The Interference of RNA Preservative and Post-Collection Interval on RNA Integrity from Different Mice Tissues
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Mice Tissues
2.2. RNA Extraction and Evaluation of RNA Integrity
2.3. Statistical Analysis
3. Results
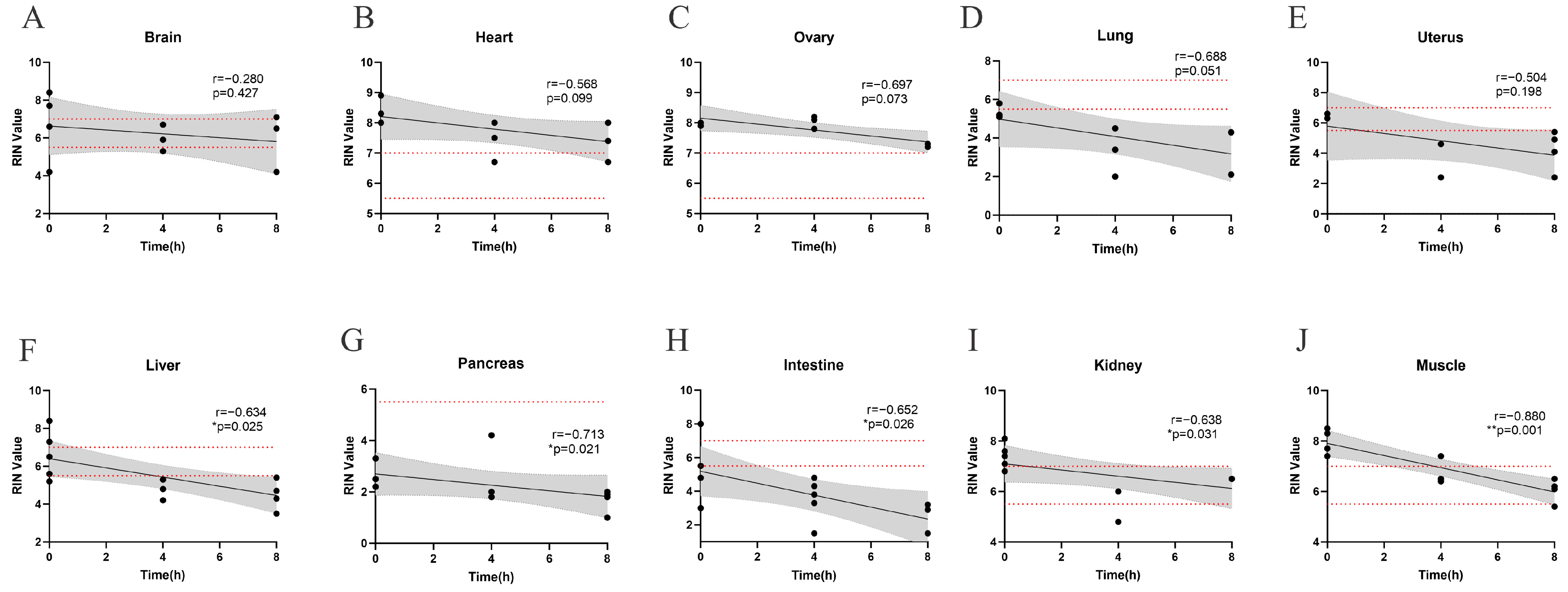
3.1. Correlation of RNA Integrity with Post-Collection Intervals
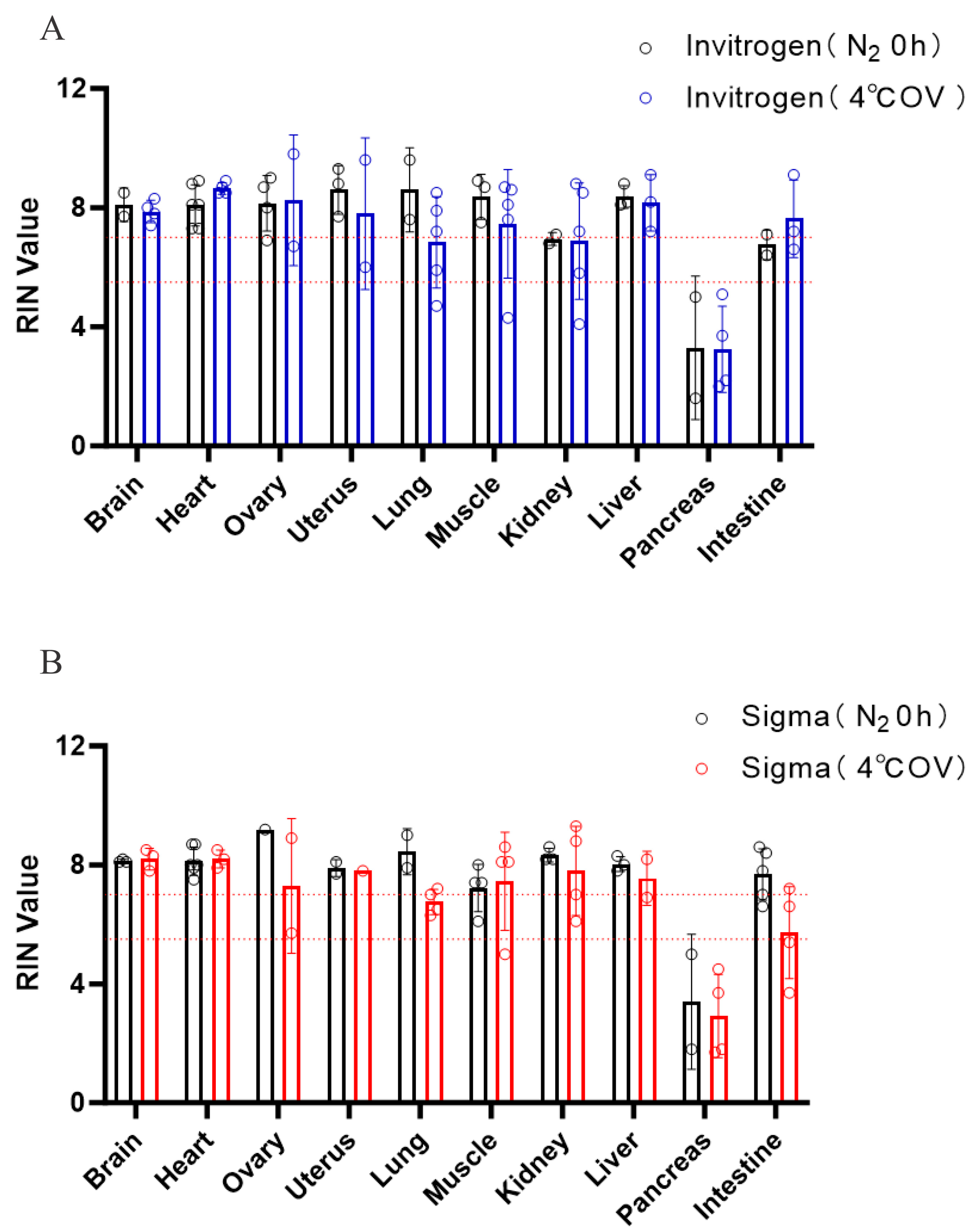
3.2. Effective Preservation: Overnight Immersion or Immediate Freezing?
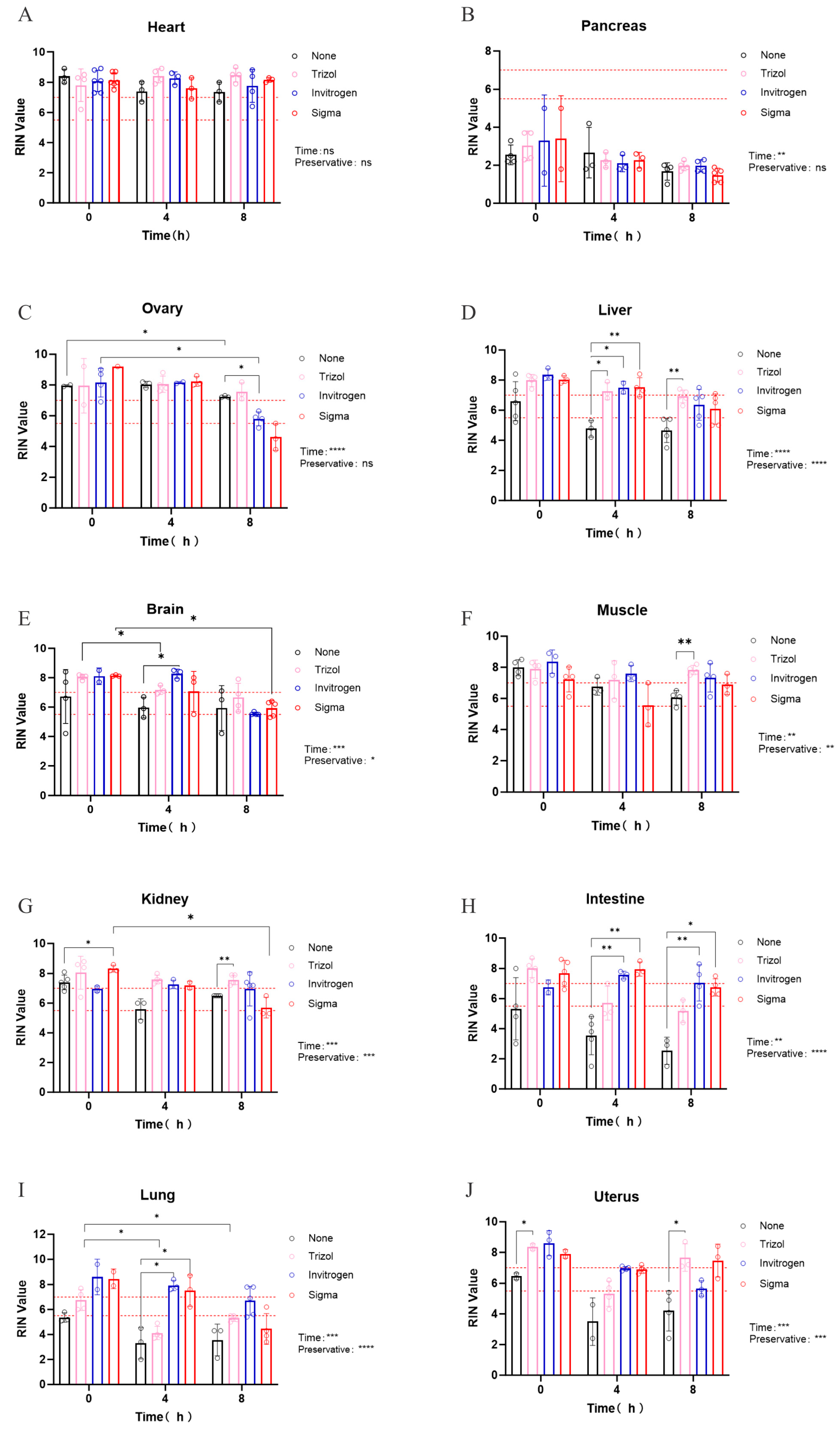
3.3. Evaluation of Preservative Efficacy in Maintaining RNA Integrity in Various Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RIN | RNA integrity number |
| ANOVA | one-way analysis of variance |
| qRT-PCR | real-time fluorescent quantitative polymerase chain reaction |
| RT | room temperature |
References
- Zeng, Y.; Tang, X.; Chen, J.; Kang, X.; Bai, D. Optimizing total RNA extraction method for human and mice samples. PeerJ 2024, 12, e18072. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.H.; Zhang, S.D.; Zhang, P.F.; Li, X.Z.; Hu, Y.Z.; Tian, T.; Zhu, L.; Wang, R.Z.; Jia, W.H. Tumor Cell Content and RNA Integrity of Surgical Tissues from Different Types of Tumors and Its Correlation with Ex Vivo and In Vivo Ischemia. Ann. Surg. Oncol. 2018, 25, 3764–3770. [Google Scholar] [CrossRef]
- Zheng, H.; Tao, Y.P.; Chen, F.Q.; Li, H.F.; Zhang, Z.D.; Zhou, X.X.; Yang, Y.; Zhou, W.P. Temporary Ischemia Time Before Snap Freezing Is Important for Maintaining High-Integrity RNA in Hepatocellular Carcinoma Tissues. Biopreserv. BioBank. 2019, 17, 425–432. [Google Scholar] [CrossRef]
- Liu, N.; Luo, Y.; Zhu, Y.; Peng, H.; Zou, C.; Zhou, Z.; Chen, W.; Wang, H.; Liu, H.; Hu, Y.; et al. Effects of Warm Ischemia Time, Cryopreservation, and Grinding Methods on RNA Quality of Mouse Kidney Tissues. Biopreserv. BioBank. 2021, 19, 306–311. [Google Scholar] [CrossRef]
- Hentze, J.L.; Kringelbach, T.M.; Novotny, G.W.; Hamid, B.H.; Ravn, V.; Christensen, I.J.; Høgdall, C.; Høgdall, E. Optimized Biobanking Procedures for Preservation of RNA in Tissue: Comparison of Snap-Freezing and RNAlater-Fixation Methods. Biopreserv. BioBank. 2019, 17, 562–569. [Google Scholar] [CrossRef]
- Chheda, U.; Pradeepan, S.; Esposito, E.; Strezsak, S.; Fernandez-Delgado, O.; Kranz, J. Factors Affecting Stability of RNA-Temperature, Length, Concentration, pH, and Buffering Species. J. Pharm. Sci. 2024, 113, 377–385. [Google Scholar] [CrossRef]
- Gambarino, S.; Galliano, I.; Clemente, A.; Calvi, C.; Montanari, P.; Pau, A.; Dini, M.; Bergallo, M. Characteristics of RNA Stabilizer RNApro for Peripheral Blood Collection. Diagnostics 2024, 14, 971. [Google Scholar] [CrossRef]
- Pelisek, J.; Yundung, Y.; Reutersberg, B.; Meuli, L.; Rössler, F.; Rabin, L.; Kopp, R.; Zimmermann, A. Swiss Vascular Biobank: Evaluation of Optimal Extraction Method and Admission Solution for Preserving RNA from Human Vascular Tissue. J. Clin. Med. 2023, 12, 5109. [Google Scholar] [CrossRef]
- Sun, H.; Sun, R.; Hao, M.; Wang, Y.; Zhang, X.; Liu, Y.; Cong, X. Effect of Duration of Ex Vivo Ischemia Time and Storage Period on RNA Quality in Biobanked Human Renal Cell Carcinoma Tissue. Ann. Surg. Oncol. 2016, 23, 297–304. [Google Scholar] [CrossRef]
- Neuber, A.C.; Komoto, T.T.; da Silva, E.C.A.; Duval, V.D.S.; Scapulatempo-Neto, C.; Marques, M.M.C. Quality Assessment of Cryopreserved Human Biological Samples from the Biobank of Barretos Cancer Hospital. Biopreserv. BioBank. 2023, 21, 74–80. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xu, Q.N.; Liu, X.L.; Li, C.T. Effects of Peripheral Blood Different Pretreatment Methods and Preservation Time on RNA Quality. Fa Yi Xue Za Zhi 2021, 37, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Sanchez, M.; Burraco, P.; Gomez-Mestre, I.; Leonard, J.A. Preservation of RNA and DNA from mammal samples under field conditions. Mol. Ecol. Resour. 2013, 13, 663–673. [Google Scholar] [CrossRef]
- Walker, D.G.; Whetzel, A.M.; Serrano, G.; Sue, L.I.; Lue, L.F.; Beach, T.G. Characterization of RNA isolated from eighteen different human tissues: Results from a rapid human autopsy program. Cell Tissue Bank. 2016, 17, 361–375. [Google Scholar] [CrossRef]
- Guo, D.; Wang, A.; Xie, T.; Zhang, S.; Cao, D.; Sun, J. Effects of ex vivo ischemia time and delayed processing on quality of specimens in tissue biobank. Mol. Med. Rep. 2020, 22, 4278–4288. [Google Scholar] [CrossRef]
- Assirelli, E.; Naldi, S.; Brusi, V.; Ciaffi, J.; Lisi, L.; Mancarella, L.; Pignatti, F.; Pulsatelli, L.; Faldini, C.; Ursini, F.; et al. Building a rheumatology biobank for reliable basic/translational research and precision medicine. Front. Med. 2023, 10, 1228874. [Google Scholar] [CrossRef]
- Bledsoe, M.J.; Grizzle, W.E. The Use of Human Tissues for Research: What Investigators Need to Know. Altern. Lab. Anim. 2022, 50, 265–274. [Google Scholar] [CrossRef]
- Kao, C.Y.; Chang, C.T.; Kuo, P.Y.; Lin, C.J.; Chiu, H.H.; Liao, H.W. Sequential isolation of metabolites and lipids from a single sample to achieve multiomics by using TRIzol reagent. Talanta 2023, 258, 124416. [Google Scholar] [CrossRef]
- Passow, C.N.; Kono, T.J.Y.; Stahl, B.A.; Jaggard, J.B.; Keene, A.C.; McGaugh, S.E. Nonrandom RNAseq gene expression associated with RNAlater and flash freezing storage methods. Mol. Ecol. Resour. 2019, 19, 456–464. [Google Scholar] [CrossRef]
- Opitz, L.; Salinas-Riester, G.; Grade, M.; Jung, K.; Jo, P.; Emons, G.; Ghadimi, B.M.; Beissbarth, T.; Gaedcke, J. Impact of RNA degradation on gene expression profiling. BMC Med. Genomics 2010, 3, 36. [Google Scholar] [CrossRef]
- Vermeulen, J.; De Preter, K.; Lefever, S.; Nuytens, J.; De Vloed, F.; Derveaux, S.; Hellemans, J.; Speleman, F.; Vandesompele, J. Measurable impact of RNA quality on gene expression results from quantitative PCR. Nucleic Acids Res. 2011, 39, e63. [Google Scholar] [CrossRef]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Aspects Med. 2006, 27, 126–139. [Google Scholar] [CrossRef]
- Tian, J.; Lam, T.G.; Ross, S.K.; Ciener, B.; Leskinen, S.; Sivakumar, S.; Bennett, D.A.; Menon, V.; McKhann, G.M.; Runnels, A.; et al. An analysis of RNA quality metrics in human brain tissue. J. Neuropathol. Exp. Neurol. 2025, 84, 236–243. [Google Scholar] [CrossRef]
- Jiménez-Montenegro, L.; Alfonso, L.; Soret, B.; Mendizabal, J.A.; Urrutia, O. Preservation of milk in liquid nitrogen during sample collection does not affect the RNA quality for RNA-seq analysis. BMC Genom. 2025, 26, 525. [Google Scholar] [CrossRef]
- Brown, L.G.; Haack, A.J.; Kennedy, D.S.; Adams, K.N.; Stolarczuk, J.E.; Takezawa, M.G.; Berthier, E.; Thongpang, S.; Lim, F.Y.; Chaussabel, D.; et al. At-home blood collection and stabilization in high temperature climates using homeRNA. Front. Digit. Health 2022, 4, 903153. [Google Scholar] [CrossRef]
- Fang, Y.; Peng, Z.; Wang, Y.; Yuan, X.; Gao, K.; Fan, R.; Liu, R.; Liu, Y.; Zhang, H.; Xie, Z.; et al. Improvements and challenges of tissue preparation for spatial transcriptome analysis of skull base tumors. Heliyon 2023, 9, e14133. [Google Scholar] [CrossRef]
- Kvastad, L.; Carlberg, K.; Larsson, L.; Villacampa, E.G.; Stuckey, A.; Stenbeck, L.; Mollbrink, A.; Zamboni, M.; Magnusson, J.P.; Basmaci, E.; et al. The spatial RNA integrity number assay for in situ evaluation of transcriptome quality. Commun. Biol. 2021, 4, 57. [Google Scholar] [CrossRef] [PubMed]
- Thakral, S.; Purohit, P.; Mishra, R.; Gupta, V.; Setia, P. The impact of RNA stability and degradation in different tissues to the determination of post-mortem interval: A systematic review. Forensic Sci. Int. 2023, 349, 111772. [Google Scholar] [CrossRef]
- Latorre, N.; Dorda, B.A.; Rey, I.; Roldan, E.R.S.; Sanchez-Rodriguez, A. RNA quality and protamine gene expression after storage of mouse testes under different conditions. PLoS ONE 2024, 19, e0314013. [Google Scholar] [CrossRef]
- Al-Adsani, A.M.; Barhoush, S.A.; Bastaki, N.K.; Al-Bustan, S.A.; Al-Qattan, K.K. Comparing and Optimizing RNA Extraction from the Pancreas of Diabetic and Healthy Rats for Gene Expression Analyses. Genes 2022, 13, 881. [Google Scholar] [CrossRef]
- Ahmed, S.; Shaffer, A.; Geddes, T.; Studzinski, D.; Mitton, K.; Pruetz, B.; Long, G.; Shanley, C. Evaluation of optimal RNA extraction method from human carotid atherosclerotic plaque. Cardiovasc. Pathol. 2015, 24, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Florell, S.R.; Coffin, C.M.; Holden, J.A.; Zimmermann, J.W.; Gerwels, J.W.; Summers, B.K.; Jones, D.A.; Leachman, S.A. Preservation of RNA for functional genomic studies: A multidisciplinary tumor bank protocol. Mod. Pathol. 2001, 14, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Suhovskih, A.V.; Kazanskaya, G.M.; Volkov, A.M.; Tsidulko, A.Y.; Aidagulova, S.V.; Grigorieva, E.V. Suitability of RNALater solution as a tissue-preserving reagent for immunohistochemical analysis. Histochem. Cell Biol. 2019, 152, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bennike, T.B.; Kastaniegaard, K.; Padurariu, S.; Gaihede, M.; Birkelund, S.; Andersen, V.; Stensballe, A. Comparing the proteome of snap frozen, RNAlater preserved, and formalin-fixed paraffin-embedded human tissue samples. EuPA Open Proteom. 2016, 10, 9–18. [Google Scholar] [CrossRef]
- Alyethodi, R.R.; Karthik, S.; Muniswamy, K.; Ravi, S.K.; Perumal, P.; Bhattacharya, D.; Bala, P.A.; De, A.K.; Sujatha, T.; Sunder, J.; et al. Assessment of Protein Profiles of RNAlater Stored and Fresh PBMC Cells Using Different Protein Extraction Buffers. Protein J. 2020, 39, 291–300. [Google Scholar] [CrossRef]
- Zhu, Y.; Mullen, A.M.; Rai, D.K.; Kelly, A.L.; Sheehan, D.; Cafferky, J.; Hamill, R.M. Assessment of RNAlater(®) as a Potential Method to Preserve Bovine Muscle Proteins Compared with Dry Ice in a Proteomic Study. Foods 2019, 8, 60. [Google Scholar] [CrossRef]
- Jia, E.; Zhou, Y.; Shi, H.; Pan, M.; Zhao, X.; Ge, Q. Effects of brain tissue section processing and storage time on gene expression. Anal. Chim. Acta 2021, 1142, 38–47. [Google Scholar] [CrossRef]
- Agarwal, V.; Kelley, D.R. The genetic and biochemical determinants of mRNA degradation rates in mammals. Genome Biol. 2022, 23, 245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, T.; Zhu, H.; Wang, X.; Li, F.; Wang, A.; Zhang, Y.; Zhang, S.; Guo, D. The Interference of RNA Preservative and Post-Collection Interval on RNA Integrity from Different Mice Tissues. Genes 2025, 16, 1421. https://doi.org/10.3390/genes16121421
Xie T, Zhu H, Wang X, Li F, Wang A, Zhang Y, Zhang S, Guo D. The Interference of RNA Preservative and Post-Collection Interval on RNA Integrity from Different Mice Tissues. Genes. 2025; 16(12):1421. https://doi.org/10.3390/genes16121421
Chicago/Turabian StyleXie, Ting, Hui Zhu, Xiaoxi Wang, Fangyuan Li, Anqi Wang, Yaran Zhang, Sumei Zhang, and Dan Guo. 2025. "The Interference of RNA Preservative and Post-Collection Interval on RNA Integrity from Different Mice Tissues" Genes 16, no. 12: 1421. https://doi.org/10.3390/genes16121421
APA StyleXie, T., Zhu, H., Wang, X., Li, F., Wang, A., Zhang, Y., Zhang, S., & Guo, D. (2025). The Interference of RNA Preservative and Post-Collection Interval on RNA Integrity from Different Mice Tissues. Genes, 16(12), 1421. https://doi.org/10.3390/genes16121421





