Electrophysiological Phenotyping of hiPSC-Derived Atrial Cardiomyocytes Using Automated Patch-Clamp: A Platform for Studying Atrial Inherited Arrhythmias
Highlights
- Developed an optimized dissociation and recording protocol enabling reliable automated patch-clamp recordings of major atrial ionic currents (INa, ICaL, Ito, IKur, ISK, and If) in hiPSC-derived atrial cardiomyocytes.
- Demonstrated that current profiles obtained with the automated Patchliner system resemble those of native human atrial cardiomyocytes, validating the physiological relevance of the model.
- The optimized automated patch-clamp workflow provides a robust platform for the functional characterization of ion channels and genetic variants implicated in atrial arrhythmias.
- This approach facilitates precision medicine applications and targeted drug development for atrial channelopathies.
Abstract
1. Introduction
2. Materials and Methods
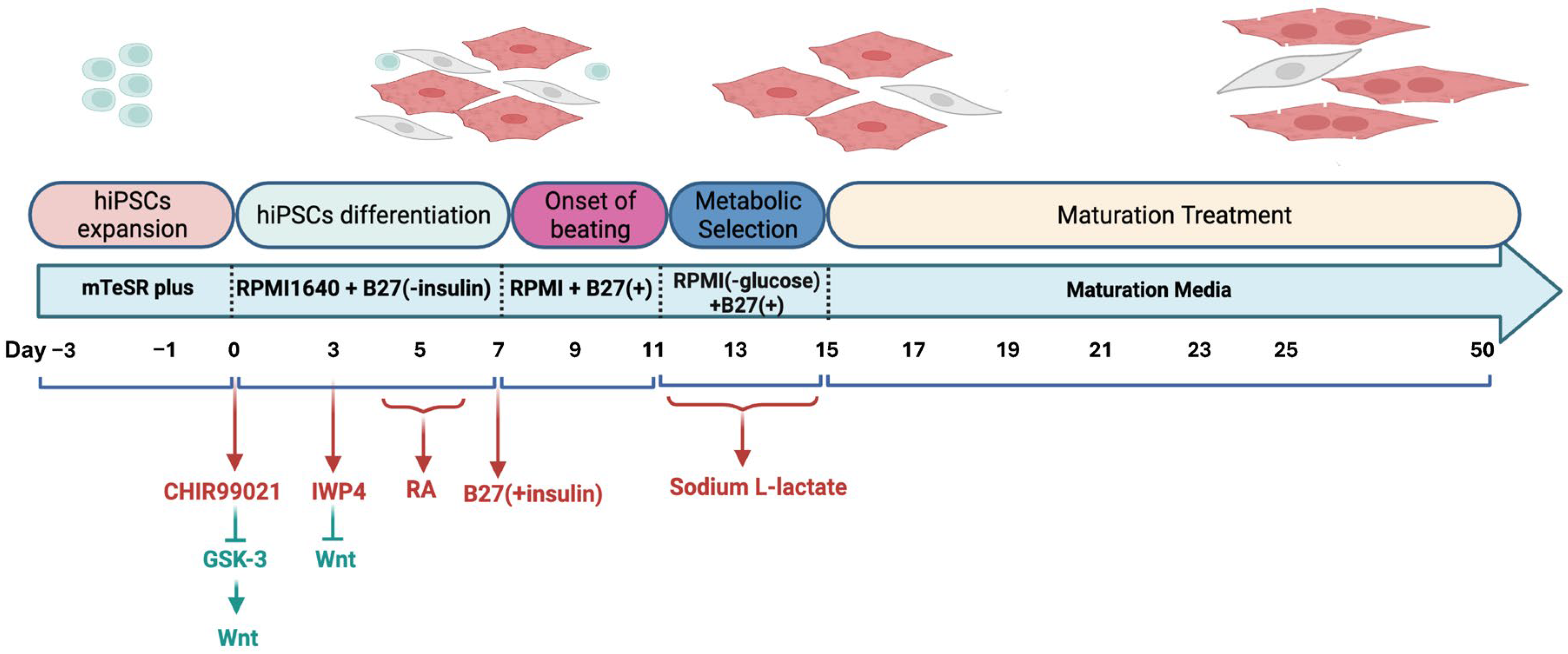
2.1. Differentiation of hiPSC into Atrial Cardiomyocytes
2.2. Preparation of hiPSC-aCMs for Automated Patch-Clamp Experiments
2.3. Automated Patch-Clamp Technique
2.3.1. Solutions and Specific Protocols Used for Electrophysiological Recordings
2.3.2. Measurement of Na+ Current
2.3.3. Measurement of L-Type Calcium Current
2.3.4. Measurement of Major Atrial Potassium Repolarizing Currents
2.3.5. Measurement of Small-Conductance Calcium-Activated Potassium Current
2.3.6. Measurement of Funny Current
2.4. Data Analysis
2.4.1. Statistical Analysis
2.4.2. Mathematical Analysis
3. Results
3.1. Sodium Current Characterization in hiPSC-aCMs
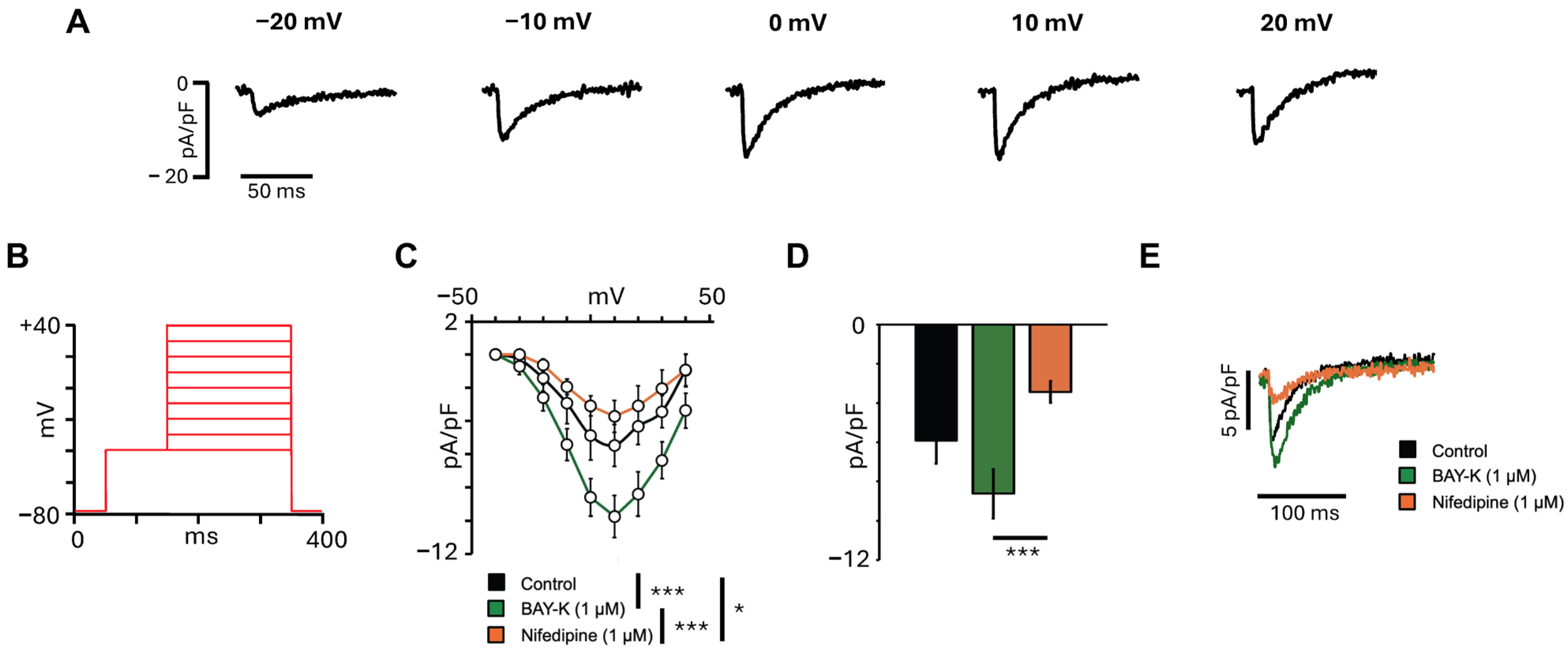
3.2. L-Type Calcium Current Characterization in hiPSC-aCMs
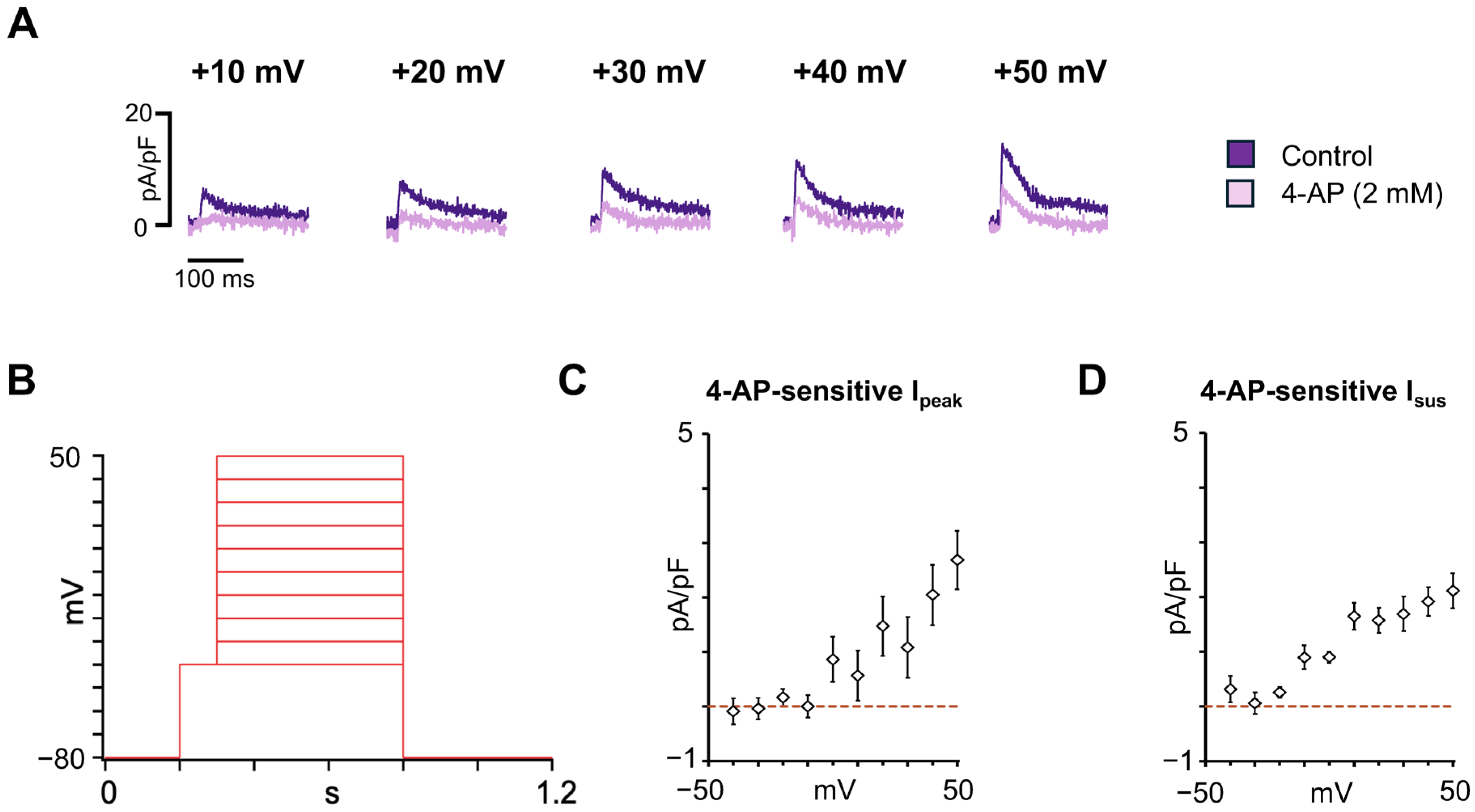
3.3. Characterization of the Major Atrial K+ Repolarizing Currents in hiPSC-aCMs
3.4. Apamin-Sensitive SK Current Characterization in hiPSC-aCMs
3.5. Funny Current Characterization in hiPSC-aCMs
4. Discussion
5. Conclusions and Clinical Relevance
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-AP | 4-aminopyridine |
| AF | Atrial fibrillation |
| AP | Action potential |
| APD | Action potential duration |
| cTnT | Cardiac troponin T |
| CPVT | Catecholaminergic polymorphic ventricular tachycardia |
| DAD | Delayed afterdepolarization |
| ENa | Sodium equilibrium potential |
| HCN | Hyperpolarization-activated cyclic nucleotide-gated |
| hiPSC-aCMs | Human-induced pluripotent stem cell-derived atrial cardiomyocytes |
| hiPSC-CMs | Human-induced pluripotent stem cell-derived cardiomyocytes |
| hiPSC-vCMs | Human-induced pluripotent stem cell-derived ventricular cardiomyocytes |
| ICaL | L-type calcium current |
| If | Funny current |
| IKur | Ultrarapid component of the delayed rectifier current |
| INa | Sodium current |
| ISK | Small-conductance calcium-activated potassium current |
| Ito | Transient outward potassium current |
| LQTS | Long QT syndrome |
| MACS | Magnetic-activated cell sorting |
| MLC2a | Myosin light chain 2a |
| SAN | Sinoatrial node |
| Vrev | Reversal potential |
References
- Arslanova, A.; Shafaattalab, S.; Lin, E.; Barszczewski, T.; Hove-Madsen, L.; Tibbits, G.F. Investigating Inherited Arrhythmias Using HiPSC-Derived Cardiomyocytes. Methods 2022, 203, 542–557. [Google Scholar] [CrossRef]
- Bezzerides, V.J.; Zhang, D.; Pu, W.T. Modeling Inherited Arrhythmia Disorders Using Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. J. 2016, 81, 12–21. [Google Scholar] [CrossRef]
- Brandão, K.O.; Tabel, V.A.; Atsma, D.E.; Mummery, C.L.; Davis, R.P. Human Pluripotent Stem Cell Models of Cardiac Disease: From Mechanisms to Therapies. Dis. Model. Mech. 2017, 10, 1039–1059. [Google Scholar] [CrossRef]
- Gharanei, M.; Shafaattalab, S.; Sangha, S.; Gunawan, M.; Laksman, Z.; Hove-Madsen, L.; Tibbits, G.F. Atrial-Specific HiPSC-Derived Cardiomyocytes in Drug Discovery and Disease Modeling. Methods 2022, 203, 364–377. [Google Scholar] [CrossRef]
- Gunawan, M.G.; Sangha, S.S.; Shafaattalab, S.; Lin, E.; Heims-Waldron, D.A.; Bezzerides, V.J.; Laksman, Z.; Tibbits, G.F. Drug Screening Platform Using Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes and Optical Mapping. Stem Cells Transl. Med. 2021, 10, 68–82. [Google Scholar] [CrossRef]
- Matsa, E.; Burridge, P.W.; Wu, J.C. Human Stem Cells for Modeling Heart Disease and for Drug Discovery. Sci. Transl. Med. 2014, 6, 239ps6. [Google Scholar] [CrossRef]
- Kolanowski, T.J.; Antos, C.L.; Guan, K. Making Human Cardiomyocytes up to Date: Derivation, Maturation State and Perspectives. Int. J. Cardiol. 2017, 241, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Garg, V.; Shrestha, R.; Sanguinetti, M.C.; Kamp, T.J.; Wu, J.C. Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes as Models for Cardiac Channelopathies: A Primer for Non-Electrophysiologists. Circ. Res. 2018, 123, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Kaese, S.; Verheule, S. Cardiac Electrophysiology in Mice: A Matter of Size. Front. Physiol. 2012, 3, 345. [Google Scholar] [CrossRef] [PubMed]
- Cyganek, L.; Tiburcy, M.; Sekeres, K.; Gerstenberg, K.; Bohnenberger, H.; Lenz, C.; Henze, S.; Stauske, M.; Salinas, G.; Zimmermann, W.H.; et al. Deep Phenotyping of Human Induced Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes. JCI Insight 2018, 3, e99941. [Google Scholar] [CrossRef]
- Lian, X.; Zhang, J.; Azarin, S.M.; Zhu, K.; Hazeltine, L.B.; Bao, X.; Hsiao, C.; Kamp, T.J.; Palecek, S.P. Directed Cardiomyocyte Differentiation from Human Pluripotent Stem Cells by Modulating Wnt/β-Catenin Signaling under Fully Defined Conditions. Nat. Protoc. 2013, 8, 162–175. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Bellin, M.; Welling, A.; Jung, C.B.; Lam, J.T.; Bott-Flügel, L.; Dorn, T.; Goedel, A.; Höhnke, C.; Hofmann, F.; et al. Patient-Specific Induced Pluripotent Stem-Cell Models for Long-QT Syndrome. N. Engl. J. Med. 2010, 363, 1397–1409. [Google Scholar] [CrossRef]
- Venkateshappa, R.; Hunter, D.V.; Muralidharan, P.; Nagalingam, R.S.; Huen, G.; Faizi, S.; Luthra, S.; Lin, E.; Cheng, Y.M.; Hughes, J.; et al. Targeted Activation of Human Ether-à-Go-Go-Related Gene Channels Rescues Electrical Instability Induced by the R56Q+/- Long QT Syndrome Variant. Cardiovasc. Res. 2023, 119, 2522–2535. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Sallam, K.; Wu, H.; Li, Y.; Itzhaki, I.; Garg, P.; Zhang, Y.; Termglichan, V.; Lan, F.; Gu, M.; et al. Patient-Specific and Genome-Edited Induced Pluripotent Stem Cell–Derived Cardiomyocytes Elucidate Single-Cell Phenotype of Brugada Syndrome. J. Am. Coll. Cardiol. 2016, 68, 2086–2096. [Google Scholar] [CrossRef]
- Arslanova, A.; Shafaattalab, S.; Ye, K.; Asghari, P.; Lin, L.; Kim, B.; Roston, T.M.; Hove-Madsen, L.; van Petegem, F.; Sanatani, S.; et al. Using HiPSC-CMs to Examine Mechanisms of Catecholaminergic Polymorphic Ventricular Tachycardia. Curr. Protoc. 2021, 1, e320. [Google Scholar] [CrossRef]
- Zhang, X.-H.; Haviland, S.; Wei, H.; Šarić, T.; Fatima, A.; Hescheler, J.; Cleemann, L.; Morad, M. Ca2+ Signaling in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes (IPS-CM) from Normal and Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT)-Afflicted Subjects. Cell Calcium 2013, 54, 57–70. [Google Scholar] [CrossRef]
- Yazawa, M.; Hsueh, B.; Jia, X.; Pasca, A.M.; Bernstein, J.A.; Hallmayer, J.; Dolmetsch, R.E. Using Induced Pluripotent Stem Cells to Investigate Cardiac Phenotypes in Timothy Syndrome. Nature 2011, 471, 230–234. [Google Scholar] [CrossRef]
- Babini, H.; Jiménez-Sábado, V.; Stogova, E.; Arslanova, A.; Butt, M.; Dababneh, S.; Asghari, P.; Moore, E.D.W.; Claydon, T.W.; Chiamvimonvat, N.; et al. HiPSC-Derived Cardiomyocytes as a Model to Study the Role of Small-Conductance Ca2+-Activated K+ (SK) Ion Channel Variants Associated with Atrial Fibrillation. Front. Cell Dev. Biol. 2024, 12, 1298007. [Google Scholar] [CrossRef]
- Seibertz, F.; Rubio, T.; Springer, R.; Popp, F.; Ritter, M.; Liutkute, A.; Bartelt, L.; Stelzer, L.; Haghighi, F.; Pietras, J.; et al. Atrial Fibrillation-Associated Electrical Remodelling in Human Induced Pluripotent Stem Cell-Derived Atrial Cardiomyocytes: A Novel Pathway for Antiarrhythmic Therapy Development. Cardiovasc. Res. 2023, 119, 2623–2637. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Lahrouchi, N.; Priori, S.G. Genetics of Sudden Cardiac Death. Circ. Res. 2015, 116, 1919–1936. [Google Scholar] [CrossRef]
- Dunlop, J.; Bowlby, M.; Peri, R.; Vasilyev, D.; Arias, R. High-Throughput Electrophysiology: An Emerging Paradigm for Ion-Channel Screening and Physiology. Nat. Rev. Drug Discov. 2008, 7, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Fertig, N.; Blick, R.H.; Behrends, J.C. Whole Cell Patch Clamp Recording Performed on a Planar Glass Chip. Biophys. J. 2002, 82, 3056–3062. [Google Scholar] [CrossRef] [PubMed]
- González, J.; Oades, K.; Leychkis, Y.; Harootunian, A.; Negulescu, P. Cell-Based Assays and Instrumentation for Screening Ion-Channel Targets. Drug Discov. Today 1999, 4, 431–439. [Google Scholar] [CrossRef]
- Ma, J.G.; Vandenberg, J.I.; Ng, C.-A. Development of Automated Patch Clamp Assays to Overcome the Burden of Variants of Uncertain Significance in Inheritable Arrhythmia Syndromes. Front. Physiol. 2023, 14, 1294741. [Google Scholar] [CrossRef]
- Milligan, C.J.; Li, J.; Sukumar, P.; Majeed, Y.; Dallas, M.L.; English, A.; Emery, P.; Porter, K.E.; Smith, A.M.; McFadzean, I.; et al. Robotic Multiwell Planar Patch-Clamp for Native and Primary Mammalian Cells. Nat. Protoc. 2009, 4, 244–255. [Google Scholar] [CrossRef]
- Li, W.; Luo, X.; Ulbricht, Y.; Wagner, M.; Piorkowski, C.; El-Armouche, A.; Guan, K. Establishment of an Automated Patch-Clamp Platform for Electrophysiological and Pharmacological Evaluation of HiPSC-CMs. Stem Cell Res. 2019, 41, 101662. [Google Scholar] [CrossRef]
- Melgari, D.; Calamaio, S.; Frosio, A.; Prevostini, R.; Anastasia, L.; Pappone, C.; Rivolta, I. Automated Patch-Clamp and Induced Pluripotent Stem Cell-Derived Cardiomyocytes: A Synergistic Approach in the Study of Brugada Syndrome. Int. J. Mol. Sci. 2023, 24, 6687. [Google Scholar] [CrossRef]
- Rajamohan, D.; Kalra, S.; Duc Hoang, M.; George, V.; Staniforth, A.; Russell, H.; Yang, X.; Denning, C. Automated Electrophysiological and Pharmacological Evaluation of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells Dev. 2016, 25, 439–452. [Google Scholar] [CrossRef]
- Goversen, B.; Becker, N.; Stoelzle-Feix, S.; Obergrussberger, A.; Vos, M.A.; van Veen, T.A.B.; Fertig, N.; de Boer, T.P. A Hybrid Model for Safety Pharmacology on an Automated Patch Clamp Platform: Using Dynamic Clamp to Join IPSC-Derived Cardiomyocytes and Simulations of IK1 Ion Channels in Real-Time. Front. Physiol. 2018, 8, 1094. [Google Scholar] [CrossRef]
- Cordeiro, J.M.; Nesterenko, V.V.; Sicouri, S.; Goodrow, R.J.; Treat, J.A.; Desai, M.; Wu, Y.; Doss, M.X.; Antzelevitch, C.; Di Diego, J.M. Identification and Characterization of a Transient Outward K+ Current in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. J. Mol. Cell Cardiol. 2013, 60, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Devalla, H.D.; Schwach, V.; Ford, J.W.; Milnes, J.T.; El-Haou, S.; Jackson, C.; Gkatzis, K.; Elliott, D.A.; Chuva de Sousa Lopes, S.M.; Mummery, C.L.; et al. Atrial-like Cardiomyocytes from Human Pluripotent Stem Cells Are a Robust Preclinical Model for Assessing Atrial-Selective Pharmacology. EMBO Mol. Med. 2015, 7, 394–410. [Google Scholar] [CrossRef]
- Hilderink, S.; Devalla, H.D.; Bosch, L.; Wilders, R.; Verkerk, A.O. Ultrarapid Delayed Rectifier K+ Channelopathies in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 536. [Google Scholar] [CrossRef] [PubMed]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hörmann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human IPSC-Derived Cardiomyocytes. Cell Rep. 2020, 32, 107925. [Google Scholar] [CrossRef]
- Caballero, R.; de la Fuente, M.G.; Gómez, R.; Barana, A.; Amorós, I.; Dolz-Gaitón, P.; Osuna, L.; Almendral, J.; Atienza, F.; Fernández-Avilés, F.; et al. In Humans, Chronic Atrial Fibrillation Decreases the Transient Outward Current and Ultrarapid Component of the Delayed Rectifier Current Differentially on Each Atria and Increases the Slow Component of the Delayed Rectifier Current in Both. J. Am. Coll. Cardiol. 2010, 55, 2346–2354. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. The Dual Effect of Membrane Potential on Sodium Conductance in the Giant Axon of Loligo. J. Physiol. 1952, 116, 497–506. [Google Scholar] [CrossRef]
- Duhme, N.; Schweizer, P.A.; Thomas, D.; Becker, R.; Schröter, J.; Barends, T.R.M.; Schlichting, I.; Draguhn, A.; Bruehl, C.; Katus, H.A.; et al. Altered HCN4 Channel C-Linker Interaction Is Associated with Familial Tachycardia-Bradycardia Syndrome and Atrial Fibrillation. Eur. Heart J. 2013, 34, 2768–2775. [Google Scholar] [CrossRef]
- Macri, V.; Mahida, S.N.; Zhang, M.L.; Sinner, M.F.; Dolmatova, E.V.; Tucker, N.R.; McLellan, M.; Shea, M.A.; Milan, D.J.; Lunetta, K.L.; et al. A Novel Trafficking-Defective HCN4 Mutation Is Associated with Early-Onset Atrial Fibrillation. Heart Rhythm 2014, 11, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. Block and Activation of the Pace-maker Channel in Calf Purkinje Fibres: Effects of Potassium, Caesium and Rubidium. J. Physiol. 1982, 329, 485–507. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D.; Ferroni, A.; Mazzanti, M.; Tromba, C. Properties of the Hyperpolarizing-activated Current (If) in Cells Isolated from the Rabbit Sino-atrial Node. J. Physiol. 1986, 377, 61–88. [Google Scholar] [CrossRef]
- Macri, V.; Proenza, C.; Agranovich, E.; Angoli, D.; Accili, E.A. Separable Gating Mechanisms in a Mammalian Pacemaker Channel. J. Biol. Chem. 2002, 277, 35939–35946. [Google Scholar] [CrossRef]
- Moroni, A.; Barbuti, A.; Altomare, C.; Viscomi, C.; Morgan, J.; Baruscotti, M.; DiFrancesco, D. Kinetic and Ionic Properties of the Human HCN2 Pacemaker Channel. Pflügers Arch. 2000, 439, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Bucchi, A.; Barbuti, A.; DiFrancesco, D.; Baruscotti, M. Funny Current and Cardiac Rhythm: Insights from HCN Knockout and Transgenic Mouse Models. Front. Physiol. 2012, 3, 240. [Google Scholar] [CrossRef]
- Olson, T.M.; Michels, V.V.; Ballew, J.D.; Reyna, S.P.; Karst, M.L.; Herron, K.J.; Horton, S.C.; Rodeheffer, R.J.; Anderson, J.L. Sodium Channel Mutations and Susceptibility to Heart Failure and Atrial Fibrillation. JAMA 2005, 293, 447–454. [Google Scholar] [CrossRef]
- Olesen, M.S.; Refsgaard, L.; Holst, A.G.; Larsen, A.P.; Grubb, S.; Haunsø, S.; Svendsen, J.H.; Olesen, S.-P.; Schmitt, N.; Calloe, K. A Novel KCND3 Gain-of-Function Mutation Associated with Early-Onset of Persistent Lone Atrial Fibrillation. Cardiovasc. Res. 2013, 98, 488–495. [Google Scholar] [CrossRef]
- Ellinor, P.T.; Lunetta, K.L.; Glazer, N.L.; Pfeufer, A.; Alonso, A.; Chung, M.K.; Sinner, M.F.; de Bakker, P.I.W.; Smith, N.L.; Smith, J.D.; et al. Common Variants in KCNN3 Are Associated with Lone Atrial Fibrillation. Nat. Genet. 2010, 42, 240–244. [Google Scholar] [CrossRef]
- Olson, T.M.; Alekseev, A.E.; Liu, X.K.; Park, S.; Zingman, L.V.; Bienengraeber, M.; Sattiraju, S.; Ballew, J.D.; Jahangir, A.; Terzic, A. Kv1.5 Channelopathy Due to KCNA5 Loss-of-Function Mutation Causes Human Atrial Fibrillation. Hum. Mol. Genet. 2006, 15, 2185–2191. [Google Scholar] [CrossRef]
- Christophersen, I.E.; Olesen, M.S.; Liang, B.; Andersen, M.N.; Larsen, A.P.; Nielsen, J.B.; Haunsø, S.; Olesen, S.-P.; Tveit, A.; Svendsen, J.H.; et al. Genetic Variation in KCNA5: Impact on the Atrial-Specific Potassium Current IKur in Patients with Lone Atrial Fibrillation. Eur. Heart J. 2013, 34, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Weeke, P.; Muhammad, R.; Delaney, J.T.; Shaffer, C.; Mosley, J.D.; Blair, M.; Short, L.; Stubblefield, T.; Roden, D.M.; Darbar, D. Whole-Exome Sequencing in Familial Atrial Fibrillation. Eur. Heart J. 2014, 35, 2477–2483. [Google Scholar] [CrossRef]
- Li, X.-H.; Hu, Y.-M.; Yin, G.-L.; Wu, P. Correlation between HCN4 Gene Polymorphisms and Lone Atrial Fibrillation Risk. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2989–2993. [Google Scholar] [CrossRef] [PubMed]
- Haythornthwaite, A.; Stoelzle, S.; Hasler, A.; Kiss, A.; Mosbacher, J.; George, M.; Brüggemann, A.; Fertig, N. Characterizing Human Ion Channels in Induced Pluripotent Stem Cell–Derived Neurons. SLAS Discov. 2012, 17, 1264–1272. [Google Scholar] [CrossRef]
- Lodola, F.; De Giusti, V.C.; Maniezzi, C.; Martone, D.; Stadiotti, I.; Sommariva, E.; Maione, A.S. Modeling Cardiomyopathies in a Dish: State-of-the-Art and Novel Perspectives on HiPSC-Derived Cardiomyocytes Maturation. Biology 2021, 10, 730. [Google Scholar] [CrossRef]
- Hove-Madsen, L.; Llach, A.; Bayes-Geniís, A.; Roura, S.; Font, E.R.; Ariís, A.; Cinca, J. Atrial Fibrillation Is Associated with Increased Spontaneous Calcium Release from the Sarcoplasmic Reticulum in Human Atrial Myocytes. Circulation 2004, 110, 1358–1363. [Google Scholar] [CrossRef]
- Fozzard, H.A.; Hanck, D.A. Structure and Function of Voltage-Dependent Sodium Channels: Comparison of Brain II and Cardiac Isoforms. Physiol. Rev. 1996, 76, 887–926. [Google Scholar] [CrossRef]
- Caves, R.E.; Cheng, H.; Choisy, S.C.; Gadeberg, H.C.; Bryant, S.M.; Hancox, J.C.; James, A.F. Atrial-Ventricular Differences in Rabbit Cardiac Voltage-Gated Na+ Currents: Basis for Atrial-Selective Block by Ranolazine. Heart Rhythm 2017, 14, 1657–1664. [Google Scholar] [CrossRef]
- Burashnikov, A.; Di Diego, J.M.; Zygmunt, A.C.; Belardinelli, L.; Antzelevitch, C. Atrium-Selective Sodium Channel Block as a Strategy for Suppression of Atrial Fibrillation. Circulation 2007, 116, 1449–1457. [Google Scholar] [CrossRef]
- Caves, R.E.; Carpenter, A.; Choisy, S.C.; Clennell, B.; Cheng, H.; McNiff, C.; Mann, B.; Milnes, J.T.; Hancox, J.C.; James, A.F. Inhibition of Voltage-Gated Na+ Currents by Eleclazine in Rat Atrial and Ventricular Myocytes. Heart Rhythm O2 2020, 1, 206–214. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Wasserstrom, J.A.; Furukawa, T.; Jia, H.; Arentzen, C.E.; Hartz, R.S.; Singer, D.H. Characterization of the Sodium Current in Single Human Atrial Myocytes. Circ. Res. 1992, 71, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rook, M.B.; Evers, M.M.; Vos, M.A.; Bierhuizen, M.F.A. Biology of Cardiac Sodium Channel Nav1.5 Expression. Cardiovasc. Res. 2012, 93, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, D.O.; Pizzo, E.; Ketkar, H.; Parambath, S.P.; Tang, S.; Cianflone, E.; Cannata, A.; Vinukonda, G.; Jain, S.; Jacobson, J.T.; et al. Scn1b Expression in the Adult Mouse Heart Modulates Na+ Influx in Myocytes and Reveals a Mechanistic Link between Na+ Entry and Diastolic Function. Am. J. Physiol. -Heart Circ. Physiol. 2022, 322, H975–H993. [Google Scholar] [CrossRef] [PubMed]
- Chapotte-Baldacci, C.-A.; Pierre, M.; Djemai, M.; Pouliot, V.; Chahine, M. Biophysical Properties of NaV1.5 Channels from Atrial-like and Ventricular-like Cardiomyocytes Derived from Human Induced Pluripotent Stem Cells. Sci. Rep. 2023, 13, 20685. [Google Scholar] [CrossRef]
- Jansen, J.A.; Noorman, M.; Musa, H.; Stein, M.; de Jong, S.; van der Nagel, R.; Hund, T.J.; Mohler, P.J.; Vos, M.A.; van Veen, T.A.; et al. Reduced Heterogeneous Expression of Cx43 Results in Decreased Nav1.5 Expression and Reduced Sodium Current That Accounts for Arrhythmia Vulnerability in Conditional Cx43 Knockout Mice. Heart Rhythm 2012, 9, 600–607. [Google Scholar] [CrossRef]
- Hofmann, F.; Flockerzi, V.; Kahl, S.; Wegener, J.W. L-Type Cav1.2 Calcium Channels: From In Vitro Findings to In Vivo Function. Physiol. Rev. 2014, 94, 303–326. [Google Scholar] [CrossRef]
- Seibertz, F.; Rapedius, M.; Fakuade, F.E.; Tomsits, P.; Liutkute, A.; Cyganek, L.; Becker, N.; Majumder, R.; Clauß, S.; Fertig, N.; et al. A Modern Automated Patch-Clamp Approach for High Throughput Electrophysiology Recordings in Native Cardiomyocytes. Commun. Biol. 2022, 5, 969. [Google Scholar] [CrossRef]
- Eroglu, T.E.; Mohr, G.H.; Blom, M.T.; Verkerk, A.O.; Souverein, P.C.; Torp-Pedersen, C.; Folke, F.; Wissenberg, M.; van den Brink, L.; Davis, R.P.; et al. Differential Effects on Out-of-Hospital Cardiac Arrest of Dihydropyridines: Real-World Data from Population-Based Cohorts across Two European Countries. Eur. Heart J. Cardiovasc. Pharmacother. 2020, 6, 347–355. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Marchal, G.A.; Zegers, J.G.; Kawasaki, M.; Driessen, A.H.G.; Remme, C.A.; de Groot, J.R.; Wilders, R. Patch-Clamp Recordings of Action Potentials From Human Atrial Myocytes: Optimization Through Dynamic Clamp. Front. Pharmacol. 2021, 12, 649414. [Google Scholar] [CrossRef]
- Uzun, A.U.; Mannhardt, I.; Breckwoldt, K.; Horváth, A.; Johannsen, S.S.; Hansen, A.; Eschenhagen, T.; Christ, T. Ca(2+)-Currents in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Effects of Two Different Culture Conditions. Front. Pharmacol. 2016, 7, 300. [Google Scholar] [CrossRef]
- Niwa, N.; Nerbonne, J.M. Molecular Determinants of Cardiac Transient Outward Potassium Current (Ito) Expression and Regulation. J. Mol. Cell Cardiol. 2010, 48, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Wettwer, E.; Amos, G.J.; Posival, H.; Ravens, U. Transient Outward Current in Human Ventricular Myocytes of Subepicardial and Subendocardial Origin. Circ. Res. 1994, 75, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Chiamvimonvat, N.; Chen-Izu, Y.; Clancy, C.E.; Deschenes, I.; Dobrev, D.; Heijman, J.; Izu, L.; Qu, Z.; Ripplinger, C.M.; Vandenberg, J.I.; et al. Potassium Currents in the Heart: Functional Roles in Repolarization, Arrhythmia and Therapeutics. J. Physiol. 2017, 595, 2229–2252. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.F.; Drury, T.; Refsum, H.; Aldrete, V.; Giles, W. Contributions of a Transient Outward Current to Repolarization in Human Atrium. Am. J. Physiol. -Heart Circ. Physiol. 1989, 257, H1773–H1781. [Google Scholar] [CrossRef]
- Ellinghaus, P.; Scheubel, R.J.; Dobrev, D.; Ravens, U.; Holtz, J.; Huetter, J.; Nielsch, U.; Morawietz, H. Comparing the Global MRNA Expression Profile of Human Atrial and Ventricular Myocardium with High-Density Oligonucleotide Arrays. J. Thorac. Cardiovasc. Surg. 2005, 129, 1383–1390. [Google Scholar] [CrossRef]
- Wettwer, E.; Hála, O.; Christ, T.; Heubach, J.F.; Dobrev, D.; Knaut, M.; Varró, A.; Ravens, U. Role of IKur in Controlling Action Potential Shape and Contractility in the Human Atrium: Influence of Chronic Atrial Fibrillation. Circulation 2004, 110, 2299–2306. [Google Scholar] [CrossRef]
- Johnson, E.K.; Springer, S.J.; Wang, W.; Dranoff, E.J.; Zhang, Y.; Kanter, E.M.; Yamada, K.A.; Nerbonne, J.M. Differential Expression and Remodeling of Transient Outward Potassium Currents in Human Left Ventricles. Circ. Arrhythm. Electrophysiol. 2018, 11, e005914. [Google Scholar] [CrossRef]
- Van Wagoner, D.R.; Pond, A.L.; McCarthy, P.M.; Trimmer, J.S.; Nerbonne, J.M. Outward K+ Current Densities and Kv1.5 Expression Are Reduced in Chronic Human Atrial Fibrillation. Circ. Res. 1997, 80, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Veerman, C.C.; Mengarelli, I.; Guan, K.; Stauske, M.; Barc, J.; Tan, H.L.; Wilde, A.A.M.; Verkerk, A.O.; Bezzina, C.R. HiPSC-Derived Cardiomyocytes from Brugada Syndrome Patients without Identified Mutations Do Not Exhibit Clear Cellular Electrophysiological Abnormalities. Sci. Rep. 2016, 6, 30967. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Zhou, W.; Hamrick, S.K.; Tester, D.J.; Kim, C.S.J.; Barajas-Martinez, H.; Hu, D.; Giudicessi, J.R.; Antzelevitch, C.; Ackerman, M.J. Acacetin, a Potent Transient Outward Current Blocker, May Be a Novel Therapeutic for KCND3-Encoded Kv4.3 Gain-of-Function-Associated J-Wave Syndromes. Circ. Genom. Precis. Med. 2022, 15, e003238. [Google Scholar] [CrossRef]
- Schulz, C.; Sönmez, M.; Krause, J.; Schwedhelm, E.; Bangfen, P.; Alihodzic, D.; Hansen, A.; Eschenhagen, T.; Christ, T. A Critical Role of Retinoic Acid Concentration for the Induction of a Fully Human-like Atrial Action Potential Phenotype in HiPSC-CM. Stem Cell Rep. 2023, 18, 2096–2107. [Google Scholar] [CrossRef]
- Diness, J.G.; Kirchhoff, J.E.; Speerschneider, T.; Abildgaard, L.; Edvardsson, N.; Sørensen, U.S.; Grunnet, M.; Bentzen, B.H. The KCa2 Channel Inhibitor AP30663 Selectively Increases Atrial Refractoriness, Converts Vernakalant-Resistant Atrial Fibrillation and Prevents Its Reinduction in Conscious Pigs. Front. Pharmacol. 2020, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.M.; Qian, L.L.; Wang, R.X. Modulation of SK Channels: Insight into Therapeutics of Atrial Fibrillation. Heart Lung Circ. 2021, 30, 1130–1139. [Google Scholar] [CrossRef]
- Zhang, X.-D.; Timofeyev, V.; Li, N.; Myers, R.E.; Zhang, D.-M.; Singapuri, A.; Lau, V.C.; Bond, C.T.; Adelman, J.; Lieu, D.K.; et al. Critical Roles of a Small Conductance Ca2+-Activated K+ Channel (SK3) in the Repolarization Process of Atrial Myocytes. Cardiovasc. Res. 2014, 101, 317–325. [Google Scholar] [CrossRef]
- Zhao, Z.; Lan, H.; El-Battrawy, I.; Li, X.; Buljubasic, F.; Sattler, K.; Yücel, G.; Lang, S.; Tiburcy, M.; Zimmermann, W.-H.; et al. Ion Channel Expression and Characterization in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells Int. 2018, 2018, 6067096. [Google Scholar] [CrossRef]
- Butler, A.S.; Ascione, R.; Marrion, N.V.; Harmer, S.C.; Hancox, J.C. In Situ Monolayer Patch Clamp of Acutely Stimulated Human IPSC-Derived Cardiomyocytes Promotes Consistent Electrophysiological Responses to SK Channel Inhibition. Sci. Rep. 2024, 14, 3185. [Google Scholar] [CrossRef]
- Jimenez-Sabado, V.; Tarifa, C.; Casabella-Ramon, S.; Montiel, J.; Rodriguez-Font, E.; Olesen, M.S.; Hove-Madsen, L. The Rs13376333 Risk Allele Mimics the Effect of Atrial Fibrillation on SK-Current Density and Afterdepolarizations in Human Atrial Myocytes. Eur. Heart J. 2022, 43 (Suppl. 2), ehac544.2978. [Google Scholar] [CrossRef]
- Fan, X.; Yu, Y.; Lan, H.; Ou, X.; Yang, L.; Li, T.; Cao, J.; Zeng, X.; Li, M. Ca2+/Calmodulin-Dependent Protein Kinase II (CaMKII) Increases Small-Conductance Ca2+-Activated K+ Current in Patients with Chronic Atrial Fibrillation. Med. Sci. Monit. 2018, 24, 3011–3023. [Google Scholar] [CrossRef]
- Heijman, J.; Zhou, X.; Morotti, S.; Molina, C.E.; Abu-Taha, I.H.; Tekook, M.; Jespersen, T.; Zhang, Y.; Dobrev, S.; Milting, H.; et al. Enhanced Ca2+-Dependent SK-Channel Gating and Membrane Trafficking in Human Atrial Fibrillation. Circ. Res. 2023, 132, e116–e133. [Google Scholar] [CrossRef] [PubMed]
- Benzoni, P.; Campostrini, G.; Landi, S.; Bertini, V.; Marchina, E.; Iascone, M.; Ahlberg, G.; Olesen, M.S.; Crescini, E.; Mora, C.; et al. Human IPSC Modelling of a Familial Form of Atrial Fibrillation Reveals a Gain of Function of If and ICaL in Patient-Derived Cardiomyocytes. Cardiovasc. Res. 2020, 116, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, D. HCN4, Sinus Bradycardia and Atrial Fibrillation. Arrhythm. Electrophysiol. Rev. 2015, 4, 9. [Google Scholar] [CrossRef]
- Sartiani, L.; Mannaioni, G.; Masi, A.; Novella Romanelli, M.; Cerbai, E. The Hyperpolarization-Activated Cyclic Nucleotide–Gated Channels: From Biophysics to Pharmacology of a Unique Family of Ion Channels. Pharmacol. Rev. 2017, 69, 354–395. [Google Scholar] [CrossRef]
- Fan, W.; Sun, X.; Yuan, R.; Hou, X.; Wan, J.; Liao, B. HCN4 and Arrhythmias: Insights into Base Mutations. Mutat. Res. Rev. Mutat. Res. 2025, 795, 108534. [Google Scholar] [CrossRef]
- Verkerk, A.O.; Wilders, R. Pacemaker Activity of the Human Sinoatrial Node: Effects of HCN4 Mutations on the Hyperpolarization-Activated Current. EP Eur. 2014, 16, 384–395. [Google Scholar] [CrossRef]
- DiFrancesco, D. Funny Channel Gene Mutations Associated with Arrhythmias. J. Physiol. 2013, 591, 4117–4124. [Google Scholar] [CrossRef]
- Weng, L.-C.; Rämö, J.T.; Jurgens, S.J.; Khurshid, S.; Chaffin, M.; Hall, A.W.; Morrill, V.N.; Wang, X.; Nauffal, V.; Sun, Y.V.; et al. The Impact of Common and Rare Genetic Variants on Bradyarrhythmia Development. Nat. Genet. 2025, 57, 53–64. [Google Scholar] [CrossRef]
- Munk, A.A.; Adjemian, R.A.; Zhao, J.; Ogbaghebriel, A.; Shrier, A. Electrophysiological Properties of Morphologically Distinct Cells Isolated from the Rabbit Atrioventricular Node. J. Physiol. 1996, 493, 801–818. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Noble, D. A Model of Cardiac Electrical Activity Incorporating Ionic Pumps and Concentration Changes. Philos. Trans. R. Soc. London. B Biol. Sci. 1985, 307, 353–398. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, F.; Benzoni, P.; Campostrini, G.; Milanesi, R.; Bucchi, A.; Baruscotti, M.; Dell’Era, P.; Rossini, A.; Barbuti, A. A Detailed Characterization of the Hyperpolarization-Activated “Funny” Current (If) in Human-Induced Pluripotent Stem Cell (IPSC)–Derived Cardiomyocytes with Pacemaker Activity. Pflug. Arch. 2021, 473, 1009–1021. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Guo, L.; Fiene, S.J.; Anson, B.D.; Thomson, J.A.; Kamp, T.J.; Kolaja, K.L.; Swanson, B.J.; January, C.T. High Purity Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes: Electrophysiological Properties of Action Potentials and Ionic Currents. Am. J. Physiol. -Heart Circ. Physiol. 2011, 301, H2006–H2017. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.; Sartiani, L.; Spinelli, V.; Del Lungo, M.; Stillitano, F.; Nosi, D.; Mugelli, A.; Cerbai, E.; Jaconi, M. Molecular and Functional Evidence of HCN4 and Caveolin-3 Interaction during Cardiomyocyte Differentiation from Human Embryonic Stem Cells. Stem Cells Dev. 2013, 22, 1717–1727. [Google Scholar] [CrossRef]
- Linscheid, N.; Logantha, S.J.R.J.; Poulsen, P.C.; Zhang, S.; Schrölkamp, M.; Egerod, K.L.; Thompson, J.J.; Kitmitto, A.; Galli, G.; Humphries, M.J.; et al. Quantitative Proteomics and Single-Nucleus Transcriptomics of the Sinus Node Elucidates the Foundation of Cardiac Pacemaking. Nat. Commun. 2019, 10, 2889. [Google Scholar] [CrossRef]
- Yin, Z.; Torre, E.; Marrot, M.; Peters, C.H.; Feather, A.; Nichols, W.G.; Logantha, S.J.R.J.; Arshad, A.; Martis, S.A.; Ozturk, N.T.; et al. Identifying Sex Similarities and Differences in Structure and Function of the Sinoatrial Node in the Mouse Heart. Front. Med. 2024, 11, 1488478. [Google Scholar] [CrossRef]
- El Khoury, N.; Mathieu, S.; Marger, L.; Ross, J.; El Gebeily, G.; Ethier, N.; Fiset, C. Upregulation of the Hyperpolarization-Activated Current Increases Pacemaker Activity of the Sinoatrial Node and Heart Rate during Pregnancy in Mice. Circulation 2013, 127, 2009–2020. [Google Scholar] [CrossRef]
- D’Souza, A.; Bucchi, A.; Johnsen, A.B.; Logantha, S.J.R.J.; Monfredi, O.; Yanni, J.; Prehar, S.; Hart, G.; Cartwright, E.; Wisloff, U.; et al. Exercise Training Reduces Resting Heart Rate via Downregulation of the Funny Channel HCN4. Nat. Commun. 2014, 5, 3775. [Google Scholar] [CrossRef] [PubMed]
- Stieber, J.; Stöckl, G.; Herrmann, S.; Hassfurth, B.; Hofmann, F. Functional Expression of the Human HCN3 Channel. J. Biol. Chem. 2005, 280, 34635–34643. [Google Scholar] [CrossRef] [PubMed]
- Altomare, C.; Terragni, B.; Brioschi, C.; Milanesi, R.; Pagliuca, C.; Viscomi, C.; Moroni, A.; Baruscotti, M.; DiFrancesco, D. Heteromeric HCN1-HCN4 Channels: A Comparison with Native Pacemaker Channels from the Rabbit Sinoatrial Node. J. Physiol. 2003, 549, 347–359. [Google Scholar] [CrossRef]
- Altomare, C.; Bucchi, A.; Camatini, E.; Baruscotti, M.; Viscomi, C.; Moroni, A.; DiFrancesco, D. Integrated Allosteric Model of Voltage Gating of HCN Channels. J. Gen. Physiol. 2001, 117, 519–532. [Google Scholar] [CrossRef]
- Qu, Y.; Whitaker, G.M.; Hove-Madsen, L.; Tibbits, G.F.; Accili, E.A. Hyperpolarization-activated Cyclic Nucleotide-modulated ‘HCN’ Channels Confer Regular and Faster Rhythmicity to Beating Mouse Embryonic Stem Cells. J. Physiol. 2008, 586, 701–716. [Google Scholar] [CrossRef] [PubMed]




| Parameters (N) | INa | ICaL | Ito/IKur | ISK | If |
|---|---|---|---|---|---|
| Cells expressing current, % | 14/22, 64% | 12/25, 48% | 10/22, 45% | 23/40, 58% | 15/33, 45% |
| Seal resistance, GΩ | 0.5 ± 0.1 (11) | 3.7 ± 1.5 (9) | 1.0 ± 0.4 (6) | 0.9 ± 0.2 (16) | 0.4 ± 0.2 (11) |
| Series resistance, MΩ | 5.5 ± 0.5 (11) | 5.8 ± 1.0 (9) | 6.2 ± 1.3 (6) | 6.4 ± 0.5 (16) | 6.5 ± 0.7 (11) |
| Cell capacitance, pF | 35.8 ± 0.5 (11) | 32.8 ± 6.0 (9) | 36.4 ± 6.3 (6) | 36.6 ± 5.0 (16) | 55.2 ± 9.7 (11) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Sábado, V.; Babini, H.; Ruben, P.C.; Accili, E.A.; Claydon, T.W.; Hove-Madsen, L.; Tibbits, G.F. Electrophysiological Phenotyping of hiPSC-Derived Atrial Cardiomyocytes Using Automated Patch-Clamp: A Platform for Studying Atrial Inherited Arrhythmias. Cells 2025, 14, 1941. https://doi.org/10.3390/cells14241941
Jiménez-Sábado V, Babini H, Ruben PC, Accili EA, Claydon TW, Hove-Madsen L, Tibbits GF. Electrophysiological Phenotyping of hiPSC-Derived Atrial Cardiomyocytes Using Automated Patch-Clamp: A Platform for Studying Atrial Inherited Arrhythmias. Cells. 2025; 14(24):1941. https://doi.org/10.3390/cells14241941
Chicago/Turabian StyleJiménez-Sábado, Verónica, Hosna Babini, Peter C. Ruben, Eric A. Accili, Thomas W. Claydon, Leif Hove-Madsen, and Glen F. Tibbits. 2025. "Electrophysiological Phenotyping of hiPSC-Derived Atrial Cardiomyocytes Using Automated Patch-Clamp: A Platform for Studying Atrial Inherited Arrhythmias" Cells 14, no. 24: 1941. https://doi.org/10.3390/cells14241941
APA StyleJiménez-Sábado, V., Babini, H., Ruben, P. C., Accili, E. A., Claydon, T. W., Hove-Madsen, L., & Tibbits, G. F. (2025). Electrophysiological Phenotyping of hiPSC-Derived Atrial Cardiomyocytes Using Automated Patch-Clamp: A Platform for Studying Atrial Inherited Arrhythmias. Cells, 14(24), 1941. https://doi.org/10.3390/cells14241941






