Secondary Ophthalmic Features Represent Diagnostic Clues and Potential Points of Intervention for Inherited Retinal Diseases (Target 5000 Report 3)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Demographics, BCVA and Amblyopia
3.2. Cataract
3.3. Refractive Error
3.4. Macular Pathology
3.5. Retinal Detachment
3.6. Glaucoma
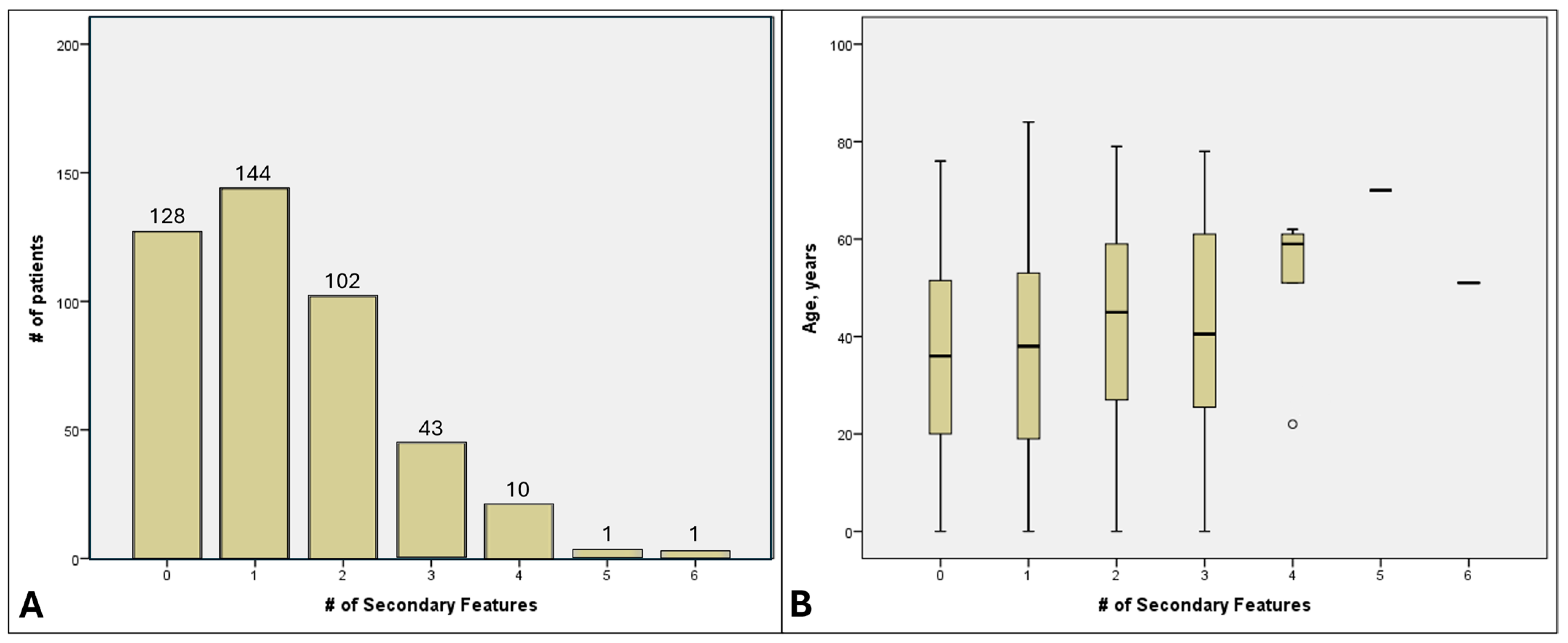
3.7. Cumulative SOFs per Patient
4. Discussion
4.1. BCVA and Amblyopia
4.2. Cataract
4.3. Refractive Error
4.4. Keratoconus
4.5. Macular Pathology
4.6. Retinal Detachment
4.7. Glaucoma
4.8. Rare SOFs
4.9. Limitations and Strengths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBS | Bardet–Biedl Syndrome |
| BCVA | Best corrected visual acuity |
| CML | cystoid macular lesions |
| CNV | choroidal neovascularisation |
| CSNB | congenital stationary night blindness |
| ERM | epiretinal membrane |
| IRDs | inherited retinal degenerations |
| KC | keratoconus |
| LCA | Leber congenital amaurosis |
| nsRP | non-syndromic retinitis pigmentosa |
| RP | retinitis pigmentosa |
| RRD | rhegmatogenous retinal detachment |
| SOFs | secondary ophthalmic features |
| USH | Usher syndrome |
| XLRS | X-linked retinoschisis |
References
- Liew, G.; Michaelides, M.; Bunce, C. A comparison of the causes of blindness certifications in England and Wales in working age adults (16–64 years), 1999–2000 with 2009–2010. BMJ Open 2014, 4, e004015. [Google Scholar] [CrossRef]
- Jeffery, R.C.H.; Mukhtar, S.A.; McAllister, I.L.; Morgan, W.H.; Mackey, D.A.; Chen, F.K. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021, 42, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Daiger, S.P. Summaries of Genes and Loci Causing Retinal Diseases (RetNet). 2020. Available online: https://sph.uth.edu/retnet/ (accessed on 28 August 2025).
- Verbakel, S.K.; van Huet, R.A.C.; Boon, C.J.F.; den Hollander, A.I.; Collin, R.W.J.; Klaver, C.C.W.; Hoyng, C.B.; Roepman, R.; Klevering, B.J. Non-syndromic retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 66, 157–186. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Ayala-Ramirez, R.; Graue-Wiechers, F.; Robredo, V.; Amato-Almanza, M.; Horta-Diez, I.; Zenteno, J.C. A new autosomal recessive syndrome consisting of posterior microphthalmos, retinitis pigmentosa, foveoschisis, and optic disc drusen is caused by a MFRP gene mutation. Mol. Vis. 2006, 12, 1483–1489. [Google Scholar]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Viriato, D.; Bennett, N.; Sidhu, R.; Hancock, E.; Lomax, H.; Trueman, D.; MacLaren, R.E. An Economic Evaluation of Voretigene Neparvovec for the Treatment of Biallelic RPE65-Mediated Inherited Retinal Dystrophies in the UK. Adv. Ther. 2020, 37, 1233–1247. [Google Scholar] [CrossRef] [PubMed]
- Cukras, C.; Wiley, H.E.; Jeffrey, B.G.; Sen, H.N.; Turriff, A.; Zeng, Y.; Vijayasarathy, C.; Marangoni, D.; Ziccardi, L.; Kjellstrom, S.; et al. Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery. Mol. Ther. 2018, 26, 2282–2294. [Google Scholar] [CrossRef] [PubMed]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef]
- Russell, S.R.; Drack, A.V.; Cideciyan, A.V.; Jacobson, S.G.; Leroy, B.P.; Van Cauwenbergh, C.; Ho, A.C.; Dumitrescu, A.V.; Han, I.C.; Martin, M.; et al. Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: A phase 1b/2 trial. Nat. Med. 2022, 28, 1014–1021. [Google Scholar] [CrossRef]
- Stefanov, A.; Flannery, J.G. A Systematic Review of Optogenetic Vision Restoration: History, Challenges, and New Inventions from Bench to Bedside. Cold Spring Harb. Perspect. Med. 2023, 13, a041304. [Google Scholar] [CrossRef] [PubMed]
- Aleman, T.S.; Uyhazi, K.E.; Roman, A.J.; Weber, M.L.; O’Neil, E.C.; Swider, M.; Sumaroka, A.; Maguire, K.H.; Aleman, E.M.; Santos, A.J.; et al. Recovery of cone-mediated vision in Lebercilin associated retinal ciliopathy after gene therapy: One-year results of a phase I/II trial. Mol. Ther. 2025, 33, 4784–4798. [Google Scholar] [CrossRef]
- Michaelides, M.; Laich, Y.; Wong, S.C.; Oluonye, N.; Zaman, S.; Kumaran, N.; Kalitzeos, A.; Petrushkin, H.; Georgiou, M.; Tailor, V.; et al. Gene therapy in children with AIPL1-associated severe retinal dystrophy: An open-label, first-in-human interventional study. Lancet 2025, 405, 648–657. [Google Scholar] [CrossRef]
- Stephenson, K.A.J.; Zhu, J.; Wynne, N.; Dockery, A.; Cairns, R.M.; Duignan, E.; Whelan, L.; Malone, C.P.; Dempsey, H.; Collins, K.; et al. Target 5000: A standardized all-Ireland pathway for the diagnosis and management of inherited retinal degenerations. Orphanet J. Rare Dis. 2021, 16, 200. [Google Scholar] [CrossRef]
- Dockery, A.; Stephenson, K.; Keegan, D.; Wynne, N.; Silvestri, G.; Humphries, P.; Kenna, P.F.; Carrigan, M.; Farrar, G.J. Target 5000: Target Capture Sequencing for Inherited Retinal Degenerations. Genes 2017, 8, 304. [Google Scholar] [CrossRef]
- Whelan, L.; Dockery, A.; Wynne, N.; Zhu, J.; Stephenson, K.; Silvestri, G.; Turner, J.; O’Byrne, J.J.; Carrigan, M.; Humphries, P.; et al. Findings from a Genotyping Study of Over 1000 People with Inherited Retinal Disorders in Ireland. Genes 2020, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Carrigan, M.; Duignan, E.; Malone, C.P.; Stephenson, K.; Saad, T.; McDermott, C.; Green, A.; Keegan, D.; Humphries, P.; Kenna, P.F.; et al. Panel-Based Population Next-Generation Sequencing for Inherited Retinal Degenerations. Sci. Rep. 2016, 6, 33248. [Google Scholar] [CrossRef]
- Stephenson, K.A.J.; Whelan, L.; Zhu, J.; Dockery, A.; Wynne, N.C.; Cairns, R.M.; Kirk, C.; Turner, J.; Duignan, E.S.; O’Byrne, J.J.; et al. Usher Syndrome on the Island of Ireland: A Genotype-Phenotype Review. Investig. Ophthalmol. Vis. Sci. 2023, 64, 23. [Google Scholar] [CrossRef]
- Zhu, J.; Stephenson, K.A.J.; Dockery, A.; Turner, J.; O’Byrne, J.J.; Fitzsimon, S.; Farrar, G.J.; Flitcroft, D.I.; Keegan, D.J. Electrophysiology-Guided Genetic Characterisation Maximises Molecular Diagnosis in an Irish Paediatric Inherited Retinal Degeneration Population. Genes 2022, 13, 615. [Google Scholar] [CrossRef]
- Lange, C.; Feltgen, N.; Junker, B.; Schulze-Bonsel, K.; Bach, M. Resolving the clinical acuity categories “hand motion” and “counting fingers” using the Freiburg Visual Acuity Test (FrACT). Graefes Arch. Clin. Exp. Ophthalmol. 2009, 247, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pierrache, L.H.; Hartel, B.P.; van Wijk, E.; Meester-Smoor, M.A.; Cremers, F.P.; de Baere, E.; de Zaeytijd, J.; van Schooneveld, M.J.; Cremers, C.W.; Dagnelie, G.; et al. Visual Prognosis in USH2A-Associated Retinitis Pigmentosa Is Worse for Patients with Usher Syndrome Type IIa Than for Those with Nonsyndromic Retinitis Pigmentosa. Ophthalmology 2016, 123, 1151–1160. [Google Scholar] [CrossRef]
- Nanda, A.; Salvetti, A.P.; Clouston, P.; Downes, S.M.; MacLaren, R.E. Exploring the Variable Phenotypes of RPGR Carrier Females in Assessing their Potential for Retinal Gene Therapy. Genes 2018, 9, 643. [Google Scholar] [CrossRef] [PubMed]
- Gocuk, S.A.; Edwards, T.L.; Jolly, J.K.; Chen, F.K.; Sousa, D.C.; McGuinness, M.B.; McLaren, T.L.; Lamey, T.M.; Thompson, J.A.; Ayton, L.N. Retinal Disease Variability in Female Carriers of RPGR Variants Associated with Retinitis Pigmentosa: Clinical and Genetic Parameters. Genes 2025, 16, 221. [Google Scholar] [CrossRef]
- Hong, Y.; Li, H.; Sun, Y.; Ji, Y. A Review of Complicated Cataract in Retinitis Pigmentosa: Pathogenesis and Cataract Surgery. J. Ophthalmol. 2020, 2020, 6699103. [Google Scholar] [CrossRef]
- Nguyen, X.T.; Thiadens, A.; Fiocco, M.; Tan, W.; McKibbin, M.; Klaver, C.C.W.; Meester-Smoor, M.A.; Van Cauwenbergh, C.; Strubbe, I.; Vergaro, A.; et al. Outcome of Cataract Surgery in Patients With Retinitis Pigmentosa. Am. J. Ophthalmol. 2022, 246, 1–9. [Google Scholar] [CrossRef]
- Mu, J.; Xu, F.; Guo, W.; Sun, C.; Peng, B.; Huang, Q.; Fan, W. Updated study on demographic and ocular biometric characteristics of cataract patients indicates new trends in cataract surgery. Sci. Rep. 2025, 15, 17289. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Agrawal, D.; Agrawal, D.; Parchand, S.M.; Sahu, A. Cataract surgery in retinitis pigmentosa. Indian J. Ophthalmol. 2021, 69, 1753–1757. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, H.; Matsuo, K.; Nakao, F.; Hayashi, F. Anterior capsule contraction and intraocular lens dislocation after implant surgery in eyes with retinitis pigmentosa. Ophthalmology 1998, 105, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- De Rojas, J.O.; Schuerch, K.; Mathews, P.M.; Cabral, T.; Hazan, A.; Sparrow, J.; Tsang, S.H.; Suh, L.H. Evaluating Structural Progression of Retinitis Pigmentosa After Cataract Surgery. Am. J. Ophthalmol. 2017, 180, 117–123. [Google Scholar] [CrossRef]
- Georgiou, M.; Shakarchi, A.F.; Elhusseiny, A.M.; Michaelides, M.; Sallam, A.B. Cataract Surgery Outcomes in Retinitis Pigmentosa A Comparative Clinical Database Study. Am. J. Ophthalmol. 2024, 262, 34–39. [Google Scholar] [CrossRef]
- He, H.; Song, H.; Meng, X.; Cao, K.; Liu, Y.X.; Wang, J.; Wan, X.; Jin, Z.B. Effects and Prognosis of Cataract Surgery in Patients with Retinitis Pigmentosa. Ophthalmol. Ther. 2022, 11, 1975–1989. [Google Scholar] [CrossRef]
- Nakamura, S.; Fujiwara, K.; Yoshida, N.; Murakami, Y.; Shimokawa, S.; Koyanagi, Y.; Ikeda, Y.; Sonoda, K.H. Long-term Outcomes of Cataract Surgery in Patients with Retinitis Pigmentosa. Ophthalmol. Retin. 2022, 6, 268–272. [Google Scholar] [CrossRef]
- Dikopf, M.S.; Chow, C.C.; Mieler, W.F.; Tu, E.Y. Cataract extraction outcomes and the prevalence of zonular insufficiency in retinitis pigmentosa. Am. J. Ophthalmol. 2013, 156, 82–88.e82. [Google Scholar] [CrossRef]
- Sakai, D.; Takagi, S.; Hirami, Y.; Nakamura, M.; Kurimoto, Y. Use of ellipsoid zone width for predicting visual prognosis after cataract surgery in patients with retinitis pigmentosa. Eye 2023, 37, 42–47. [Google Scholar] [CrossRef]
- Hepworth, L.R.; Rowe, F.J.; Burnside, G. Development of a patient reported outcome measures for measuring the impact of visual impairment following stroke. BMC Health Serv. Res. 2019, 19, 348. [Google Scholar] [CrossRef]
- Lacy, G.D.; Abalem, M.F.; Musch, D.C.; Jayasundera, K.T. Patient-reported outcome measures in inherited retinal degeneration gene therapy trials. Ophthalmic Genet. 2020, 41, 1–6. [Google Scholar] [CrossRef]
- Sieving, P.A.; Fishman, G.A. Refractive errors of retinitis pigmentosa patients. Br. J. Ophthalmol. 1978, 62, 163–167. [Google Scholar] [CrossRef]
- Flitcroft, D.I.; Adams, G.G.; Robson, A.G.; Holder, G.E. Retinal dysfunction and refractive errors: An electrophysiological study of children. Br. J. Ophthalmol. 2005, 89, 484–488. [Google Scholar] [CrossRef]
- Igelman, A.D.; White, E.; Tayyib, A.; Everett, L.; Vincent, A.; Heon, E.; Zeitz, C.; Michaelides, M.; Mahroo, O.A.; Katta, M.; et al. Characterising the refractive error in paediatric patients with congenital stationary night blindness: A multicentre study. Br. J. Ophthalmol. 2025, 109, 286–292. [Google Scholar] [CrossRef]
- Yassin, S.H.; Wagner, N.E.; Khuu, T.; Schmidt, R.; Igelman, A.D.; Marra, M.; Schwartz, H.; Walker, E.; Nagiel, A.; Yang, P.; et al. Refractive Error in Inherited Retinal Disease. Am. J. Ophthalmol. 2025, 269, 381–392. [Google Scholar] [CrossRef]
- Carricondo, P.C.; Andrade, T.; Prasov, L.; Ayres, B.M.; Moroi, S.E. Nanophthalmos: A Review of the Clinical Spectrum and Genetics. J. Ophthalmol. 2018, 2018, 2735465. [Google Scholar] [CrossRef]
- Flitcroft, D.I.; Loughman, J.; Wildsoet, C.F.; Williams, C.; Guggenheim, J.A. Novel Myopia Genes and Pathways Identified from Syndromic Forms of Myopia. Investig. Ophthalmol. Vis. Sci. 2018, 59, 338–348. [Google Scholar] [CrossRef]
- Troilo, D.; Smith, E.L., 3rd; Nickla, D.L.; Ashby, R.; Tkatchenko, A.V.; Ostrin, L.A.; Gawne, T.J.; Pardue, M.T.; Summers, J.A.; Kee, C.S.; et al. IMI—Report on Experimental Models of Emmetropization and Myopia. Investig. Ophthalmol. Vis. Sci. 2019, 60, M31–M88. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Chen, L.J.; Tham, C.C.; Yam, J.C.; Pang, C.P. Genes for childhood myopia. Asia Pac. J. Ophthalmol. 2025, 14, 100139. [Google Scholar] [CrossRef]
- Pan, W.; Saw, S.M.; Wong, T.Y.; Morgan, I.; Yang, Z.; Lan, W. Prevalence and temporal trends in myopia and high myopia children in China: A systematic review and meta-analysis with projections from 2020 to 2050. Lancet Reg. Health West. Pac. 2025, 55, 101484. [Google Scholar] [CrossRef]
- Tran, M.; Kolesnikova, M.; Kim, A.H.; Kowal, T.; Ning, K.; Mahajan, V.B.; Tsang, S.H.; Sun, Y. Clinical characteristics of high myopia in female carriers of pathogenic RPGR mutations: A case series and review of the literature. Ophthalmic Genet. 2023, 44, 295–303. [Google Scholar] [CrossRef]
- Kurata, K.; Hosono, K.; Hayashi, T.; Mizobuchi, K.; Katagiri, S.; Miyamichi, D.; Nishina, S.; Sato, M.; Azuma, N.; Nakano, T.; et al. X-linked Retinitis Pigmentosa in Japan: Clinical and Genetic Findings in Male Patients and Female Carriers. Int. J. Mol. Sci. 2019, 20, 1518. [Google Scholar] [CrossRef]
- González-Iglesias, E.; López-Vázquez, A.; Noval, S.; Nieves-Moreno, M.; Granados-Fernández, M.; Arruti, N.; Rosa-Pérez, I.; Pacio-Míguez, M.; Montaño, V.E.F.; Rodríguez-Solana, P.; et al. Next-Generation Sequencing Screening of 43 Families with Non-Syndromic Early-Onset High Myopia: A Clinical and Genetic Study. Int. J. Mol. Sci. 2022, 23, 4233. [Google Scholar] [CrossRef]
- Hendriks, M.; Verhoeven, V.J.M.; Buitendijk, G.H.S.; Polling, J.R.; Meester-Smoor, M.A.; Hofman, A.; Kamermans, M.; van den Born, L.I.; Klaver, C.C.W. Development of Refractive Errors-What Can We Learn from Inherited Retinal Dystrophies? Am. J. Ophthalmol. 2017, 182, 81–89. [Google Scholar] [CrossRef]
- Chassine, T.; Bocquet, B.; Daien, V.; Avila-Fernandez, A.; Ayuso, C.; Collin, R.W.; Corton, M.; Hejtmancik, J.F.; van den Born, L.I.; Klevering, B.J.; et al. Autosomal recessive retinitis pigmentosa with RP1 mutations is associated with myopia. Br. J. Ophthalmol. 2015, 99, 1360–1365. [Google Scholar] [CrossRef]
- Kurata, K.; Hosono, K.; Hotta, Y. Clinical and genetic findings of a Japanese patient with RP1-related autosomal recessive retinitis pigmentosa. Doc. Ophthalmol. 2018, 137, 47–56. [Google Scholar] [CrossRef]
- Hartong, D.T.; Berson, E.L.; Dryja, T.P. Retinitis pigmentosa. Lancet 2006, 368, 1795–1809. [Google Scholar] [CrossRef]
- Coussa, R.G.; Binkley, E.M.; Wilkinson, M.E.; Andorf, J.L.; Tucker, B.A.; Mullins, R.F.; Sohn, E.H.; Yannuzzi, L.A.; Stone, E.M.; Han, I.C. Predominance of hyperopia in autosomal dominant Best vitelliform macular dystrophy. Br. J. Ophthalmol. 2022, 106, 522–527. [Google Scholar] [CrossRef]
- Wagner, R.S.; Caputo, A.R.; Nelson, L.B.; Zanoni, D. High hyperopia in Leber’s congenital amaurosis. Arch. Ophthalmol. 1985, 103, 1507–1509. [Google Scholar] [CrossRef]
- Sundin, O.H.; Dharmaraj, S.; Bhutto, I.A.; Hasegawa, T.; McLeod, D.S.; Merges, C.A.; Silval, E.D.; Maumenee, I.H.; Lutty, G.A. Developmental basis of nanophthalmos: MFRP Is required for both prenatal ocular growth and postnatal emmetropization. Ophthalmic Genet. 2008, 29, 1–9. [Google Scholar] [CrossRef]
- O’Connell, A.; Zhu, J.; Stephenson, K.A.J.; Whelan, L.; Dockery, A.; Turner, J.; O’Byrne, J.J.; Farrar, G.J.; Keegan, D. MFRP-Associated Retinopathy and Nanophthalmos in Two Irish Probands: A Case Report. Case Rep. Ophthalmol. 2022, 13, 1015–1023. [Google Scholar] [CrossRef]
- Rabinowitz, Y.S. Keratoconus. Surv. Ophthalmol. 1998, 42, 297–319. [Google Scholar] [CrossRef]
- Karel, I. Keratoconus in congenital diffuse tapetoretinal degeneration. Ophthalmologica 1968, 155, 8–15. [Google Scholar] [CrossRef]
- den Hollander, A.I.; Roepman, R.; Koenekoop, R.K.; Cremers, F.P. Leber congenital amaurosis: Genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008, 27, 391–419. [Google Scholar] [CrossRef]
- Coyle, J.T. Keratoconus and eye rubbing. Am. J. Ophthalmol. 1984, 97, 527–528. [Google Scholar] [CrossRef]
- Koller, B.; Neuhann, T.F.; Neuhann, I.M. Keratoplasty in patients with intellectual disability. Cornea 2014, 33, 10–13. [Google Scholar] [CrossRef]
- Ben-Avi, R.; Rivera, A.; Hendler, K.; Sharon, D.; Banin, E.; Khateb, S.; Yahalom, C. Prevalence and associated factors of cystoid macular edema in children with early onset inherited retinal dystrophies. Eur. J. Ophthalmol. 2022, 33, 11206721221136318. [Google Scholar] [CrossRef]
- Liew, G.; Strong, S.; Bradley, P.; Severn, P.; Moore, A.T.; Webster, A.R.; Mitchell, P.; Kifley, A.; Michaelides, M. Prevalence of cystoid macular oedema, epiretinal membrane and cataract in retinitis pigmentosa. Br. J. Ophthalmol. 2019, 103, 1163–1166. [Google Scholar] [CrossRef]
- Liew, G.; Moore, A.T.; Webster, A.R.; Michaelides, M. Efficacy and prognostic factors of response to carbonic anhydrase inhibitors in management of cystoid macular edema in retinitis pigmentosa. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1531–1536. [Google Scholar] [CrossRef]
- Strong, S.; Liew, G.; Michaelides, M. Retinitis pigmentosa-associated cystoid macular oedema: Pathogenesis and avenues of intervention. Br. J. Ophthalmol. 2017, 101, 31–37. [Google Scholar] [CrossRef]
- Ng, C.H.; Cheung, N.; Wang, J.J.; Islam, A.F.; Kawasaki, R.; Meuer, S.M.; Cotch, M.F.; Klein, B.E.; Klein, R.; Wong, T.Y. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology 2011, 118, 694–699. [Google Scholar] [CrossRef]
- Testa, F.; Rossi, S.; Colucci, R.; Gallo, B.; Di Iorio, V.; della Corte, M.; Azzolini, C.; Melillo, P.; Simonelli, F. Macular abnormalities in Italian patients with retinitis pigmentosa. Br. J. Ophthalmol. 2014, 98, 946–950. [Google Scholar] [CrossRef]
- Hogg, P.A.; Grierson, I.; Hiscott, P. Direct comparison of the migration of three cell types involved in epiretinal membrane formation. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2749–2757. [Google Scholar]
- Vedantham, V.; Ramasamy, K. Pigmented epiretinal membranes caused by RPE migration: OCT-based observational case reports. Indian J. Ophthalmol. 2007, 55, 148–149. [Google Scholar] [CrossRef]
- Jaissle, G.B.; May, C.A.; van de Pavert, S.A.; Wenzel, A.; Claes-May, E.; Giessl, A.; Szurman, P.; Wolfrum, U.; Wijnholds, J.; Fischer, M.D.; et al. Bone spicule pigment formation in retinitis pigmentosa: Insights from a mouse model. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1063–1070. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ikeda, Y.; Murakami, Y.; Nakatake, S.; Tachibana, T.; Yoshida, N.; Nakao, S.; Hisatomi, T.; Yoshida, S.; Yoshitomi, T.; et al. Association Between Aqueous Flare and Epiretinal Membrane in Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4282–4286. [Google Scholar] [CrossRef]
- Pruett, R.C. Retinitis pigmentosa: Clinical observations and correlations. Trans. Am. Ophthalmol. Soc. 1983, 81, 693–735. [Google Scholar]
- Ikeda, Y.; Yoshida, N.; Murakami, Y.; Nakatake, S.; Notomi, S.; Hisatomi, T.; Enaida, H.; Ishibashi, T. Long-term Surgical Outcomes of Epiretinal Membrane in Patients with Retinitis Pigmentosa. Sci. Rep. 2015, 5, 13078. [Google Scholar] [CrossRef]
- Fishman, G.A.; Maggiano, J.M.; Fishman, M. Foveal lesions seen in retinitis pigmentosa. Arch. Ophthalmol. 1977, 95, 1993–1996. [Google Scholar] [CrossRef]
- Mitry, D.; Charteris, D.G.; Fleck, B.W.; Campbell, H.; Singh, J. The epidemiology of rhegmatogenous retinal detachment: Geographical variation and clinical associations. Br. J. Ophthalmol. 2010, 94, 678–684. [Google Scholar] [CrossRef]
- Ogawa, A.; Tanaka, M. The relationship between refractive errors and retinal detachment—Analysis of 1166 retinal detachment cases. Jpn. J. Ophthalmol. 1988, 32, 310–315. [Google Scholar]
- Burton, T.C. The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans. Am. Ophthalmol. Soc. 1989, 87, 143–155; discussion 155–157. [Google Scholar]
- Johnston, T.; Chandra, A.; Hewitt, A.W. Current Understanding of the Genetic Architecture of Rhegmatogenous Retinal Detachment. Ophthalmic Genet. 2016, 37, 121–129. [Google Scholar] [CrossRef]
- Ang, A.; Poulson, A.V.; Goodburn, S.F.; Richards, A.J.; Scott, J.D.; Snead, M.P. Retinal detachment and prophylaxis in type 1 Stickler syndrome. Ophthalmology 2008, 115, 164–168. [Google Scholar] [CrossRef]
- Georgiou, M.; Finocchio, L.; Fujinami, K.; Fujinami-Yokokawa, Y.; Virgili, G.; Mahroo, O.A.; Webster, A.R.; Michaelides, M. X-Linked Retinoschisis: Deep Phenotyping and Genetic Characterization. Ophthalmology 2021, 129, 542–551. [Google Scholar] [CrossRef]
- Alexander, P.; Fincham, G.S.; Brown, S.; Collins, D.; McNinch, A.M.; Poulson, A.V.; Richards, A.; Martin, H.; Wareham, N.; Snead, M.P. Cambridge Prophylactic Protocol, Retinal Detachment, and Stickler Syndrome. N. Engl. J. Med. 2023, 388, 1337–1339. [Google Scholar] [CrossRef]
- Richards, A.J.; Snead, M.P. Molecular Basis of Pathogenic Variants in the Fibrillar Collagens. Genes 2022, 13, 1199. [Google Scholar] [CrossRef] [PubMed]
- Meguro, A.; Ideta, H.; Ota, M.; Ito, N.; Ideta, R.; Yonemoto, J.; Takeuchi, M.; Uemoto, R.; Nishide, T.; Iijima, Y.; et al. Common variants in the COL4A4 gene confer susceptibility to lattice degeneration of the retina. PLoS ONE 2012, 7, e39300. [Google Scholar] [CrossRef]
- Chan, W.O.; Brennan, N.; Webster, A.R.; Michealides, M.; Muqit, M.M.K. Retinal detachment in retinitis pigmentosa. BMJ Open Ophthalmol. 2020, 5, e000454. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Fang, Y.; Duan, X.; Chen, L.; Shi, J.; Liu, J.; Sun, Y.; Wang, J.; Li, Y.; Tang, X. Combination of Trabeculectomy and Primary Pars Plana Vitrectomy in the Successful Treatment of Angle-Closure Glaucoma with BEST1 Mutations: Self-Controlled Case Series. Ophthalmol. Ther. 2022, 11, 2271–2284. [Google Scholar] [CrossRef]
- Uliss, A.E.; Gregor, Z.J.; Bird, A.C. Retinitis pigmentosa and retinal neovascularization. Ophthalmology 1986, 93, 1599–1603. [Google Scholar] [CrossRef]
- O’Connell, A.; Stephenson, K.A.J.; Zhu, J.; FitzSimon, S. Coats-like exudative vitreoretinopathy (CLEVER) in CEP290 inherited retinal degeneration. BMJ Case Rep. 2022, 15, e247229. [Google Scholar] [CrossRef]
- Moinuddin, O.; Sathrasala, S.; Jayasundera, K.T.; Branham, K.H.; Chang, E.Y.; Qian, C.X.; Recchia, F.M.; Fahim, A.T.; Besirli, C.G. Coats-like Exudative Vitreoretinopathy in Retinitis Pigmentosa: Ocular Manifestations and Treatment Outcomes. Ophthalmol. Retin. 2021, 5, 86–96. [Google Scholar] [CrossRef]
- Magliyah, M.; Alshamrani, A.A.; Schatz, P.; Taskintuna, I.; Alzahrani, Y.; Nowilaty, S.R. Clinical spectrum, genetic associations and management outcomes of Coats-like exudative retinal vasculopathy in autosomal recessive retinitis pigmentosa. Ophthalmic Genet. 2021, 42, 178–185. [Google Scholar] [CrossRef]
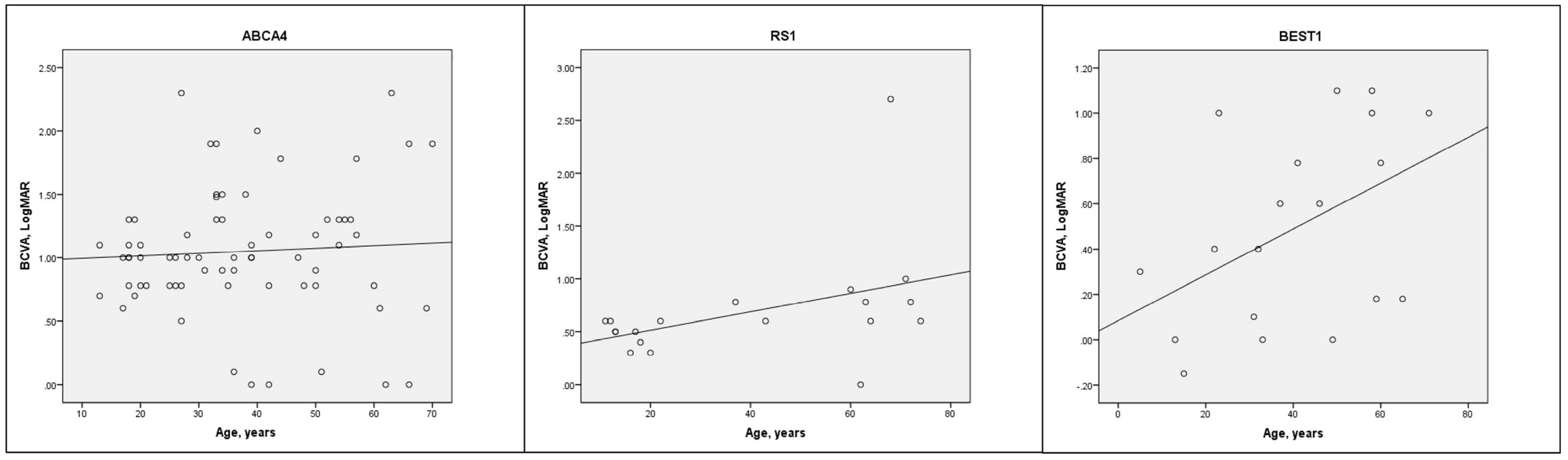

| Ranking | Genotype | n = (% of Total) | Age, Years ± SD | Female, n = (%) | BCVA, LogMAR Mean ± SD |
| Total | 429 (100) | 39.8 ± 19.3 | 191 (44.5) | 0.79 ± 0.73 | |
| 1 | ABCA4 | 69 (16.2) | 37.4 ± 15.5 | 40 (58.0) | 1.08 ± 0.49 |
| 2 | RS1 | 21 (4.9) | 37.9 ± 24.9 | 0 | 0.72 ± 0.53 |
| 3 | BEST1 | 20 (4.7) | 39.5 ± 19.1 | 5 (25) | 0.52 ± 0.44 |
| 3 | RPGR (Total) | 20 (4.7) | 43.2 ± 21.3 | 8 (40) | 0.69 ± 0.84 |
| 3M | RPGR (Males) | 12 (2.8) | 38.3 ± 25.7 | 0 | 0.79 ± 0.88 |
| 3F | RPGR (Females) | 8 (1.9) | 50.9 ± 13.3 | 8 (100) | 0.57 ± 0.89 |
| 4 | USH2A | 19 (4.4) | 46.4 ± 19.3 | 5 (26.3) | 0.35 ± 0.41 |
| 5 | RHO | 16 (3.7) | 42.3 ± 16.7 | 10 (62.5) | 0.37 ± 0.25 |
| 6 | COL2A1 | 13 (3) | 27.0 ± 14.8 | 6 (46.2) | 0.41 ± 0.39 |
| 7 | FBN1 | 12 (2.8) | 50.6 ± 17.5 | 6 (50) | 0.69 ± 0.99 |
| 7 | PRPH2 | 12 (2.8) | 54.0 ± 14.3 | 4 (33.3) | 0.32 ± 0.23 |
| 7 | RP1 | 12 (2.8) | 57.2 ± 13.5 | 8 (66.7) | 0.48 ± 0.82 |
| Refractive Group | Genotype | Cataract, n = (%) | IOL, n = (%) | Refraction of n = | SE, Mean ± SD (D) | Cyl, Mean ± SD (D) | ≤−6.00D, n = (%) | ≥+5.00D, n = (%) |
|---|---|---|---|---|---|---|---|---|
| Myopia | COL2A1 | 1 (7.7) | 4 (30.8) | 4 | −10.84 ± 7.64 | 2.44 ± 3.25 | 3 (75) | 0 |
| GUCY2D | 3 (27.3) | 1 (9.1) | 3 | −10.63 ± 6.51 | 1.08 ± 1.13 | 2 (66.7) | 0 | |
| TRPM1 | 0 | 0 | 3 | −8.92 ± 2.63 | 2.00 ± 0.50 | 3 (100) | 0 | |
| FBN1 | 3 (25.0) | 8 (66.7) | 3 | −8.58 ± 7.26 | 1.83 ± 2.02 | 2 (66.7) | 0 | |
| NYX | 0 | 0 | 4 | −8.34 ± 1.75 | 2.56 ± 0.66 | 4 (100) | 0 | |
| Hyperopia | TULP1 | 3 (100) | 0 | 3 | +4.67 ± 2.67 | 1.33 ± 0.76 | 0 | 1 |
| RS1 | 5 (23.8) | 4 (19) | 10 | +4.21 ± 2.83 | 1.03 ± 1.20 | 0 | 4 | |
| BEST1 | 5 (25.0) | 0 | 12 | +3.80 ± 2.38 | 0.73 ± 0.72 | 0 | 4 (33.3) | |
| CHM (Female) | 0 | 1 (20.0) | 3 | +3.08 ± 0.69 | 0.67 ± 1.55 | 0 | 0 | |
| CNGA3 | 0 | 0 | 3 | +2.29 ± 2.35 | 0.75 ± 0.66 | 0 | 1 (33.3) |
| Phenotype | Total n = | Age (Years), Mean ± SD, (Range) | Male, n = (%) | BCVA, Mean ± SD, (Range) | Cataract, n = (%) | IOL, n = (%) | Refraction n = (%) | SE, in D, Mean ± SD (Range) | Cyl, Mean ± SD (D) | ≤−6.00D, n = (%) | ≥+5.00D, n = (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ACHM | 15 | 20.3 ± 20.0 (2–64) | 6 (40) | 0.98 ± 0.28 (0.60–1.78) | 1 (6.7) | 0 | 13 (86.7) | +0.95 ± 4.26 (−7.5–+6.5) | 1.29 ± 1.14 | 2 (15.4) | 2 (15.4) |
| BBS | 21 | 31.5 ± 11.9 (16–54) | 13 (61.9) | 1.11 ± 0.91 (0.00–2.70) | 7 (33.3) | 3 (14.3) | 11 (52.4) | −0.70 ± 2.86 (−4.75–+2.75) | 2.36 ± 1.35 | 0 | 0 |
| CSNB | 10 | 26.7 ± 21.3 (11–83) | 7 (70) | 0.39 ± 0.37 (−0.08–1.30) | 0 | 1 (10) | 8 (80) | −7.52 ± 3.53 (−0.05–−11.5) | 2.22 ± 0.65 | 7 (87.5) | 0 |
| LCA | 53 | 34.0 ± 19.2 (1–76) | 30 (56.6) | 1.37 ± 0.85 (−0.08–2.70) | 16 (30.2) | 4 (7.5) | 19 (35.8) | +1.20 ± 6.59 (−14.75–+10.00) | 1.04 ± 1.06 | 2 (10.5) | 6 (31.6) |
| nsRP | 86 | 45.3 ± 19.4 (8–78) | 40 (46.5) | 0.58 ± 0.73 (−0.18–2.70) | 34 (39.5) | 21 (24.4) | 53 (61.6) | −2.05 ± 4.63 (−14.88–+15.38) | 1.43 ± 1.05 | 7 (13.2) | 2 (3.8) |
| COL | 15 | 28.5 ± 14.9 (10–62) | 7 (46.7) | 0.20 ± 0.28 (−0.15–NPL) | 1 (6.7) | 6 (40) | 5 (33.3) | −10.84 ± 7.64 (−3.75–−21.38) | 2.44 ± 3.25 | 5 (100) | 0 |
| USH | 44 | 45.4 ± 18.3 (13–84) | 30 (68.2) | 0.49 ± 0.65 (−0.08–2.70) | 21 (47.7) | 10 (22.7) | 25 (56.8) | −0.55 ± 3.17 (−8.75–+4.25) | 1.16 ± 0.68 | 2 (8) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephenson, K.A.J.; Zhu, J.; Conway, M.; Moran, B.; Dockery, A.; Whelan, L.; Turner, J.; O’Byrne, J.J.; Flitcroft, D.I.; Farrar, G.J.; et al. Secondary Ophthalmic Features Represent Diagnostic Clues and Potential Points of Intervention for Inherited Retinal Diseases (Target 5000 Report 3). Genes 2025, 16, 1433. https://doi.org/10.3390/genes16121433
Stephenson KAJ, Zhu J, Conway M, Moran B, Dockery A, Whelan L, Turner J, O’Byrne JJ, Flitcroft DI, Farrar GJ, et al. Secondary Ophthalmic Features Represent Diagnostic Clues and Potential Points of Intervention for Inherited Retinal Diseases (Target 5000 Report 3). Genes. 2025; 16(12):1433. https://doi.org/10.3390/genes16121433
Chicago/Turabian StyleStephenson, Kirk A. J., Julia Zhu, Marcus Conway, Bridget Moran, Adrian Dockery, Laura Whelan, Jacqueline Turner, James J. O’Byrne, D. Ian Flitcroft, G. Jane Farrar, and et al. 2025. "Secondary Ophthalmic Features Represent Diagnostic Clues and Potential Points of Intervention for Inherited Retinal Diseases (Target 5000 Report 3)" Genes 16, no. 12: 1433. https://doi.org/10.3390/genes16121433
APA StyleStephenson, K. A. J., Zhu, J., Conway, M., Moran, B., Dockery, A., Whelan, L., Turner, J., O’Byrne, J. J., Flitcroft, D. I., Farrar, G. J., & Keegan, D. J. (2025). Secondary Ophthalmic Features Represent Diagnostic Clues and Potential Points of Intervention for Inherited Retinal Diseases (Target 5000 Report 3). Genes, 16(12), 1433. https://doi.org/10.3390/genes16121433






