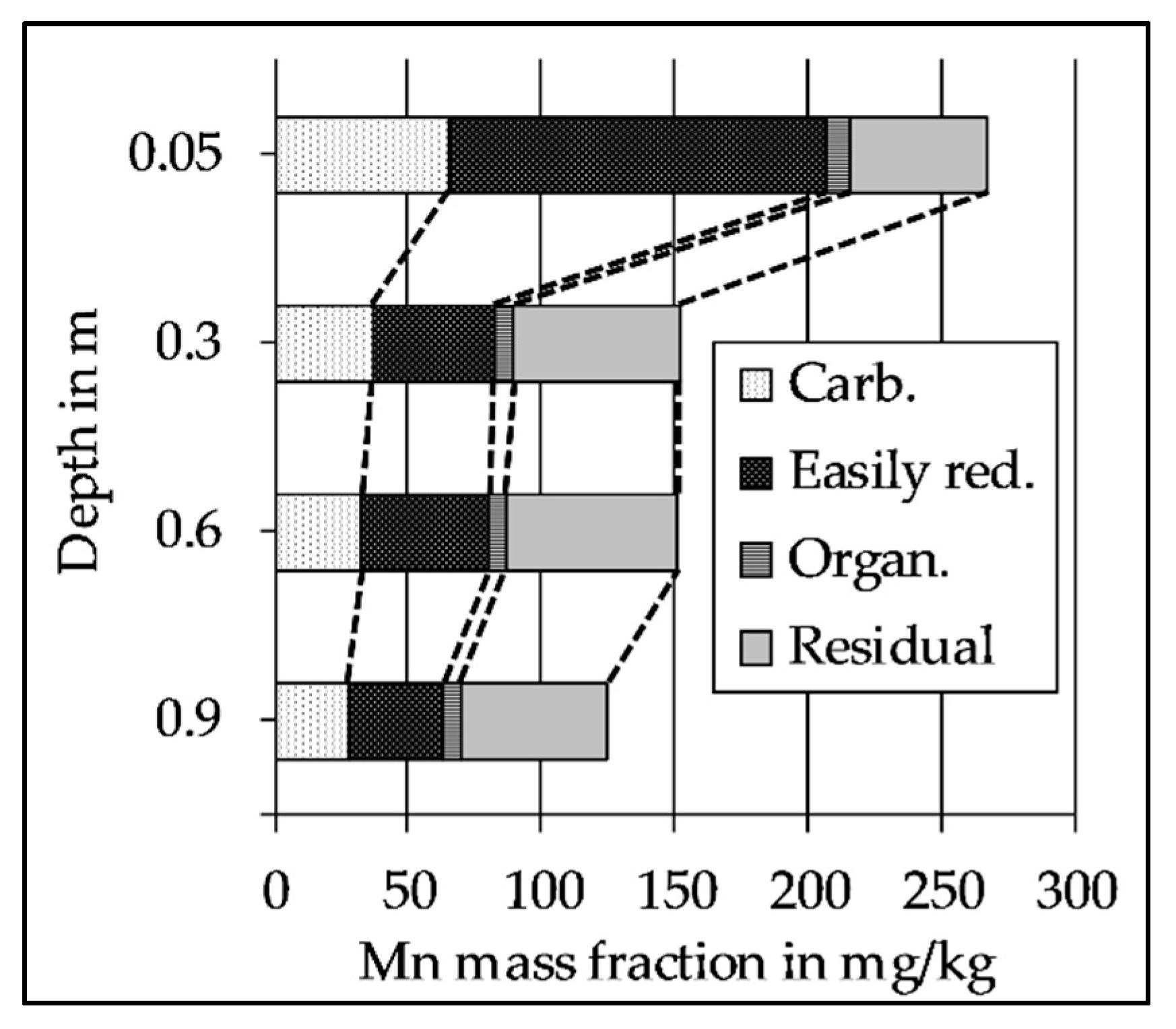
2.5. Sequential Extraction of the Riverbed Sediment
2.6. Estimation of Reduction Constants for the Elbe Riverbed with PHREEQC
3. Results
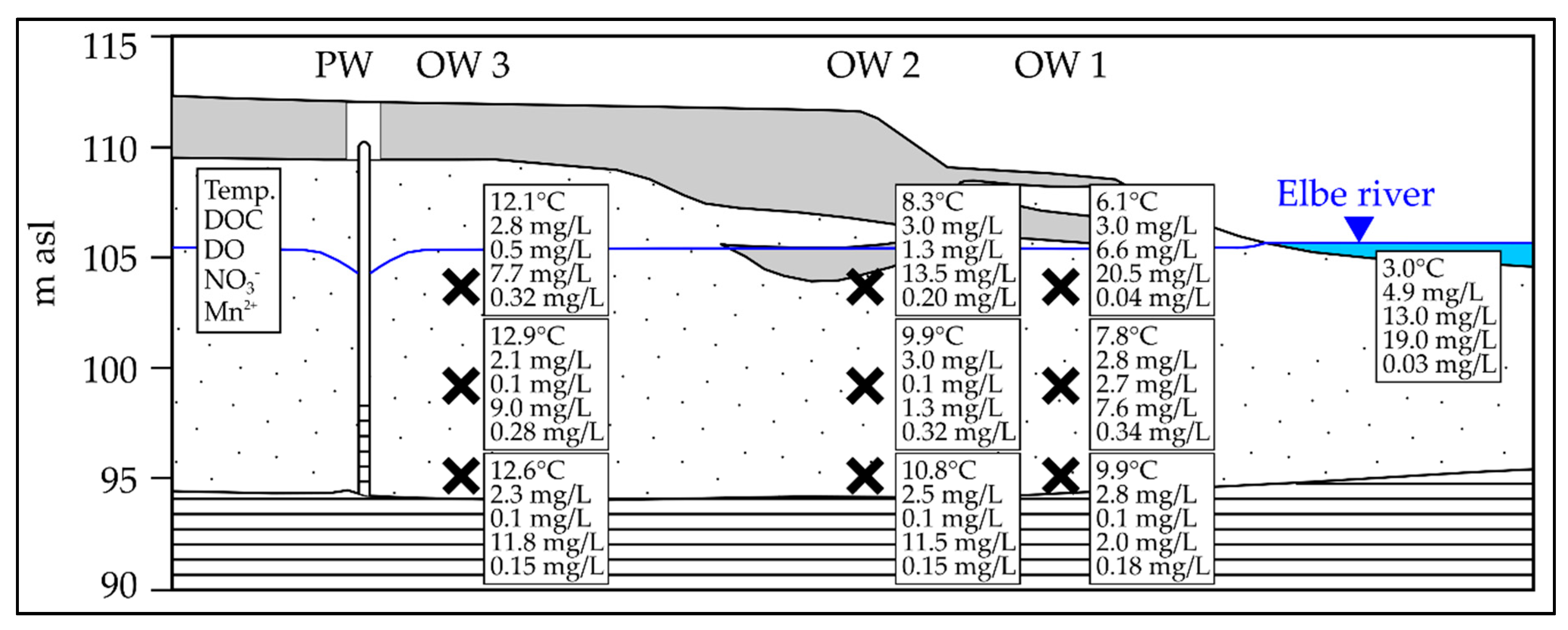
3.1. Seasonal Fluctuation of Redox-Sensitive Parameters Close to the Riverbank
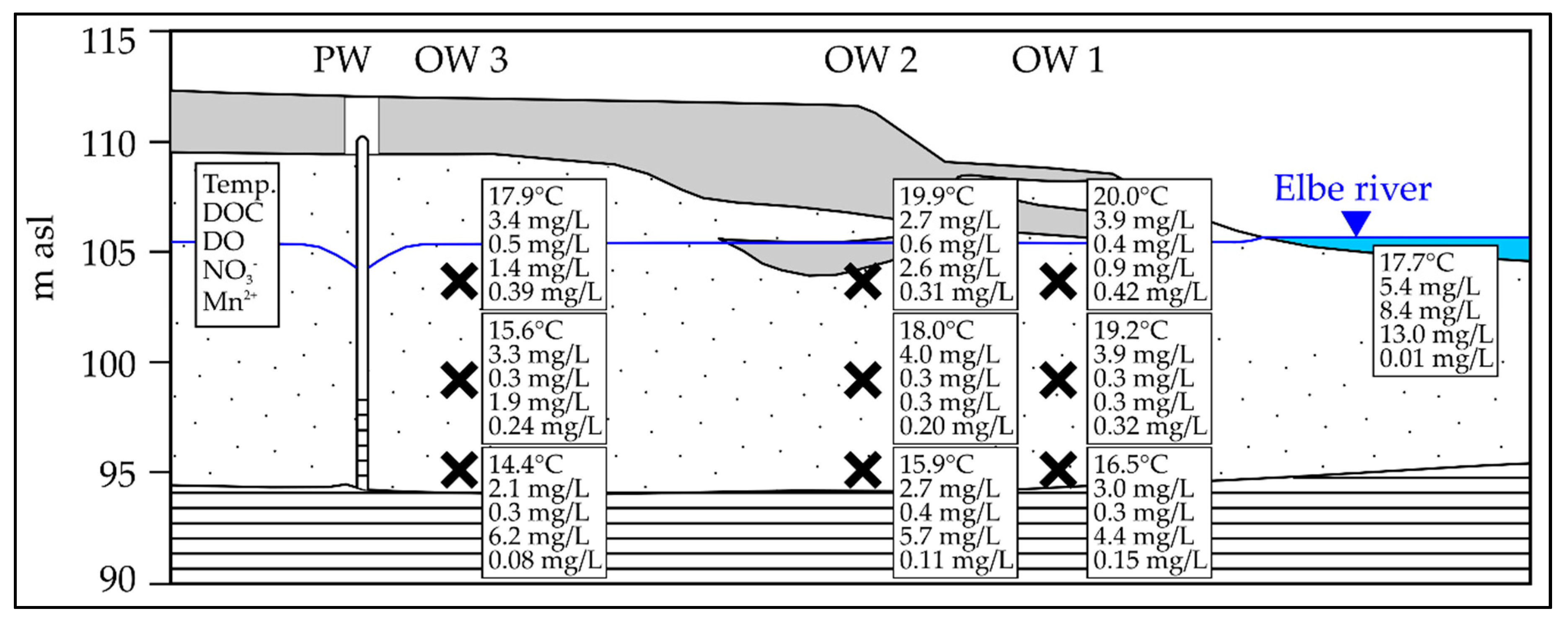
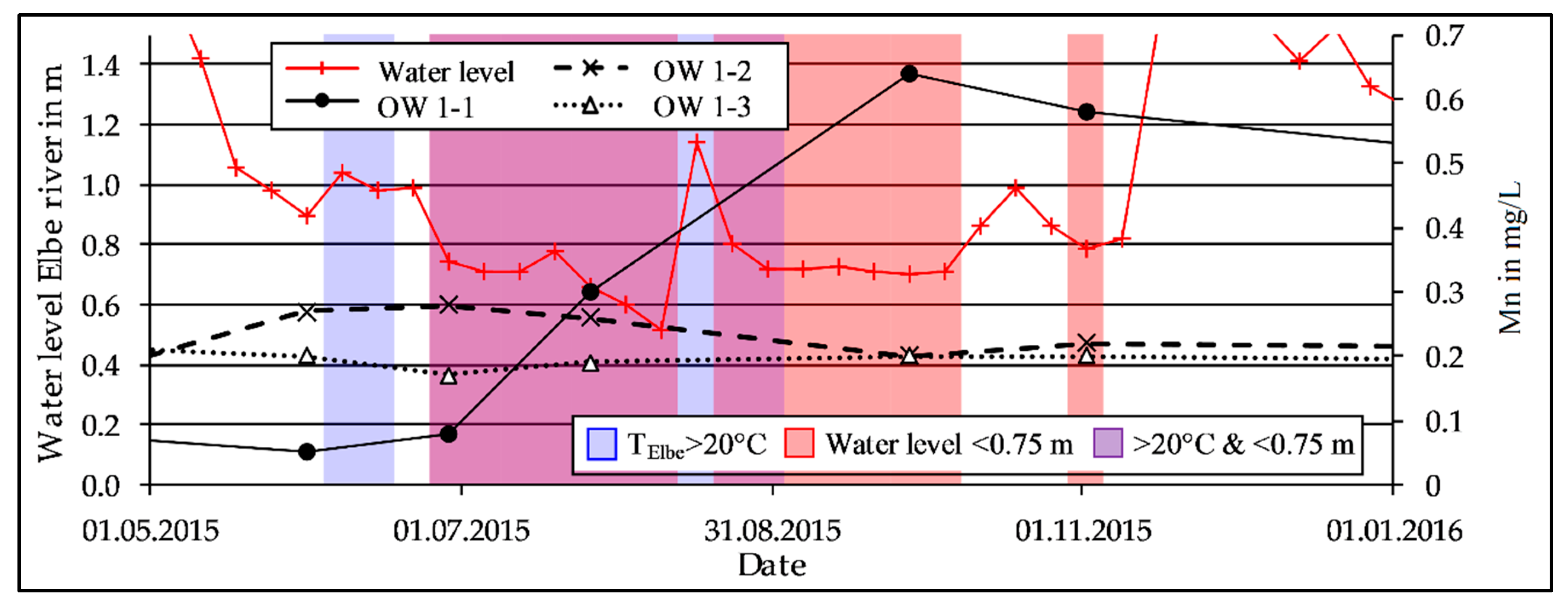
3.2. Mn Release During Low Discharge Periods of the Elbe River
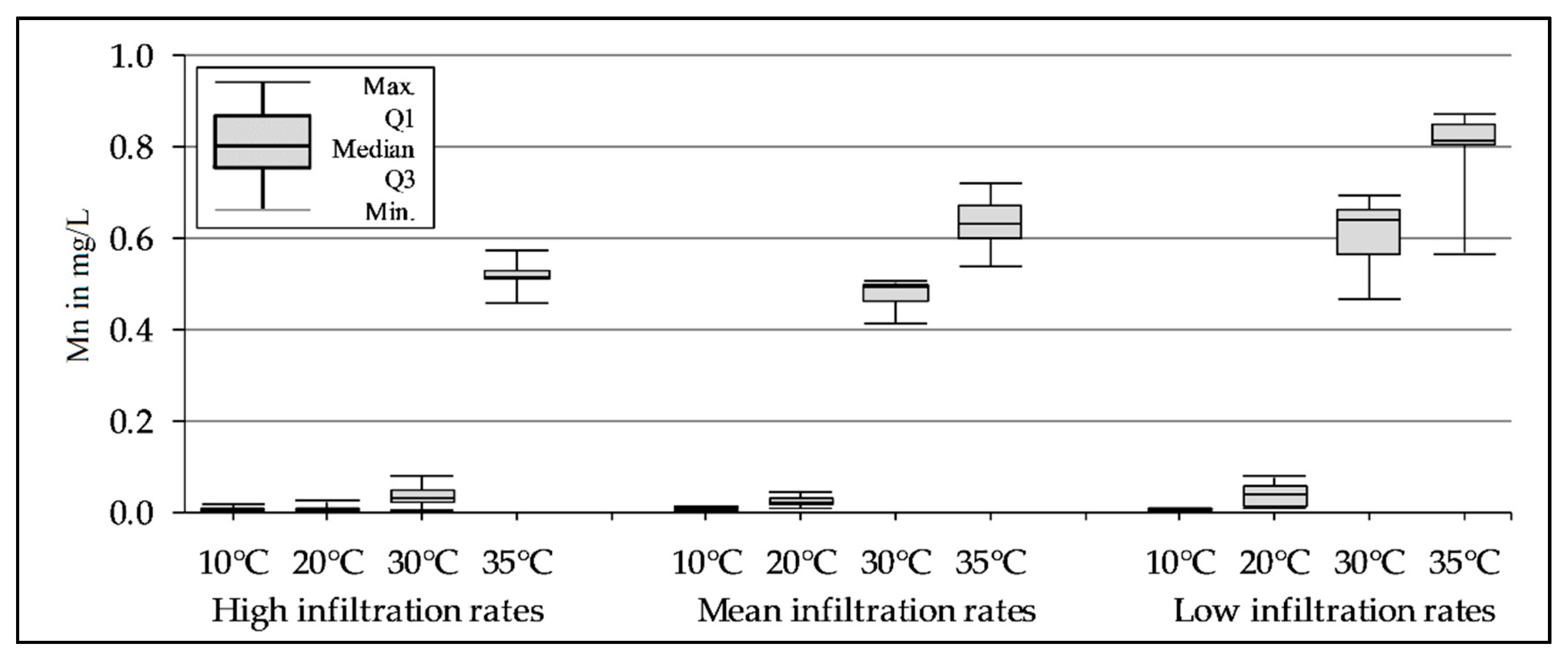
3.3. Mn Release Depending on the Temperature and Infiltration Rate During the Column Experiments
3.4. Reduction Constants of O2, NO3− and Mn(IV) as Electron Acceptors
3.5. The Decrease of Easily Reducible Mn Along the Flow Path
4. Discussion
4.1. The Significance of the Calibrated Degradation Rate Constants
4.2. Impact of the Discharge on the Observed Redox Patterns
4.3. Manganese Release Controlled by Temperature
4.4. Depletion of the Mn Reservoir Within the Riverbank
4.5. Implications for (River-)Bank Filtration Sites
5. Conclusions
Supplementary Materials
- Figure S1: The flow scheme of the column experiments inside the thermostatic cabinets;
- Figure S2: The Mn concentration at OW 2 during a low discharge period in 2015;
- Figure S3: The Mn concentration at OW 3 during a low discharge period in 2015;
- Figure S4: The increase of the Mn concentration within the first 5 PV at 30 °C and 1 mL/min after lowering from 2 mL/min, Median, 10- and 90-%ile (each n = 3);
- Figure S5: The calibrated reduction rate constants for the column experiment;
- Figure S6: The Mn concentration at OW 1 during a low discharge period in 2007;
- Figure S7: The Mn concentration at OW 2 during a low discharge period in 2007;
- Figure S8: The Mn concentration at OW 3 during a low discharge period in 2007;
- Table S2: The input data of the column in PHREEQC;
- Table S3: The statistical data of the Elbe river at Dresden for the entire observation period 2006–2016 [25];
- Table S4: The monthly and 10-year median values of selected parameters along all three OW’s of the transect in the WW Dresden-Tolkewitz for the entire observation period 2006–2016;
- Table S5: The statistical data for the low flow period in 2015;
- Table S6: The statistical data for Mn release during the column experiment with riverbed sediment from the Elbe river in Dresden-Tolkewitz, Q1 and Q3 correspond to the 1st and 3rd quartile;
- Table S7: The comparison of the measured parameter values during the column experiment and the modeled parameters from PHREEQC;
- Table S8: The results and statistical data of the sequential extraction of the riverbed sediment after the column experiment (n = 3 for each statistical data);
- Table S9: The median concentration of DO in the outflow during the column experiment.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bourg, A.C.M.; Bertin, C. Biogeochemical processes during the infiltration of river water into an alluvial aquifer. Environ. Sci. Technol. 1993, 27, 661–666. [Google Scholar] [CrossRef]
- Grischek, T.; Schoenheinz, D.; Worch, E.; Hiscock, K. Bank filtration in Europe – An overview of aquifer conditions and hydraulic controls. In Management of Aquifer Recharge for Sustainability; Dillon, P., Ed.; Balkema Publ.: Lisse, The Netherlands, 2002; pp. 485–488. [Google Scholar]
- Ray, C.; Melin, G.; Linsky, R.B. Riverbank Filtration—Improving Source Water Quality; Kluwer: Dordrecht, The Netherlands, 2003; 364p. [Google Scholar]
- Regnery, J.; Barringer, J.; Wing, A.D.; Hoppe-Jones, C.; Teerlink, J.; Drewes, J.E. Start-up performance of a full-scale riverbank filtration site regarding removal of DOC, nutrients, and trace organic chemicals. Chemosphere 2015, 127, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Blanford, W.; Boving, T.; Al-Ghazawi, Z.; Shawaqfah, M.; Al-Rashdan, J.; Saadoun, I.; Schijven, J.; Ababneh, Q. River bank filtration for protection of Jordanian surface and groundwater. In Proceedings of the World Environmental and Water Resources Congress 2010: Challenges of Change; ASCE: Providence, RI, USA, 2010; pp. 776–781. [Google Scholar]
- Ghodeif, K.; Paufler, S.; Grischek, T.; Wahaab, R.; Souaya, E.; Bakr, M.; Abogabal, A. Riverbank filtration in Cairo, Egypt—Part I: Installation of a new riverbank filtration site and first monitoring results. Environ. Earth. Sci. 2018, 77, 270. [Google Scholar] [CrossRef]
- Hu, B.; Teng, Y.; Zhai, Y.; Zuo, R.; Li, J.; Chen, H. Riverbank filtration in China: A review and perspective. J. Hydrol. 2016, 541, 914–927. [Google Scholar] [CrossRef]
- Sandhu, C.; Grischek, T.; Kumar, P.; Ray, C. Potential for riverbank filtration in India. Clean Technol. Environ. Policy 2011, 13, 295–316. [Google Scholar] [CrossRef]
- Hiscock, K.M.; Grischek, T. Attenuation of groundwater pollution by bank filtration. J. Hydrol. 2002, 266, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Kuehn, W.; Mueller, U. Riverbank filtration—An overview. J. Am. Water Works Assoc. 2000, 92, 60–69. [Google Scholar] [CrossRef]
- Bertelkamp, C.; Reungoat, J.; Cornelissen, E.R.; Singhal, N.; Reynisson, J.; Cabo, A.J.; van der Hoek, J.P.; Verliefde, A.R.D. Sorption and biodegradation of organic micropollutants during river bank filtration: A laboratory column study. Water Res. 2014, 52, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Bourg, A.C.M.; Richard-Raymond, F. Spatial and temporal variability in the water redox chemistry of the M27 experimental site in the Drac river calcareous alluvial aquifer (Grenoble, France). J. Contam. Hydrol. 1994, 15, 93–105. [Google Scholar] [CrossRef]
- Von Gunten, H.R.; Karametaxas, G.; Krähenbühl, U.; Kuslys, M.; Giovanoli, R.; Hoehn, E.; Keil, R. Seasonal biogeochemical cycles in riverborne groundwater. Geochim. Cosmochim. Acta 1991, 55, 3597–3609. [Google Scholar] [CrossRef]
- Henzler, A.F.; Greskowiak, J.; Massmann, G. Seasonality of temperatures and redox zonations during bank filtration—A modeling approach. J. Hydrol. 2016, 535, 282–292. [Google Scholar] [CrossRef]
- Gross-Wittke, A.; Gunkel, G.; Hoffmann, A. Temperature effects on bank filtration: Redox conditions and physical–chemical parameters of pore water at Lake Tegel, Berlin, Germany. J. Water Clim. Chang. 2010, 1, 55–66. [Google Scholar] [CrossRef]
- Van’t Hoff, J.H.; Cohen, E. Studies on Chemical Dynamics; Studien zur chemischen Dynamik, Etudes de dynamique chemique; Frederik Muller & Co.: Amsterdam, The Netherlands, 1896; p. 128. [Google Scholar]
- Diem, S.; von Rohr, M.R.; Hering, J.G.; Kohler, H.-P.E.; Schirmer, M.; von Gunten, U. NOM degradation during river infiltration: Effects of the climate variables temperature and discharge. Water Res. 2013, 47, 6585–6595. [Google Scholar] [CrossRef] [PubMed]
- Schoenheinz, D.; Grischek, T. Behavior of dissolved organic carbon during bank filtration under extreme climate conditions. In Riverbank Filtration for Water Security in Desert Countries; Shamrukh, M., Ed.; NATO Science for Peace and Security Series C: Environmental Security; Springer: Dordrecht, The Netherlands, 2011; pp. 151–168. ISBN 978-94-007-0025-3. [Google Scholar]
- Sprenger, C.; Lorenzen, G.; Hulshoff, I.; Gruetzmacher, G.; Ronghang, M.; Pekdeger, A. Vulnerability of bank filtration systems to climate change. Sci. Total Environ. 2011, 409, 655–663. [Google Scholar] [CrossRef] [PubMed]
- EEA European Environment Agency. Climate Change, Impacts and Vulnerability in Europe 2016; An Indicator-Based Report; Publications Office of the European Union: Luxembourg, 2017; ISBN 978-92-9213-835-6. [Google Scholar]
- Kreienkamp, F.; Spekat, A.; Enke, W. WEREX V: Provision of an Ensemble of Regional Climate Projections for Saxony, 1st ed.; Saxon State Office for the Environment, Agriculture and Geology: Dresden, Germany, 2011; 71p. (In German) [Google Scholar]
- Grischek, T.; Bartak, R. Riverbed clogging and sustainability of riverbank filtration. Water 2016, 8, 604. [Google Scholar] [CrossRef]
- Ahrns, J. Modeling the Exchange between Surface Water and Groundwater Using Temperature as Tracer. Diploma Thesis, Dresden University of Applied Sciences, Dresden, Germany, 2008. (In German). [Google Scholar]
- DVGW German Technical and Scientific Association for Gas and Water. Guideline DVGW W 112 (A), Principles of Groundwater Sampling from Groundwater Monitoring Wells; WVGW: Bonn, Germany, 2011; ISSN 0176-3504. (In German) [Google Scholar]
- SMUL Saxon State Ministry for Environment and Agriculture. Water Quality Data for the Elbe River. 2018. Available online: www.umwelt.sachsen.de/umwelt/wasser/ (accessed on 15 June 2018).
- WSV Federal Waterways and Shipping Administration. Hydrological Information System. 2018. Available online: www.wsv.de (accessed on 14 June 2018).
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.A.; Tack, F.M.G. Determination of Al, Cu, Fe, Mn, Pb and Zn in certified reference materials using the optimized BCR sequential extraction procedure. Anal. Chim. Acta 2002, 454, 249–257. [Google Scholar] [CrossRef]
- Farnsworth, C.E.; Hering, J.G. Inorganic geochemistry and redox dynamics in bank filtration settings. Environ. Sci. Technol. 2011, 45, 5079–5087. [Google Scholar] [CrossRef] [PubMed]
- Parkhurst, D.L.; Appelo, C.A.J. Description of Input and Examples for Phreeqc Version 3: A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. U.S. Geological Survey Techniques and Methods, book 6, chap. A43; 2013. Available online: http://pubs.usgs.gov/tm/06/a43 (accessed on 14 June 2018).
- Greskowiak, J.; Prommer, H.; Massmann, G.; Nutzmann, G. Modeling seasonal redox dynamics and the corresponding fate of the pharmaceutical residue phenazone during artificial recharge of groundwater. Environ. Sci. Technol. 2006, 40, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Diem, S.; Cirpka, O.A.; Schirmer, M. Modeling the dynamics of oxygen consumption upon riverbank filtration by a stochastic–convective approach. J. Hydrol. 2013, 505, 352–363. [Google Scholar] [CrossRef]
- Sharma, L.; Greskowiak, J.; Ray, C.; Eckert, P.; Prommer, H. Elucidating the effects on seasonal variations of biogeochemical turnover rates during riverbank filtration. J. Contam. Hydrol. 2012, 428–429, 104–115. [Google Scholar] [CrossRef]
- Doherty, J. PEST. Model-Independent Parameter Estimation, 7th ed.; Watermark Numerical Computing: Brisbane, Australia, 2018. [Google Scholar]
- Matsunaga, T.; Karametaxas, G.; von Gunten, H.R.; Lichtner, P.C. Redox chemistry of iron and manganese minerals in river-recharged aquifers: A model interpretation of a column experiment. Geochim. Cosmochim. Acta 1993, 57, 1691–1704. [Google Scholar] [CrossRef]
- Mayer, K.U.; Benner, S.G.; Frind, E.O.; Thornton, S.F.; Lerner, D.N. Reactive transport modeling of processes controlling the distribution and natural attenuation of phenolic compounds in a deep sandstone aquifer. J. Contam. Hydrol. 2001, 53, 341–368. [Google Scholar] [CrossRef]
- Prommer, H.; Stuyfzand, P.J. Identification of temperature-dependent water quality changes during a deep well injection experiment in a pyritic aquifer. Environ. Sci. Technol. 2005, 39, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Massmann, G.; Pekdeger, A.; Merz, C. Redox processes in the Oderbruch polder groundwater flow system in Germany. Appl. Geochem. 2004, 19, 863–886. [Google Scholar] [CrossRef]
- Jensen, D.L.; Boddum, J.K.; Tjell, J.C.; Christensen, T.H. The solubility of rhodochrosite (MnCO3) and siderite (FeCO3) in anaerobic aquatic environments. Appl. Geochem. 2002, 17, 503–511. [Google Scholar] [CrossRef]
- Heeger, D. Investigation of Clogging in Rivers. Ph.D. Thesis, Dresden University of Technology, Dresden, Germany, 1987. (In German). [Google Scholar]
- Grischek, T. Management of bank filtration sites along the Elbe River. Ph.D. Thesis, Faculty of Forestry, Geo and Hydro Sciences, Dresden University of Technology, Dresden, Germany, 2003. (In German). [Google Scholar]
- Eckert, P.; Rohns, H.P.; Irmscher, R. Dynamic processes during bank filtration and their impact on raw water quality. In Proceedings of the Aquifer Recharge, Recharge Systems for Protecting and Enhancing Groundwater Resources, Berlin, Germany, 11–16 June 2005; pp. 17–22. [Google Scholar]
- Eckert, P.; Irmscher, R. Over 130 years of experience with riverbank filtration in Düsseldorf, Germany. Aqua 2006, 55, 283–291. [Google Scholar] [CrossRef]
- Von Rohr, R.M.; Hering, J.G.; Kohler, H.P.; von Gunten, U. Column studies to assess the effects of climate variables on redox processes during riverbank filtration. Water Res. 2014, 61, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Groffman, A.R.; Crossey, L.J. Transient redox regimes in a shallow alluvial aquifer. Chem. Geol. 1999, 161, 415–442. [Google Scholar] [CrossRef]
- Bourg, A.C.M.; Darmendrail, D.; Ricour, J. Geochemical filtration of riverbank and migration of heavy metals between the Deûle River and the Ansereuilles alluvion-chalk aquifer (Nord, France). Geoderma 1989, 44, 229–244. [Google Scholar] [CrossRef]
- Jacobs, L.A.; von Gunten, H.R.; Keil, R.; Kuslys, M. Geochemical changes along a river-groundwater infiltration flow path: Glattfelden, Switzerland. Geochim. Cosmochim. Acta 1988, 52, 2693–2706. [Google Scholar] [CrossRef]
- Kedziorek, M.; Geoffriau, S.; Bourg, A.C.M. Organic matter and modeling redox reactions during river bank filtration in an alluvial aquifer of the Lot River, France. Environ. Sci. Technol. 2008, 42, 2793–2798. [Google Scholar] [CrossRef] [PubMed]
- Maeng, S.K.; Ameda, E.; Sharma, S.K.; Grützmacher, G.; Amy, G.L. Organic micro-pollutant removal from wastewater effluent-impacted drinking water sources during bank filtration and artificial recharge. Water Res. 2010, 44, 4003–4014. [Google Scholar] [CrossRef] [PubMed]
- Von Gunten, H.R.; Karametaxas, G.; Keil, R. Chemical processes in infiltrated riverbed sediments. Environ. Sci. Technol. 1994, 28, 2087–2093. [Google Scholar] [CrossRef] [PubMed]
- Bourg, A.C.M.; Bertin, C. Seasonal and spatial trends in manganese solubility in an alluvial aquifer. Environ. Sci. Technol. 1994, 28, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, E.; Zobrist, J.; Schwarzenbach, R.P. Infiltration of river water into groundwater—Hydrogeological and hydrochemical investigations in the Glattal. Gas Wasser Abwasser 1983, 63, 401–410. (In German) [Google Scholar]
- Martin, J.M.; Meybeck, M. Elemental mass-balance of material carried by major world rivers. Mar. Chem. 1979, 7, 173–206. [Google Scholar] [CrossRef]
- Jain, C.K.; Ram, D. Adsorption of lead and zinc on bed sediments of the river Kali. Water Res. 1997, 31, 154–162. [Google Scholar] [CrossRef]
- Jain, C.K.; Sharma, M.K. Adsorption of Cadmium on bed sediments of River Hindon: Adsorption models and kinetics. Water Air Soil Poll. 2001, 137, 1–19. [Google Scholar] [CrossRef]
- Förstner, U.; Salomons, W. Trace metal analysis on polluted sediments, II. Evaluation of environmental impact. Environ. Technol. Lett. 1980, 1, 506–517. [Google Scholar] [CrossRef]
- Grischek, T.; Dehnert, J.; Nestler, W.; Treutler, H.C.; Freyer, K. Description of system conditions during bank filtration through accompanying drilling core investigations. In Proceedings of the 2nd Dresdner Grundwasserforschungstage, Dresden, Germany, 25 March 1993; pp. 207–220. (In German). [Google Scholar]
- Paufler, S. Management of Riverbank Filtration Sites to Reduce Manganese Concentrations in Raw Water. Diploma Thesis, Dresden University of Applied Sciences, Dresden, Germany, 2015. (In German). [Google Scholar]
- Nirel, P.M.V.; Morel, F.M.M. Pitfalls of sequential extractions. Water Res. 1990, 24, 1055–1056. [Google Scholar] [CrossRef]
- Van Vliet, M.T.H.; Franssen, W.H.P.; Yearsley, J.R.; Ludwig, F.; Haddeland, I.; Lettenmaier, D.P.; Kabat, P. Global river discharge and water temperature under climate change. Glob. Environ. Chang. 2013, 23, 450–464. [Google Scholar] [CrossRef]
- Abdalla, F.A.; Shamrukh, M. Quantification of river Nile/Quaternary aquifer exchanges via riverbank filtration by hydrochemical and biological indicators, Assiut, Egypt. J. Earth Syst. Sci. 2016, 125, 1697–1711. [Google Scholar] [CrossRef]
- Buschmann, J.; Berg, M.; Stengel, C.; Sampson, M.L. Arsenic and manganese contamination of drinking water resources in Cambodia: Coincidence of risk areas with low relief topography. Environ. Sci. Technol. 2007, 41, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Brandhuber, P.; Clark, S.; Knocke, W.; Tobiason, J. (Eds.) Guidance for the Treatment of Manganese; Water Research Foundation: Denver, Colorado, 2013; 148p, ISBN 978-1-60573-187-2. [Google Scholar]
- Rohns, H.P.; Forner, C.; Eckert, P.; Irmscher, R. Efficiency of riverbank filtration considering the removal of pathogenic microorganisms of the River Rhine. In Recent Progress in Slow Sand and Alternative Biofiltration Processes; Gimbel, R., Graham, N.J.D., Collins, M.R., Eds.; IWA Publishing: London, UK, 2006; pp. 539–546. ISBN 9781843391203. [Google Scholar]
- Grischek, T.; Paufler, S. Prediction of iron release during riverbank filtration. Water 2017, 9, 317. [Google Scholar] [CrossRef]
- Grischek, T.; Schoenheinz, D.; Eckert, P.; Ray, C. Sustainability of river bank filtration: Examples from Germany. In Groundwater Quality Sustainability; Maloszewski, P., Witczak, S., Malina, G., Eds.; Taylor & Francis Group: London, UK, 2012; pp. 213–227. [Google Scholar]
- Soares, M. The influence of high infiltration rates, suspended sediment concentration and sediment grain size on river and lake bed clogging. Ph.D. Thesis, Technical University of Berlin, Berlin, Germany, 2015. [Google Scholar]
| Column | 1, 2, and 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature in °C | 10 | 20 | 30 | 35 | ||||||||
| Flow in mL/min | 1 | 2 | 4 | 1 | 2 | 4 | 1 | 2 | 4 | 1 | 2 | 4 |
| n Samples/event | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| This Study | |||||
| Temperature | va | kox | knit | kmn | Notes |
| m/s | mol/(L·s) | mol/(L·s) | mol/(L·s) | ||
| 10 °C | 8.60 × 10−6 | 5.18 × 10−10 | 2.00 × 10−12 | 2.50 × 10−10 | Low infiltration rate (1 mL/min) |
| 1.72 × 10−5 | 8.65 × 10−10 | 7.00 × 10−12 | 2.00 × 10−10 | Mean infiltration rate (2 mL/min) | |
| 3.44 × 10−5 | 1.80 × 10−9 | 5.00 × 10−12 | 1.00 × 10−10 | High infiltration rate (4 mL/min) | |
| 20 °C | 8.60 × 10−6 | 2.17 × 10−10 | 1.53 × 10−10 | 1.10 × 10−10 | Low infiltration rate (1 mL/min) |
| 1.72 × 10−5 | 4.50 × 10−10 | 5.50 × 10−11 | 1.50 × 10−10 | Mean infiltration rate (2 mL/min) | |
| 3.44 × 10−5 | 8.90 × 10−10 | 5.00 × 10−12 | 1.00 × 10−10 | High infiltration rate (4 mL/min) | |
| 30 °C | 8.60 × 10−6 | 1.02 × 10−10 | 1.88 × 10−10 | 1.90 × 10−9 | Low infiltration rate (1 mL/min) |
| 1.72 × 10−5 | 2.14 × 10−10 | 2.80 × 10−10 | 2.20 × 10−9 | Mean infiltration rate (2 mL/min) | |
| 3.44 × 10−5 | 4.70 × 10−10 | 5.00 × 10−12 | 1.00 × 10−10 | High infiltration rate (4 mL/min) | |
| 35 °C | 8.60 × 10−6 | 9.70 × 10−11 | 1.35 × 10−10 | 1.62 × 10−9 | Low infiltration rate (1 mL/min) |
| 1.72 × 10−5 | 2.14 × 10−10 | 3.50 × 10−11 | 1.40 × 10−9 | Mean infiltration rate (2 mL/min) | |
| 3.44 × 10−5 | 4.28 × 10−10 | 5.00 × 10−12 | 1.95 × 10−9 | High infiltration rate (4 mL/min) | |
| 10%ile | 1.13 × 10−10 | 5.00 × 10−12 | 1.00 × 10−10 | ||
| Median | 4.39 × 10−10 | 2.10 × 10−11 | 2.25 × 10−10 | ||
| 90%ile | 8.88 × 10−10 | 1.85 × 10−10 | 1.95 × 10−9 | ||
| n | 12 | 12 | 12 | ||
| Literature Data | |||||
| Temperature | va | kox | knit | kmn | Source |
| m/s | mol/(L·s) | mol/(L·s) | mol/(L·s) | ||
| Variable | Variable | 1.52 × 10−10 | 3.81 × 10−11 | 8.91 × 10−13 | [31] |
| Variable | Variable | 2.00 × 10−10 | 1.00 × 10−10 | 1.70 × 10−12 | [14] |
| 22 °C | 7.60 × 10−6 | 3.50 × 10−8 | 3.40 × 10−8 | 3.00 × 10−13 | [35] |
| n.a. | n.a. | 3.98 × 10−10 | 3.98 × 10−11 | 6.31 × 10−14 | [36] |
| Variable | Variable | 1.57 × 10−9 | 1.00 × 10−11 | n.a. | [37] |
| Variable | Variable | 1.30 × 10−9 | 8.00 × 10−10 | n.a. | [33] for DOC |
| Variable | Variable | 1.90 × 10−11 | 1.20 × 10−11 | n.a. | [33] for SOM |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paufler, S.; Grischek, T.; Benso, M.R.; Seidel, N.; Fischer, T. The Impact of River Discharge and Water Temperature on Manganese Release from the Riverbed during Riverbank Filtration: A Case Study from Dresden, Germany. Water 2018, 10, 1476. https://doi.org/10.3390/w10101476
Paufler S, Grischek T, Benso MR, Seidel N, Fischer T. The Impact of River Discharge and Water Temperature on Manganese Release from the Riverbed during Riverbank Filtration: A Case Study from Dresden, Germany. Water. 2018; 10(10):1476. https://doi.org/10.3390/w10101476
Chicago/Turabian StylePaufler, Sebastian, Thomas Grischek, Marcos Roberto Benso, Nadine Seidel, and Thomas Fischer. 2018. "The Impact of River Discharge and Water Temperature on Manganese Release from the Riverbed during Riverbank Filtration: A Case Study from Dresden, Germany" Water 10, no. 10: 1476. https://doi.org/10.3390/w10101476





