Greenhouse Evaluation of the Agronomic Potential of Urban Wastewater-Based Fertilizers: Sewage Sludge and Struvite for Lettuce Production in Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin and Characterization of Sewage Sludge, Struvite, and Soil
2.2. Experimental Setup
2.3. Soil Physicochemical Characterization
2.4. Plant Analysis
2.5. Statistical Analysis
3. Results and Discussion
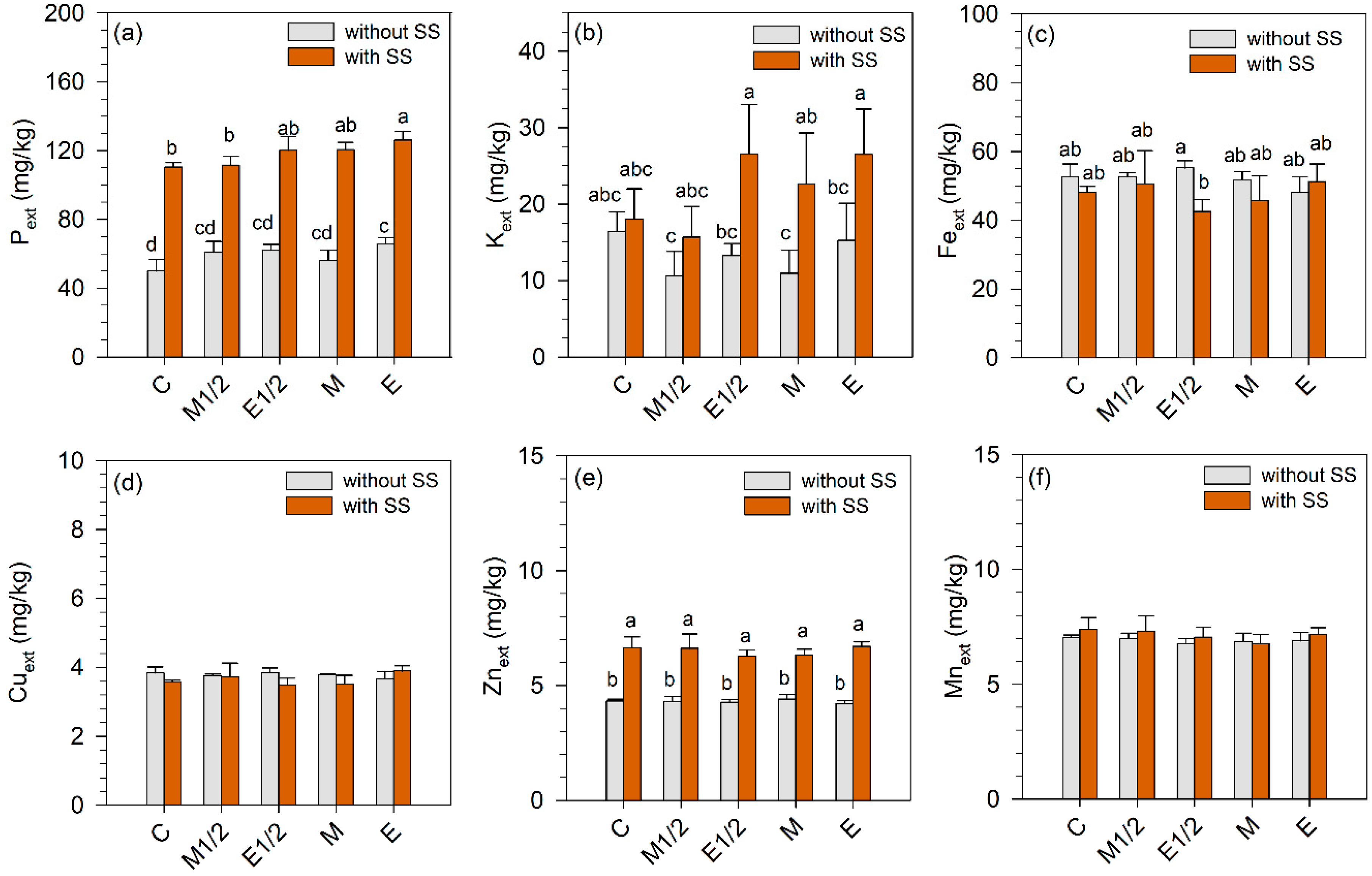
3.1. Effects of Sewage Sludge and Fertilizer Application on Soil Properties
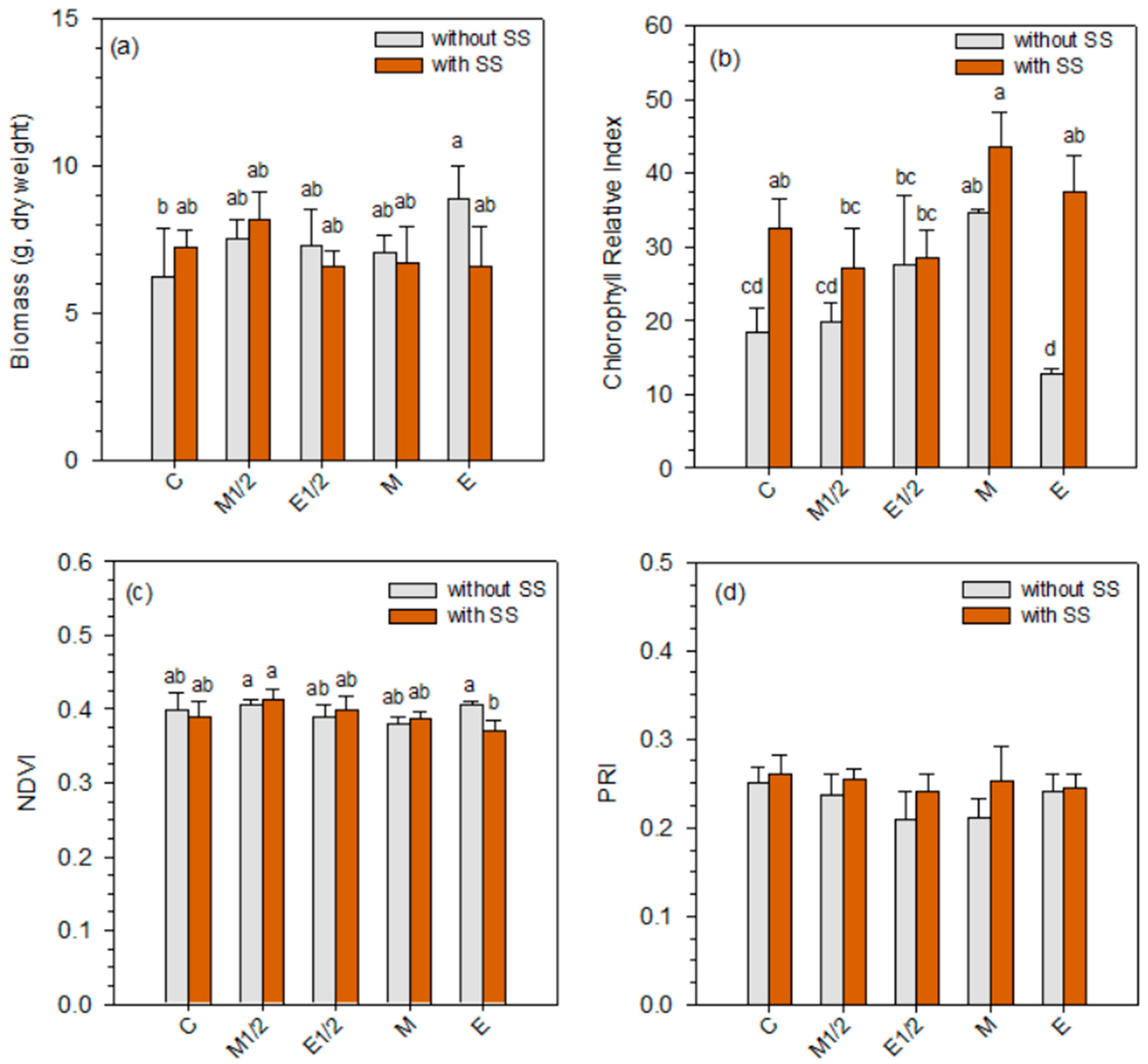
3.2. Effect of Sludge and Fertilizer Application on Lettuce Growth and Physiological Parameters
3.3. Effects of Sludge and Fertilizer Application on Lettuce Nutritional Status
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferreira, C.S.S.; Seifollahi-Aghmiuni, S.; Destouni, G.; Ghajarnia, N.; Kalantari, Z. Soil Degradation in the European Mediterranean Region: Processes, Status and Consequences. Sci. Total Environ. 2022, 805, 150106. [Google Scholar] [CrossRef]
- EIP-Agri. Soil Organic Matter in Mediterranean Regions; EIP-Agri: Saint-Josse-ten-Noode, Belgium, 2015. [Google Scholar]
- Withers, P.J.A.; Nash, D.M.; Laboski, C.A.M. Environmental Management of Phosphorus Fertilizers. Phosphorus Agric. Environ. 2005, 46, 781–827. [Google Scholar]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic Phosphorus Imbalances across the World’s Croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef]
- Muntwyler, A.; Panagos, P.; Pfister, S.; Lugato, E. Assessing the Phosphorus Cycle in European Agricultural Soils: Looking beyond Current National Phosphorus Budgets. Sci. Total Environ. 2024, 906, 167143. [Google Scholar] [CrossRef]
- Li, Q.; Li, J.; Cui, X.; Wei, D.; Ma, Y. On-Farm Assessment of Biosolids Effects on Nitrogen and Phosphorus Accumulation in Soils. J. Integr. Agric. 2012, 11, 1545–1554. [Google Scholar] [CrossRef]
- International Fertilizer Association. Reducing Emissions from Fertilizer Use—Executive Summary; International Fertilizer Association: Paris, France, 2022. [Google Scholar]
- Mabrouk, O.; Hamdi, H.; Sayadi, S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H.; Zouari, N. Reuse of Sludge as Organic Soil Amendment: Insights into the Current Situation and Potential Challenges. Sustainability 2023, 15, 6773. [Google Scholar] [CrossRef]
- Lucia, C.; Badalucco, L.; Corsino, S.F.; Galati, A.; Iovino, M.; Muscarella, S.M.; Paliaga, S.; Torregrossa, M.; Laudicina, V.A. Management and Valorisation of Sewage Sludge to Foster the Circular Economy in the Agricultural Sector. Discov. Soil 2025, 2, 80. [Google Scholar] [CrossRef]
- European Commission. Commission Staff Working Document Evaluation—Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture SWD/2023/0157 Final; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- Bünemann, E.K.; Reimer, M.; Smolders, E.; Smith, S.R.; Bigalke, M.; Palmqvist, A.; Brandt, K.K.; Möller, K.; Harder, R.; Hermann, L.; et al. Do Contaminants Compromise the Use of Recycled Nutrients in Organic Agriculture? A Review and Synthesis of Current Knowledge on Contaminant Concentrations, Fate in the Environment and Risk Assessment. Sci. Total Environ. 2024, 912, 168901. [Google Scholar] [CrossRef]
- European Commission. Executive Summary of the Evalution: Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge Is Used in Agriculture; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- López-Rayo, S.; Laursen, K.H.; Lekfeldt, J.D.S.; Delle Grazie, F.; Magid, J. Long-Term Amendment of Urban and Animal Wastes Equivalent to More than 100 Years of Application Had Minimal Effect on Plant Uptake of Potentially Toxic Elements. Agric. Ecosyst. Environ. 2016, 231, 44–53. [Google Scholar] [CrossRef]
- Riber, L.; Poulsen, P.H.B.; Al-Soud, W.A.; Skov Hansen, L.B.; Bergmark, L.; Brejnrod, A.; Norman, A.; Hansen, L.H.; Magid, J.; Sørensen, S.J. Exploring the Immediate and Long-Term Impact on Bacterial Communities in Soil Amended with Animal and Urban Organic Waste Fertilizers Using Pyrosequencing and Screening for Horizontal Transfer of Antibiotic Resistance. FEMS Microbiol. Ecol. 2014, 90, 206–224. [Google Scholar] [CrossRef]
- Rede, D.; Teixeira, I.; Delerue-Matos, C.; Fernandes, V.C. Assessing Emerging and Priority Micropollutants in Sewage Sludge: Environmental Insights and Analytical Approaches. Environ. Sci. Pollut. Res. 2023, 31, 3152–3168. [Google Scholar] [CrossRef]
- Muys, M.; Phukan, R.; Brader, G.; Samad, A.; Moretti, M.; Haiden, B.; Pluchon, S.; Roest, K.; Vlaeminck, S.E.; Spiller, M. A Systematic Comparison of Commercially Produced Struvite: Quantities, Qualities and Soil-Maize Phosphorus Availability. Sci. Total Environ. 2021, 756, 143726. [Google Scholar] [CrossRef]
- Galamini, G.; Ferretti, G.; Medoro, V.; Eftekhari, N.; Favero, M.; Faccini, B.; Coltorti, M. Applying Natural and K-Enriched Zeolite Before Struvite Precipitation Improved the Recovery of NH4+ from Liquid Digestate and the Reagent Use Efficiency. Int. J. Environ. Res. 2024, 18, 44. [Google Scholar] [CrossRef]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in Struvite Precipitation Technologies for Nutrients Removal and Recovery from Aqueous Waste and Wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Otieno, B.; Funani, C.K.; Khune, S.M.; Kabuba, J.; Osifo, P. Struvite Recovery from Anaerobically Digested Waste-Activated Sludge: A Short Review. J. Mater. Res. 2023, 38, 3815–3826. [Google Scholar] [CrossRef]
- Santos, A.F.; Almeida, P.V.; Alvarenga, P.; Gando-Ferreira, L.M.; Quina, M.J. From Wastewater to Fertilizer Products: Alternative Paths to Mitigate Phosphorus Demand in European Countries. Chemosphere 2021, 284, 131258. [Google Scholar] [CrossRef]
- ISO 6579:2002; Microbiology of Food and Animal Feeding Stuff—Horizontal Method for the Detection of Salmonella spp. ISO: Geneva, Switzerland, 2002.
- ISO 16649-2:2001; Microbiology of Food and animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli, Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-chloro-3-indolyl Beta-D-Glucuronide. ISO: Geneva, Switzerland, 2001.
- WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; WRB: Vienna, Austria, 2022. [Google Scholar]
- Veloso, A.; Sempiterno, C.; Calouro, F.; Rebelo, F.; Pedra, F.; Castro, I.; Gonçalves, M.C.; Marcelo, M.E.; Pereira, P.; Fareleira, P.; et al. Manual de Fertilização Das Culturas, 3rd ed.; Instituto Nacional de Investigação Agrária e Veterinária, I.P.-INIAV., Ed.: Lisbon, Portugal, 2022; ISBN 978-972-579-063-2. (In Portuguese) [Google Scholar]
- ISO 3696:1987; Water for Analytical Laboratory Use—Specification and Test Methods. ISO: Geneva, Switzerland, 1987.
- Riehm, H. Die Ammoniumlaktatessigsaure-Methode Zur Bestimmung Der Leichtloeslichen Phosphosaure in Karbonathaltigen Boden. Agrochimica 1958, 3, 49–65. [Google Scholar]
- Korzeniowska, J.; Stanislawska-Glubiak, E. Evaluation of the Egner–Riehm DL and Mehlich 3 Tests for the Determination of Phosphorus: The Influence of Soil Properties on Extraction Efficiency and Test Conversion. Agronomy 2024, 14, 2921. [Google Scholar] [CrossRef]
- EN 13654; Soil Improvers and Growing Media-Determination of Nitrogen—Part 2: Dumas Method. CEN: Brussels, Belgium, 2001.
- CEN. Soil Improvers and Growing Media—Extraction of Aqua Regia Soluble Elements; CEN: Brussels, Belgium, 2001. [Google Scholar]
- Gómez-Suárez, A.D.; Nobile, C.; Faucon, M.-P.; Pourret, O.; Houben, D. Fertilizer Potential of Struvite as Affected by Nitrogen Form in the Rhizosphere. Sustainability 2020, 12, 2212. [Google Scholar] [CrossRef]
- Antoniadis, V.; Koutroubas, S.D.; Fotiadis, S. Nitrogen, Phosphorus, and Potassium Availability in Manure- and Sewage Sludge–Applied Soil. Commun. Soil Sci. Plant Anal. 2015, 46, 393–404. [Google Scholar] [CrossRef]
- Achkir, A.; Aouragh, A.; El Mahi, M.; Lotfi, E.M.; Labjar, N.; EL Bouch, M.; Ouahidi, M.L.; Badza, T.; Farhane, H.; EL Moussaoui, T. Implication of Sewage Sludge Increased Application Rates on Soil Fertility and Heavy Metals Contamination Risk. Emerg. Contam. 2023, 9, 100200. [Google Scholar] [CrossRef]
- Zuzunaga-Rosas, J.; Calone, R.; Mircea, D.M.; Shakya, R.; Ibáñez-Asensio, S.; Boscaiu, M.; Fita, A.; Moreno-Ramón, H.; Vicente, O. Mitigation of Salt Stress in Lettuce by a Biostimulant That Protects the Root Absorption Zone and Improves Biochemical Responses. Front. Plant Sci. 2024, 15, 1341714. [Google Scholar] [CrossRef]
- Kurunc, A. Effects of Water and Salinity Stresses on Growth, Yield, and Water Use of Iceberg Lettuce. J. Sci. Food Agric. 2021, 101, 5688–5696. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Mourinha, C.; Farto, M.; Dôres, J.; Patanita, M.; Cunha-Queda, C.; Natal-da-Luz, T.; Renaud, M.; Sousa, J.P. Recycling Organic Wastes to Agricultural Land as a Way to Improve Its Quality: A Field Study to Evaluate Benefits and Risks. Waste Manag. 2017, 61, 582–592. [Google Scholar] [CrossRef]
- Almási, C.; Orosz, V.; Tóth, T.; Mansour, M.M.; Demeter, I.; Henzsel, I.; Bogdányi, Z.; Szegi, T.A.; Makádi, M. Effects of Sewage Sludge Compost on Carbon, Nitrogen, Phosphorus, and Sulfur Ratios and Soil Enzyme Activities in a Long-Term Experiment. Agronomy 2025, 15, 143. [Google Scholar] [CrossRef]
- Zayed, O.; Hewedy, O.A.; Abdelmoteleb, A.; Ali, M.; Youssef, M.S.; Roumia, A.F.; Seymour, D.; Yuan, Z.-C. Nitrogen Journey in Plants: From Uptake to Metabolism, Stress Response, and Microbe Interaction. Biomolecules 2023, 13, 1443. [Google Scholar] [CrossRef]
- Norton, J.; Ouyang, Y. Controls and Adaptive Management of Nitrification in Agricultural Soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef]
- Blombäck, K.; Bolster, C.H.; Lindsjö, A.; Hesse, K.; Linefur, H.; Parvage, M.M. Comparing Measures for Determination of Phosphorus Saturation as a Method to Estimate Dissolved P in Soil Solution. Geoderma 2021, 383, 114708. [Google Scholar] [CrossRef]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in Agriculture—Status and Perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Havlin, J.L. Fertility. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Pérez-Piqueres, A.; Ribó, M.; Rodríguez-Carretero, I.; Quiñones, A.; Canet, R. Struvite as a Sustainable Fertilizer in Mediterranean Soils. Agronomy 2023, 13, 1391. [Google Scholar] [CrossRef]
- Bastida, F.; Jehmlich, N.; Martínez-Navarro, J.; Bayona, V.; García, C.; Moreno, J.L. The Effects of Struvite and Sewage Sludge on Plant Yield and the Microbial Community of a Semiarid Mediterranean Soil. Geoderma 2019, 337, 1051–1057. [Google Scholar] [CrossRef]
- Li, Y.; He, N.; Hou, J.; Xu, L.; Liu, C.; Zhang, J.; Wang, Q.; Zhang, X.; Wu, X. Factors Influencing Leaf Chlorophyll Content in Natural Forests at the Biome Scale. Front. Ecol. Evol. 2018, 6, 64. [Google Scholar] [CrossRef]
- He, J.; Ma, J.; Cao, Q.; Wang, X.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Development of Critical Nitrogen Dilution Curves for Different Leaf Layers within the Rice Canopy. Eur. J. Agron. 2022, 132, 126414. [Google Scholar] [CrossRef]
- Kizilgeci, F.; Yildirim, M.; Islam, M.S.; Ratnasekera, D.; Iqbal, M.A.; Sabagh, A. EL Normalized Difference Vegetation Index and Chlorophyll Content for Precision Nitrogen Management in Durum Wheat Cultivars under Semi-Arid Conditions. Sustainability 2021, 13, 3725. [Google Scholar] [CrossRef]
- Sukhova, E.; Sukhov, V. Analysis of Light-Induced Changes in the Photochemical Reflectance Index (PRI) in Leaves of Pea, Wheat, and Pumpkin Using Pulses of Green-Yellow Measuring Light. Remote Sens. 2019, 11, 810. [Google Scholar] [CrossRef]
- Wang, J.; Xue, L.; Hou, P.; Hao, T.; Xue, L.; Zhang, X.; Sun, T.; Lobanov, S.; Yang, L. Struvite as P Fertilizer on Yield, Nutrient Uptake and Soil Nutrient Status in the Rice–Wheat Rotation System: A Two-Year Field Observation. Agronomy 2023, 13, 2948. [Google Scholar] [CrossRef]
| Struvite | Sewage Sludge | ||||
|---|---|---|---|---|---|
| pH | 9.38 | 6.43 | Heavy metal per hectare load after 12 t/ha SS application | ||
| EC (mS/cm) | 0.11 ± 0.05 | 7.23 ± 0.59 | |||
| OM (%) | 39.1 ± 0.25 | 70.1 ± 1.23 | |||
| TP (mg P/gdb) | 83.8 ± 4.94 | 15.1 ± 0.85 | |||
| N (mg TKN/gdb) | 28.3 ± 1.20 | 58.2 ± 0.22 | |||
| Mg (mg/gdb) | 27.1 ± 2.36 | 3.85 ± 0.06 | |||
| Ca (mg/gdb) | 24.7 ± 3.21 | 16.4 ± 0.63 | |||
| Heavy metals (mg/kgdb) | Value | Value | Legal limits according to Council Directive 86/278/EEC * | (kg/ha) ** | Legal limit (kg/ha/year) * |
| Pb | n.d. | 28.1 ± 0.25 | 750–1200 | 0.34 | 15 |
| Cr | n.d. | 36.8 ± 1.52 | - | 0.45 | - |
| Zn | 12.0 ± 1.35 | 970 ± 56.1 | 2500–4000 | 11.8 | 30 |
| Cd | n.d. | 1.2 ± 0.05 | 20–40 | 0.01 | 0.15 |
| Cu | n.d. | 340 ± 32.1 | 1000–1750 | 4.15 | 12 |
| Ni | n.d. | 20.9 ± 1.63 | 300–400 | 0.25 | 3 |
| Treatment Designation | Sludge (t/ha) | Mineral P Fertilizer (kg P2O5/ha) | Struvite (kg P2O5/ha) |
|---|---|---|---|
| C | 0 | 0 | 0 |
| CS | 12 | 0 | 0 |
| M1/2 | 0 | 30 | 0 |
| M1/2S | 12 | 30 | 0 |
| E1/2 | 0 | 0 | 30 |
| E1/2S | 12 | 0 | 30 |
| M | 0 | 60 | 0 |
| MS | 12 | 60 | 0 |
| E | 0 | 0 | 60 |
| ES | 12 | 0 | 60 |
| Treatment | pH | EC (µS/cm) | OM (g/kg) | NH4+-N (mg/kg) | NO3−-N (mg/kg) | Nmin (mg/kg) |
|---|---|---|---|---|---|---|
| C | 5.14 ± 0.12 | 542 ± 63 ab | 6.7 ± 0.2 b | 49 ± 13 | 79 ± 6 | 127 ± 19 |
| CS | 5.19 ± 0.14 | 620 ± 96 ab | 7.5 ± 0.8 ab | 37 ± 18 | 69 ± 10 | 105 ± 27 |
| M1/2 | 5.20 ± 0.32 | 470 ± 53 ab | 6.8 ± 0.4 b | 31 ± 9 | 61 ± 6 | 92 ± 14 |
| M1/2S | 5.00 ± 0.05 | 625 ± 51 ab | 8.4 ± 0.3 a | 37 ± 13 | 67 ± 3 | 104 ± 14 |
| E1/2 | 5.39 ± 0.40 | 443 ± 112 b | 6.6 ± 0.2 b | 29 ± 16 | 55 ± 11 | 83 ± 26 |
| E1/2S | 5.15 ± 0.06 | 681 ± 129 a | 8.3 ± 0.4 a | 55 ± 20 | 69 ± 14 | 124 ± 32 |
| M | 5.20 ± 0.10 | 512 ± 102 ab | 6.5 ± 0.2 b | 38 ± 15 | 66 ± 12 | 103 ± 27 |
| MS | 5.10 ± 0.04 | 586 ± 79 ab | 7.8 ± 0.7 ab | 45 ± 8 | 61 ± 11 | 105 ± 16 |
| E | 5.08 ± 0.20 | 513 ± 122 ab | 6.9 ± 0.7 b | 41 ± 21 | 66 ± 16 | 107 ± 36 |
| ES | 5.22 ± 0.15 | 533 ± 71 ab | 8.6 ± 1.0 a | 47 ± 20 | 56 ± 8 | 103 ± 28 |
| Two-way ANOVA (F values and significance) | ||||||
| Sludge (S) | 1.26 n.s. | 15.14 *** | 63.05 *** | 1.69 n.s. | 0.06 n.s. | 0.52 n.s. |
| Fertilizer (F) | 0.87 n.s. | 0.43 n.s. | 2.18 n.s. | 0.51 n.s. | 1.85 n.s. | 0.57 n.s. |
| S × F | 1.43 n.s. | 1.72 n.s. | 1.03 n.s. | 1.42 n.s. | 2.21 n.s. | 1.71 n.s. |
| F Values and Significance | ||||||
|---|---|---|---|---|---|---|
| Origin of the variation | Pext | Kext | Feext | Cuext | Znext | Mnext |
| Main factors | ||||||
| Sludge (S) | 1204.04 *** | 35.82 *** | 8.27 ** | 4.60 * | 515.89 *** | 3.61 n.s. |
| Fertilizer (F) | 9.48 *** | 3.58 * | 0.48 n.s. | 0.56 n.s. | 0.61 n.s. | 1.46 n.s |
| Interactions | ||||||
| S × F | 1.86 n.s. | 2.42 n.s. | 2.88 * | 2.85 * | 1.25 n.s. | 0.50 n.s |
| Non-Acid Exchangeable Cations (cmol(+)/kg) | Exchangeable Acidity (cmol(+)/kg) | CEC (cmol(+)/kg) | ||||
|---|---|---|---|---|---|---|
| Treat. | K+ | Ca2+ | Mg2+ | Na+ | H+ | |
| C | 0.042 ± 0.007 abc | 1.70 ± 0.06 c | 0.50 ± 0.03 c | 0.036 ± 0.006 b | 0.113 ± 0.010 d | 2.40 ± 0.06 b |
| CS | 0.046 ± 0.010 abc | 2.29 ± 0.17 a | 0.61 ± 0.05 abc | 0.067 ± 0.019 a | 0.153 ± 0.027 abcd | 3.17 ± 0.21 a |
| M1/2 | 0.027 ± 0.008 c | 1.81 ± 0.14 bc | 0.51 ± 0.06 bc | 0.027 ± 0.004 b | 0.131 ± 0.006 cd | 2.50 ± 0.19 b |
| M1/2S | 0.040 ± 0.010 abc | 2.57 ± 0.29 a | 0.64 ± 0.06 ab | 0.069 ± 0.005 a | 0.152 ± 0.016 abcd | 3.47 ± 0.28 a |
| E1/2 | 0.034 ± 0.004 bc | 1.70 ± 0.10 c | 0.48 ± 0.05 c | 0.024 ± 0.006 b | 0.136 ± 0.034 bcd | 2.38 ± 0.13 b |
| E1/2S | 0.068 ± 0.016 a | 2.28 ± 0.12 a | 0.69 ± 0.07 a | 0.082 ± 0.021 a | 0.191 ± 0.021 a | 3.30 ± 0.21 a |
| M | 0.028 ± 0.008 c | 1.77 ± 0.15 bc | 0.49 ± 0.05 c | 0.027 ± 0.005 b | 0.116 ± 0.022 d | 2.43 ± 0.21 b |
| MS | 0.058 ± 0.017 ab | 2.37 ± 0.30 a | 0.59 ± 0.08 abc | 0.073 ± 0.011 a | 0.162 ± 0.002 abc | 3.26 ± 0.20 a |
| E | 0.039 ± 0.012 bc | 1.75 ± 0.10 c | 0.59 ± 0.05 abc | 0.027 ± 0.005 b | 0.141 ± 0.015 bcd | 2.54 ± 0.16 b |
| ES | 0.068 ± 0.015 a | 2.16 ± 0.09 ab | 0.67 ± 0.06 a | 0.068 ± 0.006 a | 0.180 ± 0.016 ab | 3.15 ± 0.15 a |
| Two-way ANOVA (F values and significance) | ||||||
| S | 35.82 *** | 119.75 *** | 50.69 *** | 170.80 *** | 44.07 *** | 190.18 *** |
| F | 3.58 * | 2.38 n.s | 2.89 * | 0.41 n.s. | 4.08 ** | 1.27 n.s. |
| S × F | 2.42 n.s | 1.04 n.s | 1.46 n.s | 1.71 n.s. | 0.81 n.s. | 1.18 n.s. |
| F Values and Significance | ||||
|---|---|---|---|---|
| Origin of the Variation | Dry Biomass | Chlorophyll Relative Index | NDVI | PRI |
| Main factors | ||||
| Sludge (S) | 1.32 n.s. | 59.49 *** | 1.11 n.s. | 7.89 * |
| Fertilizer (F) | 2.34 n.s. | 14.92 *** | 3.34 * | 2.09 n.s. |
| Interactions | ||||
| S × F | 3.48 * | 7.42 *** | 3.31 * | 0.88 n.s. |
| Macronutrients (g/kgdb) | ||||||
|---|---|---|---|---|---|---|
| Treatment | P | N | K | Ca | Mg | S |
| C | 3.16 ± 0.28 b | 70.3 ± 8.9 | 24.8 ± 5.0 | 13.6 ± 2.6 b | 5.5 ± 0.5 b | 3.7 ± 0.7 bc |
| CS | 4.03 ± 0.71 ab | 67.3 ± 3.4 | 18.7 ± 3.9 | 13.8 ± 3.4 b | 5.4 ± 1.0 b | 4.0 ± 0.9 bc |
| M1/2 | 3.53 ± 0.52 b | 63.6 ± 4.7 | 24.0 ± 4.1 | 13.8 ± 1.6 b | 5.7 ± 0.4 b | 3.8 ± 0.6 bc |
| M1/2S | 5.23 ± 0.68 a | 65.8 ± 3.5 | 23.0 ± 2.8 | 16.9 ± 1.5 ab | 6.6 ± 0.5 ab | 4.8 ± 0.4 abc |
| E1/2 | 3.40 ± 0.47 b | 65.1 ± 6.9 | 20.2 ± 4.9 | 12.5 ± 1.3 b | 5.6 ± 0.4 b | 3.5 ± 0.5 bc |
| E1/2S | 4.98 ± 0.46 a | 72.1 ± 5.3 | 24.0 ± 1.3 | 18.0 ± 2.4 ab | 6.7 ± 0.9 ab | 5.2 ± 0.5 ab |
| M | 3.92 ± 0.07 ab | 68.2 ± 4.9 | 24.3 ± 0.6 | 15.3 ± 1.1 ab | 6.1 ± 0.2 b | 4.2 ± 0.3 abc |
| MS | 4.56 ± 0.92 ab | 73.1 ± 3.2 | 21.7 ± 5.1 | 15.6 ± 2.9 ab | 6.0 ± 0.8 b | 4.9 ± 0.7 abc |
| E | 3.47 ± 0.75 b | 67.9 ± 9.3 | 16.5 ± 6.4 | 13.4 ± 3.4 b | 5.7 ± 1.1 b | 3.3 ± 1.2 c |
| ES | 5.10 ± 0.44 a | 70.3 ± 8.9 | 23.1 ± 3.1 | 20.5 ± 2.2 a | 7.9 ± 0.9 a | 5.8 ± 0.9 a |
| Two-way ANOVA (F values and significance) | ||||||
| Sludge (S) | 49.06 *** | 1.235 n.s. | 0.012 n.s. | 18.23 *** | 12.73 ** | 30.88 *** |
| Fertilizer (F) | 2.29 n.s. | 1.218 n.s. | 1.000 n.s. | 1.89 n.s. | 3.51 * | 1.25 n.s. |
| S × F | 1.44 n.s. | 1.073 n.s. | 3.110 * | 3.34 * | 3.60 * | 3.17 * |
| Micronutrients (mg/kgdb) | ||||||
| Treatment | Fe | Cu | Zn | Mn | B | Mo |
| C | 265 ± 86 | 12.7 ± 1.6 | 217 ± 34 | 264 ± 39 | 44.3 ± 4.8 ab | 0.34 ± 0.22 |
| CS | 193 ± 63 | 11.8 ± 2.0 | 202 ± 43 | 240 ± 57 | 38.3 ± 8.5 ab | <0.25 |
| M1/2 | 258 ± 96 | 13.5 ± 2.2 | 231 ± 43 | 289 ± 35 | 47.0 ± 5.1 ab | <0.25 |
| M1/2S | 201 ± 21 | 14.3 ± 1.1 | 251 ± 45 | 321 ± 19 | 50.6 ± 3.6 a | <0.25 |
| E1/2 | 202 ± 47 | 12.0 ± 1.1 | 207 ± 37 | 274 ± 32 | 40.7 ± 4.5 ab | <0.25 |
| E1/2S | 284 ± 78 | 14.4 ± 1.0 | 222 ± 44 | 289 ± 50 | 47.2 ± 6.2 ab | <0.25 |
| M | 289 ± 89 | 14.5 ± 1.8 | 233 ± 6 | 300 ± 7 | 45.9 ± 2.4 ab | 0.35 ± 0.13 |
| MS | 143 ± 28 | 13.4 ± 2.2 | 209 ± 53 | 297 ± 34 | 47.0 ± 0.9 ab | 0.29 ± 0.09 |
| E | 148 ± 49 | 8.9 ± 2.0 | 139 ± 55 | 183 ± 59 | 32.9 ± 8.0 b | <0.25 |
| ES | 242 ± 66 | 12.7 ± 1.1 | 156 ± 28 | 256 ± 41 | 44.9 ± 4.1 ab | <0.25 |
| Two-way ANOVA (F values and significance) | ||||||
| Sludge (S) | 0.626 n.s. | 0.847 n.s. | 0.104 n.s. | 0.380 n.s. | 1.537 n.s. | n.a. |
| Fertilizer (F) | 0.085 n.s. | 1.986 n.s. | 4.429 ** | 3.527 * | 2.771 * | n.a. |
| S × F | 1.678 n.s. | 1.600 n.s. | 0.655 n.s. | 1.375 n.s. | 2.517 n.s. | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.F.; Carreira, G.; Mota, M.; Gando-Ferreira, L.M.; Quina, M.J.; Alvarenga, P. Greenhouse Evaluation of the Agronomic Potential of Urban Wastewater-Based Fertilizers: Sewage Sludge and Struvite for Lettuce Production in Sandy Soil. Agronomy 2025, 15, 2589. https://doi.org/10.3390/agronomy15112589
Santos AF, Carreira G, Mota M, Gando-Ferreira LM, Quina MJ, Alvarenga P. Greenhouse Evaluation of the Agronomic Potential of Urban Wastewater-Based Fertilizers: Sewage Sludge and Struvite for Lettuce Production in Sandy Soil. Agronomy. 2025; 15(11):2589. https://doi.org/10.3390/agronomy15112589
Chicago/Turabian StyleSantos, Andreia F., Gonçalo Carreira, Mariana Mota, Licínio M. Gando-Ferreira, Margarida J. Quina, and Paula Alvarenga. 2025. "Greenhouse Evaluation of the Agronomic Potential of Urban Wastewater-Based Fertilizers: Sewage Sludge and Struvite for Lettuce Production in Sandy Soil" Agronomy 15, no. 11: 2589. https://doi.org/10.3390/agronomy15112589
APA StyleSantos, A. F., Carreira, G., Mota, M., Gando-Ferreira, L. M., Quina, M. J., & Alvarenga, P. (2025). Greenhouse Evaluation of the Agronomic Potential of Urban Wastewater-Based Fertilizers: Sewage Sludge and Struvite for Lettuce Production in Sandy Soil. Agronomy, 15(11), 2589. https://doi.org/10.3390/agronomy15112589









