Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Collagen-Induced Arthritis Model
2.3. Preparation of BM-Derived MSCs
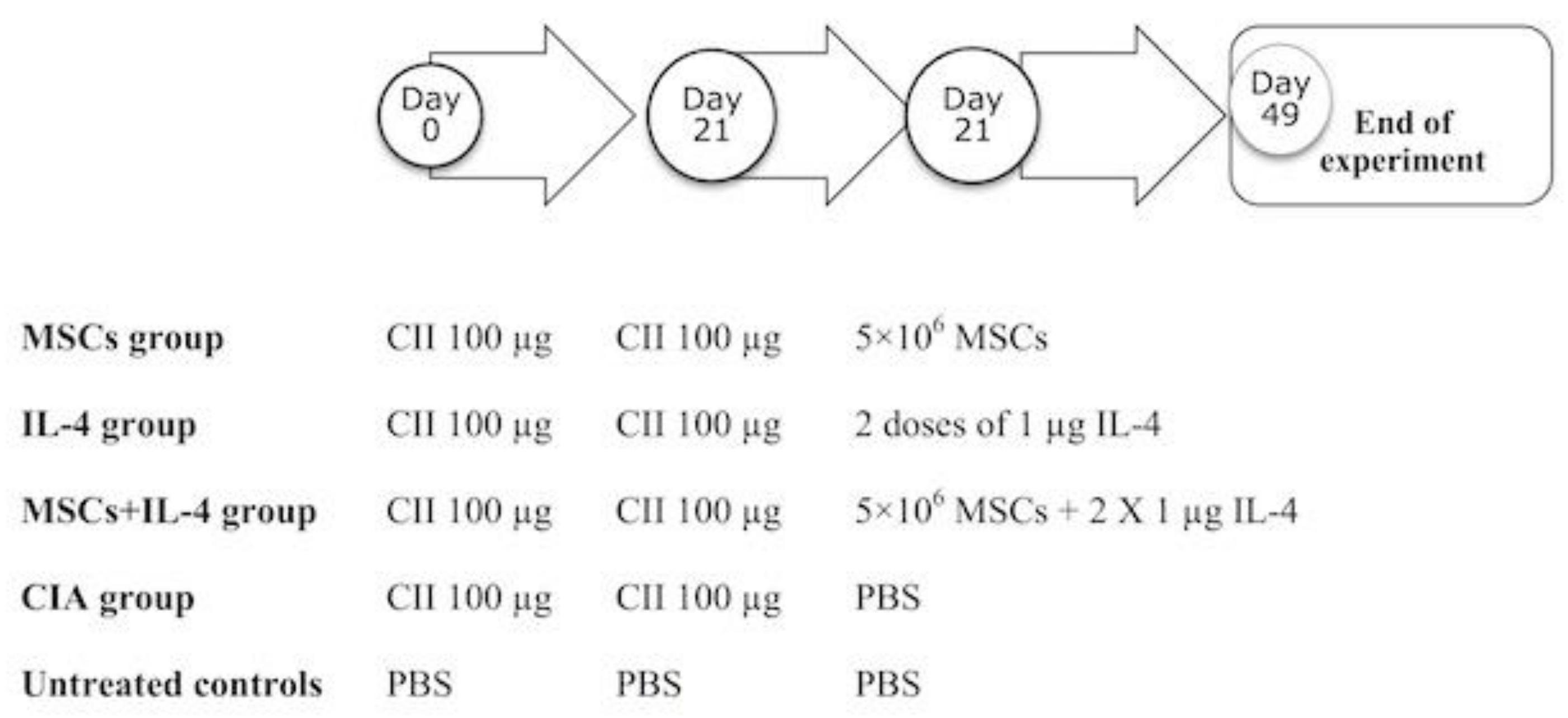
2.4. Experimental Design
2.5. Biochemical Analysis of Rheumatoid Markers
2.6. RNA Isolation and Reverse Transcription
2.7. Quantitative Real-Time PCR
2.8. Analysis of Knee Joint Histopathology
2.9. Statistical Analysis
3. Results
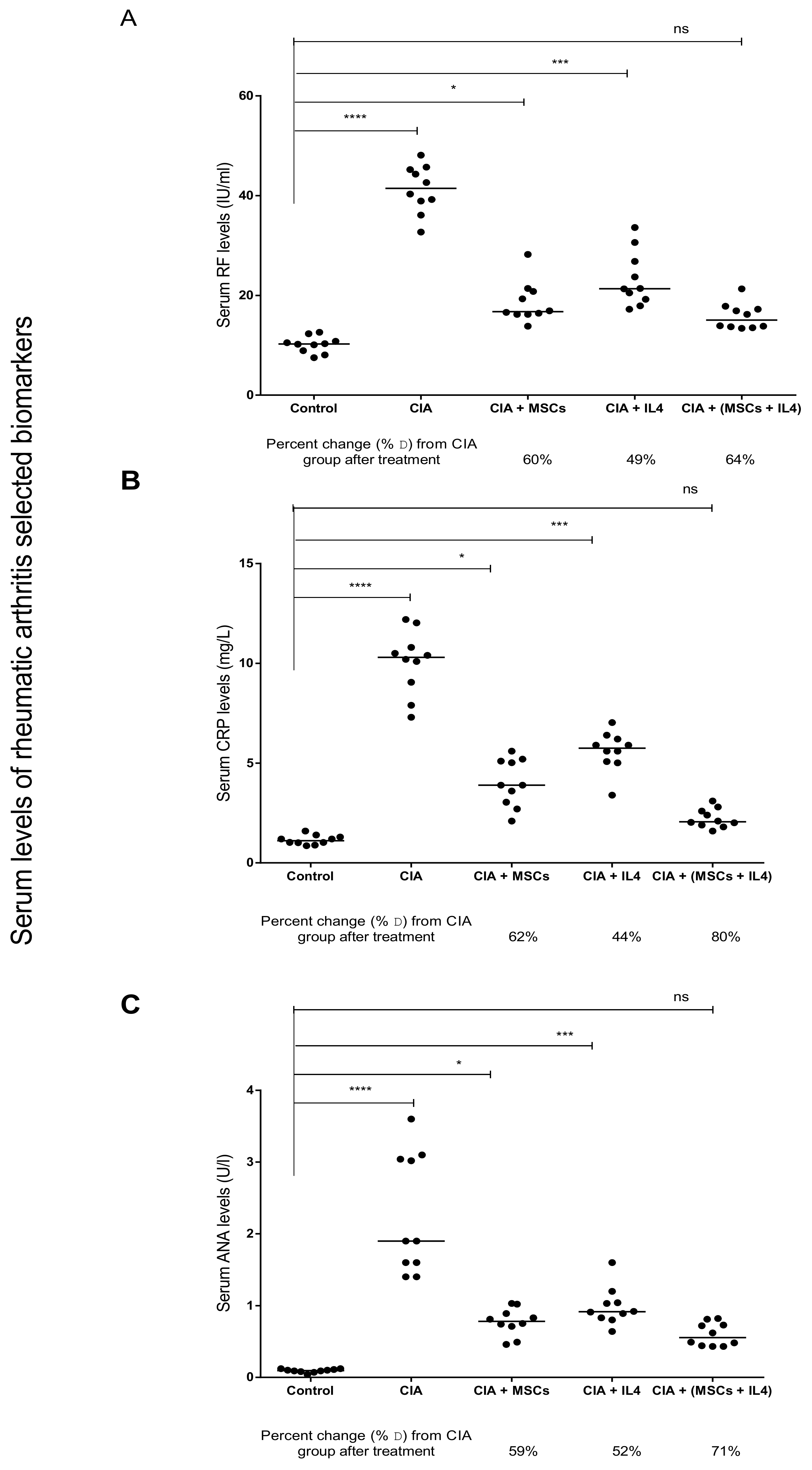
3.1. Combined MSCs and IL-4 Treatment Showed Best Improvement in Biochemical Markers of RA
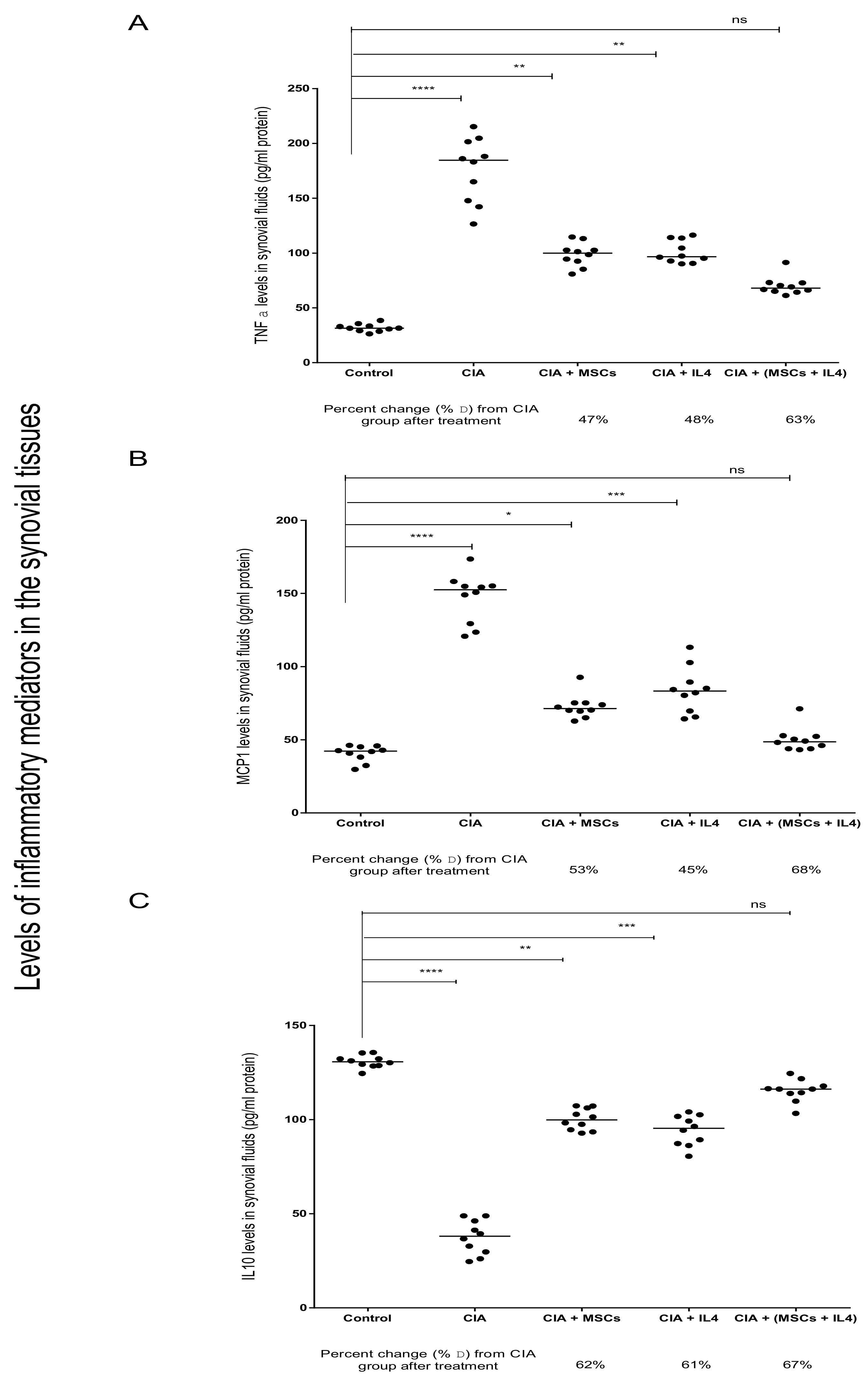
3.2. Combined MSCs and IL-4 Attenuated RA via Anti-Inflammatory Action
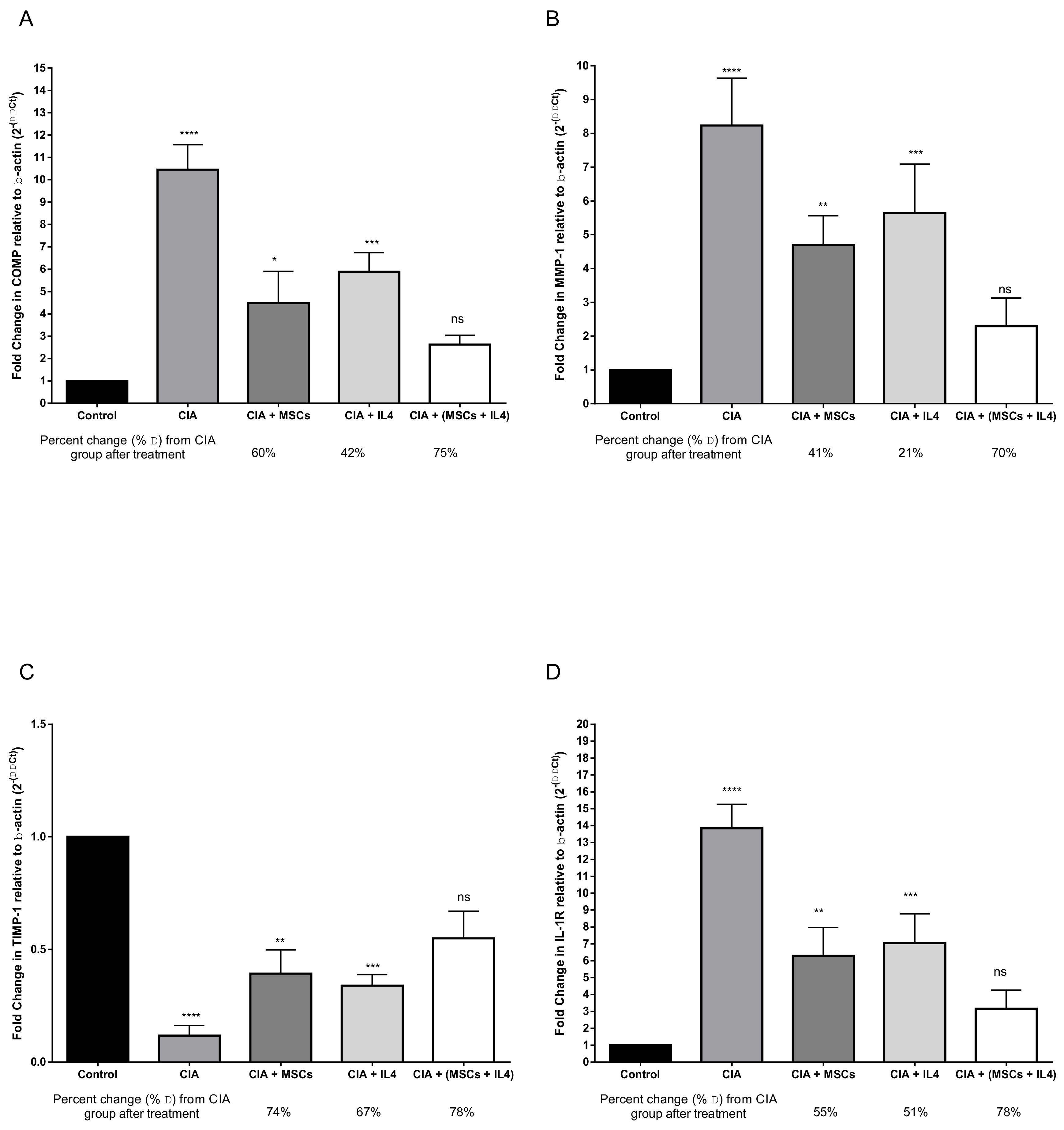
3.3. Combined MSCs and IL-4 Attenuated Articular Cartilage Degradation and Positively Affected Connective Tissue Remodeling of Synovial Tissue
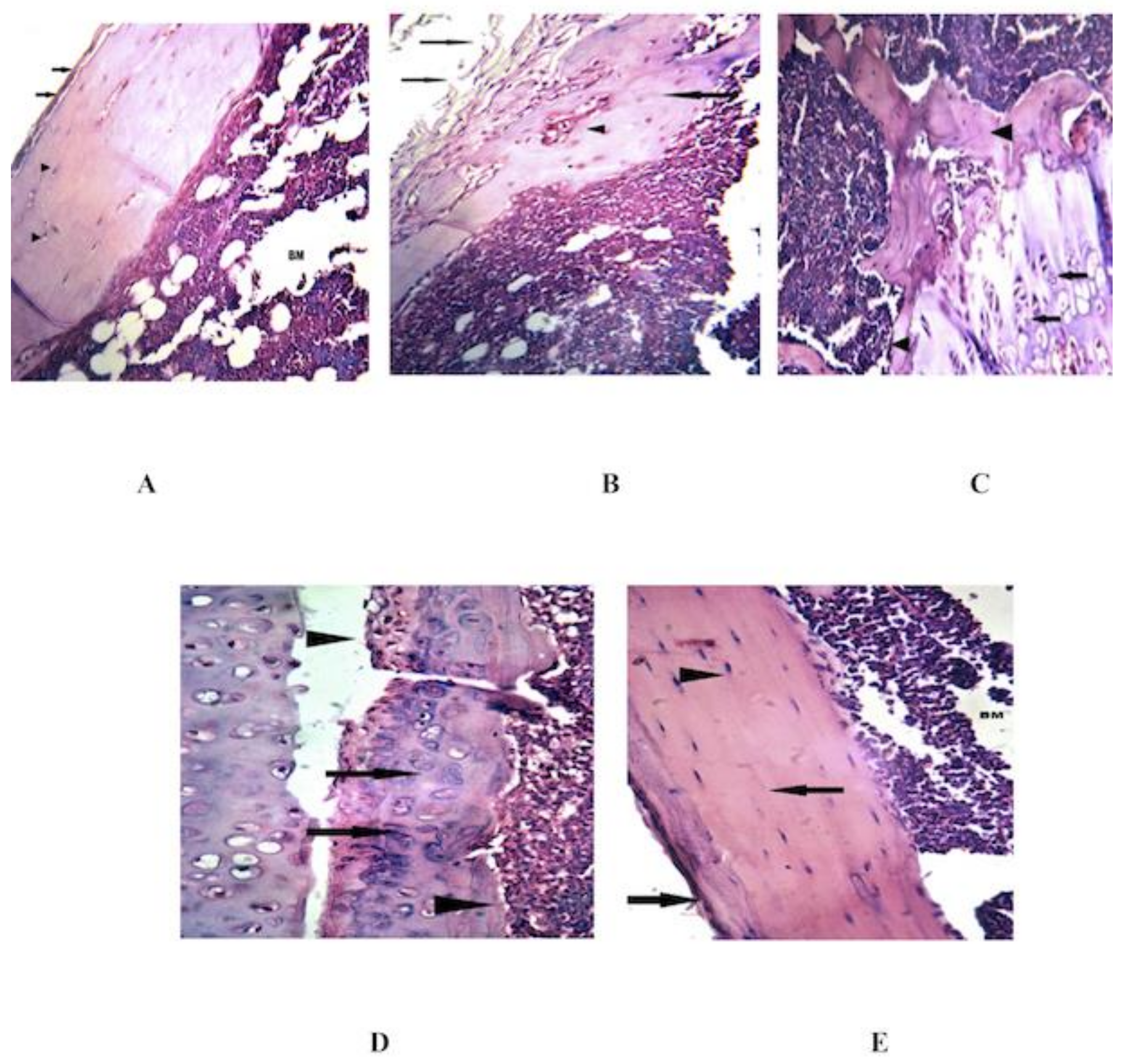
3.4. Combined MSCs and IL-4 Improve the Histopathological Changes in the Knee Joint
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhou, B.; Yuan, J.; Zhou, Y.; Ghawji, M.; Deng, Y.P.; Lee, A.J.; Yoo, T.J. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin. Immunol. 2011, 141, 328–337. [Google Scholar] [CrossRef] [PubMed]
- González, M.A.; Gonzalez_Rey, E.; Rico, L.; Büscher, D.; Delgado, M. Treatment of experimental arthritis by inducing immune tolerance with human adipose-derived mesenchymal stem cells. Arthritis Rheum. 2009, 60, 1006–1019. [Google Scholar] [CrossRef] [PubMed]
- Luque-Campos, N.; Contreras-López, R.A.; Paredes-Martínez, M.J.; Torres, M.J.; Bahraoui, S.; Wei, M.; Luz-Crawford, P. Mesenchymal stem cells improve rheumatoid arthritis progression by controlling memory T cell response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yuan, Q.; Xie, L. Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Satija, N.K.; Singh, V.K.; Verma, Y.K.; Gupta, P.; Sharma, S.; Afrin, F.; Gurudutta, G.U. Mesenchymal stem cell-based therapy: A new paradigm in regenerative medicine. J. Cell. Mol. Med. 2009, 13, 4385–4402. [Google Scholar] [CrossRef]
- Ben-Ami, E.; Berrih-Aknin, S.; Miller, A. Mesenchymal stem cells as an immunomodulatory therapeutic strategy for autoimmune diseases. Autoimmun. Rev. 2011, 10, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef]
- Finnegan, A.; Grusby, M.J.; Kaplan, C.D.; O’Neill, S.K.; Eibel, H.; Koreny, T.; Czipri, M.; Mikecz, K.; Zhang, J. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J. Immunol. 2002, 169, 3345–3352. [Google Scholar] [CrossRef]
- Cao, Y.; Brombacher, F.; Tunyogi-Csapo, M.; Glant, T.T.; Finnegan, A. Interleukin-4 regulates proteoglycan-induced arthritis by specifically suppressing the innate immune response. Arthritis Rheum. 2007, 56, 861–870. [Google Scholar] [CrossRef]
- Lubberts, E.; Joosten, L.A.; Van Den Bersselaar, L.; Helsen, M.M.; Bakker, A.C.; Van Meurs, J.B.; Graham, F.L.; Richards, C.D.; van den Berg, W.B. Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. J. Immunol. 1999, 163, 4546–4556. [Google Scholar]
- Lubberts, E.; Joosten, L.A.; Chabaud, M.; van den Bersselaar, L.; Oppers, B.; Coenen-de Roo, C.J.; Richards, C.D.; Miossec, P.; van den Berg, W.B. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J. Clin. Investig. 2000, 105, 1697–1710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemmerle, T.; Doll, F.; Neri, D. Antibody-based delivery of IL4 to the neovasculature cures mice with arthritis. Proc. Natl. Acad. Sci. USA 2014, 111, 12008–12012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.; Qiu, M.; Chen, J. Il-4 modulates macrophage polarization in ankylosing spondylitis. Cell. Physiol. Biochem. 2015, 35, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Augello, A.; Tasso, R.; Negrini, S.M.; Cancedda, R.; Pennesi, G. Cell therapy using allogeneic bone marrow mesenchymal stem cells prevents tissue damage in collagen-induced arthritis. Arthritis Rheum. 2017, 56, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.H.; Del Prete, A.; Bock, C.B.; Haribabu, B. Targeted disruption of leukotriene B4 receptors BLT1 and BLT2: A critical role for BLT1 in collagen-induced arthritis in mice. J. Immunol. 2006, 176, 6254–6261. [Google Scholar] [CrossRef]
- El-denshary, E.S.M.; Rashed, L.A.; Elhussiny, M. Mesenchymal stromal cells versus betamethasone can dampen disease activity in the collagen arthritis mouse model. Clin. Exp. Med. 2014, 14, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Alhadlaq, A.; Mao, J.J. Mesenchymal stem cells: Isolation and therapeutics. Stem Cells Dev. 2004, 13, 436–448. [Google Scholar] [CrossRef]
- Maccario, R.; Podestà, M.; Moretta, A.; Cometa, A.; Comoli, P.; Montagna, D.; Frassoni, F.; Locatelli, F. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005, 90, 516–525. [Google Scholar]
- Joosten, L.A.; Lubberts, E.; Helsen, M.M.; Saxne, T.; Coenen-de Roo, C.J.; Heinegård, D.; van den Berg, W.B. Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis. Arthritis Res. Ther. 1999, 1, 1. [Google Scholar]
- Zhao, C.; Zhang, L.; Kong, W.; Liang, J.; Xu, X.; Wu, H.; Sun, L. Umbilical Cord-Derived Mesenchymal Stem Cells Inhibit Cadherin-11 Expression by Fibroblast-Like Synoviocytes in Rheumatoid Arthritis. J. Immunol. Res. 2015. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef]
- Simon, J.; Surber, R.; Kleinstäuber, G.; Petrow, P.K.; Henzgen, S.; Kinne, R.W.; Bräuer, R. Systemic macrophage activation in locally-induced experimental arthritis. J. Autoimmun. 2001, 17, 127–136. [Google Scholar] [CrossRef]
- Fournier, C. Where do T cells stand in rheumatoid arthritis? Jt. Bone Spine 2005, 72, 527–532. [Google Scholar] [CrossRef]
- Kannan, K.; Ortmann, R.A.; Kimpel, D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 2005, 12, 167–181. [Google Scholar] [CrossRef]
- Wooley, P.H. The usefulness and the limitations of animal models in identifying targets for therapy in arthritis. Best Pract. Res. Clin. Rheumatol. 2004, 18, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Hegen, M.; Keith, J.C.; Collins, M.; Nickerson-Nutter, C.L. Utility of animal models for identification of potential therapeutics for rheumatoid arthritis. Ann. Rheum. Dis. 2008, 67, 1505–1515. [Google Scholar] [CrossRef]
- Baddack, U.; Hartmann, S.; Bang, H.; Grobe, J.; Loddenkemper, C.; Lipp, M.; Müller, G. A chronic model of arthritis supported by a strain-specific periarticular lymph node in BALB/c mice. Nat. Commun. 2013, 4, 1644. [Google Scholar] [CrossRef] [Green Version]
- Ghannam, S.; Bouffi, C.; Djouad, F.; Jorgensen, C.; Noël, D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010, 1, 2. [Google Scholar] [CrossRef]
- Roberts, S.; Genever, P.; McCaskie, A.; De Bari, C. Prospects of stem cell therapy in osteoarthritis. Regen. Med. 2011, 6, 351–366. [Google Scholar] [CrossRef] [Green Version]
- De Bari, C. Are mesenchymal stem cells in rheumatoid arthritis the good or bad guys? Arthritis Res. Ther. 2015, 17, 113. [Google Scholar] [CrossRef]
- Lee, K.; Park, N.; Jung, H.; Rim, Y.A.; Nam, Y.; Lee, J.; Park, S.; Ju, J.H. Mesenchymal stem cells ameliorate experimental arthritis via expression of interleukin-1 receptor antagonist. PLoS ONE 2018, 13, e0193086. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Jung, S.M.; Rim, Y.A.; Jung, H.; Lee, K.; Park, N.; Ju, J.H. Intraperitoneal infusion of mesenchymal stem cell attenuates severity of collagen antibody induced arthritis. PLoS ONE 2018, 13, e0198740. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.; Rivas, M.D.; Perez, G. Interleukin-4: A multifunctional cytokine. Immunologia 2003, 22, 215–224. [Google Scholar]
- Newkirk, M.M. Rheumatoid factors: Host resistance or autoimmunity? Clin. Immunol. 2002, 104, 1–13. [Google Scholar] [CrossRef]
- Machold, K.P.; Stamm, T.A.; Nell, V.P.K.; Pflugbeil, S.; Aletaha, D.; Steiner, G.; Smolen, J.S. Very recent onset rheumatoid arthritis: Clinical and serological patient characteristics associated with radiographic progression over the first years of disease. Rheumatology 2007, 46, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Loyer, P.; Ilyin, G.; Razzak, Z.A.; Banchereau, J.; Dezier, J.F.; Campion, J.P.; Guillouzo, A. Interleukin 4 inhibits the production of some acute-phase proteins by human hepatocytes in primary culture. FEBS Lett. 1993, 336, 215–220. [Google Scholar] [CrossRef] [Green Version]
- Walravens, M. Systemic diseases and the detection of antinuclear and anticytoplasmic antibodies. An hystorical review. Clin. Rheumatol. 1987, 6, 9–17. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef]
- Derksen, V.; Huizinga, T.; van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef]
- Khandpur, R.; Carmona-Rivera, C.; Vivekanandan-Giri, A.; Gizinski, A.; Yalavarthi, S.; Knight, J.S.; Friday, S.; Li, S.; Patel, R.M.; Subramanian, V.; et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 2013, 5, 178ra40. [Google Scholar] [CrossRef]
- Hecht, C.; Englbrecht, M.; Rech, J.; Schmidt, S.; Araujo, E.; Engelke, K.; Finzel, S.; Schett, G. Additive effect of anti-citrullinated protein antibodies and rheumatoid factor on bone erosions in patients with RA. Ann. Rheum. Dis. 2015, 74, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
- Van Steenbergen, H.W.; Ajeganova, S.; Forslind, K.; Svensson, B.; Van Der Helm-van Mil, A.H.M. The effects of rheumatoid factor and anticitrullinated peptide antibodies on bone erosions in rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, e3. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Yu, X.P.; Zhang, Y.; Tian, Q.; Song, H.; Mucignat, M.T.; Liu, C.; Greenberg, J.D. Enhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISA. Osteoarthr. Cartil. 2012, 20, 854–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mündermann, A.; Dyrby, C.O.; Andriacchi, T.P.; King, K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthr. Cartil. 2005, 13, 34–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincenti, M.P. The matrix metalloproteinase (MMP) and tissue inhibitor of metalloproteinase (TIMP) genes. In Matrix Metalloproteinase Protocols; Methods in Molecular Biology, vol 151; Humana Press: New York, NY, USA, 2001; pp. 121–148. [Google Scholar]
- Konttinen, Y.T.; Ainola, M.; Valleala, H.; Ma, J.; Ida, H.; Mandelin, J.; Takagi, M. Analysis of 16 different matrix metalloproteinases (MMP-1 to MMP-20) in the synovial membrane: Different profiles in trauma and rheumatoid arthritis. Ann. Rheum. Dis. 1999, 58, 691–697. [Google Scholar] [CrossRef]
- Kim, W.S.; Park, B.S.; Sung, J.H.; Yang, J.M.; Park, S.B.; Kwak, S.J.; Park, J.S. Wound healing effect of adipose-derived stem cells: A critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 2007, 48, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, A.; Cortivo, R.; Fregona, I.; Plebani, M.; Secchi, A.G.; Abatangelo, G. Effects of Th2 cytokines on expression of collagen, MMP-1, and TIMP-1 in conjunctival fibroblasts. Investig. Ophthalmol. Vis. Sci. 2003, 44, 183–189. [Google Scholar] [CrossRef]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [CrossRef]
- Bokarewa, M.; Dahlberg, L.; Tarkowski, A. Expression and functional properties of antibodies to tissue inhibitors of metalloproteinases (TIMPs) in rheumatoid arthritis. Arthritis Res. Ther. 2005, 7, R1014. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Nakamura, H.; Obata, K.I.; Yamada, H.; Hayakawa, T.; Fujikawa, K.; Okada, Y. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in synovial fluids from patients with rheumatoid arthritis or osteoarthritis. Ann. Rheum. Dis. 2000, 59, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Tondreau, T.; Meuleman, N.; Stamatopoulos, B.; De Bruyn, C.; Delforge, A.; Dejeneffe, M.; Lagneaux, L. In vitro study of matrix metalloproteinase/tissue inhibitor of metalloproteinase production by mesenchymal stromal cells in response to inflammatory cytokines: The role of their migration in injured tissues. Cytotherapy 2009, 11, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Kuno, K.; Matsushima, K. The IL-1 receptor signaling pathway. J. Leukoc. Biol. 1994, 56, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Arend, W.P. Interleukin 1 receptor antagonist. A new member of the interleukin 1 family. J. Clin. Investig. 1991, 88, 1445. [Google Scholar] [CrossRef] [PubMed]
- Fujikado, N.; Saijo, S.; Iwakura, Y. Identification of arthritis-related gene clusters by microarray analysis of two independent mouse models for rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R100. [Google Scholar] [CrossRef] [PubMed]
- Woods, J.M.; Katschke, K.J.; Volin, M.V.; Ruth, J.H.; Woodruff, D.C.; Amin, M.A.; Kumar, P.; Koch, A.E. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J. Immunol. 2001, 166, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Chomarat, P.; Vannier, E.; Dechanet, J.; Rissoan, M.C.; Banchereau, J.; Dinarello, C.A.; Miossec, P. Balance of IL-1 receptor antagonist/IL-1 beta in rheumatoid synovium and its regulation by IL-4 and IL-10. J. Immunol. 1995, 154, 1432–1439. [Google Scholar] [PubMed]
- Ouyang, W.; Rutz, S.; Crellin, N.K.; Valdez, P.A.; Hymowitz, S.G. Regulation and functions of the IL-10 family of cytokines in inflammation and disease. Annu. Rev. Immunol. 2011, 29, 71–109. [Google Scholar] [CrossRef] [PubMed]
- Kambayashi, T.; Jacob, C.O.; Strassmann, G. IL-4 and IL-13 modulate IL-10 release in endotoxin-stimulated murine peritoneal mononuclear phagocytes. Cell. Immunol. 1996, 171, 153–158. [Google Scholar] [CrossRef]
- Idriss, H.T.; Naismith, J.H. TNF and the TNF receptor superfamily: Structure_function relationship (s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Wooley, P.H.; Luthra, H.S.; Stuart, J.M.; David, C.S. Type II collagen-induced arthritis in mice. I. Major histocompatibility complex (I region) linkage and antibody correlates. J. Exp. Med. 1981, 154, 688–700. [Google Scholar] [CrossRef]
- Mao, F.; Xu, W.R.; Qian, H.; Zhu, W.; Yan, Y.M.; Shao, Q.X.; Xu, H.X. Immunosuppressive effects of mesenchymal stem cells in collagen-induced mouse arthritis. Inflamm. Res. 2010, 59, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.; Evans, C.H.; Ghivizzani, S.C.; Oligino, T.; Robbins, P.D. Effective treatment of established murine collagen-induced arthritis by systemic administration of dendritic cells genetically modified to express IL-4. J. Immunol. 2001, 166, 3499–3505. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Sequence | |
|---|---|---|
| Forward Primer | Reverse Primer | |
| Comp1 | 5′- ATGGTCTTACGGGGAGATGCC -3′ | 5′- GAGAGGTTTCTGGAGCCTTTTGG -3′ |
| Mmp1 | 5′- TTGTTGCTGCCCATGAGCTT -3′ | 5′- ACTTTGTCGCCAATTCCAGG -3′ |
| Timp1 | 5′- GCATCTGGCATCCTCTTGTT-3′ | 5′- TGGGGAACCCATGAATTTAG-3′ |
| Il1r | 5′- TGAGGTCTTGGAGGGACAGT -3′ | 5′- TGGCCCAACATGACTAAGGG -3′ |
| β-actin | 5′- ACTGCCGCATCCTCTTCCTC – 3′ | 5′- ACTCCTGCTTGCTGATCCACAT -3′ |
| Histopathological Alterations | Groups | ||||
|---|---|---|---|---|---|
| Healthy Control | CIA | MSCs | IL-4 | MSCs+ IL-4 | |
| Hyperplasia of synovial lining layer | 0 | 3 | 0 | 0 | 0 |
| Infiltration of leukocytes into synovial membrane | 0 | 3 | 1 | 1 | 0 |
| Pannus formation | 0 | 2 | 0 | 1 | 0 |
| Necrosis/erosion of cartilage | 0 | 3 | 0 | 1 | 0 |
| Final Arthritis Score | 0 | 11 | 1 | 3 | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haikal, S.M.; Abdeltawab, N.F.; Rashed, L.A.; Abd El-Galil, T.I.; Elmalt, H.A.; Amin, M.A. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells 2019, 8, 823. https://doi.org/10.3390/cells8080823
Haikal SM, Abdeltawab NF, Rashed LA, Abd El-Galil TI, Elmalt HA, Amin MA. Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model. Cells. 2019; 8(8):823. https://doi.org/10.3390/cells8080823
Chicago/Turabian StyleHaikal, Shaimaa M., Nourtan F. Abdeltawab, Laila A. Rashed, Tarek I. Abd El-Galil, Heba A. Elmalt, and Magdy A. Amin. 2019. "Combination Therapy of Mesenchymal Stromal Cells and Interleukin-4 Attenuates Rheumatoid Arthritis in a Collagen-Induced Murine Model" Cells 8, no. 8: 823. https://doi.org/10.3390/cells8080823






