HPLC-MS/MS Shows That the Cellular Uptake of All-Trans-Retinoic Acid under Hypoxia Is Downregulated by the Novel Active Agent 5-Methoxyleoligin
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. HPLC-MS/MS and HPLC-IMS/MS Analysis of Retinoid and Leoligin Species
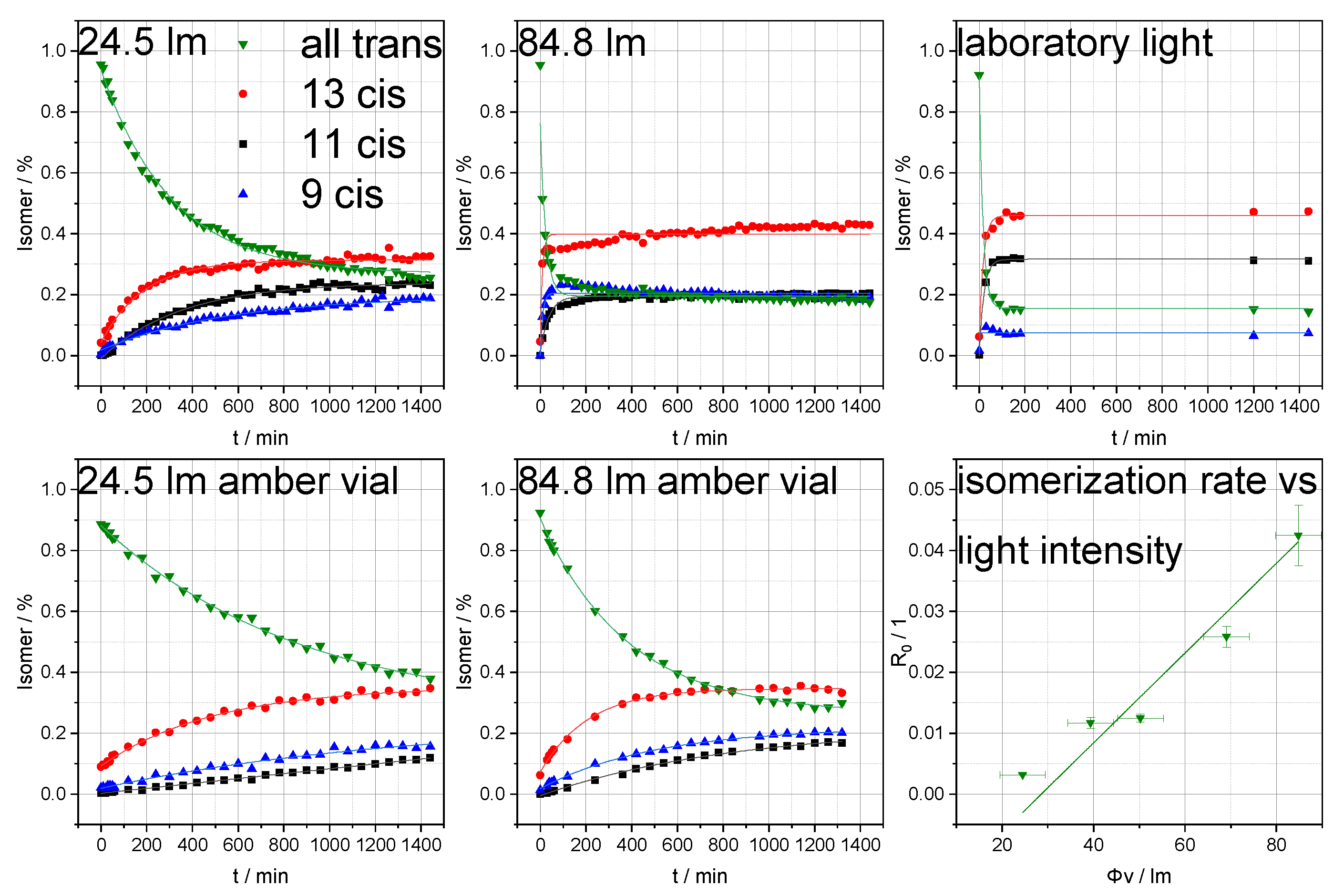
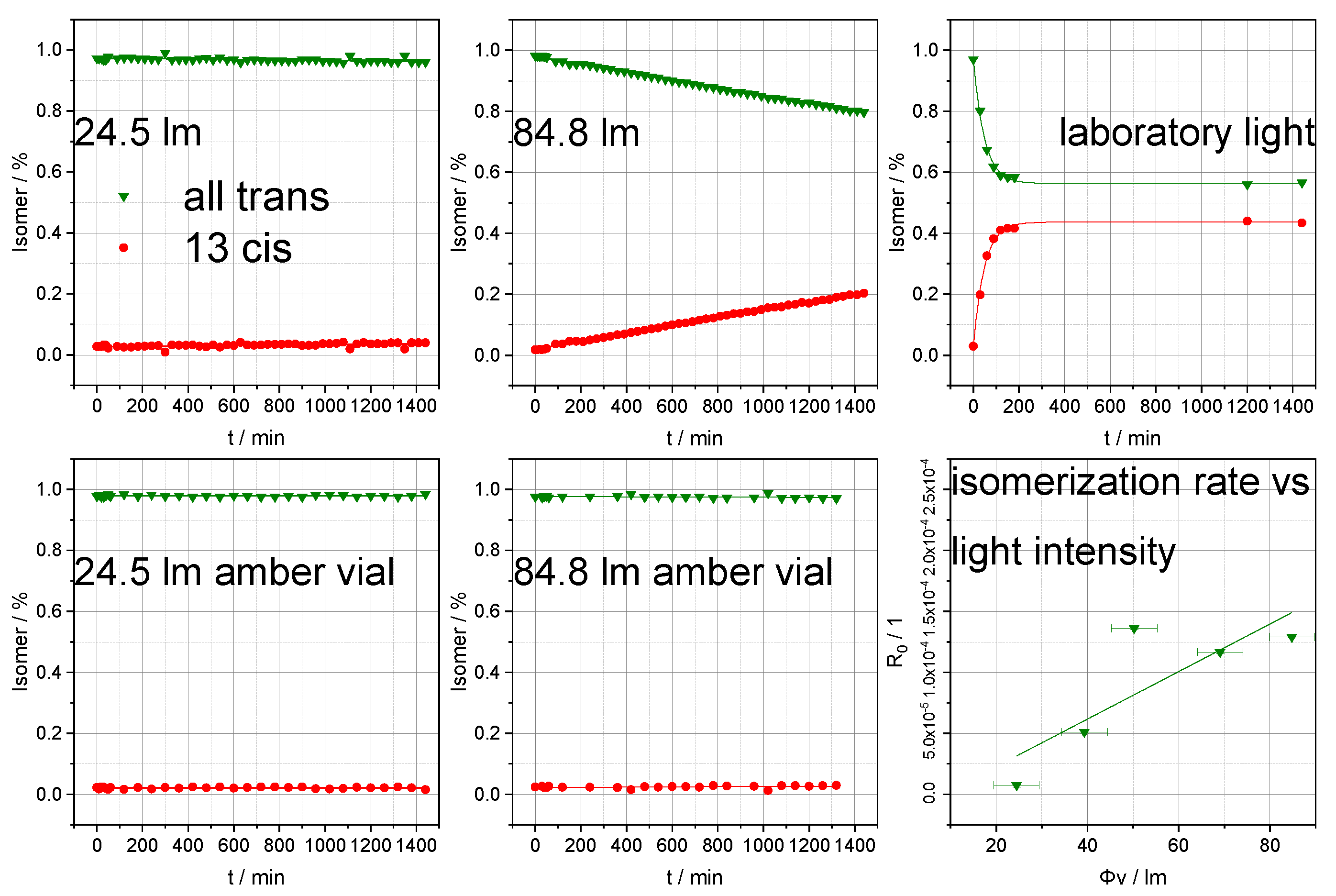
3.2. Photoisomerization Analysis of Retinol and Retinoic Acid
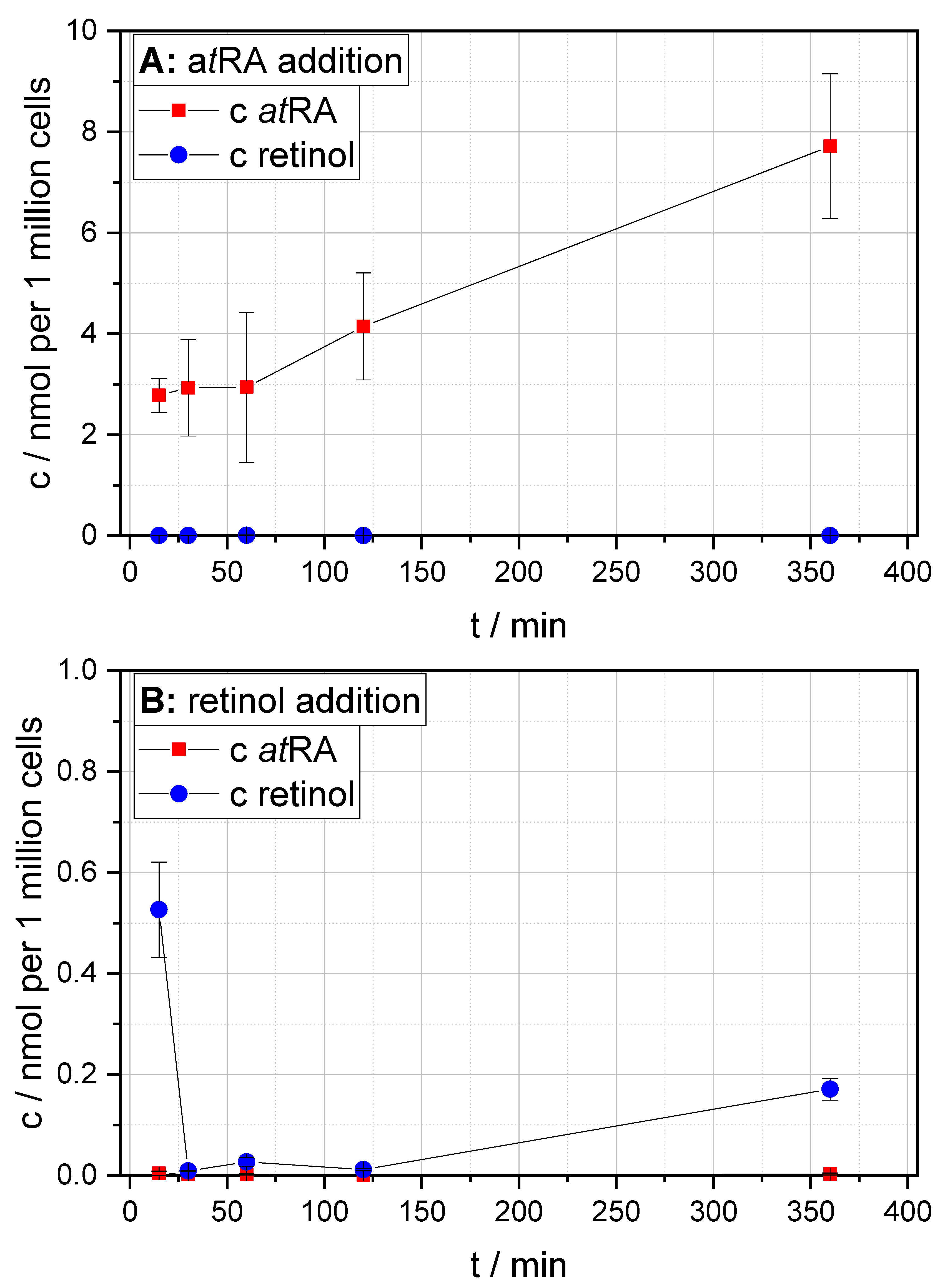
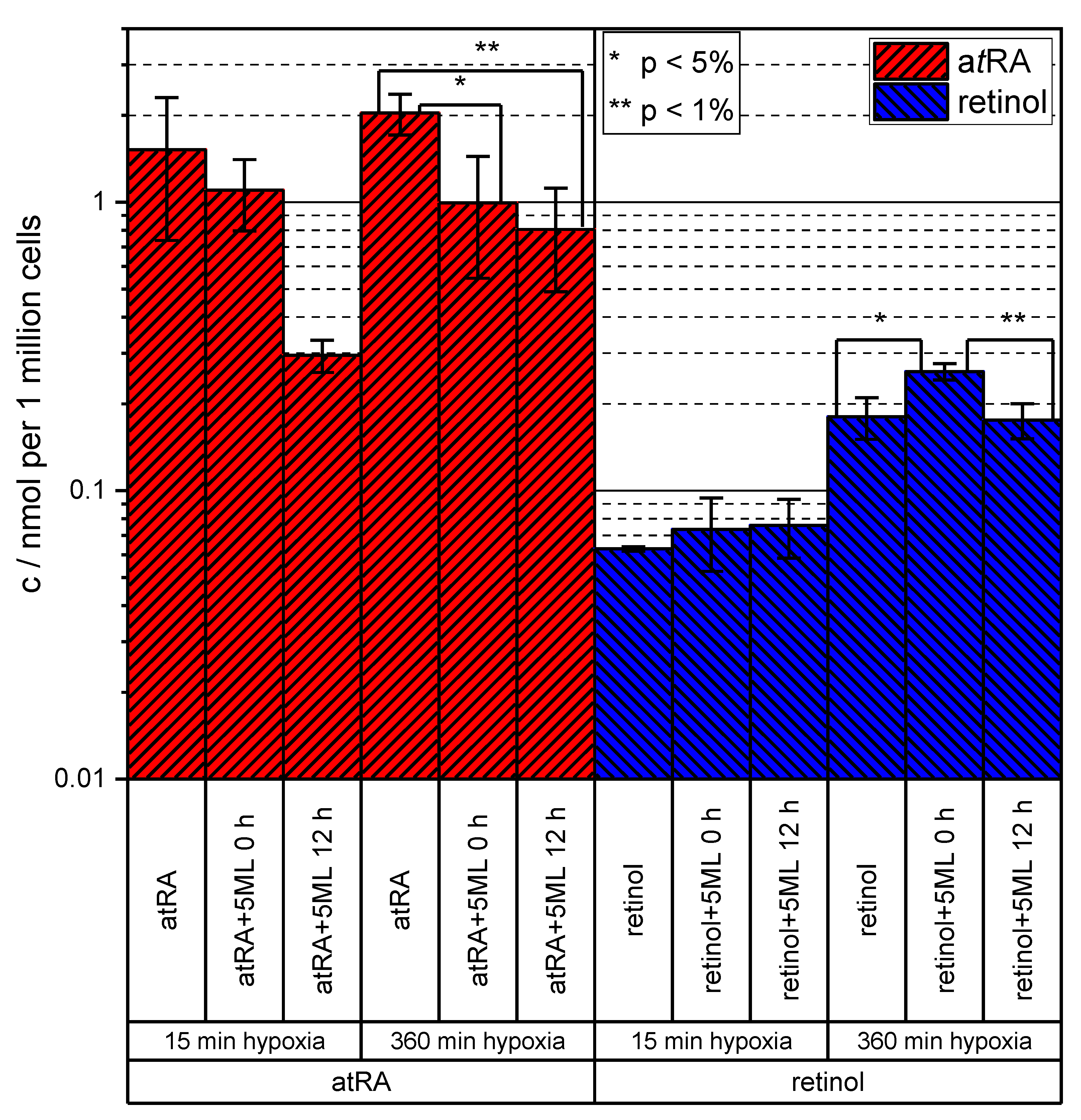
3.3. Results of Targeted Retinoid Analysis in HUVEC Experiments Including 5ML Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Obrochta, K.M.; Kane, M.A.; Napoli, J.L. Effects of diet and strain on mouse serum and tissue retinoid concentrations. PLoS ONE 2014, 9, e99435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilbija, D.; Haugen, F.; Sagave, J.; Baysa, A.; Bastani, N.; Levy, F.O.; Sirsjö, A.; Blomhoff, R.; Valen, G. Retinoic acid signalling is activated in the postischemic heart and may influence remodelling. PLoS ONE 2012, 7, e44740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, F.; Li, L.-S.; Li, R.; Deng, Q.; Yu, Q.-X.; Yang, X.; Pan, M.; Han, J.; Zhen, L.; Zhang, L.-N.; et al. All-trans-retinoid acid induces the differentiation of P19 cells into neurons involved in the PI3K/Akt/GSK3β signaling pathway. J Cell Biochem 2020. [Google Scholar] [CrossRef]
- Pino-Lagos, K.; Benson, M.J.; Noelle, R.J. Retinoic acid in the immune system. Ann. N. Y. Acad. Sci. 2008, 1143, 170–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutierrez Gossweiler, A.; Martinez-Mier, E.A. Chapter 6: Vitamins and Oral Health. Monogr. Oral Sci. 2020, 28, 59–67. [Google Scholar] [CrossRef] [PubMed]
- D’Ambrosio, D.N.; Clugston, R.D.; Blaner, W.S. Vitamin A metabolism: An update. Nutrients 2011, 3, 63–103. [Google Scholar] [CrossRef] [Green Version]
- Sirbu, I.O.; Chiş, A.R.; Moise, A.R. Role of carotenoids and retinoids during heart development. BBA-Mol. Cell Biol. L 2020, 158636. [Google Scholar] [CrossRef]
- Arnold, S.L.M.; Amory, J.K.; Walsh, T.J.; Isoherranen, N. A sensitive and specific method for measurement of multiple retinoids in human serum with UHPLC-MS/MS. J. Lipid Res. 2012, 53, 587–598. [Google Scholar] [CrossRef] [Green Version]
- Idres, N.; Marill, J.; Flexor, M.A.; Chabot, G.G. Activation of retinoic acid receptor-dependent transcription by all-trans-retinoic acid metabolites and isomers. J. Biol. Chem. 2002, 277, 31491–31498. [Google Scholar] [CrossRef] [Green Version]
- Siddikuzzaman; Guruvayoorappan, C.; Berlin Grace, V.M. All trans retinoic acid and cancer. Immunopharm. Immunot. 2011, 33, 241–249. [Google Scholar] [CrossRef]
- Perlmann, T. Retinoid metabolism: A balancing act. Nat. Genet. 2002, 31, 7–8. [Google Scholar] [CrossRef] [PubMed]
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease. Circ. Cardiovasc. Qual. 2019, 12, e005375. [Google Scholar] [CrossRef] [PubMed]
- Müller-Nordhorn, J.; Willich, S.N. Coronary Heart Disease. In International Encyclopedia of Public Health; Elsevier: Amsterdam, The Netherlands, 2017; pp. 159–167. ISBN 9780128037089. [Google Scholar]
- VandeWerf, F. Management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2003, 24, 28–66. [Google Scholar] [CrossRef] [Green Version]
- Danzl, K.; Messner, B.; Doppler, C.; Nebert, C.; Abfalterer, A.; Sakic, A.; Temml, V.; Heinz, K.; Streitwieser, R.; Edelmann, T.; et al. Early inhibition of endothelial retinoid uptake upon myocardial infarction restores cardiac function and prevents cell, tissue, and animal death. J. Mol. Cell Cardiol. 2019, 126, 105–117. [Google Scholar] [CrossRef] [Green Version]
- Bilbija, D.; Elmabsout, A.A.; Sagave, J.; Haugen, F.; Bastani, N.; Dahl, C.P.; Gullestad, L.; Sirsjö, A.; Blomhoff, R.; Valen, G. Expression of retinoic acid target genes in coronary artery disease. Int. J. Mol. Med. 2014, 33, 677–686. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Quadro, L. Reverse-phase high-performance liquid chromatography (HPLC) analysis of retinol and retinyl esters in mouse serum and tissues. Methods Mol. Biol. 2010, 652, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Kane, M.A.; Folias, A.E.; Wang, C.; Napoli, J.L. Quantitative profiling of endogenous retinoic acid in vivo and in vitro by tandem mass spectrometry. Anal. Chem. 2008, 80, 1702–1708. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, T.E.; Bastani, N.E.; Blomhoff, R. Quantitative high-throughput determination of endogenous retinoids in human plasma using triple-stage liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 1176–1186. [Google Scholar] [CrossRef]
- Kane, M.A.; Chen, N.; Sparks, S.; Napoli, J.L. Quantification of endogenous retinoic acid in limited biological samples by LC/MS/MS. Biochem. J. 2005, 388, 363–369. [Google Scholar] [CrossRef] [Green Version]
- Rühl, R. Method to determine 4-oxo-retinoic acids, retinoic acids and retinol in serum and cell extracts by liquid chromatography/diode-array detection atmospheric pressure chemical ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2497–2504. [Google Scholar] [CrossRef]
- Anderson, M. Ensuring biological sample integrity from collection to analysis for LC-MS workflows: Case studies illustrating challenges in clinical trials. Bioanalysis 2019, 11, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Murayama, A.; Suzuki, T.; Matsui, M. Photoisomerization of retinoic acids in ethanol under room light: A warning for cell biological study of geometrical isomers of retinoids. J. Nutr. Sci. Vitaminol. 1997, 43, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Motto, M.G.; Facchine, K.L.; Hamburg, P.F.; Burinsky, D.J.; Dunphy, R.; Oyler, A.R.; Cotter, M.L. Separation and identification of retinoic acid photo-isomers. J. Chromatogr. A 1989, 481, 255–262. [Google Scholar] [CrossRef]
- Jensen, N.H.; Wilbrandt, R.; Bensasson, R.V. Sensitized photoisomerization of all-trans- and 11-cis-retinal. J. Am. Chem. Soc. 1989, 111, 7877–7888. [Google Scholar] [CrossRef]
- Ioele, G.; Cione, E.; Risoli, A.; Genchi, G.; Ragno, G. Accelerated photostability study of tretinoin and isotretinoin in liposome formulations. Int. J. Pharm. 2005, 293, 251–260. [Google Scholar] [CrossRef]
- Kim, J.E.; Tauber, M.J.; Mathies, R.A. Wavelength dependent cis-trans isomerization in vision. Biochemistry 2001, 40, 13774–13778. [Google Scholar] [CrossRef]
- Schwaiger, S.; Adams, M.; Seger, C.; Ellmerer, E.P.; Bauer, R.; Stuppner, H. New constituents of Leontopodium alpinum and their in vitro leukotriene biosynthesis inhibitory activity. Planta Med. 2004, 70, 978–985. [Google Scholar] [CrossRef]
- Wawrosch, C.; Schwaiger, S.; Stuppner, H.; Kopp, B. Lignan formation in hairy root cultures of Edelweiss (Leontopodium nivale ssp. alpinum (Cass.) Greuter). Fitoterapia 2014, 97, 219–223. [Google Scholar] [CrossRef] [Green Version]
- Morrison, K.A.; Clowers, B.H. Fundamentals and applications of incorporating chromatographic separations with ion mobility-mass spectrometry. TRAC-Trend Anal. Chem. 2019, 119, 115625. [Google Scholar] [CrossRef]
- Bull, J.N.; West, C.W.; Anstöter, C.S.; da Silva, G.; Bieske, E.J.; Verlet, J.R.R. Ultrafast photoisomerisation of an isolated retinoid. Phys. Chem. Chem. Phys. 2019, 21, 10567–10579. [Google Scholar] [CrossRef] [PubMed]
- Dilger, J.; Musbat, L.; Sheves, M.; Bochenkova, A.V.; Clemmer, D.E.; Toker, Y. Direct Measurement of the Isomerization Barrier of the Isolated Retinal Chromophore. Angew. Chem. 2015, 127, 4830–4834. [Google Scholar] [CrossRef]
- Toker, Y.; Rahbek, D.B.; Kiefer, H.V.; Rajput, J.; Antoine, R.; Dugourd, P.; Brøndsted Nielsen, S.; Bochenkova, A.V.; Andersen, L.H. Photoresponse of the protonated Schiff-base retinal chromophore in the gas phase. Phys. Chem. Chem. Phys. 2013, 15, 19566–19569. [Google Scholar] [CrossRef] [PubMed]
- Guntner, A.S.; Thalhamer, B.; Klampfl, C.; Buchberger, W. Collision cross sections obtained with ion mobility mass spectrometry as new descriptor to predict blood-brain barrier permeation by drugs. Sci. Rep. 2019, 9, 19182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Persico, M.; Granucci, G. Photochemistry. A Modern Theoretical Perspective; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-89971-8. [Google Scholar]
- Engineering ToolBox. Illuminance—Recommended Light Level. Available online: https://www.engineeringtoolbox.com/light-level-rooms-d_708.html (accessed on 28 April 2020).
- Aphalo, P.J. Photon Emission Spectra. Available online: https://www.mv.helsinki.fi/home/aphalo/photobio/lamps.html (accessed on 28 April 2020).
- Furr, H.C. Analysis of retinoids and carotenoids: Problems resolved and unsolved. J. Nutr. 2004, 134, 281S–285S. [Google Scholar] [CrossRef] [PubMed]


| Compound | Precursor Ion m/z | Product Ion m/z | Dwell Time/ms | Fragmentor Voltage/V | Collision Energy/eV |
|---|---|---|---|---|---|
| 5ML | 523.33 | 423.2 | 150 | 106 | 18 |
| 181.1 | 34 | ||||
| 151.1 | 50 | ||||
| leoligin | 493.2 | 393.2 | 150 | 162 | 14 |
| 151.0 | 22 | ||||
| 107.0 | 90 | ||||
| atRA | 301.22 | 205.0 | 150 | 76 | 10 |
| 123.1 | 18 | ||||
| 91.0 | 58 | ||||
| retinal | 285.2 | 161.1 | 250 | 102 | 10 |
| 105.1 | 34 | ||||
| 91.1 | 58 | ||||
| retinol | 269.3 | 119.2 | 150 | 114 | 18 |
| 93.0 | 26 | ||||
| 78.9 | 26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guntner, A.S.; Doppler, C.; Wechselberger, C.; Bernhard, D.; Buchberger, W. HPLC-MS/MS Shows That the Cellular Uptake of All-Trans-Retinoic Acid under Hypoxia Is Downregulated by the Novel Active Agent 5-Methoxyleoligin. Cells 2020, 9, 2048. https://doi.org/10.3390/cells9092048
Guntner AS, Doppler C, Wechselberger C, Bernhard D, Buchberger W. HPLC-MS/MS Shows That the Cellular Uptake of All-Trans-Retinoic Acid under Hypoxia Is Downregulated by the Novel Active Agent 5-Methoxyleoligin. Cells. 2020; 9(9):2048. https://doi.org/10.3390/cells9092048
Chicago/Turabian StyleGuntner, Armin Sebastian, Christian Doppler, Christian Wechselberger, David Bernhard, and Wolfgang Buchberger. 2020. "HPLC-MS/MS Shows That the Cellular Uptake of All-Trans-Retinoic Acid under Hypoxia Is Downregulated by the Novel Active Agent 5-Methoxyleoligin" Cells 9, no. 9: 2048. https://doi.org/10.3390/cells9092048






