Formation of βTC3 and MIN6 Pseudoislets Changes the Expression Pattern of Gpr40, Gpr55, and Gpr119 Receptors and Improves Lysophosphatidylcholines-Potentiated Glucose-Stimulated Insulin Secretion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture Conditions
2.2.1. βTC3 Cell Line
2.2.2. MIN6 Cell Line
2.2.3. MS1 Cell Line
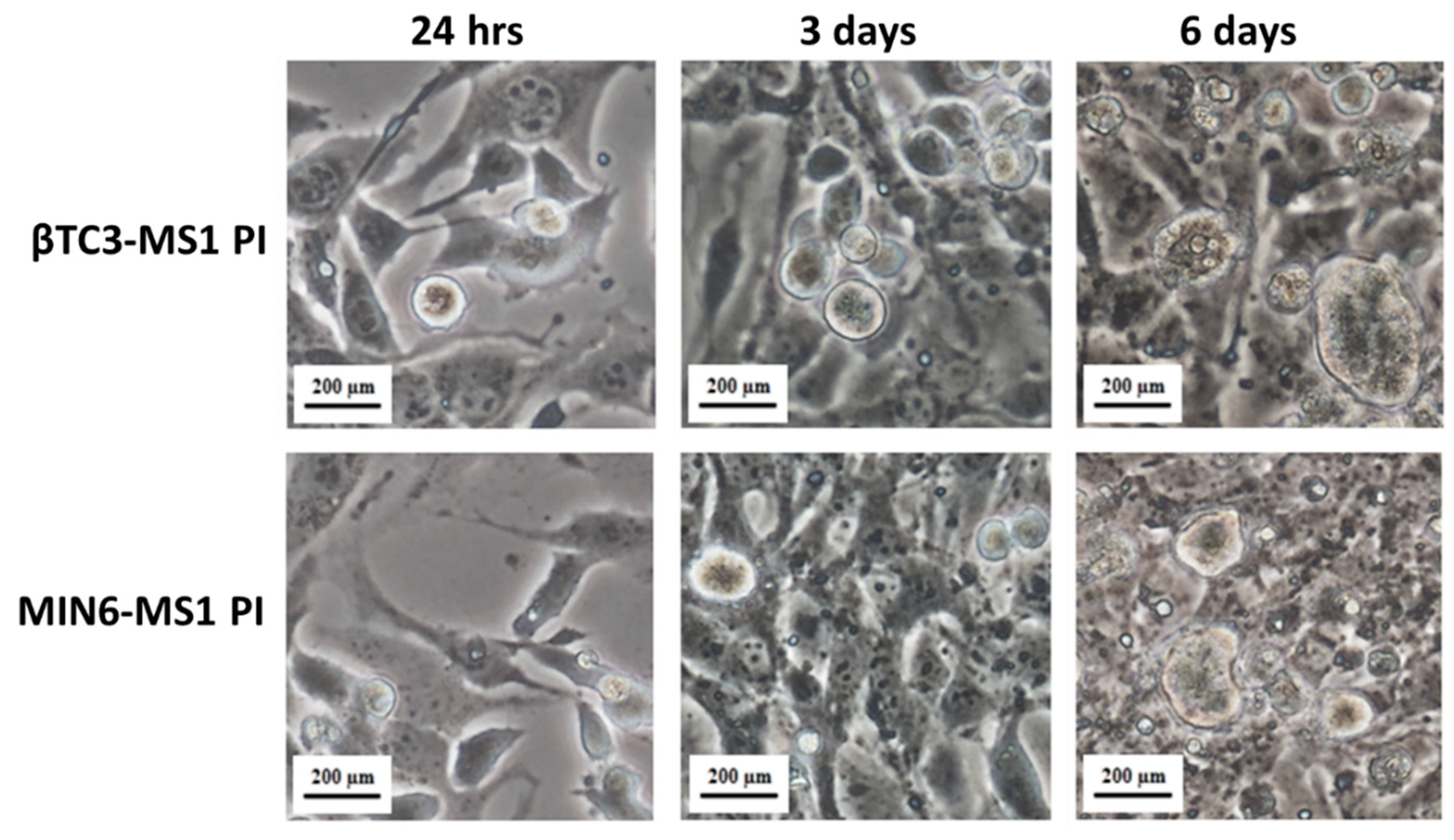
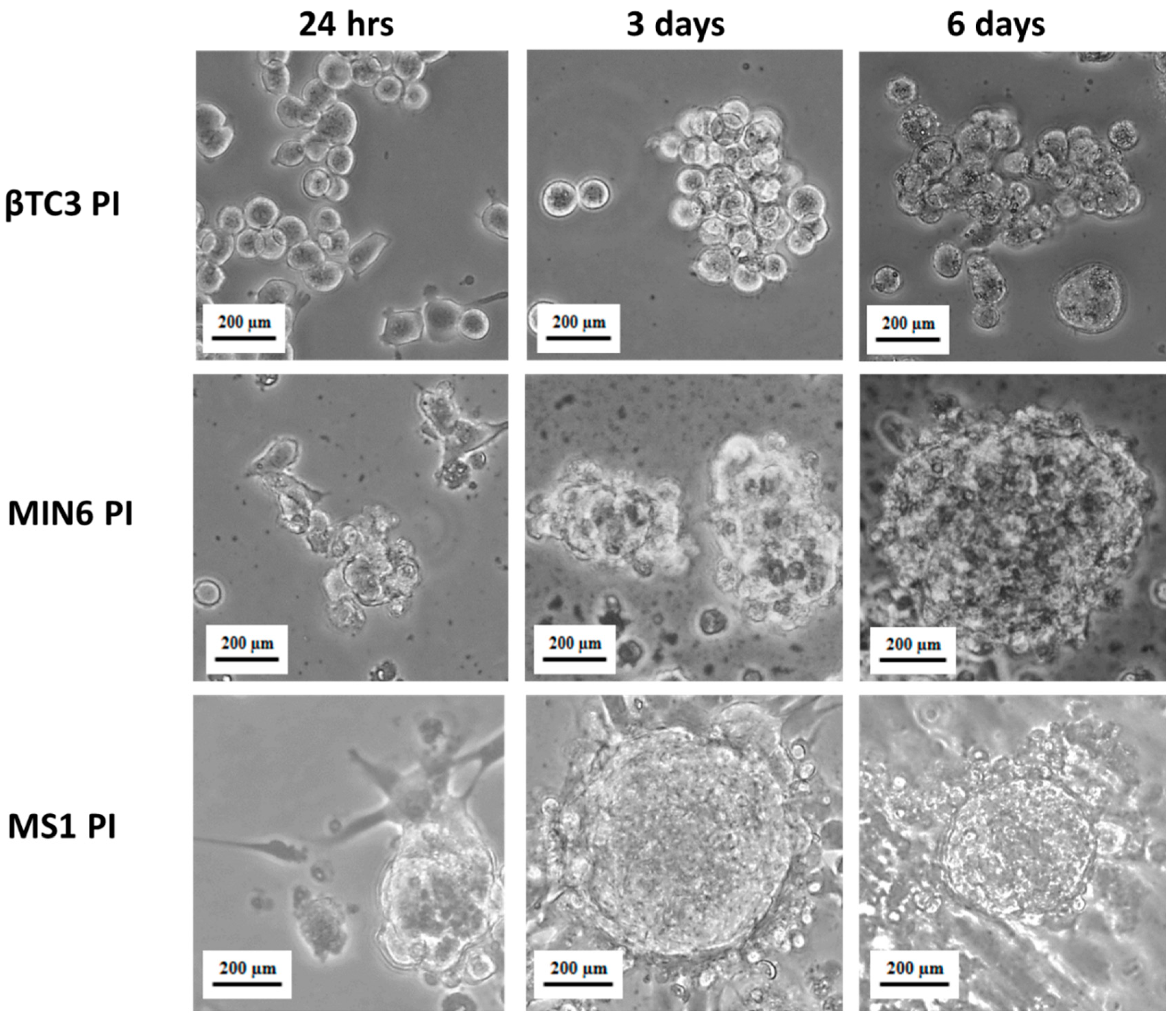
2.3. Pseudoislets Formation
2.4. Stimulation of Ins1 Expression in Adherent βTC3 and βTC3-MS1 Pseudoislets
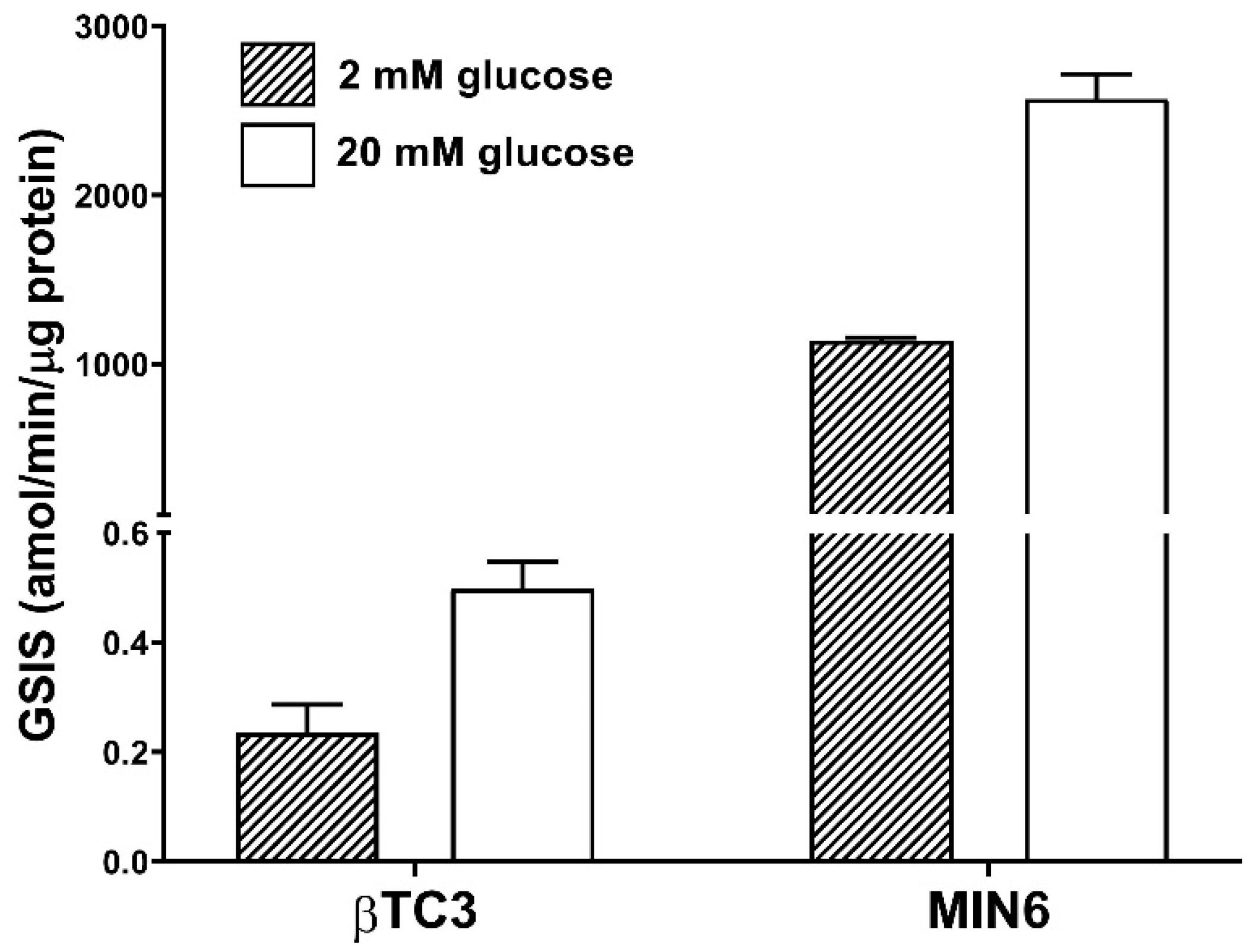
2.5. Glucose-Stimulated Insulin Secretion (GSIS)
2.6. RNA Isolation and Quantitative Reverse Transcription PCR Analysis
2.7. Statistical Analysis
3. Results
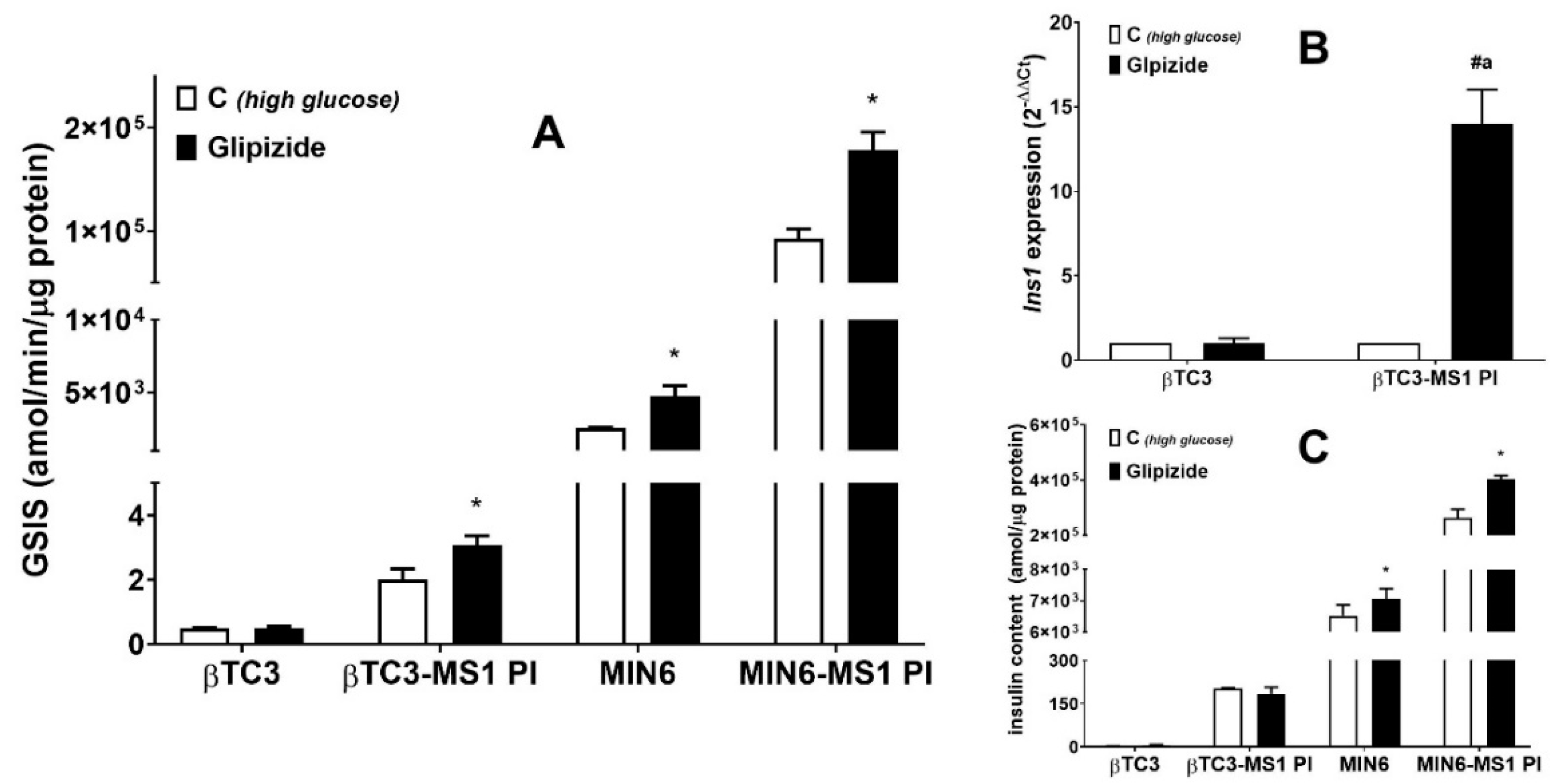
3.1. Comparison of the Responsiveness of βTC3 and MIN6 Monolayers as Well as Pseudoislets to Glipizide
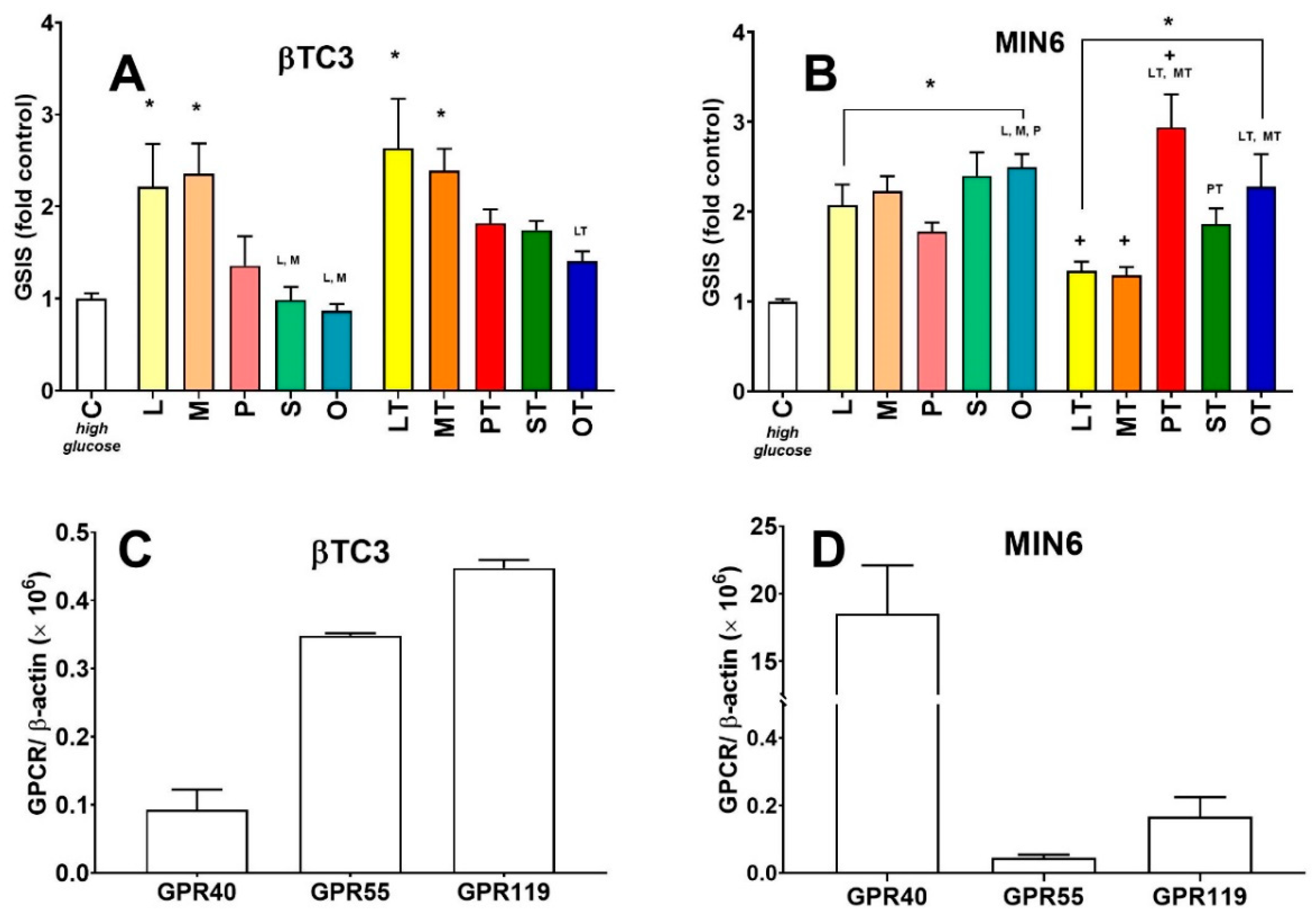
3.2. Comparison of Insulin Secretory Activity of βTC3 and MIN6 Monolayers in Response to LPC with Various Acyl Chain Lengths
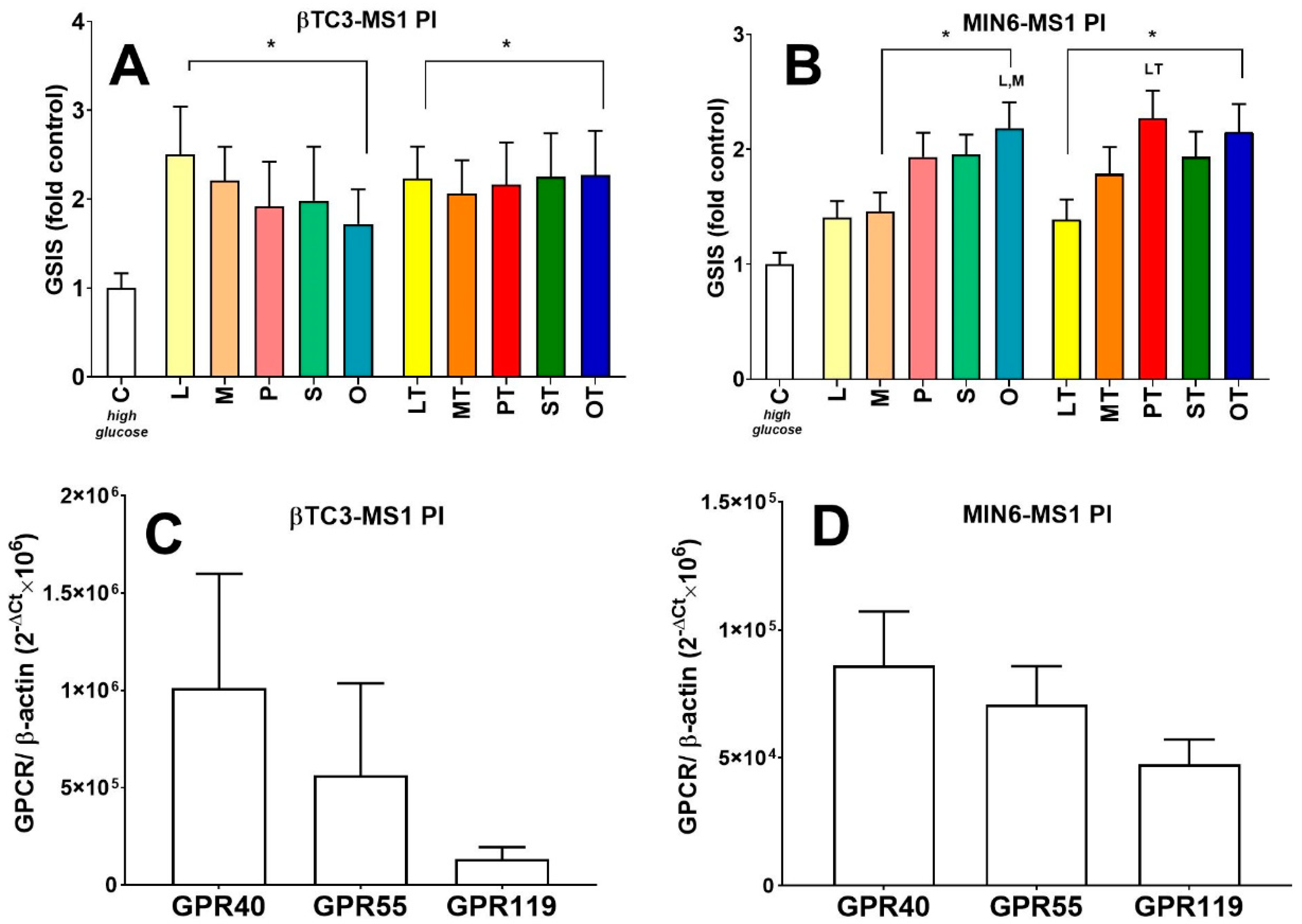
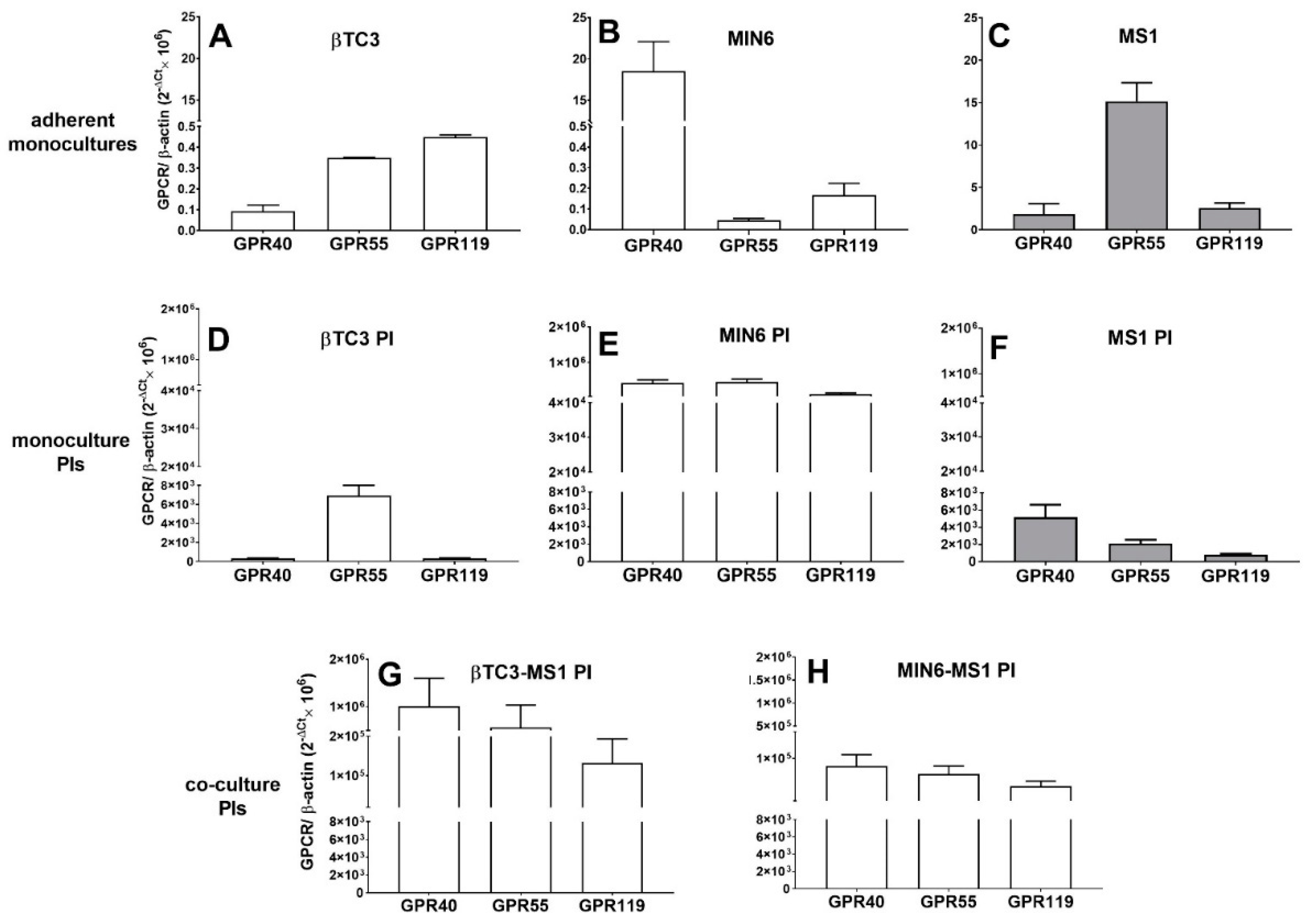
3.3. Comparison of Insulin Secretory Activity of βTC3 and MIN6 Pseudoislets in Response to LPC with Various Acyl Chain Lengths
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Inzucchi, S.E.; Bergenstal, R.M.; Buse, J.B.; Diamant, M.; Ferrannini, E.; Nauck, M.; Peters, A.L.; Tsapas, A.; Wender, R.; Matthews, D.R. Management of Hyperglycemia in Type 2 Diabetes, 2015: A Patient-Centered Approach: Update to a position statement of the american diabetes association and the european association for the study of diabetes. Diabetes Care 2015, 38, 140–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Green, A.D.; Vasu, S.; Flatt, P.R. Cellular models for beta-cell function and diabetes gene therapy. Acta Physiol. 2018, 222, e13012. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Asano, T.; Tsukuda, K.; Katagiri, H.; Inukai, K.; Anai, M.; Kikuchi, M.; Yazaki, Y.; Miyazaki, J.I.; Oka, Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia 1993, 36, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Carrington, C.A.; Rubery, E.D.; Pearson, E.C.; Hales, C.N. Five new insulin-producing cell lines with differing secretory properties. J. Endocrynol. 1985, 109, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Soga, T.; Ohishi, T.; Matsui, T.; Saito, T.; Matsumoto, M.; Takasaki, J.; Matsumoto, S.; Kamohara, M.; Hiyama, H.; Yoshida, S.; et al. Lysophosphatidylcholine enhances glucose-dependent insulin secretion via an orphan G-protein-coupled receptor. Biochem. Biophys. Res. Commun. 2005, 326, 744–751. [Google Scholar] [CrossRef]
- Spelios, M.G.; Olsen, J.A.; Kenna, L.A.; Akirav, E.M. Islet Endothelial Cells Induce Glycosylation and Increase Cell-surface Expression of Integrin β1 in β Cells*. J. Biol. Chem. 2015, 290, 15250–15259. [Google Scholar] [CrossRef] [Green Version]
- Efrat, S.; Lindet, S.; Kofodt, H.; Spector, D.; Delannoy, M.; Grant, S.; Hanahan, D.; Baekkeskovt, S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin. Proc. Natl. Acad. Sci. USA 1988, 85, 9037–9041. [Google Scholar] [CrossRef] [Green Version]
- Radvanyi, F.; Christgau, S.; Baekkeskov, S.; Jolicoeur, C.; Hanahan, D. Pancreatic Beta Cells Cultured from Individual Preneoplastic Foci in a Multistage Tumorigenesis Pathway: A Potentially General Technique for Isolating Physiologically Representative Cell Lines. Mol. Cell. Biol. 1993, 13, 4223–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, G.; Digiacomo, L.; Lavagnino, Z.; Occhipinti, M.; Bugliani, M.; Cappello, V.; Caracciolo, G.; Marchetti, P.; Piston, D.W.; Cardarelli, F. Insulin secretory granules labelled with phogrin-fluorescent proteins show alterations in size, mobility and responsiveness to glucose stimulation in living β-cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, J.; Yamato, E.; Asano, T.; Yamamura, K. Establishment of a pancreatic beta cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hauge-Evans, A.C.; Squires, P.E.; Persaud, S.J.; Jones, P.M. Pancreatic β-cell-to-β-cell interactions are required for integrated responses to nutrient stimuli: Enhanced Ca2+ and insulin secretory responses of MIN6 pseudoislets. Diabetes 1999, 48, 1402–1408. [Google Scholar] [CrossRef]
- Kelly, C.; Guo, H.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. Comparison of insulin release from MIN6 pseudoislets and pancreatic islets of Langerhans reveals importance of homotypic cell interactions. Pancreas 2010, 39, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Satagopam, V.P.; Manukyan, L.; Artemenko, K.A.; Fung, Y.M.E.; Schneider, R.; Bergquist, J.; Bergsten, P. Signaling in insulin-secreting MIN6 pseudoislets and monolayer cells. J. Proteome Res. 2013, 12, 5954–5962. [Google Scholar] [CrossRef] [PubMed]
- Lock, L.T.; Laychock, S.G.; Tzanakakis, E.S. Pseudoislets in stirred-suspension culture exhibit enhanced cell survival, propagation and insulin secretion. J. Biotechnol. 2011, 151, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Spelios, M.G.; Kenna, L.A.; Wall, B.; Akirav, E.M. In Vitro Formation of b Cell Pseudoislets Using Islet- Derived Endothelial Cells. PLoS ONE 2013, 8, e72260. [Google Scholar] [CrossRef]
- Kelly, C.; Flatt, P.R.; McClenaghan, N.H. Cell-to-cell communication and cellular environment alter the somatostatin status of delta cells. Biochem. Biophys. Res. Commun. 2010, 399, 162–166. [Google Scholar] [CrossRef]
- Green, A.D.; Vasu, S.; Flatt, P.R. Functionality and antidiabetic utility of β- and L-cell containing pseudoislets. Exp. Cell Res. 2016, 344, 201–209. [Google Scholar] [CrossRef]
- Teraoku, H.; Lenzen, S. Dynamics of Insulin Secretion from EndoC-β H1 β-Cell Pseudoislets in Response to Glucose and Other Nutrient and Nonnutrient Secretagogues. J. Diabetes Res. 2017, 2017, 2309630. [Google Scholar] [CrossRef]
- Gendaszewska-Darmach, E.; Drzazga, A.; Koziołkiewicz, M. Targeting GPCRs Activated by Fatty Acid-Derived Lipids in Type 2 Diabetes. Trends Mol. Med. 2019, 25, 915–929. [Google Scholar] [CrossRef] [Green Version]
- Green, A.D.; Vasu, S.; McClenaghan, N.H.; Flatt, P.R. Implanting 1.1B4 human β-cell pseudoislets improves glycaemic control in diabetic severe combined immune deficient mice. World J. Diabetes 2016, 7, 523. [Google Scholar] [CrossRef]
- Spelios, M.G.; Afinowicz, L.A.; Tipon, R.C.; Akirav, E.M. Human EndoC-βH1 β-cells form pseudoislets with improved glucose sensitivity and enhanced GLP-1 signaling in the presence of islet-derived endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2018, 314, E512–E521. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Yang, J.; Zhou, J.; Ye, Z.; Feng, D. European Journal of Medicinal Chemistry Design, synthesis and biological evaluation of novel FFA1/GPR40 agonists: New breakthrough in an old scaffold. Eur. J. Med. Chem. 2019, 179, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Deng, L.; Chen, H.; Liao, R.; Li, Y.; Zeng, X. Bioorganic & Medicinal Chemistry Letters Design, synthesis and biological activity of deuterium-based FFA1 agonists with improved pharmacokinetic profiles. Bioorg. Med. Chem. Lett. 2019, 29, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Hassing, H.A.; Fares, S.; Larsen, O.; Pad, H.; Hauge, M.; Jones, R.M.; Schwartz, T.W.; Hansen, H.S.; Rosenkilde, M.M. Biased signaling of lipids and allosteric actions of synthetic molecules for GPR119. Biochem. Pharmacol. 2016, 119, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Mandøe, M.J.; Hansen, K.B.; Windeløv, J.A.; Knop, F.K.; Rehfeld, J.F.; Rosenkilde, M.M.; Holst, J.J.; Hansen, H.S. Comparing olive oil and C4-dietary oil, a prodrug for the GPR119 agonist, 2-oleoyl glycerol, less energy intake of the latter is needed to stimulate incretin hormone secretion in overweight subjects with type 2 diabetes. Nutr. Diabetes 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Sakuma, K.; Yabuki, C.; Maruyama, M.; Abiru, A.; Komatsu, H. Fasiglifam (TAK-875) has dual potentiating mechanisms via G a q-GPR40/FFAR1 signaling branches on glucose-dependent insulin secretion. Pharmacol. Res. Perspect. 2016, 4. [Google Scholar] [CrossRef]
- Drzazga, A.; Kristinsson, H.; Sałaga, M.; Zatorski, H.; Koziołkiewicz, M.; Gendaszewska-Darmach, E.; Bergsten, P. Lysophosphatidylcholine and its phosphorothioate analogues potentiate insulin secretion via GPR40 (FFAR1), GPR55 and GPR119 receptors in a different manner. Mol. Cell. Endocrinol. 2017, 472, 117–125. [Google Scholar] [CrossRef]
- Drzazga, A.; Sowińska, A.; Krzemińska, A.; Okruszek, A.; Paneth, P.; Koziołkiewicz, M.; Gendaszewska-Darmach, E. 2-OMe-lysophosphatidylcholine analogues are GPR119 ligands and activate insulin secretion from βTC-3 pancreatic cells: Evaluation of structure-dependent biological activity. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 91–103. [Google Scholar] [CrossRef]
- McKillop, A.M.; Moran, B.M.; Abdel-Wahab, Y.H.A.; Flatt, P.R. Evaluation of the insulin releasing and antihyperglycaemic activities of GPR55 lipid agonists using clonal beta-cells, isolated pancreatic islets. Br. J. Pharmacol. 2013, 170, 978–990. [Google Scholar] [CrossRef] [Green Version]
- McKillop, A.M.; Moran, B.M.; Abdel-Wahab, Y.H.A.; Gormley, N.M.; Flatt, P.R. Metabolic effects of orally administered small-molecule agonists of GPR55 and GPR119 in multiple low-dose streptozotocin-induced diabetic and incretin-receptor-knockout mice. Diabetologia 2016, 59, 2674–2685. [Google Scholar] [CrossRef] [Green Version]
- Drzazga, A.; Sowinska, A.; Krzeminska, A.; Rytczak, P.; Koziolkiewicz, M.; Gendaszewska-Darmach, E. Lysophosphatidylcholine elicits intracellular calcium signaling in a GPR55-dependent manner. Biochem. Biophys. Res. Commun. 2017, 489, 242–247. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, A.G.; Miskelly, M.G.; Moore, C.B.T.; Nesbit, M.A.; Christie, K.A.; Owolabi, A.I.; Flatt, P.R.; McKillop, A.M. CRISPR/Cas9 gene editing demonstrates metabolic importance of GPR55 in the modulation of GIP release and pancreatic beta cell function. Peptides 2020, 125, 170251. [Google Scholar] [CrossRef] [PubMed]
- Rytczak, P.; Drzazga, A.; Gendaszewska-Darmach, E.; Okruszek, A. The chemical synthesis and cytotoxicity of new sulfur analogues of 2-methoxy-lysophosphatidylcholine. Bioorg. Med. Chem. Lett. 2013, 23, 6794–6798. [Google Scholar] [CrossRef] [PubMed]
- McClure, K.F.; Darout, E.; Guimarães, C.R.W.; Deninno, M.P.; Mascitti, V.; Munchhof, M.J.; Robinson, R.P.; Kohrt, J.; Harris, A.R.; Moore, D.E.; et al. Activation of the G-protein-coupled receptor 119: A conformation-base hypothesis for understanding agonist response. J. Med. Chem. 2011, 54, 1948–1952. [Google Scholar] [CrossRef] [PubMed]
- Bergsten, P.; Hellman, B. Glucose-induced amplitude regulation of pulsatile insulin secretion from individual pancreatic islets. Diabetes 1993, 42, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Harlow, E.; Lane, D. Bradford assay. CSH Protoc. 2006, 2006, 1121–1132. [Google Scholar] [CrossRef]
- Reers, C.; Hauge-Evans, A.C.; Morgan, N.G.; Willcox, A.; Persaud, S.J.; Jones, P.M. Downregulation of proliferation does not affect the secretory function of transformed β-cell lines regardless of their anatomical configuration. Islets 2011, 3, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Stendahl, J.C.; Kaufman, D.B.; Stupp, S.I. Extracellular matrix in pancreatic islets: Relevance to scaffold design and transplantation. Cell Transplant. 2009, 18, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Le, Y.; Wei, R.; Yang, K.; Lang, S.; Gu, L.; Liu, J.; Hong, T.; Yang, J. Liraglutide ameliorates palmitate-induced oxidative injury in islet microvascular endothelial cells through GLP-1 receptor/PKA and GTPCH1/eNOS signaling pathways. Peptides 2020, 124, 170212. [Google Scholar] [CrossRef]
- Jo, J.; Moo, Y.C.; Koh, D.S. Size distribution of mouse Langerhans islets. Biophys. J. 2007, 93, 2655–2666. [Google Scholar] [CrossRef] [Green Version]
- De Wet, H.; Proks, P. Molecular action of sulphonylureas on KATP channels: A real partnership between drugs and nucleotides. Biochem. Soc. Trans. 2015, 43, 901–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panten, U.; Früh, E.; Reckers, K.; Rustenbeck, I. Acute metabolic amplification of insulin secretion in mouse islets: Role of cytosolic acetyl-CoA. Metabolism 2016, 65, 1225–1229. [Google Scholar] [CrossRef] [PubMed]
- Luther, M.J.; Davies, E.; Muller, D.; Harrison, M.; Bone, A.J.; Persaud, S.J.; Jones, P.M. Cell-to-cell contact influences proliferative marker expression and apoptosis in MIN6 cells grown in islet-like structures. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E502–E509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luther, M.J.; Hauge-Evans, A.; Souza, K.L.A.; Jörns, A.; Lenzen, S.; Persaud, S.J.; Jones, P.M. MIN6 β-cell-β-cell interactions influence insulin secretory responses to nutrients and non-nutrients. Biochem. Biophys. Res. Commun. 2006, 343, 99–104. [Google Scholar] [CrossRef]
- Lecomte, M.-J.; Pechberty, S.; Machado, C.; Da Barroca, S.; Ravassard, P.; Scharfmann, R.; Czernichow, P.; Duvillié, B. Aggregation of Engineered Human β-Cells into Pseudoislets: Insulin Secretion and Gene Expression Profile in Normoxic and Hypoxic Milieu. Cell Med. 2016, 8, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Tsonkova, V.G.; Sand, F.W.; Wolf, X.A.; Grunnet, L.G.; Kirstine Ringgaard, A.; Ingvorsen, C.; Winkel, L.; Kalisz, M.; Dalgaard, K.; Bruun, C.; et al. The EndoC-βH1 cell line is a valid model of human beta cells and applicable for screenings to identify novel drug target candidates. Mol. Metab. 2018, 8, 144–157. [Google Scholar] [CrossRef]
- Hauge-Evans, A.C.; Squires, P.E.; Belin, V.D.; Roderigo-Milne, H.; Ramracheya, R.D.; Persaud, S.J.; Jones, P.M. Role of adenine nucleotides in insulin secretion from MIN6 pseudoislets. Mol. Cell. Endocrinol. 2002, 191, 167–176. [Google Scholar] [CrossRef]
- Oran, D.C.; Lokumcu, T.; Inceoglu, Y.; Akolpoglu, M.B.; Albayrak, O.; Bal, T.; Kurtoglu, M.; Erkan, M.; Can, F.; Bagci-Onder, T.; et al. Engineering human stellate cells for beta cell replacement therapy promotes in vivo recruitment of regulatory T cells. Mater. Today Bio 2019, 2, 100006. [Google Scholar] [CrossRef]
- Jiao, A.; Li, F.; Zhang, C.; Lv, W.; Chen, B.; Zhang, J. Simulated Cholinergic Reinnervation of β (INS-1) Cells: Antidiabetic Utility of Heterotypic Pseudoislets Containing β Cell and Cholinergic Cell. Int. J. Endocrinol. 2018, 2018, 1505307. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; Parke, H.G.; McCluskey, J.T.; Flatt, P.R.; McClenaghan, N.H. The role of glucagon-and somatostatin-secreting cells in the regulation of insulin release and beta-cell function in heterotypic pseudoislets. Diabetes Metab. Res. Rev. 2010, 26, 525–533. [Google Scholar] [CrossRef]
- Bal, T.; Inceoglu, Y.; Karaoz, E.; Kizilel, S. Sensitivity Study for the Key Parameters in Heterospheroid Preparation with Insulin-Secreting β-Cells and Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2019, 5, 5229–5239. [Google Scholar] [CrossRef]
- Itoh, Y.; Kawamata, Y.; Harada, M.; Kobayashi, M.; Fujii, R.; Fukusumi, S.; Ogi, K.; Hosoya, M.; Tanaka, Y.; Uejima, H.; et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 2003, 422, 173–176. [Google Scholar] [CrossRef]
- Odori, S.; Hosoda, K.; Tomita, T.; Fujikura, J.; Kusakabe, T.; Kawaguchi, Y.; Doi, R.; Takaori, K.; Ebihara, K.; Sakai, Y.; et al. GPR119 expression in normal human tissues and islet cell tumors: Evidence for its islet-gastrointestinal distribution, expression in pancreatic beta and alpha cells, and involvement in islet function. Metabolism 2013, 62, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, Y.; Inoue, H.; Kawakami, S.; Miyawaki, K.; Miyamoto, T.; Mizuta, K.; Itakura, M. Expression and distribution of Gpr119 in the pancreatic islets of mice and rats: Predominant localization in pancreatic polypeptide-secreting PP-cells. Biochem. Biophys. Res. Commun. 2006, 351, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Meidute Abaraviciene, S.; Muhammed, S.J.; Amisten, S.; Lundquist, I.; Salehi, A. GPR40 protein levels are crucial to the regulation of stimulated hormone secretion in pancreatic islets. Lessons from spontaneous obesity-prone and non-obese type 2 diabetes in rats. Mol. Cell. Endocrinol. 2013, 381, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Guerra, S.; Bugliani, M.; D’Aleo, V.; Del Prato, S.; Boggi, U.; Mosca, F.; Filipponi, F.; Lupi, R. G-protein-coupled receptor 40 (GPR40) expression and its regulation in human pancreatic islets: The role of type 2 diabetes and fatty acids. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Natalicchio, A.; Labarbuta, R.; Tortosa, F.; Biondi, G.; Marrano, N.; Peschechera, A.; Carchia, E.; Orlando, M.R.; Leonardini, A.; Cignarelli, A.; et al. Exendin-4 protects pancreatic beta cells from palmitate-induced apoptosis by interfering with GPR40 and the MKK4/7 stress kinase signalling pathway. Diabetologia 2013, 56, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Ekberg, J.H.; Hauge, M.; Kristensen, L.V.; Madsen, A.N.; Engelstoft, M.S.; Husted, A.S.; Sichlau, R.; Egerod, K.L.; Timshel, P.; Kowalski, T.J.; et al. GPR119, a major enteroendocrine sensor of dietary triglyceride metabolites coacting in synergy with FFA1 (GPR40). Endocrinology 2016, 157, 4561–4569. [Google Scholar] [CrossRef]
- Romero-Zerbo, S.Y.; Rafacho, A.; Díaz-Arteaga, A.; Suárez, J.; Quesada, I.; Imbernon, M.; Ross, R.A.; Dieguez, C.; de Fonseca, F.R.; Nogueiras, R.; et al. Role for the putative cannabinoid receptor GPR55 in the islets of Langerhans. J. Endocrinol. 2011, 211, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Song, S.; Ruz-Maldonado, I.; Pingitore, A.; Huang, G.C.; Baker, D.; Jones, P.M.; Persaud, S.J. GPR55-dependent stimulation of insulin secretion from isolated mouse and human islets of Langerhans. Diabetes Obes. Metab. 2016, 18, 1263–1273. [Google Scholar] [CrossRef] [Green Version]
- Johansson, M.; Mattsson, G.; Andersson, A.; Jansson, L.; Carlsson, P.O. Islet endothelial cells and pancreatic β-cell proliferation: Studies in vitro and during pregnancy in adult rats. Endocrinology 2006, 147, 2315–2324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gregersen, S.; Thomsen, J.L.; Brock, B.; Hermansen, K. Endothelin-1 stimulates insulin secretion by direct-action on the islets of Langerhans in mice. Diabetologia 1996, 39, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Olerud, J.; Mokhtari, D.; Johansson, M.; Christoffersson, G.; Lawler, J.; Welsh, N.; Carlsson, P.O. Thrombospondin-1: An islet endothelial cell signal of importance for β-cell function. Diabetes 2011, 60, 1946–1954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drott, C.J.; Olerud, J.; Emanuelsson, H.; Christoffersson, G.; Carlsson, P.O. Sustained Beta-Cell Dysfunction but Normalized Islet Mass in Aged Thrombospondin-1 Deficient Mice. PLoS ONE 2012, 7, e47451. [Google Scholar] [CrossRef] [Green Version]
- Parnaud, G.; Hammar, E.; Rouiller, D.G.; Armanet, M.; Halban, P.A.; Bosco, D. Blockade of β1 integrin-laminin-5 interaction affects spreading and insulin secretion of rat β-cells attached on extracellular matrix. Diabetes 2006, 55, 1413–1420. [Google Scholar] [CrossRef] [Green Version]
- Dogic, D.; Rousselle, P.; Aumailley, M. Cell adhesion to laminin 1 or 5 induces isoform-specific clustering of integrins and other focal adhesion components. J. Cell Sci. 1998, 111, 793–802. [Google Scholar]
- Waldeck-Welermair, M.; Zoratti, C.; Osibow, K.; Balenga, N.; Goessnitzer, E.; Waldhoer, M.; Malli, R.; Graier, W.F. Integrin clustering enables anandamide-induced Ca2+ signaling in endothelial cells via GPR55 by protection against CB1-receptor-triggered repression. J. Cell Sci. 2008, 121, 1704–1717. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.; McClenaghan, N.H.; Flatt, P.R. Role of islet structure and cellular interactions in the control of insulin secretion. Islets 2011, 3, 41–47. [Google Scholar] [CrossRef]
- Moonwiriyakit, A.; Wattanaphichet, P.; Chatsudthipong, V.; Muanprasat, C. GPR40 receptor activation promotes tight junction assembly in airway epithelial cells via AMPK-dependent mechanisms. Tissue Barriers 2018, 6, 1–12. [Google Scholar] [CrossRef]
- Puebla, C.; Cisterna, B.A.; Salas, D.P.; Delgado-López, F.; Lampe, P.D.; Sáez, J.C. Linoleic acid permeabilizes gastric epithelial cells by increasing connexin 43 levels in the cell membrane via a GPR40- and Akt-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2016, 1861, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.S.; Yang, S.Y.; Han, J.H.; Jung, S.H.; Park, H.S.; Myung, C.S. Differential gene expression in GPR40-overexpressing pancreatic β-cells treated with linoleic acid. Korean J. Physiol. Pharmacol. 2015, 19, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, J.; Mizukure, T.; Park, S.B.; Kishino, S.; Kimura, I.; Hirano, K.; Bergamo, P.; Rossi, M.; Suzuki, T.; Arita, M.; et al. A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway. J. Biol. Chem. 2015, 290, 2902–2918. [Google Scholar] [CrossRef] [Green Version]
- Tunaru, S.; Bonnavion, R.; Brandenburger, I.; Preussner, J.; Thomas, D.; Scholich, K.; Offermanns, S. 20-HETE promotes glucose-stimulated insulin secretion in an autocrine manner through FFAR1. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.R.; Andresen, T.L.; Feldborg, L.N.; Duelund, L.; Ipsen, J.H. Understanding detergent effects on lipid membranes: A model study of lysolipids. Biophys. J. 2010, 98, 2199–2205. [Google Scholar] [CrossRef] [Green Version]
- Drzazga, A.; Ciesielska, A.; Gendaszewska-Darmach, E. Sulfur- and Acyl Chain-Dependent Influence of 2-Methoxy-Lysophosphatidylcholine Analogues on β Pancreatic Cells. Curr. Top. Med. Chem. 2015, 15, 2395–2405. [Google Scholar] [CrossRef]

| Gene Symbol | Forward Primer | Reverse Primer | Accession No. |
|---|---|---|---|
| Ins1 | 5′-ACCTGGAGACCTTAATGGGCCAAA-3′ | 5′-ATGACCTGCTTGCTGATGGTCTCT-3′ | NM_008386.3 |
| Actβ | 5′-AAGAGCTATGAGCTGCCTGA-3 | 5′-TACGGATGTCAACGTCACAC-3′ | NM_007393.5 |
| Gpr40 | 5′-TCTGCCTGGGGCCCTATAAT-3′ | 5′-TCCAGGACCTGTTCCCAAGT-3′ | NM_194057.3 |
| Gpr55 | 5′-AGCCTTCTGACTTGGACAGC-3′ | 5′-CCTCATCCCCTTCATACTGG-3′ | NM_001033290.2 |
| Gpr119 | 5′-CTTCTACTGTGACATGCTCAAGATTG-3 | 5′-CCATGGCTCCTGCATGTTC-3 | NM_181751.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drzazga, A.; Cichońska, E.; Koziołkiewicz, M.; Gendaszewska-Darmach, E. Formation of βTC3 and MIN6 Pseudoislets Changes the Expression Pattern of Gpr40, Gpr55, and Gpr119 Receptors and Improves Lysophosphatidylcholines-Potentiated Glucose-Stimulated Insulin Secretion. Cells 2020, 9, 2062. https://doi.org/10.3390/cells9092062
Drzazga A, Cichońska E, Koziołkiewicz M, Gendaszewska-Darmach E. Formation of βTC3 and MIN6 Pseudoislets Changes the Expression Pattern of Gpr40, Gpr55, and Gpr119 Receptors and Improves Lysophosphatidylcholines-Potentiated Glucose-Stimulated Insulin Secretion. Cells. 2020; 9(9):2062. https://doi.org/10.3390/cells9092062
Chicago/Turabian StyleDrzazga, Anna, Eliza Cichońska, Maria Koziołkiewicz, and Edyta Gendaszewska-Darmach. 2020. "Formation of βTC3 and MIN6 Pseudoislets Changes the Expression Pattern of Gpr40, Gpr55, and Gpr119 Receptors and Improves Lysophosphatidylcholines-Potentiated Glucose-Stimulated Insulin Secretion" Cells 9, no. 9: 2062. https://doi.org/10.3390/cells9092062






