Human Breast Milk miRNAs: Investigation of Association Between Breastfeeding Children and Maternal Obesity in Obesity Development in Offspring
Abstract
1. Introduction
1.1. Human Breast Milk Benefits
1.2. Childhood Obesity Risks
1.3. Epigenetic Regulators in Human Breast Milk
1.4. Vertical Transmission of Milk Exosomal MicroRNAs
1.5. Epigenetic Aspects of Adipogenesis
1.5.1. The Wnt Signaling Pathway
1.5.2. The FTO Protein
1.6. Epigenetic Impact of Maternal Lifestyle and Nutrition During Pregnancy and Lactation in Children
2. Materials and Methods
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Randomized controlled trials, cohort studies, observational studies, case reports. | Reviews, meta-analyses, or any other type of study. |
| Studies performed in humans. | Studies not performed in humans. |
| Studies conducted on breastfeeding women. | Studies including men and women. |
| Free full texts. | Inaccessible articles. |
3. Results
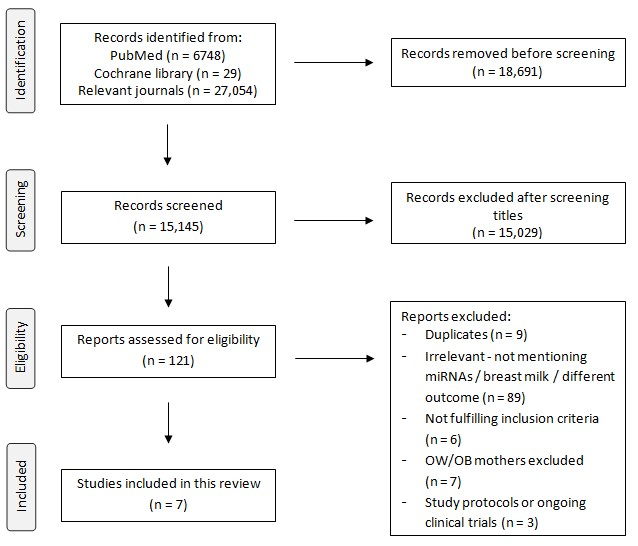
3.1. General Results of the Study Process
3.2. General Characteristics of Included Studies
3.3. MiRNAs in Human Breast Milk Related to Maternal Obesity and Obesity Development in Offspring
3.3.1. Role of miRNAs in Obesity Development
3.3.2. Main Results Associated with the Most Abundant miRNAs
4. Discussion
4.1. Interfering Factors
4.1.1. Types of Samples
4.1.2. Gestational Age
4.2. The miR-30 Family
4.3. The Let-7 Family
4.4. Importance of miR-148a
4.5. Association Between MicroRNAs and Breast Milk Constituents
4.6. Breast Milk miRNAs and the Wnt-FTO Metabolic Axis
4.7. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADIPOR1 | Adiponectin receptor 1 |
| ADIPOR2 | Adiponectin receptor 2 |
| AGA | Appropriate for gestational age |
| AGO2 | Argonaute-2 |
| bEV | Breast milk extracellular vesicle |
| BF | Breastfeeding |
| BM | Breast milk |
| BMI | Body mass index |
| BPA | Bishpenol A |
| CD | Celiac disease |
| CHD | Coronary heart disease |
| CIDEC | Cell-death-inducing DFFA-like effector C |
| CVD | Cardiovascular disease |
| CWG | Conditional weight gain |
| C/EBPα | CCAAT-enhancer binding protein alpha |
| DNA | Deoxyribonucleic acid |
| DNMTs | DNA methyltransferases |
| d | Days |
| EV | Extracellular vesicle |
| FTO | Fat mass and obesity-associated protein |
| GDM | Gestational diabetes mellitus |
| HM | Human milk |
| HPA | Hypothalamic–pituitary–adrenal |
| HMGA2 | High-mobility-group A 2 protein |
| IF | Infant formula |
| IgA | Immunoglobulin A |
| LEPR | Leptin receptor |
| MetS | Metabolic syndrome |
| miRNA | MicroRNA |
| mo | Months |
| MUFA | Monounsaturated fatty acid |
| MW | Maternal weight |
| m6A | N6-methyladenosine |
| NICU | Neonatal intensive care unit |
| NW | Normal weight |
| OB | Obese |
| OW | Overweight |
| PCR | Polymerase chain reaction |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PUFA | Polyunsaturated fatty acid |
| qPCR | Quantitative PCR |
| RCT | Randomized controlled trial |
| RNA | Ribonucleic acid |
| RT-PCR | Reverse-transcription PCR |
| sEVs | Small extracellular vesicles |
| SFA | Saturated fatty acid |
| SGA | Small for gestational age |
| SREBP1c | Sterol regulatory element binding protein 1c |
| T1D | Type 1 diabetes |
| T2D | Type 2 diabetes |
| WFL | Weight-for-length |
| wks | Weeks |
| WTA | Whole transcriptome assay |
References
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin. Exp. Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef]
- Heinig, M.J.; Dewey, K.G. Health Advantages of Breast Feeding for Infants: A Critical Review. Nutr. Res. Rev. 1996, 9, 89–110. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, P.S.; Sankar, M.J.; Walker, N.; Rollins, N.C. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Walker, A. Breast milk as the gold standard for protective nutrients. J. Pediatr. 2010, 156, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Anatolitou, F. Human milk benefits and breastfeeding. J. Pediatr. Neonatal Individ. Med. 2012, 1, 11–18. [Google Scholar]
- Ballard, O.; Morrow, A.L. Human milk composition. Pediatr. Clin. North Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Horta, B.L.; De Mola, C.L.; Victora, C.G. Long-term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta-analysis. Acta Paediatr. 2015, 104, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Gillman, M.W.; Kleinman, K.; Rich-Edwards, J.W.; Rifas-Shiman, S.L. Racial/Ethnic Differences in Early-Life Risk factors for childhood obesity. Pediatrics 2010, 125, 686–695. [Google Scholar] [CrossRef]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Juonala, M.; Magnussen, C.G.; Berenson, G.S.; Venn, A.; Burns, T.L.; Sabin, M.A.; Srinivasan, S.R.; Daniels, S.R.; Davis, P.H.; Chen, W.; et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N. Engl. J. Med. 2011, 365, 1876–1885. [Google Scholar] [CrossRef]
- Reilly, J.J.; Kelly, J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: Systematic review. Int. J. Obes. 2010, 35, 891–898. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Alshaer, W.; Hasan, H.; Albakri, K.A.; Alkhafaji, E.; Issa, N.N.; Al-Holy, M.A.; Abderrahman, S.M.; et al. Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects. Biomedicines 2022, 10, 1219. [Google Scholar] [CrossRef]
- Tingö, L.; Ahlberg, E.; Johansson, L.; Pedersen, S.A.; Chawla, K.; Sætrom, P.; Cione, E.; Simpson, M.R. Non-Coding RNAs in Human Breast Milk: A Systematic Review. Front. Immunol. 2021, 12, 725323. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, M.; Wang, X.; Li, Q.; Wang, T.; Zhu, Q.; Zhou, X.; Wang, X.; Gao, X.; Li, X. Immune-related MicroRNAs are Abundant in Breast Milk Exosomes. Int. J. Biol. Sci. 2012, 8, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Aguilar-Lozano, A.; Sadri, M.; Sukreet, S.; Manca, S.; Wu, D.; Zhou, F.; Mutai, E. Biological Activities of Extracellular Vesicles and Their Cargos from Bovine and Human Milk in Humans and Implications for Infants. J. Nutr. 2017, 147, 3–10. [Google Scholar] [CrossRef]
- Melnik, B.C.; Kakulas, F.; Geddes, D.T.; Hartmann, P.E.; John, S.M.; Carrera-Bastos, P.; Cordain, L.; Schmitz, G. Milk miRNAs: Simple nutrients or systemic functional regulators? Nutr. Metab. 2016, 13, 42. [Google Scholar] [CrossRef]
- Cristóbal-Cañadas, D.; Parrón-Carrillo, R.; Parrón-Carreño, T. Exosomes in Breast Milk: Their Impact on the Intestinal Microbiota of the Newborn and Therapeutic Perspectives for High-Risk Neonates. Int. J. Mol. Sci. 2025, 26, 3421. [Google Scholar] [CrossRef]
- Yung, C.; Zhang, Y.; Kuhn, M.; Armstrong, R.J.; Olyaei, A.; Aloia, M.; Scottoline, B.; Andres, S.F. Neonatal enteroids absorb extracellular vesicles from human milk-fed infant digestive fluid. J. Extracell. Vesicles 2024, 13, e12422. [Google Scholar] [CrossRef]
- Weil, P.P.; Reincke, S.; Hirsch, C.A.; Giachero, F.; Aydin, M.; Scholz, J.; Jönsson, F.; Hagedorn, C.; Nguyen, D.N.; Thymann, T.; et al. Uncovering the gastrointestinal passage, intestinal epithelial cellular uptake, and AGO2 loading of milk miRNAs in neonates using xenomiRs as tracers. Am. J. Clin. Nutr. 2023, 117, 1195–1210. [Google Scholar] [CrossRef]
- Luo, Y.; Bi, J.; Lin, Y.; He, J.; Wu, S.; Zhang, Y.; Wang, Y.; Song, S.; Guo, H. Milk-derived small extracellular vesicles promote bifidobacteria growth by accelerating carbohydrate metabolism. LWT 2023, 182, 114866. [Google Scholar] [CrossRef]
- Prestwich, T.C.; Macdougald, O.A. Wnt/β-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007, 19, 612–617. [Google Scholar] [CrossRef]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- D’Alimonte, I.; Lannutti, A.; Pipino, C.; Di Tomo, P.; Pierdomenico, L.; Cianci, E.; Antonucci, I.; Marchisio, M.; Romano, M.; Stuppia, L.; et al. Wnt signaling behaves as a “master regulator” in the osteogenic and adipogenic commitment of human amniotic fluid mesenchymal stem cells. Stem Cell Rev. Rep. 2013, 9, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.P.; MacDougald, O.A. Wnt Signaling: From Mesenchymal Cell Fate to Lipogenesis and Other Mature Adipocyte Functions. Diabetes 2021, 70, 1419–1430. [Google Scholar] [CrossRef]
- Yang, Z.; Yu, G.L.; Zhu, X.; Peng, T.H.; Lv, Y.C. Critical roles of FTO-mediated mRNA m6A demethylation in regulating adipogenesis and lipid metabolism: Implications in lipid metabolic disorders. Genes Dis. 2021, 9, 51–61. [Google Scholar] [CrossRef]
- Melnik, B.C.; Schmitz, G. MicroRNAs: Milk’s epigenetic regulators. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 427–442. [Google Scholar] [CrossRef]
- Golan-Gerstl, R.; Shiff, Y.E.; Moshayoff, V.; Schecter, D.; Leshkowitz, D.; Reif, S. Characterization and biological function of milk-derived miRNAs. Mol. Nutr. Food Res. 2017, 61, 1700009. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Weiskirchen, R.; Weiskirchen, S.; Stremmel, W.; John, S.M.; Leitzmann, C.; Schmitz, G. Diabetes-preventive molecular mechanisms of breast versus formula feeding: New insights into the impact of milk on stem cell Wnt signaling. Front. Nutr. 2025, 12, 1652297. [Google Scholar] [CrossRef]
- Sheng, W.; Jiang, H.; Yuan, H.; Li, S. miR-148a-3p facilitates osteogenic differentiation of fibroblasts in ankylosing spondylitis by activating the Wnt pathway and targeting DKK1. Exp. Ther. Med. 2022, 23, 365. [Google Scholar] [CrossRef]
- Boyle, K.E.; Patinkin, Z.W.; Shapiro, A.L.; Baker, P.R.; Dabelea, D.; Friedman, J.E. Mesenchymal stem cells from infants born to obese mothers exhibit greater potential for adipogenesis: The Healthy Start BabyBUMP Project. Diabetes 2016, 65, 647–659. [Google Scholar] [CrossRef]
- Melnik, B.C.; Weiskirchen, R.; John, S.M.; Stremmel, W.; Leitzmann, C.; Weiskirchen, S.; Schmitz, G. White adipocyte stem cell expansion through infant formula feeding: New insights into epigenetic programming explaining the early protein hypothesis of obesity. Int. J. Mol. Sci. 2025, 26, 4493. [Google Scholar] [CrossRef]
- Martin Carli, J.F.; LeDuc, C.A.; Zhang, Y.; Stratigopoulos, G.; Leibel, R.L. FTO mediates cell-autonomous effects on adipogenesis and adipocyte lipid content by regulating gene expression via 6mA DNA modifications. J. Lipid Res. 2018, 59, 1446–1460. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Chen, X.; Cheng, S.; Shu, L.; Yan, M.; Yao, L.; Wang, B.; Huang, S.; Zhou, L.; Yang, Z.; et al. FTO promotes SREBP1c maturation and enhances CIDEC transcription during lipid accumulation in HepG2 cells. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Melnik, B.C.; Weiskirchen, R.; Stremmel, W.; John, S.M.; Schmitz, G. Risk of Fat Mass- and Obesity-Associated Gene-Dependent Obesogenic Programming by Formula Feeding Compared to Breastfeeding. Nutrients 2024, 16, 2451. [Google Scholar] [CrossRef]
- Chiba, T.; Kooka, A.; Kowatari, K.; Yoshizawa, M.; Chiba, N.; Takaguri, A.; Fukushi, Y.; Hongo, F.; Sato, H.; Wada, S. Expression profiles of hsa-miR-148a-3p and hsa-miR-125b-5p in human breast milk and infant formulae. Int. Breastfeed. J. 2022, 17, 1. [Google Scholar] [CrossRef]
- Cemali, Ö.; Çelik, E.; Deveci, G.; Hirfanoğlu, İ.M.; Önal, E.E.; Ağagündüz, D. Detection and quantification of miRNA 148a expression in infant formulas. J. Food Sci. 2025, 90, e17648. [Google Scholar] [CrossRef]
- Choi, S.W.; Friso, S. Epigenetics: A New Bridge between Nutrition and Health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef]
- Waterland, R.A.; Michels, K.B. Epigenetic Epidemiology of the Developmental Origins hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef]
- Niculescu, M.D.; Lupu, D.S. Nutritional influence on epigenetics and effects on longevity. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 35–40. [Google Scholar] [CrossRef]
- Wadhwa, P.; Buss, C.; Entringer, S.; Swanson, J. Developmental Origins of Health and Disease: Brief history of the approach and current focus on epigenetic mechanisms. Semin. Reprod. Med. 2009, 27, 358–368. [Google Scholar] [CrossRef]
- Jimenez-Chillaron, J.C.; Diaz, R.; Martinez, D.; Pentint, T.; Ramon-Krauel, M.; Ribo, S.; Plosch, T. The role of nutrition on epigenetic modifications and their implications on health. Biochimie 2012, 94, 2242–2263. [Google Scholar] [CrossRef]
- Weaver, I.C.G.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Spengler, D. Epigenetics of early child development. Front. Psychiatry 2011, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Aiken, C.E.; Ozanne, S.E. Sex differences in developmental programming models. Reproduction 2013, 145, R1–R13. [Google Scholar] [CrossRef] [PubMed]
- Denham, J. Exercise and epigenetic inheritance of disease risk. Acta Physiol. 2017, 222, e12881. [Google Scholar] [CrossRef]
- Zhu, Y.; Stevens, R.G.; Hoffman, A.E.; Tjonnel, A.; Vogel, U.B.; Zheng, T.; Hansen, J. Epigenetic Impact of Long-Term Shiftwork: Pilot evidence from Circadian Genes and Whole-Genome Methylation analysis. Chronobiol. Int. 2011, 28, 852–861. [Google Scholar] [CrossRef]
- Zamanillo, R.; Sánchez, J.; Serra, F.; Palou, A. Breast Milk Supply of MicroRNA Associated with Leptin and Adiponectin Is Affected by Maternal Overweight/Obesity and Influences Infancy BMI. Nutrients 2019, 11, 2589. [Google Scholar] [CrossRef]
- Shah, K.B.; Chernausek, S.D.; Garman, L.D.; Pezant, N.P.; Plows, J.F.; Kharoud, H.K.; Demerath, E.W.; Fields, D.A. Human Milk Exosomal MicroRNA: Associations with Maternal Overweight/Obesity and Infant Body Composition at 1 Month of Life. Nutrients 2021, 13, 1091. [Google Scholar] [CrossRef]
- Xi, Y.; Jiang, X.; Li, R.; Chen, M.; Song, W.; Li, X. The levels of human milk microRNAs and their association with maternal weight characteristics. Eur. J. Clin. Nutr. 2015, 70, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Kupsco, A.; Prada, D.; Valvi, D.; Hu, L.; Petersen, M.S.; Coull, B.; Grandjean, P.; Weihe, P.; Baccarelli, A.A. Human milk extracellular vesicle miRNA expression and associations with maternal characteristics in a population-based cohort from the Faroe Islands. Sci. Rep. 2021, 11, 5840. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.E.; Vorn, R.; Chimenti, M.; Crouch, K.; Shaoshuai, C.; Narayanaswamy, J.; Harken, A.; Schmidt, R.; Gill, J.; Lee, H. Extracellular vesicle miRNAs in breast milk of obese mothers. Front. Nutr. 2022, 9, 976886. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.B.; Song, J.H.; Le, L.N.H.; Kim, H.; Koh, J.W.; Seo, Y.; Jeong, H.R.; Kim, H.-T.; Ryu, S. Characterization of exosomal microRNAs in preterm infants fed with breast milk and infant formula. Front. Nutr. 2024, 11, 1339919. [Google Scholar] [CrossRef]
- Van Syoc, E.; Stegman, M.; Sullivan, R.; Confair, A.; Warren, K.; Hicks, S.D. Associations of maternal breastmilk microRNAs and infant obesity status at 1 year. Genes 2024, 15, 813. [Google Scholar] [CrossRef]
- Zaragosi, L.E.; Wdziekonski, B.; Brigand, K.; Villageois, P.; Mari, B.; Waldmann, R.; Dani, C.; Barbry, P. Small RNA sequencing reveals miR-642a-3p as a novel adipocyte-specific microRNA and miR-30 as a key regulator of human adipogenesis. Genome Biol. 2011, 12, R64. [Google Scholar] [CrossRef]
- Chartoumpekis, D.V.; Zaravinos, A.; Ziros, P.G.; Iskrenova, R.P.; Psyrogiannis, A.I.; Kyriazopoulou, V.E.; Habeos, I.G. Differential Expression of MicroRNAs in Adipose Tissue after Long-Term High-Fat Diet-Induced Obesity in Mice. PLoS ONE 2012, 7, e34872. [Google Scholar] [CrossRef]
- Jia, Y.Z.; Liu, J.; Wang, G.Q.; Song, Z.F. MIR-484: A potential biomarker in health and disease. Front. Oncol. 2022, 12, 830420. [Google Scholar] [CrossRef]
- Sun, T.; Fu, M.; Bookout, A.L.; Kliewer, S.A.; Mangelsdorf, D.J. MicroRNA let-7 Regulates 3T3-L1 Adipogenesis. Mol. Endocrinol. 2009, 23, 925–931. [Google Scholar] [CrossRef]
- Carney, M.; Tarasiuk, A.; DiAngelo, S.; Silveyra, P.; Podany, A.; Birch, L.L.; Paul, I.M.; Kelleher, S.; Hicks, S.D. Metabolism-related microRNAs in maternal breast milk are influenced by premature delivery. Pediatr. Res. 2017, 82, 226–236. [Google Scholar] [CrossRef]
- Geddes, D.; Kakulas, F. 7 Human Milk: Bioactive Components and Their Effects on the Infant and Beyond. In Breastfeeding and Breast Milk—From Biochemistry to Impact; Family Larson-Rosenquist Foundation, Ed.; Georg Thieme Verlag KG: Stuttgart, Germany; The Global Health Network: Oxford, UK, 2018. [Google Scholar]
- Gutman-Ido, E.; Reif, S.; Musseri, M.; Schabes, T.; Golan-Gerstl, R. Oxytocin Regulates the Expression of Selected Colostrum-derived microRNAs. J. Pediatr. Gastroenterol. Nutr. 2022, 74, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Lacerda, J.Z.; Ferreira, L.C.; Lopes, B.C.; Aristizábal-Pachón, A.F.; Bajgelman, M.C.; Borin, T.F.; Zuccari, D.A.P.C. Therapeutic Potential of Melatonin in the Regulation of MiR-148a-3p and Angiogenic Factors in Breast Cancer. MicroRNA 2019, 8, 237–247. [Google Scholar] [CrossRef] [PubMed]
| Author and Publication Date | Type of Study | Sample Characteristics | Method of miRNA Analysis | Maternal Characteristics | Infant Characteristics | |
|---|---|---|---|---|---|---|
| Health Status BMI (kg/m2) Health Issues: GDM, Preeclampsia, T1D, Hypertension, etc. (%) | Breastfeeding Status (%) | Gestational Age (wks) Gender (Female, %) | ||||
| Zamanillo R., 2019 [48] | Cohort study |
| RT-qPCR |
|
|
|
| Shah K.B., 2021 [49] | Cohort study |
| Real-time PCR |
|
|
|
| Xi Y., 2015 [50] | Observational study |
| Real-time PCR |
| 100%—3 mo |
|
| Kupsco A., 2021 [51] | Cohort study |
| MiRNA WTA (sequencing) |
| N/A |
|
| Eun Y. Cho, 2022 [52] | Observational study |
| Nanostring nCounter method |
|
|
|
| Kim E.-B., 2024 [53] | Cohort study |
| Small RNA library preparation; small RNA sequencing |
| 2 groups of study, breast-fed and formula-fed |
|
| Van Syoc E., 2024 [54] | Cohort study |
| Small RNA library preparation; small RNA sequencing |
| 69%—6 mo | Term infants: 37–42 |
| miRNAs | Role in Obesity Development |
|---|---|
| miR-17, miR-146b | Putative target of LEP and associated with obesity development and adipogenesis [48] |
| miR-27b, miR-34a, miR-128, miR-130a | Targets PPARγ in mice and can inhibit adipogenesis in vitro [51] |
| miR-30a | Target of LEP, LEPR, ADIPOR1, ADIPOR2 [48] Key regulatory role in human adipogenesis [55] |
| miR-30b | Exposure to overexpressed miR-30b may act along with fat mass increase in infants [49] Role in pathogenesis of adipogenesis, obesity, and metabolism [50] Key regulatory role in human adipogenesis [55] |
| miR-30c | Correlation to obesity and adipogenesis by disturbing the infant’s metabolism or protecting them from harmful results [52] Key regulatory role in human adipogenesis [55] |
| miR-103 | Associated with obesity development and adipogenesis [48] |
| miR-148a | Target genes are involved in important pathways related to energy metabolism, insulin signaling, and adipogenesis [49] |
| miR-200c | Promotes adipogenesis in mouse models [56] |
| miR-222 | Target of ADIPOR1, ADIPOR2, and LEPR [48] |
| miR-224 | Participates in differentiation of adipocytes and metabolism of fatty acids [54] |
| miR-378 | Role in pathogenesis of adipogenesis, obesity, and metabolism [50] |
| miR-642a, miR-448, miR-302b | Correlation to obesity and adipogenesis by disturbing the infant’s metabolism or protecting them from harmful results [52] |
| miR-484 | Regulated glucolipid metabolism Potential regulator of insulin gene expression [57] |
| miR-let-7a | Target of ADIPOR1, ADIPOR2, and LEPR [48] Regulation of adipogenesis and obesity and important role in metabolism in both mice and humans [50] |
| miR-let-7b, miR-let-7c | Target of ADIPOR1, ADIPOR2, and LEPR [48] Important regulation of adipogenesis [58] |
| miR-let-7g | Important regulation of adipogenesis [58] |
| Most Abundant miRNAs | Significant Association | Positive Association | Negative Association | No Association | Downregulation |
|---|---|---|---|---|---|
| miR-30b | Infant body measurements | Maternal weight gain during pregnancy | Maternal pre-pregnancy BMI, maternal BMI late in pregnancy | - | Colostrum of OW/OB mothers |
| miR-let-7a | - | Maternal weight gain during pregnancy | Milk adiponectin in NW women, maternal weight late in pregnancy, maternal pre-pregnancy BMI, BMI late in pregnancy | - | Colostrum and mature milk in OW/OB mothers |
| miR-148a | - | - | Milk adiponectin in NW women, infant body measurements of NW women | OW/OB mothers | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chondrogianni, M.; Lithoxopoulou, M.; Ververi, A.; Lampropoulos, A.; Sotiriadis, A.; Kolibianakis, E. Human Breast Milk miRNAs: Investigation of Association Between Breastfeeding Children and Maternal Obesity in Obesity Development in Offspring. Genes 2025, 16, 1373. https://doi.org/10.3390/genes16111373
Chondrogianni M, Lithoxopoulou M, Ververi A, Lampropoulos A, Sotiriadis A, Kolibianakis E. Human Breast Milk miRNAs: Investigation of Association Between Breastfeeding Children and Maternal Obesity in Obesity Development in Offspring. Genes. 2025; 16(11):1373. https://doi.org/10.3390/genes16111373
Chicago/Turabian StyleChondrogianni, Marina, Maria Lithoxopoulou, Athina Ververi, Alexandros Lampropoulos, Alexandros Sotiriadis, and Eystratios Kolibianakis. 2025. "Human Breast Milk miRNAs: Investigation of Association Between Breastfeeding Children and Maternal Obesity in Obesity Development in Offspring" Genes 16, no. 11: 1373. https://doi.org/10.3390/genes16111373
APA StyleChondrogianni, M., Lithoxopoulou, M., Ververi, A., Lampropoulos, A., Sotiriadis, A., & Kolibianakis, E. (2025). Human Breast Milk miRNAs: Investigation of Association Between Breastfeeding Children and Maternal Obesity in Obesity Development in Offspring. Genes, 16(11), 1373. https://doi.org/10.3390/genes16111373






