Immunohistochemical Characterization and CT-Derived Volume of Epicardial Adipose Tissue in Patients with Coronary Artery Disease
Highlights
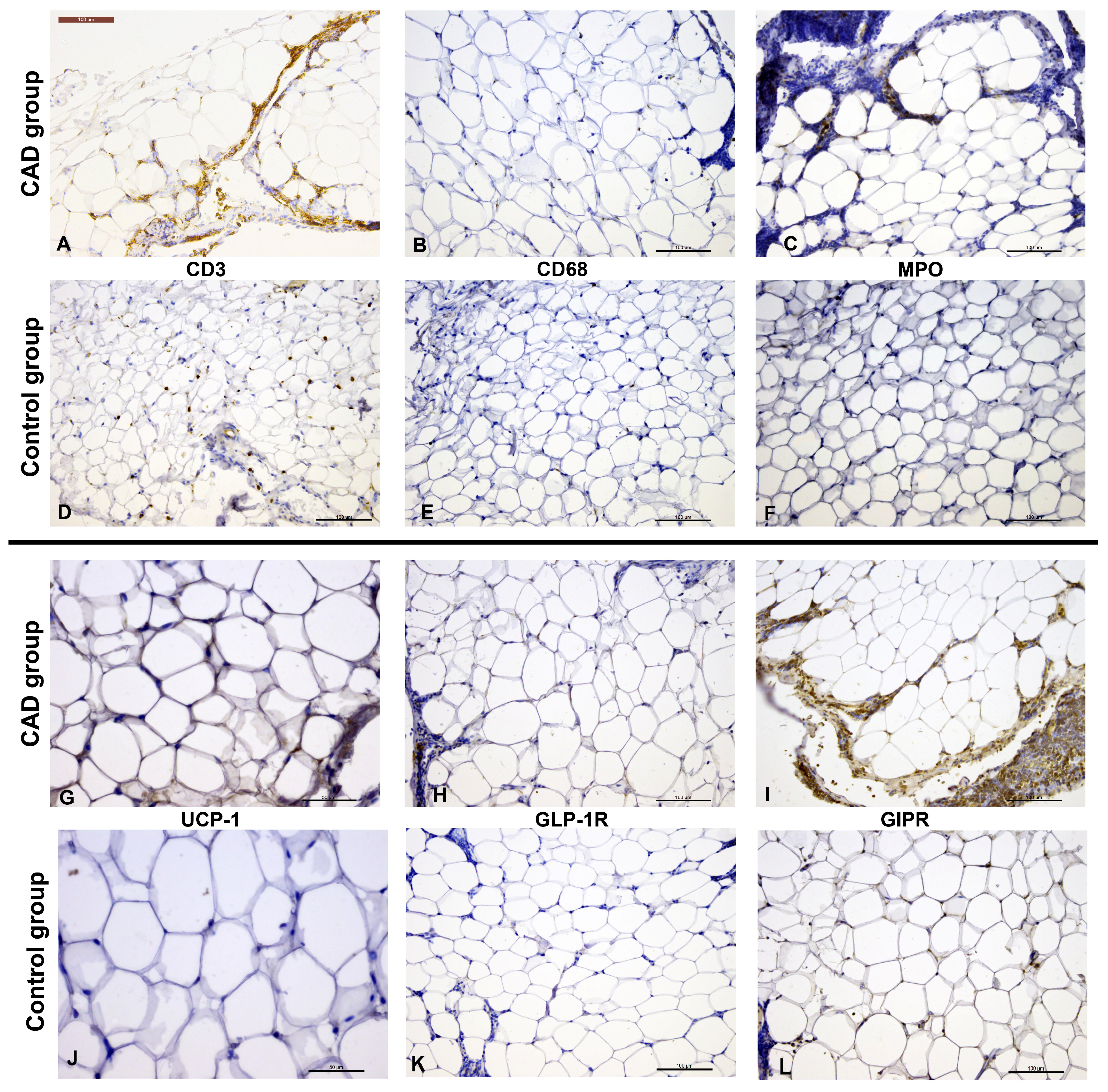
- Patients with coronary artery disease (CAD) showed increased T-cell infiltration and elevated UCP-1 expression in epicardial adipose tissue (EAT).
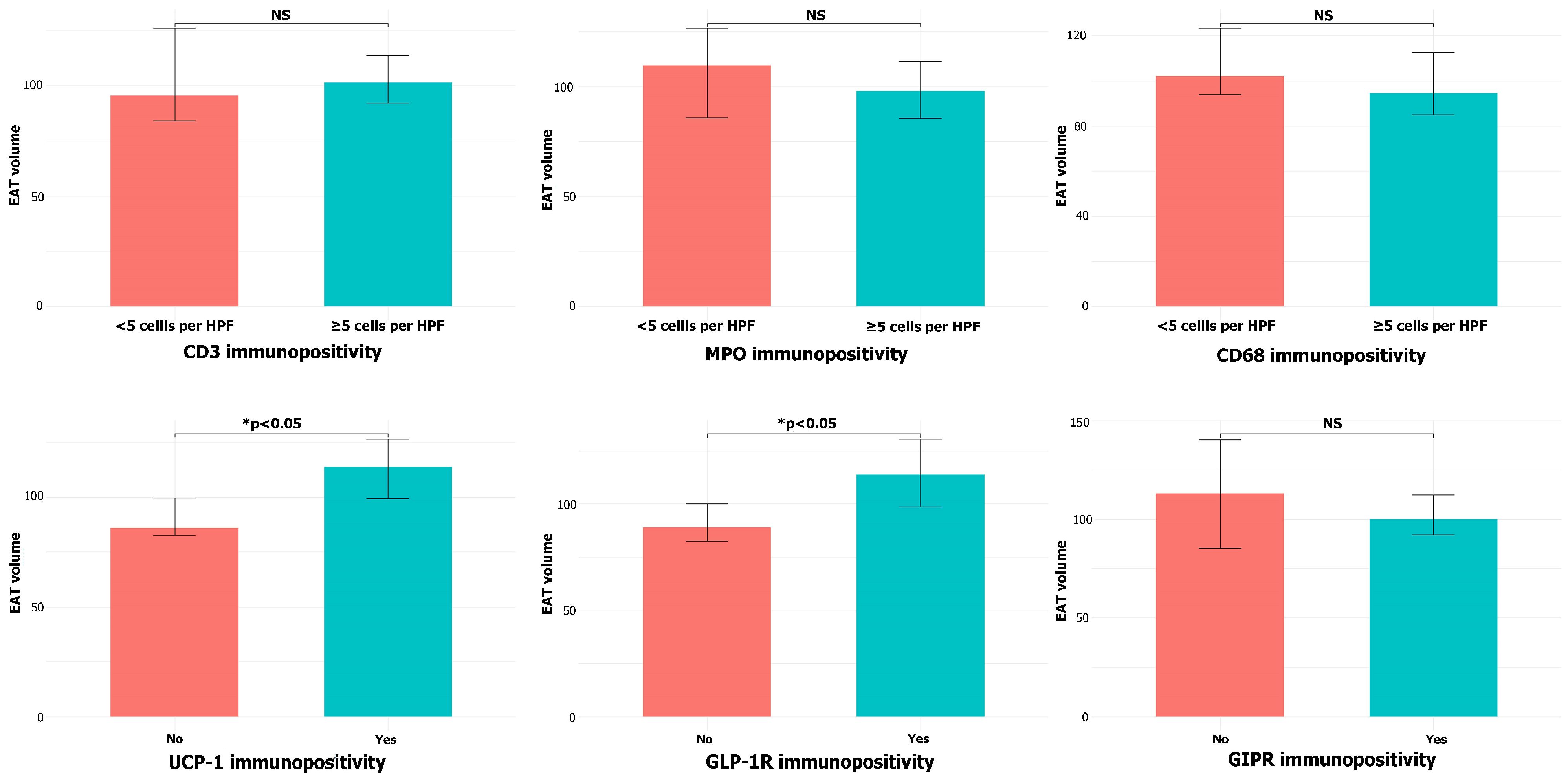
- EAT volume did not differ between CAD and non-CAD patients but correlated with BMI and was positively associated with UCP-1 and GLP-1R immunopositivity.
- The findings suggest that EAT in CAD exhibits a dual profile—both inflammatory and metabolically active.
- The association of EAT volume with UCP-1 and GLP-1R expression highlights the potential immunometabolic role of EAT as a therapeutic target in CAD.
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Characteristics and Laboratory Analyses
2.2. Epicardial Adipose Tissue Sampling and Preparation
2.3. Histomorphometric Analysis of Epicardial Adipocyte Volume
2.4. Immunohistochemical Analysis
2.5. Ehocardiographic Imaging and CT-Derived EAT Volume
2.6. Statistical Analysis
3. Results
3.1. Histomorphometric and Immunohistochemical Analysis of EAT
3.2. CT-Derived EAT Volume
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAD | Coronary artery disease |
| CABG | Coronary artery bypass grafting |
| CLS | Crown-like structure |
| CVD | Cardiovascular disease |
| EAT | Epicardial adipose tissue |
| GIPR | Glucose-dependent insulinotropic polypeptide receptor |
| GLP1-R | Glucagon-like peptide receptor |
| HLP | Hyperlipoproteinemia |
| HTA | Hypertension |
| LVEDD | Left ventricular end-diastolic diameter |
| LVEF | Left ventricle ejection fraction |
| LVESD | Left ventricular end-systolic diameter |
| MI | Myocardial infarction |
| MPO | Myeloperoxidase |
| PCI | Percutaneous coronary intervention |
| NYHA | New York Heart Association classification |
| UCP-1 | Uncoupling protein-1 |
References
- Berg, G.; Miksztowicz, V.; Morales, C.; Barchuk, M. Epicardial Adipose Tissue in Cardiovascular Disease. Adv. Exp. Med. Biol. 2019, 1127, 131–143. [Google Scholar] [CrossRef]
- van Woerden, G.; van Veldhuisen, D.J.; Westenbrink, B.D.; de Boer, R.A.; Rienstra, M.; Gorter, T.M. Connecting Epicardial Adipose Tissue and Heart Failure with Preserved Ejection Fraction: Mechanisms, Management and Modern Perspectives. Eur. J. Heart Fail. 2022, 24, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Gruzdeva, O.V.; Dyleva, Y.A.; Belik, E.V.; Sinitsky, M.Y.; Stasev, A.N.; Kokov, A.N.; Brel, N.K.; Krivkina, E.O.; Bychkova, E.E.; Tarasov, R.S.; et al. Relationship between Epicardial and Coronary Adipose Tissue and the Expression of Adiponectin, Leptin, and Interleukin 6 in Patients with Coronary Artery Disease. J. Pers. Med. 2022, 12, 129. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial Fat Links Obesity to Cardiovascular Diseases. Prog. Cardiovasc. Dis. 2023, 78, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Yorgun, H.; Canpolat, U.; Hazırolan, T.; Ateş, A.H.; Sunman, H.; Dural, M.; Şahiner, L.; Kaya, E.B.; Aytemir, K.; Tokgözoğlu, L.; et al. Increased Epicardial Fat Tissue Is a Marker of Metabolic Syndrome in Adult Patients. Int. J. Cardiol. 2013, 165, 308–313. [Google Scholar] [CrossRef]
- Fan, W.; Si, Y.; Xing, E.; Feng, Z.; Ding, Z.; Liu, Y.; Wei, C.; Tian, Y.; Zhang, Y.; Liu, J.; et al. Human Epicardial Adipose Tissue Inflammation Correlates with Coronary Artery Disease. Cytokine 2023, 162, 156119. [Google Scholar] [CrossRef]
- Bodenstab, M.L.; Varghese, R.T.; Iacobellis, G. Cardio-Lipotoxicity of Epicardial Adipose Tissue. Biomolecules 2024, 14, 1465. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Holman, B.; Cheema, P.; Chary, A.; Parks, F.; Karas, J.; Optican, R.; Bahouth, S.W.; Garrett, E.; et al. Uncoupling Protein-1 and Related Messenger Ribonucleic Acids in Human Epicardial and Other Adipose Tissues: Epicardial Fat Functioning as Brown Fat. J. Clin. Endocrinol. Metab. 2009, 94, 3611–3615. [Google Scholar] [CrossRef]
- Huesca-Gómez, C.; Torres-Paz, Y.E.; Fuentevilla-Álvarez, G.; González-Moyotl, N.J.; Ramírez-Marroquín, E.S.; Vásquez-Jiménez, X.; Sainz-Escarrega, V.; Soto, M.E.; Samano, R.; Gamboa, R. Expressions of mRNA and Encoded Proteins of Mitochondrial Uncoupling Protein Genes (UCP1, UCP2, and UCP3) in Epicardial and Mediastinal Adipose Tissue and Associations with Coronary Artery Disease. Arch. Endocrinol. Metab. 2023, 67, 214–223. [Google Scholar] [CrossRef]
- Moreno-Santos, I.; Pérez-Belmonte, L.M.; Macías-González, M.; Mataró, M.J.; Castellano, D.; López-Garrido, M.; Porras-Martín, C.; Sánchez-Fernández, P.L.; Gómez-Doblas, J.J.; Cardona, F.; et al. Type 2 Diabetes Is Associated with Decreased PGC1α Expression in Epicardial Adipose Tissue of Patients with Coronary Artery Disease. J. Transl. Med. 2016, 14, 243. [Google Scholar] [CrossRef]
- Dozio, E.; Vianello, E.; Malavazos, A.E.; Tacchini, L.; Schmitz, G.; Iacobellis, G.; Corsi Romanelli, M.M. Epicardial Adipose Tissue GLP-1 Receptor Is Associated with Genes Involved in Fatty Acid Oxidation and White-to-Brown Fat Differentiation: A Target to Modulate Cardiovascular Risk? Int. J. Cardiol. 2019, 292, 218–224. [Google Scholar] [CrossRef]
- Malavazos, A.E.; Iacobellis, G.; Dozio, E.; Basilico, S.; Di Vincenzo, A.; Dubini, C.; Menicanti, L.; Vianello, E.; Meregalli, C.; Ruocco, C.; et al. Human Epicardial Adipose Tissue Expresses Glucose-Dependent Insulinotropic Polypeptide, Glucagon, and Glucagon-like Peptide-1 Receptors as Potential Targets of Pleiotropic Therapies. Eur. J. Prev. Cardiol. 2023, 30, 680–693. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Achenbach, S.; Marwan, M.; Doris, M.K.; Cadet, S.; Commandeur, F.; Chen, X.; Slomka, P.J.; Gransar, H.; Cao, J.J.; et al. Epicardial Adipose Tissue Density and Volume Are Related to Subclinical Atherosclerosis, Inflammation and Major Adverse Cardiac Events in Asymptomatic Subjects. J. Cardiovasc. Comput. Tomogr. 2018, 12, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Tarsitano, M.G.; Pandozzi, C.; Muscogiuri, G.; Sironi, S.; Pujia, A.; Lenzi, A.; Giannetta, E. Epicardial Adipose Tissue: A Novel Potential Imaging Marker of Comorbidities Caused by Chronic Inflammation. Nutrients 2022, 14, 2926. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aversana, F.; Tuccillo, R.; Monfregola, A.; De Angelis, L.; Ferrandino, G.; Tedeschi, C.; Cacciapuoti, F.; Tamburro, F.; Liguori, C. Epicardial Adipose Tissue Volume Assessment in the General Population and CAD-RADS 2.0 Score Correlation Using Dual Source Cardiac CT. Diagnostics 2025, 15, 681. [Google Scholar] [CrossRef]
- Milanese, G.; Silva, M.; Bruno, L.; Goldoni, M.; Benedetti, G.; Rossi, E.; Ferrari, C.; Grutta, L.L.; Maffei, E.; Toia, P.; et al. Quantification of Epicardial Fat with Cardiac CT Angiography and Association with Cardiovascular Risk Factors in Symptomatic Patients: From the ALTER-BIO (Alternative Cardiovascular Bio-Imaging Markers) Registry. Diagn. Interv. Radiol. 2019, 25, 35–41. [Google Scholar] [CrossRef]
- Eisenberg, E.; McElhinney, P.A.; Commandeur, F.; Chen, X.; Cadet, S.; Goeller, M.; Razipour, A.; Gransar, H.; Cantu, S.; Miller, R.J.H.; et al. Deep Learning-Based Quantification of Epicardial Adipose Tissue Volume and Attenuation Predicts Major Adverse Cardiovascular Events in Asymptomatic Subjects. Circ. Cardiovasc. Imaging 2020, 13, e009829. [Google Scholar] [CrossRef]
- Rakocevic, J.; Kojic, S.; Orlic, D.; Stankovic, G.; Ostojic, M.; Petrovic, O.; Zaletel, I.; Puskas, N.; Todorovic, V.; Labudovic-Borovic, M. Co-Expression of Vascular and Lymphatic Endothelial Cell Markers on Early Endothelial Cells Present in Aspirated Coronary Thrombi from Patients with ST-Elevation Myocardial Infarction. Exp. Mol. Pathol. 2016, 100, 31–38. [Google Scholar] [CrossRef]
- Al-Dibouni, A.; Gaspar, R.; Ige, S.; Boateng, S.; Cagampang, F.R.; Gibbins, J.; Cox, R.D.; Sellayah, D. Unique Genetic and Histological Signatures of Mouse Pericardial Adipose Tissue. Nutrients 2020, 12, 1855. [Google Scholar] [CrossRef]
- Otto, C.M.; Nishimura, R.A.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Gentile, F.; Jneid, H.; Krieger, E.V.; Mack, M.; McLeod, C.; et al. 2020 ACC/AHA Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2021, 143, e35–e71. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Wang, Q.; Chi, J.; Wang, C.; Yang, Y.; Tian, R.; Chen, X. Epicardial Adipose Tissue in Patients with Coronary Artery Disease: A Meta-Analysis. J. Cardiovasc. Dev. Dis. 2022, 9, 253. [Google Scholar] [CrossRef] [PubMed]
- Vianello, E.; Dozio, E.; Arnaboldi, F.; Marazzi, M.G.; Martinelli, C.; Lamont, J.; Tacchini, L.; Sigrüner, A.; Schmitz, G.; Corsi Romanelli, M.M. Epicardial Adipocyte Hypertrophy: Association with M1-Polarization and Toll-like Receptor Pathways in Coronary Artery Disease Patients. Nutr. Metab. Cardiovasc. Dis. NMCD 2016, 26, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial Adipose Tissue in Contemporary Cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- The Adipokine Hypothesis of Heart Failure with a Preserved Ejection Fraction: A Novel Framework to Explain Pathogenesis and Guide Treatment|JACC. Available online: https://www.jacc.org/doi/10.1016/j.jacc.2025.06.055?utm_campaign=7111978-jacc&utm_medium=email&_hsenc=p2ANqtz--4yfQ1gg16eKp6M7NVGAiBRnXAbsam84yN3DgU2JKNipBci0xpcL8-PaH4TpMgKOHYl83rpB9eC2hkmNC9EI0RTAVdlw&_hsmi=380791358&utm_content=380791358&utm_source=hs_email (accessed on 3 October 2025).
- Si, Y.; Feng, Z.; Liu, Y.; Fan, W.; Shan, W.; Zhang, Y.; Shi, F.; Xing, E.; Sun, L. Inflammatory Biomarkers, Angiogenesis and Lymphangiogenesis in Epicardial Adipose Tissue Correlate with Coronary Artery Disease. Sci. Rep. 2023, 13, 2831. [Google Scholar] [CrossRef]
- Mráz, M.; Cinkajzlová, A.; Kloučková, J.; Lacinová, Z.; Kratochvílová, H.; Lipš, M.; Pořízka, M.; Kopecký, P.; Pierzynová, A.; Kučera, T.; et al. Coronary Artery Disease Is Associated with an Increased Amount of T Lymphocytes in Human Epicardial Adipose Tissue. Mediat. Inflamm. 2019, 2019, 4075086. [Google Scholar] [CrossRef]
- Hirata, Y.; Tabata, M.; Kurobe, H.; Motoki, T.; Akaike, M.; Nishio, C.; Higashida, M.; Mikasa, H.; Nakaya, Y.; Takanashi, S.; et al. Coronary Atherosclerosis Is Associated with Macrophage Polarization in Epicardial Adipose Tissue. J. Am. Coll. Cardiol. 2011, 58, 248–255. [Google Scholar] [CrossRef]
- Papotti, B.; Opstad, T.B.; Åkra, S.; Tønnessen, T.; Braathen, B.; Hansen, C.H.; Arnesen, H.; Solheim, S.; Seljeflot, I.; Ronda, N. Macrophage Polarization Markers in Subcutaneous, Pericardial, and Epicardial Adipose Tissue Are Altered in Patients with Coronary Heart Disease. Front. Cardiovasc. Med. 2023, 10, 1055069. [Google Scholar] [CrossRef]
- Sacks, H.S.; Fain, J.N.; Bahouth, S.W.; Ojha, S.; Frontini, A.; Budge, H.; Cinti, S.; Symonds, M.E. Adult Epicardial Fat Exhibits Beige Features. J. Clin. Endocrinol. Metab. 2013, 98, E1448–E1455. [Google Scholar] [CrossRef]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial Adipose Tissue Volume Assessed by Computed Tomography and Coronary Artery Disease: A Systematic Review and Meta-Analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef]
- Yu, W.; Chen, Y.; Zhang, F.; Liu, B.; Wang, J.; Shao, X.; Yang, X.; Shi, Y.; Wang, Y. Association of Epicardial Adipose Tissue Volume with Increased Risk of Hemodynamically Significant Coronary Artery Disease. Quant. Imaging Med. Surg. 2023, 13, 2582–2593. [Google Scholar] [CrossRef]
- Jolfayi, A.G.; Beheshti, A.T.; Hosseini, S.M.; Fakhrabadi, A.A.; Mohebbi, B.; Malakootian, M.; Maleki, M.; Pouraliakbar, H.; Hosseini, S.; Arabian, M. Epicardial Adipose Tissue Features as a Biomarker and Therapeutic Target in Coronary Artery Disease. Sci. Rep. 2025, 15, 14786. [Google Scholar] [CrossRef] [PubMed]
- Chong, B.; Jayabaskaran, J.; Ruban, J.; Goh, R.; Chin, Y.H.; Kong, G.; Ng, C.H.; Lin, C.; Loong, S.; Muthiah, M.D.; et al. Epicardial Adipose Tissue Assessed by Computed Tomography and Echocardiography Are Associated with Adverse Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Circ. Cardiovasc. Imaging 2023, 16, e015159. [Google Scholar] [CrossRef] [PubMed]
- Doukbi, E.; Soghomonian, A.; Sengenès, C.; Ahmed, S.; Ancel, P.; Dutour, A.; Gaborit, B. Browning Epicardial Adipose Tissue: Friend or Foe? Cells 2022, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Nogajski, Ł.; Mazuruk, M.; Kacperska, M.; Kurpias, M.; Mączewski, M.; Nowakowski, M.; Mączewski, M.; Michałowska, I.; Leszek, P.; Paterek, A. Epicardial Fat Density Obtained with Computed Tomography Imaging—More Important than Volume? Cardiovasc. Diabetol. 2024, 23, 389. [Google Scholar] [CrossRef]
- Pop, A.S.K.; Dănilă, M.D.; Giuchici, S.; Buriman, D.G.; Lolescu, B.M.; Sturza, A.; Muntean, D.M.; Lascu, A. Epicardial Adipose Tissue as Target of the Incretin-Based Therapies in Cardio-Metabolic Pathologies: A Narrative Review. Can. J. Physiol. Pharmacol. 2025, 103, 182–192. [Google Scholar] [CrossRef]
| Characteristic | CAD Group (n = 25) | Control Group (n = 25) | p Value |
|---|---|---|---|
| Male, n | 22 (88%) | 18 (72%) | 0.157 |
| Age (years) * | 64 (50–75) | 69 (43–78) | 0.759 |
| BMI (kg/m2) ** | 29.4 ± 4.7 | 28.4 ± 4.4 | 0.936 |
| NYHA | |||
| I | 6 (24%) | 5 (20%) | |
| II | 14 (56%) | 14 (56%) | 0.913 |
| III | 5 (20%) | 6 (24%) | |
| HTA, n | 25 (100%) | 23 (92%) | 0.490 |
| HLP, n | 25 (100%) | 18 (72%) | 0.010 |
| Diabetes, n | 9 (36%) | 8 (32%) | 0.765 |
| Family history of CVD, n | 19 (76%) | 15 (60%) | 0.225 |
| Smoking, n | 17 (68%) | 13 (52%) | 0.248 |
| Angina pectoris, n | 16 (64%) | 4 (16%) | 0.001 |
| Previous MI, n | 8 (32%) | 0 | 0.004 |
| Previous PCI, n | 0 | 1 (4%) | 1.000 |
| Previous stroke, n | 1 (4%) | 1 (4%) | 1.000 |
| RAAS inhibitors, n | 18 (72%) | 23 (92%) | 0.066 |
| Statins, n | 13 (52%) | 22 (88%) | 0.0005 |
| Leukocytes (×109/L) * | 7.3 (5.2–13.6) | 7.2 (3.8–11.7) | 0.346 |
| Erythrocytes (×1012/L) ** | 4.66 ± 0.43 | 4.60 ± 0.60 | 0.664 |
| Hemoglobin (g/L) * | 143 (115–156) | 140 (101–177) | 0.763 |
| Urea (µmol/L) * | 6.50 (3.13–15.81) | 6.67 (4.49–17.87) | 0.839 |
| Creatinine (µmol/L) * | 89.4 (60.9–176.0) | 85.3 (46.0–125.9) | 0.260 |
| Glucose (mmol/L) * | 6.71 (5.38–12.34) | 5.87 (5.26–11.63) | 0.006 |
| CK (U/L) * | 95 (12–264) | 91 (51–529) | 0.930 |
| LVEF (%) * | 50 (30–60) | 55 (30–65) | 0.008 |
| LVEDD (mm) ** | 52.5 ± 5.3 | 53.4 ± 7.3 | 0.323 |
| LVESD (mm) ** | 34.0 ± 5.1 | 33.2 ± 7.3 | 0.560 |
| Variable | β (25th Quantile) | 95% CI | β (Median) | 95% CI | β (75th Quantile) | 95% CI |
|---|---|---|---|---|---|---|
| Age (years) | 0.08 | −0.61; 1.07 | 0.92 | −0.20; 1.78 | 1.12 | −1.03; 2.08 |
| Gender | 9.58 | 1.95; 23.99 | 16.72 | 2.90; 36.35 | 38.02 | −8.63; 51.74 |
| BMI (kg/m2) | 2.96 | −0.45; 4.34 | 2.55 | 0.82; 5.42 | 4.90 * | 0.56; 7.88 |
| CAD | 0.47 | −13.83; 12.12 | 3.50 | −9.93; 19.35 | 7.81 | −59.55; 26.76 |
| LVEF (%) | −0.84 * | −1.38; 0.18 | −0.60 | −1.71; 0.20 | 0.69 | −2.61; 1.45 |
| LVEDD (mm) | 2.18 * | 0.29; 4.38 | 2.81 * | 0.78; 4.45 | 4.02 * | 0.93; 4.04 |
| LVESD (mm) | 1.37 | 0.59; 2.65 | 1.21 | 0.75; 3.15 | 3.40 | −0.31; 6.57 |
| CK (U/L) | −0.08 | −0.24; 0.02 | −0.02 | 0.22; 0.08 | −0.15 | −0.42; 0.12 |
| Glucose (mmol/L) | −1.20 | −2.30; 3.57 | 1.63 | −2.64; 6.73 | −4.88 | −9.24; 15.88 |
| Urea (µmol/L) | −0.78 | −2.37; 3.19 | 1.19 | −3.71; 2.72 | −1.73 | −2.37; 1.01 |
| Creatinine (µmol/L) | 0.05 | −0.27; 0.43 | 0.23 | 0.04; 0.39 | 0.10 | −0.18; 0.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furtula, M.; Zivkovic, I.; Micovic, S.; Tabakovic, Z.; Vidovic, G.; Antonic, Z.; Vukmirovic, J.; Savic, D.; Bojic, M.; Beleslin, B.; et al. Immunohistochemical Characterization and CT-Derived Volume of Epicardial Adipose Tissue in Patients with Coronary Artery Disease. Cells 2025, 14, 1760. https://doi.org/10.3390/cells14221760
Furtula M, Zivkovic I, Micovic S, Tabakovic Z, Vidovic G, Antonic Z, Vukmirovic J, Savic D, Bojic M, Beleslin B, et al. Immunohistochemical Characterization and CT-Derived Volume of Epicardial Adipose Tissue in Patients with Coronary Artery Disease. Cells. 2025; 14(22):1760. https://doi.org/10.3390/cells14221760
Chicago/Turabian StyleFurtula, Matija, Igor Zivkovic, Slobodan Micovic, Zoran Tabakovic, Gorica Vidovic, Zelimir Antonic, Jelica Vukmirovic, David Savic, Milovan Bojic, Branko Beleslin, and et al. 2025. "Immunohistochemical Characterization and CT-Derived Volume of Epicardial Adipose Tissue in Patients with Coronary Artery Disease" Cells 14, no. 22: 1760. https://doi.org/10.3390/cells14221760
APA StyleFurtula, M., Zivkovic, I., Micovic, S., Tabakovic, Z., Vidovic, G., Antonic, Z., Vukmirovic, J., Savic, D., Bojic, M., Beleslin, B., Dobric, M., & Rakocevic, J. (2025). Immunohistochemical Characterization and CT-Derived Volume of Epicardial Adipose Tissue in Patients with Coronary Artery Disease. Cells, 14(22), 1760. https://doi.org/10.3390/cells14221760







