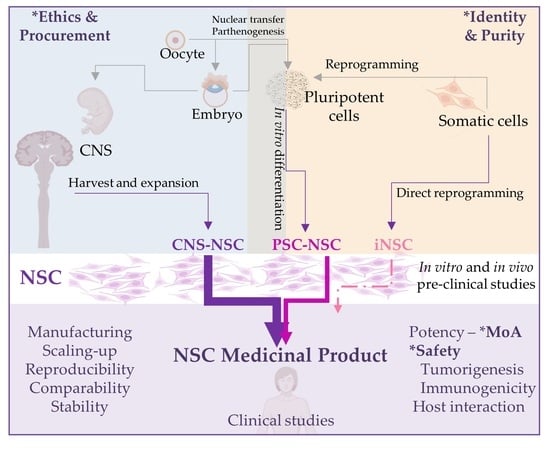
Human Neural Stem Cells for Cell-Based Medicinal Products
Abstract
:1. Neural Stem Cells
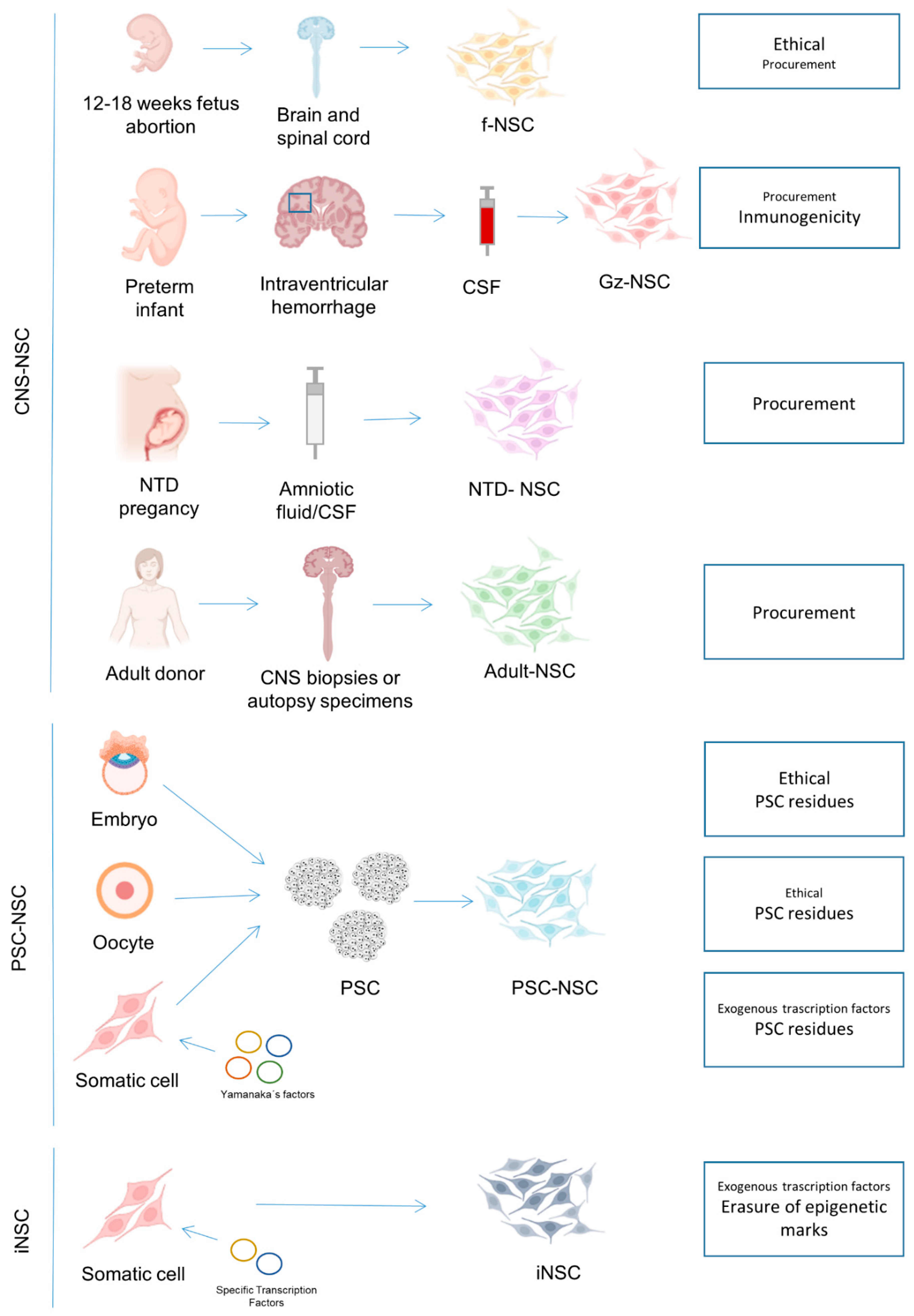
2. Neural Stem Cells from the Central Nervous System
2.1. NSC Isolated from Human Fetal Neuroectoderm
| Cell Type | Disease | ClinicalTrials.Gov Identifier | Phase | Location | Starting Year | Dose | Infusion Route | Principal Investigator | Manufacturer | References |
|---|---|---|---|---|---|---|---|---|---|---|
| fNSC | Multiple Sclerosis | NCT03269071 | Phase 1 | Italy | 2017 | 0.7–5.7 million cells/kg | Intrathecal | Gianvito Martino | Stefano Verri Laboratory of cellular and gene therapies | Not found |
| fNSC | Multiple Sclerosis | NCT03282760 | Phase 1 | Italy/Switzerland | 2017 | 5–24 million | Intracerebroventricular | Angelo L Vescovi | Laboratorio Cellule Staminali of Terni | Not found |
| fNSC | Amyotrophic Lateral Sclerosis | NCT01640067 | Phase 1 | Italy | 2011 | 2.25–4.5 million (unilateral or bilateral) | Intraspinal | Angelo L Vescovi | Laboratorio Cellule Staminali of Terni | [16,17] |
| fNSC | Amyotrophic Lateral Sclerosis | NCT01730716 | Phase 2 | United States | 2013 | 2–8 million (bilateral) | Intraspinal | Not detailed | Neuralstem Inc. | [18] |
| fNSC | Amyotrophic Lateral Sclerosis | NCT01348451 | Phase 1 | United States | 2009 | 0.5–1 million (unilateral or bilateral) | Intraspinal | Not detailed | Neuralstem Inc. | [18,19,20,21,22] |
| fNSC | Neuronal Ceroid Lipofuscinosis | NCT01238315 | Phase 1 | United States | 2010 | 500–1000 million | Intracerebral | Nathan Selden | StemCells, Inc. | Not found |
| fNSC | Neuronal Ceroid Lipofuscinosis | NCT00337636 | Phase 1 | United States | 2006 | 500–1000 million | Intracerebral | Robert Steiner | StemCells, Inc. | [23] |
| fNSC | Parkinson Disease | NCT03128450 | Phase 2/3 | China | 2017 | 4 million | Nasal | Jie Li | Shanghai Angecon Biotechnology Cooperate. | Not found |
| fNSC | Ischemic Stroke | NCT03296618 | Phase 1 | China | 2012 | 12–80 million | Intracraneal | Xu Ruxiang | Neuralstem Inc. (currently Palisade Bio, Inc.) | [24,25] |
| fNSC | Spinal Cord Injury | NCT02688049 | Phase 1/2 | China | 2016 | 10 million | Not detailed | Jianwu Dai, | Not detailed | Not found |
| fNSC | Spinal Cord Injury | NCT01772810 | Phase 1 | United States | 2014 | 1.2 million (bilateral) | Intraspinal | Joseph Ciacci | Neuralstem Inc. | [26] |
| fNSC | Spinal Cord Injury | NCT02163876 | Phase 2 | United States/Canada | 2014 | Not detailed | Intraspinal | Stephen Huhn | StemCells, Inc. | [27,28] |
| fNSC | Spinal Cord Injury | NCT01725880 | Phase 1/2 | Switzerland | 2012 | Not detailed | Intraspinal | Stephen Huhn | StemCells, Inc. | [27,28] |
| fNSC | Spinal Cord Injury | NCT01321333 | Phase 1/2 | Switzerland/Canada | 2011 | Not detailed | Intraspinal | Stephen Huhn | StemCells, Inc. | [27,28] |
| fNSC | Spinal Cord Injury | NCT03069404 | Phase 1/2 | Switzerland/Canada | 2017 | Not detailed | Intraspinal | Armin Curt | StemCells, Inc. | [27,28] |
| fNSC | Ischemic Encephalopathy | NCT02854579 | Not Applicable | China | 2016 | 4 million | Intrathecal | Zuo Luan | Not detailed | [29] |
| fNSC | Age-Related Macular Degeneration | NCT01632527 | Phase 1/2 | United States | 2012 | 0.3–1 million | Subretinal | Stephen Huhn | StemCells, Inc. | Not found |
| fNSC | Geographic atrophy age-related macular degeneration | NCT02467634 | Phase 2 | United States | 2015 | Not detailed | Subretinal | Joel Naor | StemCells, Inc. | Not found |
| fNSC | Geographic atrophy age-related macular degeneration | NCT02137915 | Phase 1/2 | United States | 2014 | Not detailed | Subretinal | David Birch | StemCells, Inc. | [30] |
| fNSC | Pelizaeus-Merzbacher Disease | NCT01005004 | Phase 1 | United States | 2009 | 300 million | Intracerebral | Stephen Huhn | StemCells, Inc. | [31] |
| fNSC | Pelizaeus-Merzbacher Disease | NCT01391637 | Phase 1 | United States | 2011 | Not detailed | Not detailed | Stephen Huhn | StemCells, Inc. | Not found |
| fNSC | Retinitis Pigmentosa | NCT04284293 | Phase 1 | United States | 2020 | 0.2–1 million | Subretinal | David Liao | Not detailed | Not found |
| fNSC? | Cerebral Palsy | NCT03005249 | Not Applicable | China | 2016 | Not detailed | Not detailed | Jing Liu | Not detailed | Not found |
| fNSC expressing GDNF | Amyotrophic Lateral Sclerosis | NCT02943850 | Phase 1 | United States | 2017 | Not detailed | Intraspinal | Robert H. Baloh | Not detailed | Not found |
| fNSC v-myc inmortalized loaded with an oncolytic virus | Malignant Glioma | NCT03072134 | Phase 1 | United States | 2017 | Not detailed | Intracerebral | Maciej S Lesniak | Not detailed | [32] |
| fNSC v-myc immortalized NSC expressing CD | Glioma | NCT01172964 | Phase 1 | United States | 2010 | 10–50 million | Intracerebral | Jana Portnow | City of Hope | [33] |
| fNSC v-myc immortalized expressing CD | Gliomas | NCT02015819 | Phase 1 | United States | 2014 | 50–150 million | Intracerebral | Jana Portnow | City of Hope | [34] |
| fNSC v-myc inmortalized expressing CE | Gliomas | NCT02192359 | Phase 1 | United States | 2016 | Not detailed | Intracerebral | Jana L Portnow | Not detailed | Not found |
| fNSC v-myc inmortalized expressing CE | Glioma | NCT02055196 | Phase 1 | United States | 2014 | Not detailed | Intracerebral | Jana Portnow | Not detailed | Not found |
| fNSC c-myc inmortalized (inducible) | Ischemic Stroke | NCT03629275 | Phase 2 | United States | 2018 | 20 million | Intracerebral | Richard Beckman | ReNeuron Limited | Not found |
| fNSC c-myc immortalized (inducible) | Stroke | NCT02117635 | Phase 2 | United Kingdom | 2014 | 20 million | Intracerebral | Keith W Muir | ReNeuron Limited | [35] |
| fNSC c-myc immortalized (inducible) | Stroke | NCT01151124 | Phase 1 | United Kingdom | 2010 | 2-million | Intracerebral | Not detailed | ReNeuron Limited | [36,37] |
| fNSC c-myc immortalized (inducible) | Peripheral Arterial Disease | NCT01916369 | Phase 1 | United Kingdom | 2014 | 20–80 million | Intramuscular | Jill JF Belch | ReNeuron Limited | Not found |
| ESC-NSC | Spinal Cord Injury | NCT04812431 | Phase 1/2 | Republic of Korea | 2021 | Not detailed | Intrathecal | Dong Ah Shin | S.Biomedics Co., Ltd. | Not found |
| ESC-NSC | Ischemic Stroke | NCT04631406 | Phase 1/2 | United States | 2021 | Not detailed | Intracerebral | Gary K Steinberg | Not detailed | Not found |
| pPSC-NSC | Parkinson Disease | NCT02452723 | Phase 1 | Australia | 2016 | 30–70 million | Intracerebral | Andrew Evans | International Stem Cell Corporation | [38] |
| iPSC-NSC | Parkinson Disease | NCT03815071 | Phase 1 | China | 2019 | Not detailed | Not detailed | Not detailed | Allife Medical Science and Technology Co., Ltd. | Not found |
2.2. NSC Isolated from the Cerebrospinal Fluid
2.3. NSC Isolated from Biopsy and Autopsy Material
3. NSC Derived from Pluripotent Stem Cells
4. Induced NSC
5. Clinical Grade NSC Therapies: Safety, Efficacy, Scalability and Quality Control Considerations
| Origin | Type of NSC | Safety | Efficacy | Immunogenicity | Ethical Concerns | Procurement | Logistics/Scalability | References |
|---|---|---|---|---|---|---|---|---|
| CNS | fNSC | Safe as confirmed by multiple clinical trials and animal models | Some clinical studies describe positive results but none demonstrating sustained recovery | Immunosuppression required (only allogeneic setting is feasible). | Derived from human fetuses, usually from elected abortions | Difficult. Ethical concerns complicate procurement being forbidden in some countries | Easy. Once obtained, cells are easy to grow and scale-up | [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,42,50,108] |
| Gz-NSC | Only confirmed in animal models | Not confirmed | No Immunosuppression required in autologous settings. High HLA expression can impede their allogenic use | No ethical issues as the original source (CSF of IVH preterm infants) is usually discarded and do not imply an extra surgery in the patient | Difficult. Few IVH cases and not all hospitals perform therapeutic neuroendoscopies to remove CSF in IVH patients. | Easy. Once obtained cells are easy to grow and scale-up. Autologous setting is costly and allogenic setting not possible due to the high HLA expression | [4] | |
| NTD-NSC | Only confirmed in animal models | Only confirmed in animal models | No Immunosuppression required in autologous settings. Immunosuppression required in allogenic treatments. | No ethical issues as the original source (CSF of NTD patients) is usually discarded and do not imply an extra surgery in the patient | Difficult. Few NTD cases | Easy. Cells are easy to grow and scale-up | [5,6,7,51] | |
| NSC from adult biopsies/autopsies | Safety confirmed in animal models and few clinical cases | Efficacy confirmed in animal models and few clinical cases | No Immunosuppression required in autologous settings. Immunosuppression required in allogenic treatments. | Biopsy procurement potentially risky for the patient | Difficult. Few cells in the source | Difficult. Few cells with probably lower growth capabilities. | [54,55,56,57,58] | |
| PSC | iPSC-NSC | Only confirmed in animal models. Safety concerns related to the use of exogenous transcription factors, the enrichment of somatic mutations, and the fact that epigenetic marks from the original somatic cell are not totally erased. Turmorigenic risk due to potential PSC residues. | Only confirmed in animal models | No Immunosuppression required in autologous settings. Immunosuppression required in allogenic treatments. | Invasive surgery in some cases to obtain the initial cell type used for reprogramming (e.g., skin fibroblast isolation) | Easy. The initial cell type used for reprogramming is usually easily accessible e.g., coming from a skin biopsy or blood sample | Easy. Establishment of iPSC lines is time-consuming and requires trained operators but once the PSC cells are obtained they are easy to grow, allowing safe production of many cell doses. | [60,61,62,64,65,66,67,68,71,74,95] |
| ESC-NSC | Only confirmed in animal models. Turmorigenic risk due to potential PSC residues. | Only confirmed in animal models | Immunosuppression required (only allogeneic setting is feasible). | Derived from human embryos | Difficult. Ethical concerns complicate procurement being forbidden in some countries | Easy. Establishment of ESC lines is time-consuming and requires trained operators but once the PSC cells are obtained they are easy to grow, allowing safe production of many cell doses. | [60,69,70,72,73,101,102] | |
| pESC-NSC | Only confirmed in animal models. Turmorigenic risk due to potential PSC residues. Haploid cells | Only confirmed in animal models | Immunosuppression required (only allogeneic setting is feasible). | Derived from human oocytes (ethical concerns about the payment to oocyte donors, the medical risks of oocyte retrieval, and with the affectation of their reproduction success as this can be compromised because fewer oocytes are available for reproductive purposes) | Difficult. Ethical concerns complicate procurement. | Easy. Establishment of pESC lines is time-consuming and requires trained operators but once the PSC cells are obtained they are easy to grow, allowing safe production of many cell doses. | [75,109] | |
| ntESC-NSC | Only confirmed in animal models. Turmorigenic risk due to potential PSC residues | Only confirmed in animal models | Immunosuppression required (only allogeneic setting is feasible). | Derived from human oocytes (ethical concerns about the payment to oocyte donors, the medical risks of oocyte retrieval, and with the affectation of their reproduction success as this can be compromised because fewer oocytes are available for reproductive purposes) | Difficult. Ethical concerns complicate procurement. | Easy. Establishment of ntESC lines is time consuming and requires trained operators but once the PSC cells are obtained they are easy to grow, allowing safe production of many cell doses. | [110] | |
| Direct reprogramming | iNSC | Only confirmed in animal models. Safety concerns related to the use of exogenous transcription factors and the fact that epigenetic marks from the original somatic cell are not erased. | Only confirmed in animal models | No immunosuppression required in autologous settings. Immunosuppression required in allogenic treatments. | Invasive surgery in some cases to obtain the initial cell type used for reprogramming (e.g., skin fibroblast isolation) | Easy. The initial cell type used for reprogramming is usually easily accessible e.g., coming from a skin biopsy or blood sample | Easy. Establishment of iNSC lines is time-consuming but, once the iNSC are obtained, they are normally easy to grow, allowing safe production of many cell doses. | [80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,96,97,98,99] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pluchino, S.; Quattrini, A.; Brambilla, E.; Gritti, A.; Salani, G.; Dina, G.; Galli, R.; Del Carro, U.; Amadio, S.; Bergami, A.; et al. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 2003, 17, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Zanotti, L.; Deleidi, M.; Martino, G. Neural stem cells and their use as therapeutic tool in neurological disorders. Brain Res. Rev. 2005, 48, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Einstein, O.; Karussis, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Reinhartz, E.; Abramsky, O.; Ben-Hur, T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol. Cell. Neurosci. 2003, 24, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Muñoz, B.; Rosell-Valle, C.; Ferrari, D.; Alba-Amador, J.; Montiel, M.Á.; Campos-Cuerva, R.; Lopez-Navas, L.; Muñoz-Escalona, M.; Martín-López, M.; Profico, D.C.; et al. Retrieval of germinal zone neural stem cells from the cerebrospinal fluid of premature infants with intraventricular hemorrhage. Stem Cells Transl. Med. 2020, 9, 1085–1101. [Google Scholar] [CrossRef]
- Turner, C.G.; Pennington, E.C.; Gray, F.L.; Ahmed, A.; Teng, Y.D.; Fauza, D.O. Intra-amniotic delivery of amniotic-derived neural stem cells in a syngeneic model of spina bifida. Fetal Diagn. Ther. 2013, 34, 38–43. [Google Scholar] [CrossRef]
- Turner, C.G.; Klein, J.D.; Wang, J.; Thakor, D.; Benedict, D.; Ahmed, A.; Teng, Y.D.; Fauza, D.O. The amniotic fluid as a source of neural stem cells in the setting of experimental neural tube defects. Stem Cells Dev. 2013, 22, 548–553. [Google Scholar] [CrossRef]
- Marotta, M.; Fernández-Martín, A.; Oria, M.; Fontecha, C.G.; Giné, C.; Martínez-Ibáñez, V.; Carreras, E.; Belfort, M.A.; Pelizzo, G.; Peiró, J.L. Isolation, characterization, and differentiation of multipotent neural progenitor cells from human cerebrospinal fluid in fetal cystic myelomeningocele. Stem Cell Res. 2017, 22, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Temple, S. Neural stem cells: Generating and regenerating the brain. Neuron 2013, 80, 588–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Y.; Yu, P.; Cheng, L. Current progress in the derivation & therapeutic application of neural stem cells. Cell Death Dis. 2017, 8, e3108. [Google Scholar] [CrossRef] [Green Version]
- Franco Lambert, A.P.; Fraga Zandonai, A.; Bonatto, D.; Cantarelli Machado, D.; Pêgas Henriques, J.A. Differentiation of human adipose-derived adult stem cells into neuronal tissue: Does it work? Differentiation 2009, 77, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Apostolova, G.; Widera, D.; Mittelbronn, M.; Dechant, G.; Kaltschmidt, B.; Rohrer, H. Alternative generation of CNS neural stem cells and PNS derivatives from neural crest-derived peripheral stem cells. Stem Cells 2015, 33, 574–588. [Google Scholar] [CrossRef]
- Lo, B.; Parham, L. Ethical issues in stem cell research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Buttery, P.C.; Barker, R.A. Gene and Cell-Based Therapies for Parkinson’s Disease: Where Are We? Neurotherapeutics 2020, 17, 1539–1562. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.R.W.; Moralí, D. National human embryo and embryoid research policies: A survey of 22 top research-intensive countries. Regen. Med. 2020, 15, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.A.; Farrell, K.; Guzman, N.V.; He, X.; Lazic, S.E.; Moore, S.; Morris, R.; Tyers, P.; Wijeyekoon, R.; Daft, D.; et al. Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med. 2019, 25, 1045–1053. [Google Scholar] [CrossRef]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sgaravizzi, G.; Projetti Pensi, M.; Muzi, G.; Ricciolini, C.; Rota Nodari, L.; Carletti, S.; Giorgi, C.; et al. Human neural stem cell transplantation in ALS: Initial results from a phase I trial. J. Transl. Med. 2015, 13, 17. [Google Scholar] [CrossRef]
- Mazzini, L.; Gelati, M.; Profico, D.C.; Sorarù, G.; Ferrari, D.; Copetti, M.; Muzi, G.; Ricciolini, C.; Carletti, S.; Giorgi, C.; et al. Results from Phase I Clinical Trial with Intraspinal Injection of Neural Stem Cells in Amyotrophic Lateral Sclerosis: A Long-Term Outcome. Stem Cells Transl. Med. 2019, 8, 887–897. [Google Scholar] [CrossRef] [Green Version]
- Glass, J.D.; Hertzberg, V.S.; Boulis, N.M.; Riley, J.; Federici, T.; Polak, M.; Bordeau, J.; Fournier, C.; Johe, K.; Hazel, T.; et al. Transplantation of spinal cord-derived neural stem cells for ALS: Analysis of phase 1 and 2 trials. Neurology 2016, 87, 392–400. [Google Scholar] [CrossRef] [Green Version]
- Tadesse, T.; Gearing, M.; Senitzer, D.; Saxe, D.; Brat, D.J.; Bray, R.; Gebel, H.; Hill, C.; Boulis, N.; Riley, J.; et al. Analysis of graft survival in a trial of stem cell transplant in ALS. Ann. Clin. Transl. Neurol. 2014, 1, 900–908. [Google Scholar] [CrossRef] [Green Version]
- Feldman, E.L.; Boulis, N.M.; Hur, J.; Johe, K.; Rutkove, S.B.; Federici, T.; Polak, M.; Bordeau, J.; Sakowski, S.A.; Glass, J.D. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: Phase 1 trial outcomes. Ann. Neurol. 2014, 75, 363–373. [Google Scholar] [CrossRef] [Green Version]
- Glass, J.D.; Boulis, N.M.; Johe, K.; Rutkove, S.B.; Federici, T.; Polak, M.; Kelly, C.; Feldman, E.L. Lumbar intraspinal injection of neural stem cells in patients with amyotrophic lateral sclerosis: Results of a phase I trial in 12 patients. Stem Cells 2012, 30, 1144–1151. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.; Glass, J.; Feldman, E.L.; Polak, M.; Bordeau, J.; Federici, T.; Johe, K.; Boulis, N.M. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: A phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery 2014, 74, 77–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selden, N.R.; Al-Uzri, A.; Huhn, S.L.; Koch, T.K.; Sikora, D.M.; Nguyen-Driver, M.D.; Guillaume, D.J.; Koh, J.L.; Gultekin, S.H.; Anderson, J.C.; et al. Central nervous system stem cell transplantation for children with neuronal ceroid lipofuscinosis. J. Neurosurg. Pediatr. 2013, 11, 643–652. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.Z.; Cunningham, M.; Zhang, H.T.; Dai, Y.W.; Zhang, P.; Ge, G.Z.; Wang, B.B.; Bai, M.C.; Hazel, T.; Johe, K.; et al. First human trial of stem cell transplantation in complex arrays for stroke patients using the intracerebral microinjection instrument. Oper. Neurosurg. 2020, 18, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Li, Y.; Reuss, J.L.; Liu, N.; Wu, C.; Li, J.; Xu, S.; Wang, F.; Hazel, T.G.; Cunningham, M.; et al. Stable Intracerebral Transplantation of Neural Stem Cells for the Treatment of Paralysis Due to Ischemic Stroke. Stem Cells Transl. Med. 2019, 8, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, E.; Martin, J.R.; Gabel, B.; Sidhu, N.; Rzesiewicz, T.K.; Mandeville, R.; Van Gorp, S.; Leerink, M.; Tadokoro, T.; Marsala, S.; et al. A First-in-Human, Phase I Study of Neural Stem Cell Transplantation for Chronic Spinal Cord Injury. Cell Stem Cell 2018, 22, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Levi, A.D.; Anderson, K.D.; Okonkwo, D.O.; Park, P.; Bryce, T.N.; Kurpad, S.N.; Aarabi, B.; Hsieh, J.; Gant, K. Clinical Outcomes from a Multi-Center Study of Human Neural Stem Cell Transplantation in Chronic Cervical Spinal Cord Injury. J. Neurotrauma 2019, 36, 891–902. [Google Scholar] [CrossRef]
- Levi, A.D.; Okonkwo, D.O.; Park, P.; Jenkins, A.L.; Kurpad, S.N.; Parr, A.M.; Ganju, A.; Aarabi, B.; Kim, D.; Casha, S.; et al. Emerging safety of intramedullary transplantation of human neural stem cells in chronic cervical and thoracic spinal cord injury. Clin. Neurosurg. 2018, 82, 562–575. [Google Scholar] [CrossRef]
- Luan, Z.; Yin, G.C.; Hu, X.H.; Qu, S.Q.; Wu, N.H.; Yan, F.Q.; Qian, Y.M.; Jin, H.Y.; Gong, X.J. Treatment of an infant with severe neonatal hypoxic-ischemic encephalopathy sequelae with transplantation of human neural stem cells into cerebral ventricle. Zhonghua Er Ke Za Zhi 2005, 43, 580–583. [Google Scholar] [PubMed]
- Nittala, M.G.; Uji, A.; Velaga, S.B.; Hariri, A.H.; Naor, J.; Birch, D.G.; Spencer, R.; Leng, T.; Capela, A.; Tsukamoto, A.; et al. Effect of Human Central Nervous System Stem Cell Subretinal Transplantation on Progression of Geographic Atrophy Secondary to Nonneovascular Age-Related Macular Degeneration. Ophthalmol. Retin. 2021, 5, 32–40. [Google Scholar] [CrossRef]
- Gupta, N.; Henry, R.G.; Kang, S.M.; Strober, J.; Lim, D.A.; Ryan, T.; Perry, R.; Farrell, J.; Ulman, M.; Rajalingam, R.; et al. Long-Term Safety, Immunologic Response, and Imaging Outcomes following Neural Stem Cell Transplantation for Pelizaeus-Merzbacher Disease. Stem Cell Rep. 2019, 13, 254–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balysnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: A first-in-human, phase 1, dose-escalation trial. Lancet Oncol. 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- Portnow, J.; Synold, T.W.; Badie, B.; Tirughana, R.; Lacey, S.F.; D’Apuzzo, M.; Metz, M.Z.; Najbauer, J.; Bedell, V.; Vo, T.; et al. Neural stem cell-based anticancer gene therapy: A first-in-human study in recurrent high-grade glioma patients. Clin. Cancer Res. 2017, 15, 2951–2960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portnow, J.; Badie, B.; Suzette Blanchard, M.; Kilpatrick, J.; Tirughana, R.; Metz, M.; Mi, S.; Tran, V.; Ressler, J.; D’Apuzzo, M.; et al. Feasibility of intracerebrally administering multiple doses of genetically modified neural stem cells to locally produce chemotherapy in glioma patients. Cancer Gene Ther. 2021, 28, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Muir, K.W.; Bulters, D.; Willmot, M.; Sprigg, N.; Dixit, A.; Ward, N.; Tyrrell, P.; Majid, A.; Dunn, L.; Bath, P.; et al. Intracerebral implantation of human neural stem cells and motor recovery after stroke: Multicentre prospective single-arm study (PISCES-2). J. Neurol. Neurosurg. Psychiatry 2020, 91, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Kalladka, D.; Sinden, J.; Pollock, K.; Haig, C.; McLean, J.; Smith, W.; McConnachie, A.; Santosh, C.; Bath, P.M.; Dunn, L.; et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. Lancet 2016, 388, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Kalladka, D.; Sinden, J.; McLean, J.; Moreton, F.C.; Huang, X.; Muir, K.W. Increased deep grey matter functional connectivity of poststroke hNSC implanted ipsilesional putamen. J. Neurol. Neurosurg. Psychiatry 2019, 90, 959–960. [Google Scholar] [CrossRef]
- Garitaonandia, I.; Gonzalez, R.; Sherman, G.; Semechkin, A.; Evans, A.; Kern, R. Novel approach to stem cell therapy in Parkinson’s disease. Stem Cells Dev. 2018, 27, 951–957. [Google Scholar] [CrossRef]
- Shin, J.C.; Kim, K.N.; Yoo, J.; Kim, I.S.; Yun, S.; Lee, H.; Jung, K.; Hwang, K.; Kim, M.; Lee, I.S.; et al. Clinical Trial of Human Fetal Brain-Derived Neural Stem/Progenitor Cell Transplantation in Patients with Traumatic Cervical Spinal Cord Injury. Neural Plast. 2015, 2015, 630932. [Google Scholar] [CrossRef] [Green Version]
- Luan, Z.; Liu, W.; Qu, S.; Du, K.; He, S.; Wang, Z.; Yang, Y.; Wang, C.; Gong, X. Effects of neural progenitor cell transplantation in children with severe cerebral palsy. Cell Transplant. 2012, 21, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Madrazo, I.; Kopyov, O.; Ávila-Rodríguez, M.A.; Ostrosky, F.; Carrasco, H.; Kopyov, A.; Avendaño-Estrada, A.; Jiménez, F.; Magallón, E.; Zamorano, C.; et al. Transplantation of Human Neural Progenitor Cells (NPC) into Putamina of Parkinsonian Patients: A Case Series Study, Safety and Efficacy Four Years after Surgery. Cell Transplant. 2019, 28, 269–285. [Google Scholar] [CrossRef]
- Qiao, L.-Y.; Huang, F.-J.; Zhao, M.; Xie, J.-H.; Shi, J.; Wang, J.; Lin, X.-Z.; Zuo, H.; Wang, Y.-L.; Geng, T.-C. A Two-Year Follow-Up Study of Cotransplantation with Neural Stem/Progenitor Cells and Mesenchymal Stromal Cells in Ischemic Stroke Patients. Cell Transplant. 2014, 23, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Morata-Tarifa, C.; Azkona, G.; Glass, J.; Mazzini, L.; Sanchez-Pernaute, R. Looking backward to move forward: A meta-analysis of stem cell therapy in amyotrophic lateral sclerosis. NPJ Regen. Med. 2021, 20. [Google Scholar] [CrossRef]
- Luan, Z.; Qu, S.; Du, K.; Liu, W.; Yang, Y.; Wang, Z.; Cui, Y.; Du, Q. Neural stem/progenitor cell transplantation for cortical visual impairment in neonatal brain injured patients. Cell Transplant. 2013, 22, S101–S112. [Google Scholar] [CrossRef] [Green Version]
- Ottoboni, L.; von Wunster, B.; Martino, G. Therapeutic Plasticity of Neural Stem Cells. Front. Neurol. 2020, 20, 148. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, L.; Rota, L.; Gelati, M. Low Immunogenic Potential of Human Neural Stem Cells. In Immunosuppression—Role in Health and Diseases; IntechOpen: London, UK, 2012. [Google Scholar]
- Laguna Goya, R.; Busch, R.; Mathur, R.; Coles, A.J.; Barker, R.A. Human fetal neural precursor cells can up-regulate MHC class I and class II expression and elicit CD4 and CD8 T cell proliferation. Neurobiol. Dis. 2011, 41, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.D.; Chang, R.C.; Leverenz, J.B.; Kopyov, O.; Perlman, S.; Hevner, R.F.; Born, D.E.; Bird, T.D.; Montine, T.J. A patient with Huntington’s disease and long-surviving fetal neural transplants that developed mass lesions. Acta Neuropathol. 2009, 117, 329–338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinarbasi, E.S.; Liu, E.A.; Yu, Z.; Kopyov, O.; Brown, N.A.; Dayalu, P.; Lieberman, A.P. Donor-containing cortical and intraventricular glioneuronal nodules in Huntington’s disease brain decades after fetal cell transplantation. Acta Neuropathol. 2021, 141, 979–981. [Google Scholar] [CrossRef]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 17, e1000029. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Su, H.L.; Hsu, L.F.; Huang, P.J.; Wang, T.H.; Cheng, F.C.; Hsu, L.W.; Tsai, M.S.; Chen, C.P.; Chang, Y.L.; et al. Isolation of Human Neural Stem Cells from the Amniotic Fluid with Diagnosed Neural Tube Defects. Stem Cells Dev. 2015, 24, 1740–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubin, A.N.; Alfonsi, F.; Humphreys, M.P.; Choi, C.K.P.; Rocha, S.F.; Kessaris, N. The germinal zones of the basal ganglia but not the septum generate GABAergic interneurons for the cortex. J. Neurosci. 2010, 30, 12050–12062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, N.D.E.; Copp, A.J. Neural tube defects. Annu. Rev. Neurosci. 2014, 37, 221–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwartz, P.H.; Bryant, P.J.; Fuja, T.J.; Su, H.; O’Dowd, D.K.; Klassen, H. Isolation and Characterization of Neural Progenitor Cells from Post-Mortem Human Cortex. J. Neurosci. Res. 2003, 74, 838–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, T.D.; Schwartz, P.H.; Taupin, P.; Kaspar, B.; Stein, S.A.; Gage, F.H. Progenitor cells from human brain after death. Nature 2001, 411, 42–43. [Google Scholar] [CrossRef]
- Cavazzin, C.; Neri, M.; Gritti, A. Isolate and culture precursor cells from the adult periventricular area. Methods Mol. Biol. 2013, 1059, 25–40. [Google Scholar] [CrossRef]
- Behnan, J.; Stangeland, B.; Langella, T.; Finocchiaro, G.; Tringali, G.; Meling, T.R.; Murrell, W. Identification and characterization of a new source of adult human neural progenitors. Cell Death Dis. 2017, 8, e2991. [Google Scholar] [CrossRef] [Green Version]
- Lévesque, M.F.; Neuman, T.; Rezak, M. Therapeutic Microinjection of Autologous Adult Human Neural Stem Cells and Differentiated Neurons for Parkinson’s Disease: Five-Year Post-Operative Outcome. Open Stem Cell J. 2009, 1, 10–19. [Google Scholar]
- Kastenberg, Z.J.; Odorico, J.S. Alternative sources of pluripotency: Science, ethics, and stem cells. Transplant. Rev. 2008, 22, 215–222. [Google Scholar] [CrossRef]
- Narsinh, K.H.; Plews, J.; Wu, J.C. Comparison of human induced pluripotent and embryonic stem cells: Fraternal or identical twins? Mol. Ther. 2011, 19, 635–638. [Google Scholar] [CrossRef]
- Kosanke, M.; Osetek, K.; Haase, A.; Wiehlmann, L.; Davenport, C.; Schwarzer, A.; Adams, F.; Kleppa, M.-J.; Schambach, A.; Merkert, S.; et al. Reprogramming enriches for somatic cell clones with small-scale mutations in cancer-associated genes. Mol. Ther. 2021, 29, 2535–2553. [Google Scholar] [CrossRef]
- Kuroda, T.; Yasuda, S.; Tachi, S.; Matsuyama, S.; Kusakawa, S.; Tano, K.; Miura, T.; Matsuyama, A.; Sato, Y. SALL3 expression balance underlies lineage biases in human induced pluripotent stem cell differentiation. Nat. Commun. 2019, 10, 2175. [Google Scholar] [CrossRef]
- Kobold, S.; Guhr, A.; Mah, N.; Bultjer, N.; Seltmann, S.; Seiler Wulczyn, A.E.M.; Stacey, G.; Jie, H.; Liu, W.; Löser, P.; et al. A Manually Curated Database on Clinical Studies Involving Cell Products Derived from Human Pluripotent Stem Cells. Stem Cell Rep. 2020, 15, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, S.; Fairchild, P.J.; Marsh, S.G.E.; Müller, C.R.; Turner, M.L.; Song, J.; Turner, D. Haplobanking induced pluripotent stem cells for clinical use. Stem Cell Res. 2020, 49, 102035. [Google Scholar] [CrossRef]
- Frederiksen, H.R.; Doehn, U.; Tveden-Nyborg, P.; Freude, K.K. Non-immunogenic Induced Pluripotent Stem Cells, a Promising Way Forward for Allogenic Transplantations for Neurological Disorders. Front. Genome Ed. 2021, 2, 30. [Google Scholar] [CrossRef]
- Hanatani, T.; Takasu, N. CiRA iPSC seed stocks (CiRA’s iPSC Stock Project). Stem Cell Res. 2021, 50, 102033. [Google Scholar] [CrossRef]
- Taylor, C.J.; Peacock, S.; Chaudhry, A.N.; Bradley, J.A.; Bolton, E.M. Generating an iPSC bank for HLA-matched tissue transplantation based on known donor and recipient hla types. Cell Stem Cell 2012, 11, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Huh, J.Y.; Turner, D.M.; Lee, S.; Robinson, J.; Stein, J.E.; Shim, S.H.; Hong, C.P.; Kang, M.S.; Nakagawa, M.; et al. Repurposing the Cord Blood Bank for Haplobanking of HLA-Homozygous iPSCs and Their Usefulness to Multiple Populations. Stem Cells 2018, 36, 1552–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdö, F.; Bührle, C.; Blunk, J.; Hoehn, M.; Xia, Y.; Fleischmann, B.; Föcking, M.; Küstermann, E.; Kolossov, E.; Hescheler, J.; et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J. Cereb. Blood Flow Metab. 2003, 23, 780–785. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Nishimura, M.; Morizane, A.; Takahashi, J.; Nozaki, K.; Hayashi, J.; Hashimoto, N. Survival and differentiation of neural progenitor cells derived from embryonic stem cells and transplanted into ischemic brain. J. Neurosurg. 2005, 103, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Duinsbergen, D.; Salvatori, D.; Eriksson, M.; Mikkers, H. Tumors Originating from Induced Pluripotent Stem Cells and Methods for Their Prevention. Ann. N. Y. Acad. Sci. 2009, 1176, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seminatore, C.; Polentes, J.; Ellman, D.; Kozubenko, N.; Itier, V.; Tine, S.; Tritschler, L.; Brenot, M.; Guidou, E.; Blondeau, J.; et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke 2010, 41, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Nori, S.; Okada, Y.; Nishimura, S.; Sasaki, T.; Itakura, G.; Kobayashi, Y.; Renault-Mihara, F.; Shimizu, A.; Koya, I.; Yoshida, R.; et al. Long-term safety issues of iPSC-based cell therapy in a spinal cord injury model: Oncogenic transformation with epithelial-mesenchymal transition. Stem Cell Rep. 2015, 4, 360–373. [Google Scholar] [CrossRef] [Green Version]
- Garitaonandia, I.; Gonzalez, R.; Christiansen-Weber, T.; Abramihina, T.; Poustovoitov, M.; Noskov, A.; Sherman, G.; Semechkin, A.; Snyder, E.; Kern, R. Neural stem cell tumorigenicity and biodistribution assessment for Phase I clinical trial in Parkinson’s disease. Sci. Rep. 2016, 6, 34478. [Google Scholar] [CrossRef] [Green Version]
- Sood, D.; Cairns, D.M.; Dabbi, J.M.; Ramakrishnan, C.; Deisseroth, K.; Black, L.D.; Santaniello, S.; Kaplan, D.L. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep. 2019, 9, 17874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammensee, S.; Kang, M.S.; Georgiou, K.; Kumar, S.; Schaffer, D.V. Dynamics of Mechanosensitive Neural Stem Cell Differentiation. Stem Cells 2017, 35, 497–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kropp, C.; Massai, D.; Zweigerdt, R. Progress and challenges in large-scale expansion of human pluripotent stem cells. Process. Biochem. 2017, 59, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Baghbaderani, B.A.; Mukhida, K.; Hong, M.; Mendez, I.; Behie, L.A. A Review of Bioreactor Protocols for Human Neural Precursor Cell Expansion in Preparation for Clinical Trials. Curr. Stem Cell Res. Ther. 2011, 6, 229–254. [Google Scholar] [CrossRef]
- Vierbuchen, T.; Ostermeier, A.; Pang, Z.P.; Kokubu, Y.; Südhof, T.C.; Wernig, M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010, 463, 1035–1041. [Google Scholar] [CrossRef] [Green Version]
- Thier, M.; Wörsdörfer, P.; Lakes, Y.B.; Gorris, R.; Herms, S.; Opitz, T.; Seiferling, D.; Quandel, T.; Hoffmann, P.; Nöthen, M.M.; et al. Direct conversion of fibroblasts into stably expandable neural stem cells. Cell Stem Cell 2012, 10, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Han, D.W.; Tapia, N.; Hermann, A.; Hemmer, K.; Höing, S.; Araúzo-Bravo, M.J.; Zaehres, H.; Wu, G.; Frank, S.; Moritz, S.; et al. Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell 2012, 10, 465–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, S.; Wörsdörfer, P.; Günther, K.; Thier, M.; Edenhofer, F. Derivation of adult human fibroblasts and their direct conversion into expandable neural progenitor cells. J. Vis. Exp. 2015, 101, e52831. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Declercq, J.; Eggermont, K.; Agirre, X.; Prosper, F.; Verfaillie, C.M. Zic3 induces conversion of human fibroblasts to stable neural progenitor-like cells. J. Mol. Cell Biol. 2012, 4, 252–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thier, M.C.; Hommerding, O.; Panten, J.; Pinna, R.; García-González, D.; Berger, T.; Wörsdörfer, P.; Assenov, Y.; Scognamiglio, R.; Przybylla, A.; et al. Identification of Embryonic Neural Plate Border Stem Cells and Their Generation by Direct Reprogramming from Adult Human Blood Cells. Cell Stem Cell 2019, 24, 166–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, R.R.; Szabo, E.; Benoit, Y.D.; Case, D.T.; Mechael, R.; Alamilla, J.; Lee, J.H.; Fiebig-Comyn, A.; Gillespie, D.C.; Bhatia, M. Activation of neural cell fate programs toward direct conversion of adult human fibroblasts into tri-potent neural progenitors using OCT-4. Stem Cells Dev. 2014, 23, 1937–1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Mitchell, R.R.; McNicol, J.D.; Shapovalova, Z.; Laronde, S.; Tanasijevic, B.; Milsom, C.; Casado, F.; Fiebig-Comyn, A.; Collins, T.J.; et al. Single Transcription Factor Conversion of Human Blood Fate to NPCs with CNS and PNS Developmental Capacity. Cell Rep. 2015, 11, 1367–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ring, K.L.; Tong, L.M.; Balestra, M.E.; Javier, R.; Andrews-Zwilling, Y.; Li, G.; Walker, D.; Zhang, W.R.; Kreitzer, A.C.; Huang, Y. Direct reprogramming of mouse and human fibroblasts into multipotent neural stem cells with a single factor. Cell Stem Cell 2012, 11, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Mirakhori, F.; Zeynali, B.; Rassouli, H.; Shahbazi, E.; Hashemizadeh, S.; Kiani, S.; Salekdeh, G.H.; Baharvand, H. Induction of neural progenitor-like cells from human fibroblasts via a genetic material-free approach. PLoS ONE 2015, 10, e0135479. [Google Scholar] [CrossRef]
- Kim, B.E.; Choi, S.W.; Shin, J.H.; Kim, J.J.; Kang, I.; Lee, B.C.; Lee, J.Y.; Kook, M.G.; Kang, K.S. Single-Factor SOX2 Mediates Direct Neural Reprogramming of Human Mesenchymal Stem Cells via Transfection of In Vitro Transcribed mRNA. Cell Transplant. 2018, 27, 1154–1167. [Google Scholar] [CrossRef] [Green Version]
- Maucksch, C.; Jones, K.S.; Connor, B. Concise Review: The Involvement of SOX2 in Direct Reprogramming of Induced Neural Stem/Precursor Cells. Stem Cells Transl. Med. 2013, 2, 579–583. [Google Scholar] [CrossRef]
- Castaño, J.; Menendez, P.; Bruzos-Cidon, C.; Straccia, M.; Sousa, A.; Zabaleta, L.; Vazquez, N.; Zubiarrain, A.; Sonntag, K.C.; Ugedo, L.; et al. Fast and efficient neural conversion of human hematopoietic cells. Stem Cell Reports 2014, 3, 1118–1131. [Google Scholar] [CrossRef] [Green Version]
- Velychko, S.; Kang, K.; Kim, S.M.; Kwak, T.H.; Kim, K.P.; Park, C.; Hong, K.; Chung, C.H.; Hyun, J.K.; MacCarthy, C.M.; et al. Fusion of Reprogramming Factors Alters the Trajectory of Somatic Lineage Conversion. Cell Rep. 2019, 27, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.P.; Li, C.; Bunina, D.; Jeong, H.W.; Ghelman, J.; Yoon, J.; Shin, B.; Park, H.; Han, D.W.; Zaugg, J.B.; et al. Donor cell memory confers a metastable state of directly converted cells. Cell Stem Cell 2021, 28, 1291–1306e10. [Google Scholar] [CrossRef] [PubMed]
- Carey, B.W.; Markoulaki, S.; Hanna, J.H.; Faddah, D.A.; Buganim, Y.; Kim, J.; Ganz, K.; Steine, E.J.; Cassady, J.P.; Creyghton, M.P.; et al. Reprogramming factor stoichiometry influences the epigenetic state and biological properties of induced pluripotent stem cells. Cell Stem Cell 2011, 9, 588–598. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Xiong, S.; Yu, P.; Liu, F.; Cheng, L. Direct Conversion of Mouse Fibroblasts into Neural Stem Cells by Chemical Cocktail Requires Stepwise Activation of Growth Factors and Nup210. Cell Rep. 2018, 24, 1355–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.C.; Lim, Y.; Duffieldl, M.D.; Li, H.; Liu, J.; Abdul Manaph, N.P.; Yang, M.; Keating, D.J.; Zhou, X.F. Direct reprogramming of mouse fibroblasts to neural stem cells by small molecules. Stem Cells Int. 2016, 2016, 4304916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, Y.H.; Sun, Y.J.; Zhu, S.; Zheng, J.; Liu, K.; Cao, N.; Li, K.; Huang, Y.; Ding, S. Pharmacological reprogramming of fibroblasts into neural stem cells by signaling-directed transcriptional activation. Cell Stem Cell 2016, 18, 653–667. [Google Scholar] [CrossRef] [Green Version]
- Erharter, A.; Rizzi, S.; Mertens, J.; Edenhofer, F. Take the shortcut—Direct conversion of somatic cells into induced neural stem cells and their biomedical applications. FEBS Lett. 2019, 593, 3353–3369. [Google Scholar] [CrossRef]
- Werbowetski-Ogilvie, T.E.; Morrison, L.C.; Fiebig-Comyn, A.; Bhatia, M. In vivo generation of neural tumors from neoplastic pluripotent stem cells models early human pediatric brain tumor formation. Stem Cells 2012, 30, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Manley, N.C.; Priest, C.A.; Denham, J.; Wirth, E.D.; Lebkowski, J.S. Human Embryonic Stem Cell-Derived Oligodendrocyte Progenitor Cells: Preclinical Efficacy and Safety in Cervical Spinal Cord Injury. Stem Cells Transl. Med. 2017, 6, 1917–1929. [Google Scholar] [CrossRef]
- Roy, N.S.; Cleren, C.; Singh, S.K.; Yang, L.; Beal, M.F.; Goldman, S.A. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nat. Med. 2006, 12, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Pendharkar, A.V.; Chua, J.Y.; Andres, R.H.; Wang, N.; Gaeta, X.; Wang, H.; De, A.; Choi, R.; Chen, S.; Rutt, B.K.; et al. Biodistribution of neural stem cells after intravascular therapy for Hypoxic-ischemia. Stroke 2010, 41, 2064–2070. [Google Scholar] [CrossRef] [Green Version]
- Melzi, R.; Antonioli, B.; Mercalli, A.; Battaglia, M.; Valle, A.; Pluchino, S.; Galli, R.; Sordi, V.; Bosi, E.; Martino, G.; et al. Co-graft of allogeneic immune regulatory neural stem cells (NPC) and pancreatic islets mediates tolerance, while inducing NPC-derived tumors in mice. PLoS ONE 2010, 5, e10357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacigaluppi, M.; Pluchino, S.; Jametti, L.P.; Kilic, E.; Kilic, Ü.; Salani, G.; Brambilla, E.; West, M.J.; Comi, G.; Martino, G.; et al. Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms. Brain 2009, 132, 2239–2251. [Google Scholar] [CrossRef] [Green Version]
- Jakubs, K.; Nanobashvili, A.; Bonde, S.; Ekdahl, C.T.; Kokaia, Z.; Kokaia, M.; Lindvall, O. Environment Matters: Synaptic Properties of Neurons Born in the Epileptic Adult Brain Develop to Reduce Excitability. Neuron 2006, 52, 1047–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darsalia, V.; Allison, S.J.; Cusulin, C.; Monni, E.; Kuzdas, D.; Kallur, T.; Lindvall, O.; Kokaia, Z. Cell number and timing of transplantation determine survival of human neural stem cell grafts in stroke-damaged rat brain. J. Cereb. Blood Flow Metab. 2011, 31, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Luan, Z.; Liu, W.P.; Qu, S.Q.; Qu, S.Q.; Hu, X.H.; Wang, Z.Y.; He, S.; Liu, C.Q.; Xiao, M. Treatment of newborns with severe injured brain with transplantation of human neural precursor cells. Zhonghua Er Ke Za Zhi 2011, 49, 445–449. [Google Scholar] [CrossRef]
- Wang, Y.K.; Zhu, W.W.; Wu, M.H.; Wu, Y.H.; Liu, Z.X.; Liang, L.M.; Sheng, C.; Hao, J.; Wang, L.; Li, W.; et al. Human Clinical-Grade Parthenogenetic ESC-Derived Dopaminergic Neurons Recover Locomotive Defects of Nonhuman Primate Models of Parkinson’s Disease. Stem Cell Rep. 2018, 11, 171–182. [Google Scholar] [CrossRef]
- Wu, D.Y.; Zhang, X.; Miao, Y.L. Reprogramming of Aged Cells into Pluripotent Stem Cells by Nuclear Transfer. Methods Mol. Biol. 2019, 2045, 271–281. [Google Scholar] [CrossRef]
- Goldman, S.A. Stem and Progenitor Cell-Based Therapy of the Central Nervous System: Hopes, Hype, and Wishful Thinking. Cell Stem Cell 2016, 18, 174–188. [Google Scholar] [CrossRef] [Green Version]
- Narbonne, P. The effect of age on stem cell function and utility for therapy. Cell Med. 2018, 10, 2155179018773756. [Google Scholar] [CrossRef] [Green Version]
- Navarro Negredo, P.; Yeo, R.W.; Brunet, A. Aging and Rejuvenation of Neural Stem Cells and Their Niches. Cell Stem Cell 2020, 27, 202–223. [Google Scholar] [CrossRef] [PubMed]
- Conti, L.; Cattaneo, E. Neural stem cell systems: Physiological players or in vitro entities? Nat. Rev. Neurosci. 2010, 11, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hu, W.W.; Jiang, Z.; Feng, M.J. Advances in treatment of neurodegenerative diseases: Perspectives for combination of stem cells with neurotrophic factors. World J. Stem Cells 2020, 12, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Tupone, M.G.; D’Angelo, M.; Castelli, V.; Catanesi, M.; Benedetti, E.; Cimini, A. A State-of-the-Art of Functional Scaffolds for 3D Nervous Tissue Regeneration. Front. Bioeng. Biotechnol. 2021, 9, 639765. [Google Scholar] [CrossRef]
- Kokaia, Z.; Martino, G.; Schwartz, M.; Lindvall, O. Cross-talk between neural stem cells and immune cells: The key to better brain repair? Nat. Neurosci. 2012, 15, 1078–1087. [Google Scholar] [CrossRef] [PubMed]
- European Commission. EudraLex—Volume 4—Good Manufacturing Practice (GMP) guidelines. In The Rules Governing Medicinal Products in the European Union; European Commission: Brussels, Belgium, 2017. [Google Scholar]
- Rossetti, T.; Nicholls, F.; Modo, M. Intracerebral cell implantation: Preparation and characterization of cell suspensions. Cell Transplant. 2016, 25, 645–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahlberg, B.; Ghuman, H.; Liu, J.R.; Modo, M. Ex vivo biomechanical characterization of syringe-needle ejections for intracerebral cell delivery. Sci. Rep. 2018, 8, 9194. [Google Scholar] [CrossRef]
- Harrison, R.; Lugo Leija, H.A.; Strohbuecker, S.; Crutchley, J.; Marsh, S.; Denning, C.; El Haj, A.; Sottile, V. Development and validation of broad-spectrum magnetic particle labelling processes for cell therapy manufacturing. Stem Cell Res. Ther. 2018, 9, 248. [Google Scholar] [CrossRef]
- Piao, J.; Zabierowski, S.; Dubose, B.N.; Hill, E.J.; Navare, M.; Claros, N.; Rosen, S.; Ramnarine, K.; Horn, C.; Fredrickson, C.; et al. Preclinical Efficacy and Safety of a Human Embryonic Stem Cell-Derived Midbrain Dopamine Progenitor Product, MSK-DA01. Cell Stem Cell 2021, 28, 217–229. [Google Scholar] [CrossRef]
- Uchida, N.; Buck, D.W.; He, D.; Reitsma, M.J.; Masek, M.; Phan, T.V.; Tsukamoto, A.S.; Gage, F.H.; Weissman, I.L. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA 2000, 97, 14720–14725. [Google Scholar] [CrossRef] [Green Version]
- Unger, C.; Skottman, H.; Blomberg, P.; Sirac dilber, M.; Hovatta, O. Good manufacturing practice and clinical-grade human embryonic stem cell lines. Hum. Mol. Genet. 2008, 17, R48–R53. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.J.; Piltti, K.M.; Hooshmand, M.J.; Nishi, R.A.; Cummings, B.J. Preclinical Efficacy Failure of Human CNS-Derived Stem Cells for Use in the Pathway Study of Cervical Spinal Cord Injury. Stem Cell Rep. 2017, 8, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Servick, K. Failed spinal cord trial offers cautionary tale. Science 2017, 355, 679. [Google Scholar] [CrossRef]
- Marsh, S.E.; Yeung, S.T.; Torres, M.; Lau, L.; Davis, J.L.; Monuki, E.S.; Poon, W.W.; Blurton-Jones, M. HuCNS-SC Human NSCs Fail to Differentiate, Form Ectopic Clusters, and Provide No Cognitive Benefits in a Transgenic Model of Alzheimer’s Disease. Stem Cell Rep. 2017, 8, 235–248. [Google Scholar] [CrossRef]
- Deleyrolle, L.P.; Reynolds, B.A. Isolation, expansion, and differentiation of adult mammalian neural stem and progenitor cells using the neurosphere assay. Methods Mol. Biol. 2009, 549, 91–101. [Google Scholar] [CrossRef]
- Yang, S.; Cao, Z.; Zhu, J.; Zhang, Z.; Zhao, H.; Zhao, L.; Sun, X.; Wang, X. In Vitro Monolayer Culture of Dispersed Neural Stem Cells on the E-Cadherin-Based Substrate with Long-Term Stemness Maintenance. ACS Omega 2019, 4, 18136–18146. [Google Scholar] [CrossRef]
- Office of Regulatory Affairs; Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. FDA Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice; Food and Drug Administration: Rockville, MD, USA, 2004.
- Center for Biologics Evaluation and Research. FDA Guidance for Industry: Manufacturing Considerations for Licensed and Investigational Cellular and Gene Therapy Products during COVID-19 Public Health Emergency (FDA-2020-D-1137); Food and Drug Administration: Rockville, MD, USA, 2021.
- Cobo, F.; Crela, D.; Concha, A. Airborne particle monitoring in clean room environments for stem cell cultures. Biotechnol. J. 2008, 3, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Dalmaso, G.; Campanella, A.; Lazzeri, P. Continuous and Effective Microbiological Air Monitoring in Critical Environments: A Comparison of Analytical Methodologies. PDA J. Pharm. Sci. Technol. 2020, 74, 446–455. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines (EDQM) 2.6.1. Sterility. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- European Directorate for the Quality of Medicines (EDQM) 2.6.27. Microbiological control of cellular products. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- European Directorate for the Quality of Medicines (EDQM) 2.6.7. Mycoplasma. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; Volume 2.6.7, ISBN 978-92-871-8921-9.
- European Directorate for the Quality of Medicines (EDQM) 2.6.14. Bacterial endotoxin. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- European Directorate for the Quality of Medicines (EDQM) 5.2.3. Cell substrates for the production of vaccines for human use. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- European Directorate for the Quality of Medicines (EDQM) 5.1.7. Viral safety. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- European Directorate for the Quality of Medicines (EDQM) 2.7.29. Nucleated cell count and viability. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- FDA Content and Review of Chemistry, Manufacturing, and Control. (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs); Rockville, MD, USA, 2008.
- European Directorate for the Quality of Medicines (EDQM) 2.7.24. Flow citometry. In European Pharmacopoeia, 9th ed.; EDQM: Strasbourg, France, 2016; ISBN 978-92-871-8134-3.
- European Directorate for the Quality of Medicines (EDQM) 2.6.21. Nucleic acid amplification techniques. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9e.
- European Directorate for the Quality of Medicines (EDQM) 2.7.1 Immunochemical methods. In European Pharmacopoeia, 7th ed.; EDQM: Strasbourg, France, 2010; ISBN 978-92-871-6700-2.
- European Medicines Agency (EMA) Guideline On Human Cell-Based Medicinal Product (Doc. Ref. EMEA/CHMP/410869/2006); EMA: London, UK, 2008.
- European Directorate for the Quality of Medicines (EDQM) 2.6.34. Host-cell protein assays. In European Pharmacopoeia, 10th ed.; EDQM: Strasbourg, France, 2020; ISBN 978-92-871-8921-9.
- Martino, G.; Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006, 7, 395–406. [Google Scholar] [CrossRef]
- FDA Potency Tests for Cellular and Gene Therapy Products (FDA-2008-D-0520); FDA: Rockville, MD, USA, 2011.
- Tiwari, S.S.; Desai, P.N. Unproven Stem Cell Therapies in India: Regulatory Challenges and Proposed Paths Forward. Cell Stem Cell 2018, 23, 649–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spits, C. Stem cell therapy: Facts and fiction. Facts Views Vis. ObGyn 2012, 4, 195–197. [Google Scholar] [PubMed]
| Test | Method | Comments |
|---|---|---|
| Sterility | Turbidity testing after growth in TSB and TG media (Ph. Eur.2.6.1) [134] or media for automated detection systems (Ph. Eur. 2.6.27) [135] | NA |
| Mycoplasma | PCR, culture (Ph. Eur. 2.6.7) [136] | NA |
| Endotoxins | LAL assay (Ph. Eur. 2.6.14) [137] | NA |
| Adventitious viruses | PCR, cytopathic effect, others (Ph. Eur. 5.2.3 and Ph. Eur. 5.1.7) [138,139] | The viruses to detect should be evaluated case by case by risk analysis (Ph. Eur. 5.1.7) [139] |
| Cell number and Population doublings | Haematocytometer, automated cell counters, flow cytometer (Ph. Eur. 2.7.29) [140] | NA |
| Viability | Trypan blue, Calcein/Ethidium, 7AAD, PI (Ph. Eur. 2.7.29) [140] | This should be higher than 70% when transplanted [141] |
| Identity/Purity | Flow cytometry (Ph. Eur. 2.7.24) [142], PCR (Ph. Eur. 2.6.21) [143], immunofluorescence (Ph. Eur. 2.7.1) [144], others (Ph. Eur. 5.2.3) [138]. | Markers of the possible contaminant cells (e.g., PSC) should be included [145] |
| Potency | Tri-lineage differentiation potential, expression of neurotrophic factors, etc. (Ph. Eur. 2.6.34) [146] | NA |
| Tumorigenicity | Karyotype, CGH array, trophic factor dependence, colony-forming assays in softagar, in vivo tumor formation assay in athymic mice (Ph. Eur. 5.2.3) [138] | NA |
| DNA Fingerprint | STR, VNTR (Ph. Eur. 5.2.3) [138] | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandez-Muñoz, B.; Garcia-Delgado, A.B.; Arribas-Arribas, B.; Sanchez-Pernaute, R. Human Neural Stem Cells for Cell-Based Medicinal Products. Cells 2021, 10, 2377. https://doi.org/10.3390/cells10092377
Fernandez-Muñoz B, Garcia-Delgado AB, Arribas-Arribas B, Sanchez-Pernaute R. Human Neural Stem Cells for Cell-Based Medicinal Products. Cells. 2021; 10(9):2377. https://doi.org/10.3390/cells10092377
Chicago/Turabian StyleFernandez-Muñoz, Beatriz, Ana Belen Garcia-Delgado, Blanca Arribas-Arribas, and Rosario Sanchez-Pernaute. 2021. "Human Neural Stem Cells for Cell-Based Medicinal Products" Cells 10, no. 9: 2377. https://doi.org/10.3390/cells10092377