Quantification of Boron Compound Concentration for BNCT Using Positron Emission Tomography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. [18. F]NaBF4 PET Tracer Production
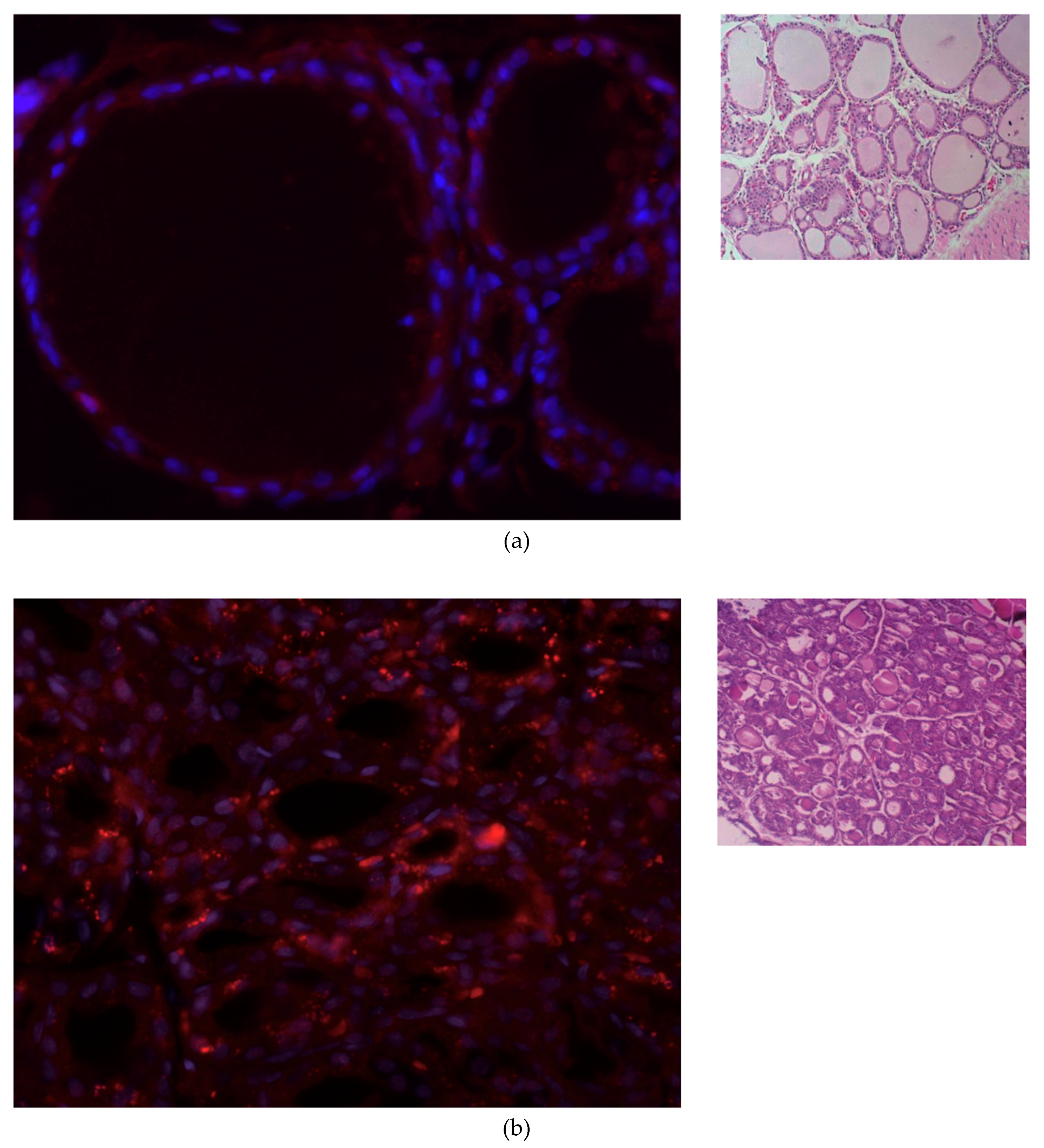
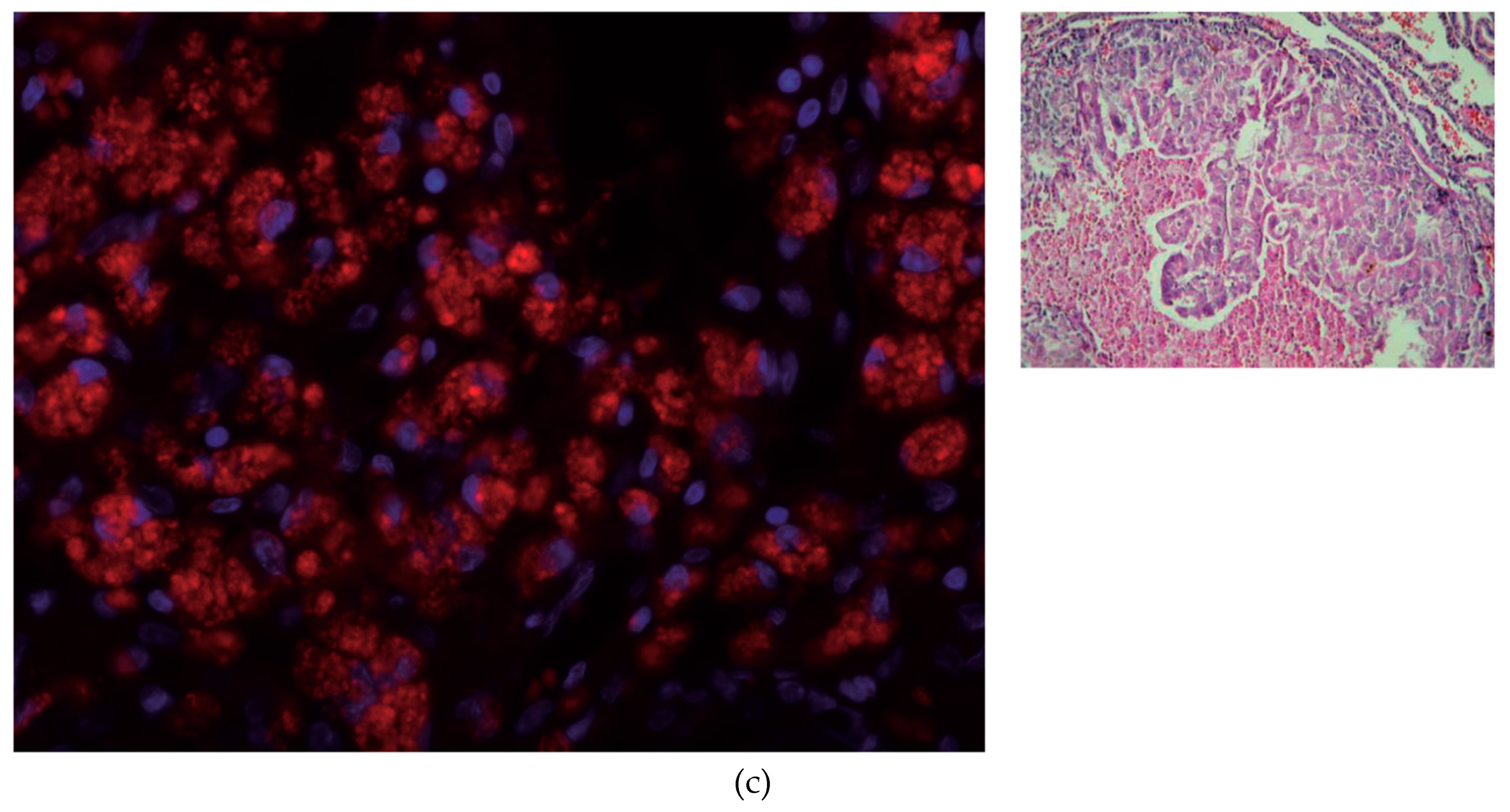
2.3. Histologic Study and NIS Immunofluorescence Staining
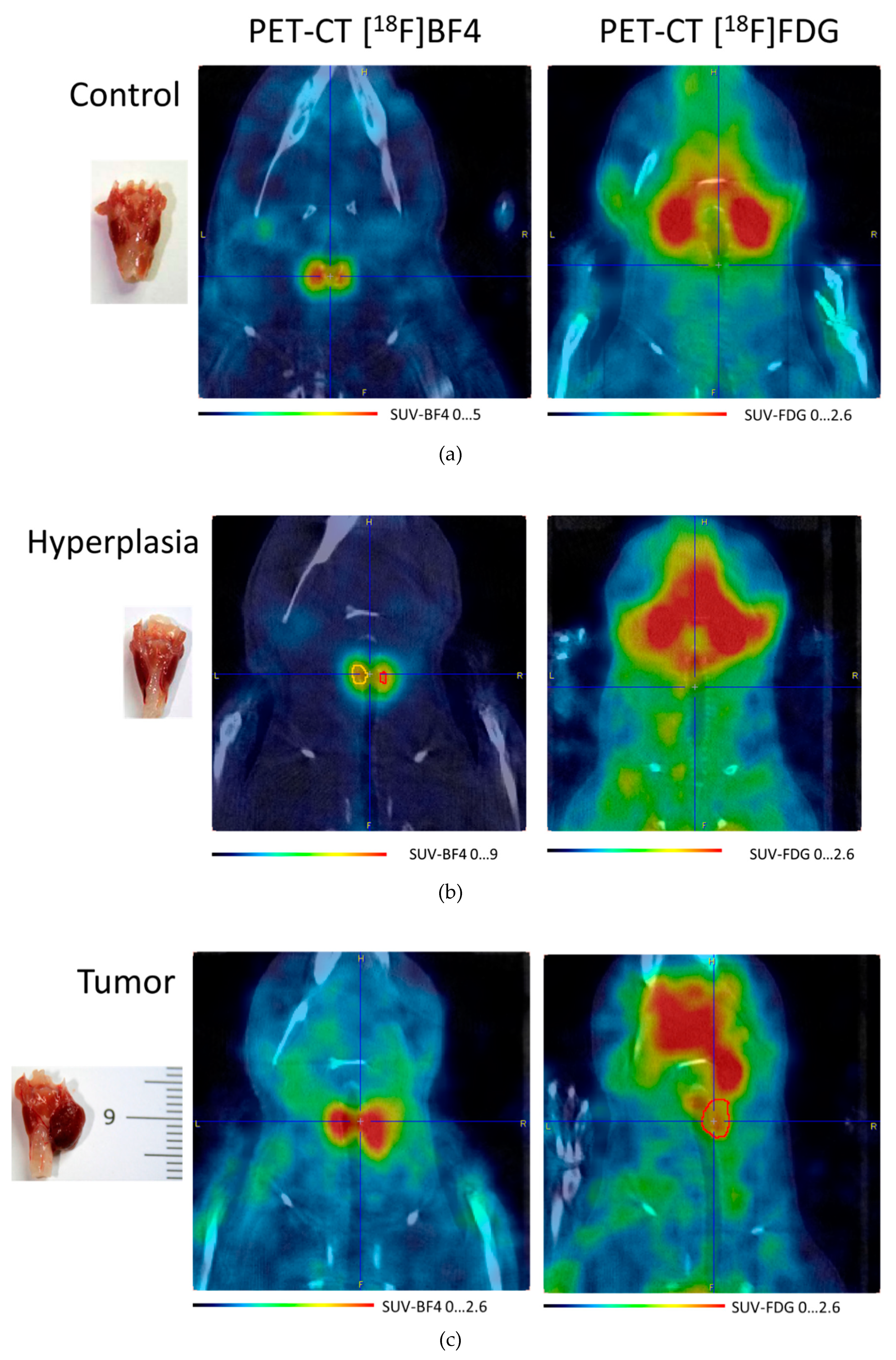
2.4. PET Imaging and Image Processing
2.5. HISPANoS as Epithermal Neutrons Irradiation Facility for BNCT
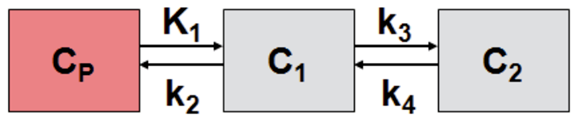
2.6. Calculation of Boron Concentration
3. Results
3.1. [18F]NaBF4 Radiotracer Production
3.2. IF Staining of NIS in Thyroid Tissue
3.3. [18F]NaBF4 PET Radiotracer Uptake in Thyroid Cells
3.4. Simulation of Epithermal Neutrons Irradiation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suzuki, M. Boron neutron capture therapy (BNCT): A unique role in radiotherapy with a view to entering the accelerator-based BNCT era. Int. J. Clin. Oncol. 2020, 25, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Irazola, L.; Praena, J.; Fernández, B.; Macías, M.; Bedogni, R.; Terrón, J.A.; Sánchez-Nieto, B.; Arias de Saavedra, F.; Porras, I.; Sánchez-Doblado, F. Using a Tandem Pelletron accelerator to produce a thermal neutron beam for detector testing purposes. Appl. Radiat. Isot. 2016, 107, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Taniguchi, E.; Fujimoto, T.; Fukumori, Y. Biodistribution of BPA and BSH after single, repeated and simultaneous administrations for neutron-capture therapy of cancer. Appl. Radiat. Isot. 2009, 67, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.H.; Chen, Y.F.; Chen, F.D.; Hwang, J.J.; Chen, J.C.; Liu, R.S.; Kai, J.J.; Chang, C.W.; Wang, H.E. Evaluation of pharmacokinetics of 4-borono-2-18F-fluoro-L- phenylalanine for boron neutron capture therapy in a glioma-bearing rat model with hyperosmolar blood-brain barrier disruption. J. Nucl. Med. 2005, 46, 1858–1865. [Google Scholar] [PubMed]
- Jauregui-Osoro, M.; Sunassee, K.; Weeks, A.J.; Berry, D.J.; Paul, R.L.; Cleij, M.; Banga, J.P.; O’Doherty, M.J.; Marsden, P.K.; Clarke, S.E.M.; et al. Synthesis and biological evaluation of [18F]tetrafluoroborate: A PET imaging agent for thyroid disease and reporter gene imaging of the sodium/iodide symporter. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2108–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balcerzyk, M.; Fernandez-Maza, L.; Mínguez, J.J.; De-Miguel, M. Preclinical [18F]tetrafluoroborate-PET/CT imaging of pituitary gland hyperplasia. Jpn. J. Clin. Oncol. 2018, 48, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Hiasa, Y.; Kitahori, Y.; Kato, Y.; Ohshima, M.; Konishi, N.; Shimoyama, T.; Sakaguchi, Y.; Hashimoto, H.; Minami, S.; Murata, Y. Potassium Perchlorate, Potassium Iodide, and Propylthiouracil: Promoting Effect on the Development of Thyroid Tumors in Rats Treated with N-Bis(2Hydroxypropyl)-nitrosamine. Jpn. J. Cancer Res. Gann 1987, 78, 1335–1340. [Google Scholar] [PubMed]
- Praena, J.; Mastinu, P.F.; Pignatari, M.; Quesada, J.M.; Capote, R.; Morilla, Y. Measurement of the MACS of 159Tb(n, γ) at kT = 30 keV by Activation. Nucl. Data Sheets 2014, 120, 205–207. [Google Scholar] [CrossRef]
- Macías, M.; Fernández, B.; Praena, J. The first neutron time-of-flight line in Spain: Commissioning and new data for the definition of a neutron standard field. Radiat. Phys. Chem. 2020, 168. [Google Scholar] [CrossRef]
- Praena, J.; Mastinu, P.F.; Pignatari, M.; Quesada, J.M.; García-López, J.; Lozano, M.; Dzysiuk, N.; Capote, R.; Martín-Hernández, G. Measurement of the MACS of Ta 181 (n, γ) at kT=30 keV as a test of a method for Maxwellian neutron spectra generation. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometersdetectors Assoc. Equip. 2013, 727, 1–6. [Google Scholar] [CrossRef]
- Malagón, D.; Bota, S.A.; Torrens, G.; Gili, X.; Praena, J.; Fernández, B.; Macías, M.; Quesada, J.M.; Sanchez, C.G.; Jiménez-Ramos, M.C.; et al. Soft error rate comparison of 6T and 8T SRAM ICs using mono-energetic proton and neutron irradiation sources. Microelectron. Reliab. 2017, 78, 38–45. [Google Scholar] [CrossRef]
- Fantidis, J.G. Beam shaping assembly study for BNCT facility based on a 2.5 MeV proton accelerator on Li target. J. Theor. Appl. Phys. 2018, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- International Atomic Energy Agency. Current Status of Neutron Capture Therapy; IAEA: Vienna, Austria, 2001. [Google Scholar]
- Balcerzyk, M. FDG and NaBF4 PET DICOM Files of Rat Thyroid Tumor Study. Available online: https://doi.org/10.5281/zenodo.3968290 (accessed on 12 September 2020).
- Brown, D.A.; Chadwick, M.B.; Capote, R.; Kahler, A.C.; Trkov, A.; Herman, M.W.; Sonzogni, A.A.; Danon, Y.; Carlson, A.D.; Dunn, M.; et al. ENDF/B-VIII.0: The 8th Major Release of the Nuclear Reaction Data Library with CIELO-project Cross Sections, New Standards and Thermal Scattering Data. Nucl. Data Sheets 2018, 148, 1–142. [Google Scholar] [CrossRef]
- Hu, N.; Tanaka, H.; Takata, T.; Endo, S.; Masunaga, S.; Suzuki, M.; Sakurai, Y. Evaluation of PHITS for microdosimetry in BNCT to support radiobiological research. Appl. Radiat. Isot. 2020, 161, 109148. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Hanaoka, K.; Naka, S.; Kanai, Y.; Ikeda, H.; Aoki, M.; Shimosegawa, E.; Kirihata, M.; Hatazawa, J. Practical calculation method to estimate the absolute boron concentration in tissues using 18F-FBPA PET. Ann. Nucl. Med. 2017, 31, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Weeks, A.J.; Jauregui-Osoro, M.; Cleij, M.; Blower, J.E.; Ballinger, J.R.; Blower, P.J. Evaluation of [18F]-tetrafluoroborate as a potential PET imaging agent for the human sodium/iodide symporter in a new colon carcinoma cell line, HCT116, expressing hNIS. Nucl. Med. Commun. 2011, 32, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.C.; Colas, C.; Finke, K.; Springer, S.; Stoner, L.; Zur, A.A.; Venteicher, B.; Campbell, J.; Hall, C.; Flint, A.; et al. Reevaluating the Substrate Specificity of the L-Type Amino Acid Transporter (LAT1). J. Med. Chem. 2018, 61, 7358–7373. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Zhang, L.; Xie, F.; Zhuang, R.; Jiang, D.; Liu, H.; Li, J.; Yang, H.; Zhang, X.; Nie, L.; et al. Rapid one-step 18F-radiolabeling of biomolecules in aqueous media by organophosphine fluoride acceptors. Nat. Commun. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| K1 | k2 | k3 | k4 | Vs | Vt | Kl/k2 | k3/k4 | Flux |
|---|---|---|---|---|---|---|---|---|
| 1.89 | 0.52 | 0.09 | 0.08 | 3.96 | 7.56 | 3.60 | 1.10 | 0.28 |
| Normal Thyroid | Tumor Thyroid | |||
|---|---|---|---|---|
| Concentration | Captured n | Captured n | Dose, Gy | Dose, Gy-e |
| 1 mM 10B | 257 | 2770 | 0.092 | 0.388 |
| 2 mM 10B | 513 | 5550 | 0.185 | 0.776 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balcerzyk, M.; De-Miguel, M.; Guerrero, C.; Fernandez, B. Quantification of Boron Compound Concentration for BNCT Using Positron Emission Tomography. Cells 2020, 9, 2084. https://doi.org/10.3390/cells9092084
Balcerzyk M, De-Miguel M, Guerrero C, Fernandez B. Quantification of Boron Compound Concentration for BNCT Using Positron Emission Tomography. Cells. 2020; 9(9):2084. https://doi.org/10.3390/cells9092084
Chicago/Turabian StyleBalcerzyk, Marcin, Manuel De-Miguel, Carlos Guerrero, and Begoña Fernandez. 2020. "Quantification of Boron Compound Concentration for BNCT Using Positron Emission Tomography" Cells 9, no. 9: 2084. https://doi.org/10.3390/cells9092084






