Physiological and Agronomic Traits of Certain Sugarcane Genotypes Grown under Field Conditions as Influenced by Early Drought Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Plant Material
2.3. Irrigation Treatments
2.4. Soil Moisture Measurement and Meteorological Conditions
2.5. Plant Growth and Physiological Measurements
2.6. Agronomic Measurements at Harvest
2.7. Statistical Analysis
3. Results
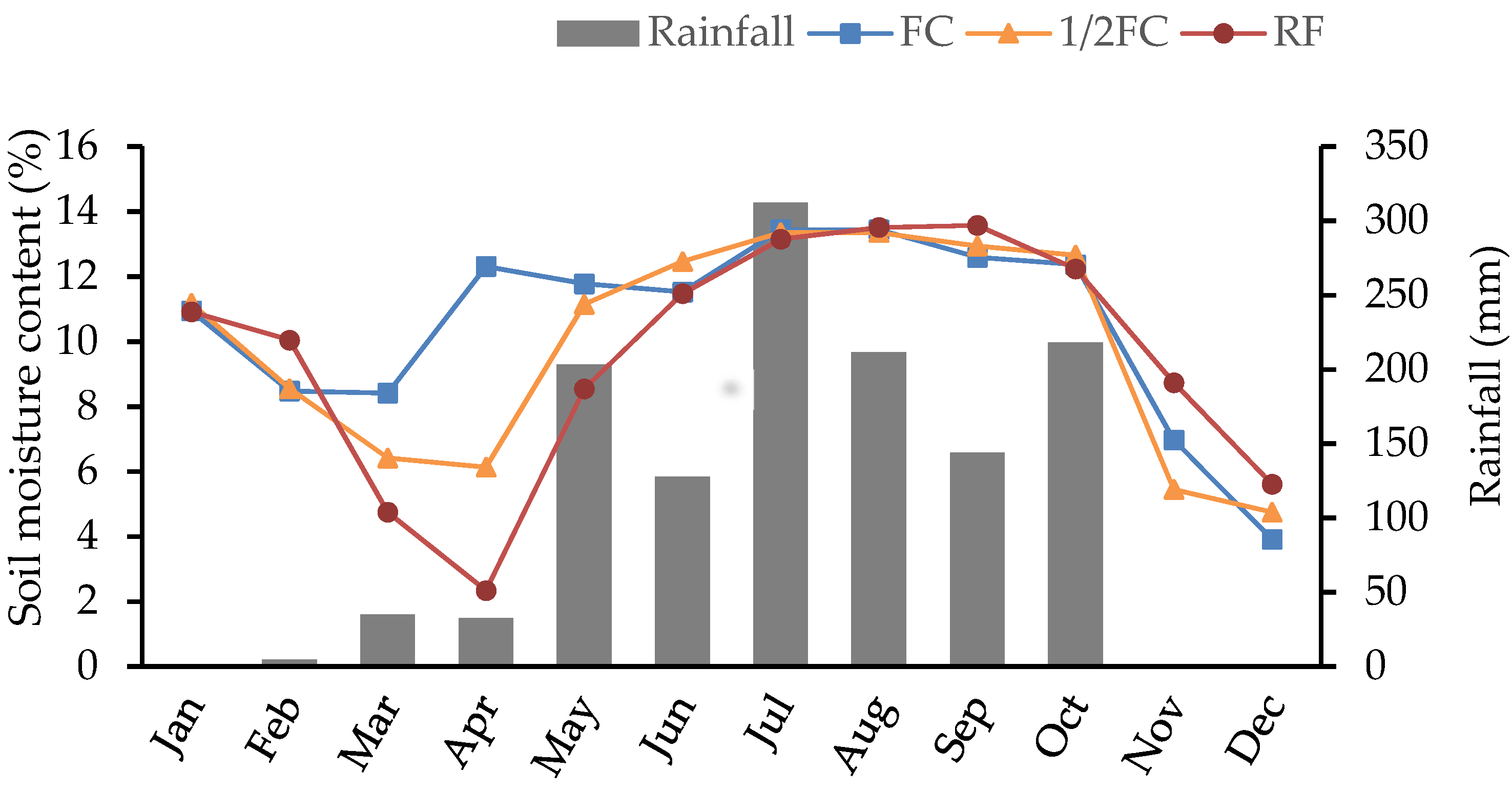
3.1. Soil Moisture Content and Meteorological Conditions
3.1.1. Soil Moisture Content
3.1.2. Meteorological Conditions
3.2. Physiological Characteristics of Six Sugarcane Genotypes
3.2.1. Effect of Early Drought Stress on Growth and Physiological Parameters
3.2.2. Effect of Early Drought Stress on Photosynthetic Parameters after 72 Days of Drought Period
3.2.3. Effect of Early Drought Stress on Photosynthetic Parameters under Recovery Conditions
3.3. Effect of Early Drought Stress on Agronomic Traits
4. Discussion
4.1. Physiological Responses to Early Drought in Six Sugarcane Genotypes
4.1.1. Effect of Early Drought Stress on Growth and Physiological Parameters
4.1.2. Effect of Drought Stress on Photosynthetic Parameters under Drought and Recovery Conditions
4.2. Effect of Drought Stress on Agronomic Traits
Effect of Drought Stress on Yield and Yield Components
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macrelli, S.; Mogensen, J.; Zacchi1, G. Techno-economic evaluation of 2nd generation bioethanol production from sugarcane bagasse and leaves integrated with the sugar-based ethanol process. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Prachaiyo, B. Farmers and forests: A changing phase in Northeast Thailand. Southeast Asian Stud. 2000, 38, 271–446. Available online: http://hdl.handle.net/2433/56758 (accessed on 1 November 2021).
- Lawlor, D.W.; Comic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [Green Version]
- Zingaretti, S.M.; Rodrigues, F.A.; Graca, J.P.; Pereira, L.M.; Lourenco, M.V. Sugarcane responses at water deficit conditions. In Water Stress, 1st ed.; Rahman, I.M.M., Ed.; IntechOpen: Shanghai, China, 2012; pp. 255–276. [Google Scholar] [CrossRef] [Green Version]
- Jangpromma, N.; Songsti, P.; Thammasirirak, S.; Jaisil, P. Rapid assessment of chlorophyll content in sugarcane using a SPAD chlorophyll meter across different water stress conditions. Asian J. Plant Sci. 2010, 9, 368–374. [Google Scholar] [CrossRef] [Green Version]
- Zhao, D.; Glaz, M.; Comstock, J.C. Sugarcane response to water-deficit stress during early growth on organic and sand soils. Am. J. Agric. Biol. Sci. 2010, 5, 403–414. [Google Scholar] [CrossRef] [Green Version]
- Cha-um, S.; Wangmoon, S.; Mongkolsiriwatana, C.; Ashraf, M.; Kirdmanee, C. Evaluating sugarcane (Saccharum sp.) cultivars for water deficit tolerance using some key physiological markers. Plant Biotechnol. 2012, 29, 431–439. [Google Scholar] [CrossRef] [Green Version]
- Inman-Bamber, N.G.; Smith, D.M. Water relations in sugarcane and response to water deficits. Field Crops Res. 2005, 92, 185–202. [Google Scholar] [CrossRef]
- Sato, A.M.; Catuchi, T.A.; Ribeiro, R.V.; Souza, G.M. The use of network analysis to uncover homeostatic responses of a drought-tolerant sugarcane cultivar under severe water deficit and phosphorus supply. Acta Physiol. Plant. 2010, 32, 1145–1151. [Google Scholar] [CrossRef]
- Griffiths, H.; Parry, M.A.J. Plant responses to water stress. Ann. Bot. 2002, 89, 801–802. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Jifon, J.L.; Santos, C.M.; Jadoski, C.J.; Silva, J.A.G. Photosynthetic capacity and water use efficiency in sugarcane genotypes subject to water deficit during early growth phase. Braz. Arch. Biol. Technol. 2013, 56, 735–748. [Google Scholar] [CrossRef] [Green Version]
- Jain, R.; Chandra, A.; Venugopaplan, V.K.; Solomon, S. Physiological changes and expression of SOD and P5CS genes in response to water deficit in sugarcane. Sugar Tech 2015, 17, 276–282. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, R.S.; Machado, E.C.; Machado, D.F.S.P.; Filho, R.M.; Landell, M.G.A. Revealing drought-resistance and productive patterns in sugarcane genotypes by evaluating both physiological responses and stalk yield. Exp. Agric. 2013, 49, 212–224. [Google Scholar] [CrossRef]
- Medeiros, D.B.; da Silva, E.C.; Nogueira, R.J.M.C.; Teixeira, M.M.; Buckeridge, M.S. Physiological limitations in two sugarcane varieties under water suppression and after recovering. Theor. Exp. Plant Physiol. 2013, 25, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, S.; Basnayake, J.; Lakshmanan, P.; Fukai, S. Genotypic variation in intrinsic transpiration efficiency correlates with sugarcane yield under rainfed and irrigated field conditions. Physiol. Plant. 2020, 172, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, R.; Vasantha, S.; Hemaprabha, G.; Alarmelu, S.; Shanthi, R.M. Evaluation of elite sugarcane clones for drought tolerance. J. Sugarcane Res. 2011, 1, 55–62. Available online: http://epubs.icar.org.in/ejournal/index.php/JSR/article/view/99240 (accessed on 1 November 2021).
- da Graca, J.P.; Rodrigues, F.A.; Farias, J.R.B.; da Oliveira, M.C.N.; Hoffmann-Campo, C.B.; Zingaretti, S.M. Physiological parameters in sugarcane cultivars submitted to water deficit. Braz. J. Plant Physiol. 2010, 22, 189–197. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M. Some important physiological selection criteria for salt tolerance in plants. Flora 2004, 199, 361–376. [Google Scholar] [CrossRef]
- Endres, L.; Silva, J.V.; Ferreira, V.M.; Souza Barbosa, G.V.D. Photosynthesis and water relations in Brazilian sugarcane. Open Agric. J. 2010, 4, 31–37. [Google Scholar] [CrossRef]
- Basnayake, J.; Jackson, P.A.; Inman-Bamber, N.G.; Lakshmanan, P. Sugarcane for water-limited environments. Variation in stomatal conductance and its genetic correlation with crop productivity. J. Exp. Bot. 2015, 66, 3945–3958. [Google Scholar] [CrossRef] [Green Version]
- Dinh, T.H.; Takaragawa, H.; Watanabe, K.; Nakabaru, M.; Kawamitsu, Y. Leaf photosynthesis response to change of soil moisture content in sugarcane. Sugar Tech 2019, 21, 949–958. [Google Scholar] [CrossRef]
- Zargar, S.M.; Gupta, N.; Nazir, M.; Mahajan, R.; Malik, F.A.; Sofi, N.R.; Shikari, A.B.; Salgotra, R.K. Impact of drought on photosynthesis: Molecular perspective. Plant Gene 2017, 11, 154–159. [Google Scholar] [CrossRef]
- Robertson, M.J.; Inman-Bamber, N.G.; Muchow, R.C.; Wood, A.W. Physiology and productivity of sugarcane with early and mid-season water deficit. Field Crops Res. 1999, 64, 211–227. [Google Scholar] [CrossRef]
- Silva, M.A.; Silva, J.A.G.D.; Enciso, J.; Sharma, V.; Jifon, J. Yield components as indicators of drought tolerance of sugarcane. Sci. Agric. 2008, 65, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Basnayake, J.; Jackson, P.A.; Inman-Bamber, N.G.; Lakshmanan, P. Sugarcane for water-limited environments: Genetic variation in cane yield and sugar content in response to water stress. J. Exp. Bot. 2012, 63, 6023–6033. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.A.; Jifon, J.L.; de Silva, J.A.G.; Sharma, V. Use of physiological parameters as fast tools to screen for drought tolerance in sugarcane. Braz. J. Plant. Physiol. 2007, 9, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.A.; Soares, R.A.B.; Landell, M.G.A.; Campana, M.P. Agronomic performance of sugarcane families in response to water stress. Bragantia 2008, 67, 655–661. [Google Scholar] [CrossRef] [Green Version]
- Hemaprabha, G.; Nagarajan, R.; Alarmelu, S.; Natarajan, U.S. Parental potential of sugarcane clones for drought resistance breeding. Sugar Tech 2006, 8, 59–62. [Google Scholar] [CrossRef]
- Lizana, C.; Wentworth, M.; Martinez, J.P.; Villegas, D.; Meneses, R.; Murchie, E.H.; Pastenes, C.; Lercari, B.; Vernieri, P.; Horton, P.; et al. Differential adaptation of two varieties of common bean to abiotic stress: I. Effects of drought on yield and photosynthesis. J. Exp. Bot. 2006, 57, 685–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filek, M.; Walas, S.; Mrowiec, H.; Rudolphy-Skόrska, E.; Sieprawska, A.; Biesaga-Kościelniak, J. Membrane permeability and micro- and microelement accumulation in spring wheat cultivars during the short-term effect of salinity and PEG-induced water stress. Acta Physiol. Plant 2012, 34, 985–995. [Google Scholar] [CrossRef] [Green Version]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving intrinsic water-use efficiency and crop yield. Crop Sci. 2002, 42, 122–131. [Google Scholar] [CrossRef]
- Ehsanullah, K.J.; Jamil, M.; Ghafar, A. Optimizing the row spacing and seeding density to improve yield and quality of sugarcane. Crop Environ. 2011, 2, 1–5. [Google Scholar]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, C.; Zhang, D.; He, C.; Zhang, J.; Li, Z. Effects of maize organ-specific drought stress response on yields from transcriptome analysis. BMC Plant Biol. 2019, 19, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martineau, J.R.; Specht, J.E.; Williams, J.H.; Sullivan, C.Y. Temperature tolerance in soybeans: Evaluation of a technique for assessing cellular membrane thermostability. Crop Sci. 1979, 19, 75–78. [Google Scholar] [CrossRef]
- Bouchemal, K.; Bouldjadj, R.; Belbekri, M.N.; Ykhlef, N.; Djekoun, A. Differences in antioxidant enzyme activities and oxidative markers in ten wheat (Triticum durum Desf.) genotypes in response to drought, heat and paraquat stress. Arch. Agron. Soil Sci. 2016, 63, 710–722. [Google Scholar] [CrossRef]
- Sudhakar, P.; Latha, P.; Babu, A.M. Evaluation of sugarcane genotypes for high water use efficiency and thermostability tolerance under imposed moisture stress at formative stage. Sugar Tech 2010, 12, 72–75. [Google Scholar] [CrossRef]
- Reyes, J.A.O.; Carpentero, A.S.; Santos, P.J.A.; Delfin, E.F. Effects of water regime, genotype, and formative stages on the agro-physiological responses of sugarcane (Saccharum officinarum L.) to drought. Plants 2020, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Xiong, L. General mechanisms to drought response and their application in drought resistance improvement in plants. Cell. Mol. Life Sci. 2015, 72, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Glaz, B.; Comstock, J.C. Sugarcane leaf photosynthesis and growth characters during development of water-deficit stress. Crop Sci. 2013, 53, 1066–1075. [Google Scholar] [CrossRef] [Green Version]
- Krause, G.H. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Biol. 1991, 43, 313–349. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Silva, M.A.; Jifon, J.L.; Silva, J.A.D.; Santos, C.M.D.; Shama, V. Relationships between physiological traits and productivity of sugarcane in response to water deficit. J. Agric. Sci. 2014, 152, 104–118. [Google Scholar] [CrossRef]
- Souza, A.P.; Grandis, A.; Arenque-Musa, B.C.; Buckeridge, M. Diurnal variation in gas exchange and nonstructural carbohydrates throughout sugarcane development. Funct. Plant Biol. 2018, 45, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.A.; Jifon, J.L.; Sharma, V.; Silva, J.A.G.; Caputo, M.M.; Damaj, M.B.; Guimaraes, E.R.; Ferro, M.I.T. Use of physiological parameters in screening drought tolerance in sugarcane genotypes. Sugar Tech 2011, 13, 191–197. [Google Scholar] [CrossRef]
- Baloch, M.J.; Dunwell, J.; Khan, N.U.; Jatoi, W.A.; Khakhwani, A.A.; Vessar, N.F.; Gul, S. Morpho-physiological characterization of spring wheat genotypes under drought stress. Int. J. Agric. Biol. 2013, 15, 945–950. [Google Scholar]
- Mir, R.R.; Zaman-Allah, M.; Sreenivasulu, N.; Trethowan, R.; Varshney, R.K. Integrated genomics, physiology and breeding approaches for improving drought tolerance in crops. Theor. Appl. Genet. 2012, 125, 625–645. [Google Scholar] [CrossRef] [Green Version]
- Endres, L.; Santos, C.M.; Silva, J.V.; Barbosa, G.V.S.; Silva, A.L.J.; Froehlich, A.; Teixeira, M.M. Inter-relationship between photosynthetic efficiency, Δ13C, antioxidant activity and sugarcane yield under drought stress in field conditions. J. Agron. Crop Sci. 2019, 205, 433–446. [Google Scholar] [CrossRef]
- Augustine, S.M.; Syamaladevi, D.P.; Premachandran, M.N.; Ravichandran, V.; Subramonian, N. Physiological and molecular insights to drought responsiveness in Erianthus spp. Sugar Tech 2015, 17, 121–129. [Google Scholar] [CrossRef]
- Irvine, J.E. Relations of photosynthetic rate and leaf and canopy characters of sugarcane yield. Crop Sci. 1975, 15, 671–676. [Google Scholar] [CrossRef]
- Silva, A.L.C.; Costa, W.A.J.M. Varietal variation in stomatal conductance, transpiration and photosynthesis of commercial sugarcane varieties under two contrasting water regimes. Trop. Agric. Res. Ext. 2009, 12, 97–102. [Google Scholar] [CrossRef]
- Hemaprabha, G.; Swapna, S.; Lavanya, D.L.; Sajitha, B.; Venkataramana, S. Evaluation of drought tolerance potential of elite genotypes and progenies of sugarcane (Saccharum sp. hybrids). Sugar Tech 2013, 15, 9–16. [Google Scholar] [CrossRef]
- Ishaq, M.N.; Olaoye, G. Cane yield attributes and heritability of juice quality characters in sugarcane under moisture deficit conditions. Sugar Tech 2009, 11, 360–367. [Google Scholar] [CrossRef]
- Klomsa-Ard, P.; Jaisil, P.; Patanothai, A. Performance and stability for yield and component traits of elite sugarcane genotypes across production environments in Thailand. Sugar Tech 2013, 15, 354–364. [Google Scholar] [CrossRef]
- Songsri, P.; Nata, J.; Bootprom, N.; Jongrungklang, N. Performances of elite sugarcane genotypes for agro-physiological traits in relation to yield potential and ratooning ability under rain-fed conditions. J. Agron. 2020, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Todd, J.; Wang, J.; Glaz, B.; Sood, S.; Ayala-Silva, T.; Nayak, S.N.; Glynn, N.C.; Gutierrez, O.A.; Kuhn, D.N.; Tahir, M.; et al. Phenotypic characterization of the Miami World Collection of sugarcane (Saccharum spp.) and related grasses for selecting a representative core. Genet. Resour. Crop Evol. 2014, 61, 1581–1596. [Google Scholar] [CrossRef]
- Bull, T.A.; Glasziou, K.T. The evolutionary significance of sugar accumulation in Saccharum. Aust. J. Biol. Sci. 1963, 16, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Nair, N.V.; Mohanraj, K.; Sunadaravelpandian, K.; Suganya, A.; Selvi, A.; Appunu, C. Characterization of an intergeneric hybrids of Erianthus procerus × Saccharum officinarum and its backcross progenies. Euphytica 2017, 213, 267–277. [Google Scholar] [CrossRef]
| Source of Variance | df | Stalk Height | Stalk Diameter | SPAD Value | EL | Fv/Fm |
|---|---|---|---|---|---|---|
| Block | 2 | 63.40 | 0.250 | 21.68 | 2.13 | 0.000085 |
| Water regime (W) | 2 | 11,496.60 ** | 0.883 ns | 156.54 ** | 2.28 ns | 0.001844 * |
| Error (a) | 4 | 50.80 | 0.219 | 9.71 | 5.42 | 0.000112 |
| Genotype (G) | 5 | 2800.10 ** | 3.084 ** | 219.61 ** | 55.46 ** | 0.000746 ** |
| W × G | 10 | 263.30 ** | 0.241 * | 24.50 ** | 21.06 ns | 0.000318 * |
| Error (b) | 30 | 112.00 | 0.099 | 8.85 | 9.93 | 0.000135 |
| Genotype | Stalk Height (cm) | Stalk Diameter (cm) | ||||
|---|---|---|---|---|---|---|
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 100.42 ± 6.60 Aa | 59.17 ± 4.37 Bb | 45.02 ± 4.75 Cbc | 1.08 ± 0.28 Ac | 1.43 ± 0.54 Ac | 1.00 ± 0.17 Ac |
| KK08-214 | 105.17 ± 14.00 Aa | 91.67 ± 12.41 ABa | 62.50 ± 18.19 Bab | 1.83 ± 0.06 Ab | 1.87 ± 0.12 Abc | 1.77 ± 0.38 Aab |
| E08-4-019 | 105.00 ± 14.86 Aa | 66.67 ± 11.43 Bb | 63.58 ± 10.36 Ba | 1.40 ± 0.20 Abc | 1.53 ± 0.25 Abc | 1.38 ± 0.26 Abc |
| KK3 | 74.67 ± 10.68 Ab | 43.83 ± 1.26 Bc | 34.08 ± 3.00 Bcd | 3.02 ± 0.51 Aa | 1.97 ± 0.41 Bb | 2.03 ± 0.23 Ba |
| Q117 | 117.25 ± 1.09 Aa | 61.00 ± 7.76 Bb | 39.70 ± 3.25 Ccd | 2.73 ± 0.58 Aa | 2.50 ± 0.43 Aa | 1.98 ± 0.36 Aa |
| LK92-11 | 61.42 ± 18.45 Ab | 32.75 ± 6.72 Bc | 24.17 ± 6.17 Bd | 3.02 ± 0.50 Aa | 2.63 ± 0.18 ABa | 2.27 ± 0.19 Ba |
| Genotype | SPAD Value | EL (%) | ||||
|---|---|---|---|---|---|---|
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 45.30 ± 0.36 Ab | 43.70 ± 5.39 Aa | 37.33 ± 5.06 Ab | 18.95 ± 3.66 Aab | 16.22 ± 2.28 Abc | 17.61 ± 5.04 Aa |
| KK08-214 | 40.10 ± 0.52 Ac | 40.23 ± 2.57 Aa | 31.97 ± 7.15 Ab | 15.64 ± 3.61 Ab | 18.37 ± 5.11 Abc | 16.79 ± 1.73 Aa |
| E08-4-019 | 51.53 ± 1.70 Aa | 43.03 ± 2.44 Ba | 48.47 ± 2.15 Aa | 24.83 ± 4.68 Aa | 20.69 ± 2.29 ABb | 18.04 ± 0.95 Ba |
| KK3 | 42.57 ± 3.01 Abc | 34.27 ± 3.62 Bb | 37.50 ± 0.90 Abb | 18.30 ± 0.40 Ab | 18.41 ± 1.83 Abc | 20.89 ± 3.61 Aa |
| Q117 | 35.10 ± 2.10 Ad | 32.80 ± 1.08 ABb | 31.30 ± 1.51 Bb | 16.10 ± 1.49 Ab | 15.76 ± 2.26 Ac | 19.14 ± 1.97 Aa |
| LK92-11 | 43.57 ± 2.72 Abc | 41.93 ± 0.84 Aa | 36.63 ± 2.93 Bb | 21.49 ± 4.22 Bab | 27.36 ± 0.80 Aa | 20.12 ± 1.22 Ba |
| Genotype | Fv/Fm | ||
|---|---|---|---|
| FC | ½FC | RF | |
| Ths98-271 | 0.796 ± 0.001 Ab | 0.800 ± 0.007 Aa | 0.798 ± 0.007 Aa |
| KK08-214 | 0.799 ± 0.001 Ab | 0.792 ± 0.007 Aab | 0.789 ± 0.011 Aa |
| E08-4-019 | 0.798 ± 0.004 Ab | 0.780 ± 0.006 Cbc | 0.788 ± 0.004 Ba |
| KK3 | 0.794 ± 0.007 Ab | 0.772 ± 0.008 Ac | 0.775 ± 0.022 Aab |
| Q117 | 0.798 ± 0.002 Ab | 0.773 ± 0.020 ABbc | 0.747 ± 0.031 Bb |
| LK92-11 | 0.806 ± 0.003 Aa | 0.775 ± 0.008 Bbc | 0.782 ± 0.003 Ba |
| Source of Variance | df | A | E | gs | WUE |
|---|---|---|---|---|---|
| Block | 2 | 7.492 | 0.26787 | 0.00035 | 6.806 |
| Water regime (W) | 2 | 127.926 ** | 1.67186 * | 0.0403 ** | 1.087 ns |
| Error (a) | 4 | 4.510 | 0.17864 | 0.0002 | 2.808 |
| Genotype (G) | 5 | 150.659 ** | 1.20583 ** | 0.00633 * | 0.911 ns |
| W × G | 10 | 71.384 ** | 0.58557 * | 0.00582 ** | 1.814 ns |
| Error (b) | 30 | 19.480 | 0.26967 | 0.00172 | 1.453 |
| Genotype | Net Photosynthetic Rate (µmol CO2 m−2s−1) | Transpiration Rate (mmol H2O m−2s−1 ) | ||||
|---|---|---|---|---|---|---|
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 40.25 ± 2.44 AB | 44.49 ± 3.33 Aa | 38.83 ± 2.42 Ba | 3.75 ± 0.23 A | 3.86 ± 0.95 Aa | 3.14 ± 0.34 Aab |
| KK08-214 | 37.42 ± 2.88 AB | 41.17 ± 2.85 Aa | 31.97 ± 3.61 Babc | 3.65 ± 0.34 A | 3.61 ± 0.25 Aab | 2.87 ± 0.34 Aabc |
| E08-4-019 | 36.67 ± 2.60 A | 33.30 ± 1.27 Ab | 39.52 ± 9.22 Aa | 3.08 ± 0.14 A | 2.88 ± 0.72 Aabc | 3.69 ± 0.95 Aa |
| KK3 | 36.76 ± 5.19 A | 35.35 ± 2.04 Ac | 25.23 ± 1.42 Bab | 3.47 ± 0.55 A | 2.13 ± 0.52 Cc | 2.82 ± 0.38 Babc |
| Q117 | 35.78 ± 4.04 A | 28.90 ± 5.42 Bbc | 24.54 ± 6.42 Bc | 3.17 ± 0.82 A | 2.70 ± 0.23 ABbc | 2.05 ± 0.41 Bc |
| LK92-11 | 40.66 ± 1.50 A | 29.99 ± 4.03 Bbc | 27.24 ± 4.95 Bbc | 3.44 ± 0.17 A | 2.75 ± 0.24 Bbc | 2.46 ± 0.41 Bbc |
| Genotype | Stomatal conductance (mol H2O m−2s−1) | Water use efficiency (µmol CO2 mmol−1 H2O) | ||||
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 0.268 ± 0.037 Aab | 0.272 ± 0.068 Aa | 0.209 ± 0.010 Aab | 10.79 ± 1.18 | 11.86 ± 2.16 | 12.49 ± 1.82 |
| KK08-214 | 0.286 ± 0.039 Aab | 0.253 ± 0.022 Aab | 0.171 ± 0.029 Bbc | 10.29 ± 0.58 | 11.48 ± 1.47 | 11.13 ± 0.26 |
| E08-4-019 | 0.234 ± 0.030 Ab | 0.193 ± 0.026 Abc | 0.244 ± 0.062 Aa | 11.93 ± 1.35 | 11.93 ± 2.27 | 10.74 ± 0.24 |
| KK3 | 0.280 ± 0.058 Aab | 0.132 ± 0.020 Cc | 0.208 ± 0.016 Bab | 10.64 ± 1.24 | 12.19 ± 2.13 | 12.70 ± 1.66 |
| Q117 | 0.243 ± 0.046 Aab | 0.155 ± 0.031 Bc | 0.124 ± 0.041 Bc | 11.59 ± 1.70 | 10.66 ± 1.05 | 11.87 ± 0.84 |
| LK92-11 | 0.314 ± 0.015 Aa | 0.175 ± 0.042 Bc | 0.141 ± 0.038 Bbc | 11.85 ± 0.91 | 10.90 ± 0.87 | 11.07 ± 0.20 |
| Source of Variance | df | A | E | gs | WUE |
|---|---|---|---|---|---|
| Block | 2 | 19.875 | 0.18221 | 0.00392 | 0.74543 |
| Water regime (W) | 2 | 4.364 ns | 0.53344 * | 0.00561 ns | 1.56514 ns |
| Error (a) | 4 | 0.699 | 0.06555 | 0.00086 | 0.36104 |
| Genotype (G) | 5 | 132.589 ** | 3.11207 ** | 0.0501 ** | 2.33369 ** |
| W × G | 10 | 15.597 ** | 0.50565 ** | 0.01002 ** | 0.71195 * |
| Error (b) | 30 | 3.475 | 0.105540 | 0.00260 | 0.29859 |
| Genotype | Net Photosynthetic Rate (µmol CO2 m−2s−1) | Transpiration Rate (mmol H2O m−2s−1) | ||||
|---|---|---|---|---|---|---|
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 39.11 ± 1.12 Ab | 37.94 ± 2.37 Ac | 37 ± 1.43 Acd | 4.77 ± 0.12 Aa | 4.13 ± 0.25 Bc | 3.82 ± 0.09 Bbc |
| KK08-214 | 41.06 ± 1.17 Aab | 37.42 ± 1.35 Ac | 39.21 ± 2.64 Abc | 4.39 ± 0.14 Aab | 4.20 ± 0.17 Ac | 4.34 ± 0.62 Aab |
| E08-4-019 | 38.60 ± 1.65 Ab | 42.11 ± 2.24 Aab | 43.13 ± 1.74 Aa | 3.99 ± 0.56 Ab | 4.92 ± 0.24 Aab | 4.86 ± 0.21 Aa |
| KK3 | 42.23 ± 1.27 Aa | 43.33 ± 1.65 Aa | 42.98 ± 0.24 Aab | 4.61 ± 0.41 ABa | 5.03 ± 0.14 Aa | 4.52 ± 0.06 Ba |
| Q117 | 31.07 ± 2.50 Ac | 32.10 ± 0.99 Ad | 32.03 ± 3.1 Ae | 3.09 ± 0.21 Ac | 3.17 ± 0.20 Ad | 2.97 ± 0.22 Ad |
| LK92-11 | 41.02 ± 2.41 Aab | 39.18 ± 3.19 Abc | 33.20 ± 2.53 Bde | 4.45 ± 0.33 Aab | 4.30 ± 0.66 Abc | 3.26 ± 0.31 Bcd |
| Genotype | Stomatal conductance (mol H2O m−2s−1) | Water use efficiency (µmol CO2 mmol−1 H2O) | ||||
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 0.398 ± 0.033 Aa | 0.317 ± 0.034 Bc | 0.293 ± 0.013 Bbc | 8.20 ± 0.16 Bb | 9.22 ± 0.82 ABb | 9.69 ± 0.51 Abc |
| KK08-214 | 0.364 ± 0.033 Aab | 0.330 ± 0.025 Ac | 0.376 ± 0.101 Aab | 9.35 ± 0.27 Aab | 8.92 ± 0.35 Ab | 9.09 ± 0.72 Ac |
| E08-4-019 | 0.305 ± 0.072 Ab | 0.440 ± 0.034 Aab | 0.441 ± 0.070 Aa | 9.78 ± 1.05 Aa | 8.56 ± 0.50 Ab | 8.88 ± 0.14 Ac |
| KK3 | 0.387 ± 0.065 Bab | 0.478 ± 0.013A a | 0.396 ± 0.014 Ba | 9.19 ± 0.56 ABab | 8.62 ± 0.35 Bb | 9.50 ± 0.13 Abc |
| Q117 | 0.205 ± 0.016 Ac | 0.217 ± 0.027 Ad | 0.200 ± 0.026 Ac | 10.05 ± 0.75 Aa | 10.17 ± 0.85 Aa | 10.78 ± 0.35 Aa |
| LK92-11 | 0.368 ± 0.041 Aab | 0.364 ± 0.102 Abc | 0.229 ± 0.032 Bc | 9.24 ± 0.42 Aab | 9.18 ± 0.77 Ab | 10.21 ± 0.52 Aab |
| Source of Variance | df | Single Stalk Weight | Stalk Height | Stalk Diameter | Internode Length | Fiber Content | Millable Cane |
|---|---|---|---|---|---|---|---|
| Block | 2 | 0.0011 | 1047.83 | 0.017 | 2.24 | 1.281 | 3.26 × 108 |
| Water regime (W) | 2 | 0.0665 * | 1807.72 ns | 0.078 ns | 5.19 ns | 10.199 ns | 1.39 × 1010 * |
| Error (a) | 4 | 0.0045 | 2286.64 | 0.087 | 2.44 | 1.479 | 1.35 × 109 |
| Genotype (G) | 5 | 2.4588 ** | 3198.10 ** | 5.681 ** | 7.63 ** | 402.445 ** | 3.71 × 1011 ** |
| W × G | 10 | 0.0357 ns | 1633.67 ns | 0.059 ns | 4.75 * | 4.507 ** | 5.84 × 109 ns |
| Error (b) | 30 | 0.0345 | 784.54 | 0.035 | 1.63 | 0.822 | 3.16 × 109 |
| Treatment | Single Stalk Weight (kg) | Stalk Height (cm) | Stalk Diameter (cm) | Millable Cane (stalks/ha) × 103 |
|---|---|---|---|---|
| Water regime (W) | ||||
| FC | 0.840 ± 0.580 A | 315.54 ± 42.98 A | 1.86 ± 0.77 A | 251.26 ± 224.59 A |
| ½FC | 0.898 ± 0.523 A | 307.88 ± 27.93 A | 1.88 ± 0.75 A | 200.22 ± 189.79 B |
| RF | 0.776 ± 0.448 B | 295.67 ± 36.32 A | 1.76 ± 0.80 A | 206.71 ± 179.60 B |
| Genotype (G) | ||||
| Ths98-271 | 0.144 ± 0.020 e | 270.79 ± 32.80 b | 0.66 ± 0.14 e | 602.75 ± 103.58 a |
| KK08-214 | 0.694 ± 0.202 c | 310.87 ± 38.48 a | 1.53 ± 0.18 c | 172.82 ± 39.08 c |
| E08-4-019 | 0.451 ± 0.171 d | 309.06 ± 39.49 a | 1.32 ± 0.35 d | 281.89 ± 101.50 b |
| KK3 | 1.475 ± 0.171 a | 305.35 ± 39.15 a | 2.59 ± 0.22 a | 101.64 ± 11.89 d |
| Q117 | 0.875 ± 0.183 b | 326.08 ± 22.74 a | 2.25 ± 0.15 b | 75.89 ± 22.62 d |
| LK92-11 | 1.392 ± 0.245 a | 316.04 ± 25.99 a | 2.65±0.16 a | 81.40 ± 13.37 d |
| F-test | ||||
| W | * | ns | ns | * |
| G | ** | ** | ** | ** |
| W × G | ns | ns | ns | ns |
| Genotype | Internode Length (cm) | Fiber Content (%) | ||||
|---|---|---|---|---|---|---|
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 14.78 ± 0.05 Aab | 13.86 ± 0.10 Aab | 13.53 ± 1.41 Aab | 27.35 ± 1.45 Ba | 26.63 ± 0.80 Ba | 31.45 ± 1.15 Aa |
| KK08-214 | 13.18 ± 0.80 ABc | 12.22 ± 1.59 Bab | 16.02 ± 2.41 Aa | 17.83 ± 0.81 Bb | 21.03 ± 2.05 Ab | 20.70 ± 1.60 ABb |
| E08-4-019 | 15.34 ± 0.88 Aa | 12.29 ± 2.32 Aab | 13.32 ± 2.79 Aab | 19.45 ± 0.65 Bb | 19.90 ± 0.76 ABb | 20.85 ± 0.45 Ab |
| KK3 | 14.63 ± 0.39 Aabc | 13.93 ± 0.19 Aa | 11.86 ± 1.74 Bb | 12.90 ± 1.25 Ac | 12.73 ± 0.50 Acd | 13.20 ± 0.70 Ac |
| Q117 | 11.11 ± 0.64 Ad | 11.71 ± 0.49 Ab | 12.21 ± 0.76 Ab | 13.23 ± 0.90 Ac | 12.97 ± 0.12 Ac | 12.80 ± 0.20 Ac |
| LK92-11 | 13.32 ± 1.41 Ac | 11.91 ± 0.37 Aab | 12.16 ± 0.10 Ab | 9.73 ± 0.64 Bd | 11.07 ± 0.38 Ad | 10.50 ± 0.36 ABd |
| Source of Variance | df | Cane Yield | Sugar Yield | CCS |
|---|---|---|---|---|
| Block | 2 | 105.70 | 4.098 | 5.41 |
| Water regime (W) | 2 | 2071.36 ** | 3.140 ns | 5.04 |
| Error (a) | 4 | 33.47 | 1.533 | 1.25 |
| Genotype (G) | 5 | 7828.81 ** | 187.044 ** | 129.47 ** |
| W × G | 10 | 962.24 ** | 7.005 ** | 3.42 ** |
| Error (b) | 30 | 127.83 | 1.755 | 0.84 |
| Genotype | Cane Yield (tons/ha) | Sugar Yield (tons/ha) | ||||
|---|---|---|---|---|---|---|
| FC | ½FC | RF | FC | ½FC | RF | |
| Ths98-271 | 82.95 ± 3.48 Acd | 89.73 ± 3.13 Acd | 81.85 ± 6.80 Ab | 0.925 ± 0.913 Ad | 0.049 ± 0.085 Ae | 1.883 ± 1.321 Ad |
| KK08-214 | 108.45 ± 10.50 Abc | 122.62 ± 11.73 Ab | 106.79 ± 3.57 Aa | 8.874 ± 2.492 Ac | 10.467 ± 2.173 Ab | 9.604 ± 1.234 Ab |
| E08-4-019 | 132.80 ± 15.65 Ab | 83.66 ± 0.98 Cde | 112.41 ± 1.16 Ba | 7.998 ± 0.843 Abc | 3.902 ± 0.463 Bd | 4.563 ± 0.065 Bc |
| KK3 | 188.66 ± 10.98 Aa | 147.23 ± 3.48 Ba | 117.32 ± 9.46 Ca | 14.697 ± 0.857 Aa | 14.314 ± 1.687 Aa | 12.53 ± 1.294 Aa |
| Q117 | 68.33 ± 15.26 Ad | 75.89 ± 2.85 Ae | 46.79 ± 0.36 Bc | 7.255 ± 2.425 Ac | 8.054 ± 1.353 Ac | 5.670 ± 0.060 Ac |
| LK92-11 | 120.48 ± 28.20 Ab | 102.92 ± 10.78 Ac | 109.11 ± 11.79 Aa | 11.424 ± 1.025 Ab | 11.100 ± 1.315 Ab | 12.002 ± 1.332 Aa |
| Genotype | CCS (%) | ||
|---|---|---|---|
| FC | ½FC | RF | |
| Ths98-271 | 1.10 ± 1.10 ABd | 0.06 ± 0.09 Bd | 2.27 ± 1.46 Ae |
| KK08-214 | 7.29 ± 1.59 Ac | 8.47 ± 1.00 Ab | 8.98 ± 0.86 Ac |
| E08-4-019 | 6.70 ± 0.17 Ac | 4.66 ± 0.50 Bc | 4.06 ± 0.10 Bd |
| KK3 | 7.79 ± 0.02 Bbc | 9.72 ± 1.04 Aab | 10.67 ± 0.36 Ab |
| Q117 | 10.49 ± 2.12 Aa | 10.58 ± 1.41 Aa | 12.12 ± 0.22 Aa |
| LK92-11 | 9.69 ± 1.44 Aab | 10.86 ± 1.62 Aa | 11.00 ± 0.36 Aab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leanasawat, N.; Kosittrakun, M.; Lontom, W.; Songsri, P. Physiological and Agronomic Traits of Certain Sugarcane Genotypes Grown under Field Conditions as Influenced by Early Drought Stress. Agronomy 2021, 11, 2319. https://doi.org/10.3390/agronomy11112319
Leanasawat N, Kosittrakun M, Lontom W, Songsri P. Physiological and Agronomic Traits of Certain Sugarcane Genotypes Grown under Field Conditions as Influenced by Early Drought Stress. Agronomy. 2021; 11(11):2319. https://doi.org/10.3390/agronomy11112319
Chicago/Turabian StyleLeanasawat, Nithaya, Manit Kosittrakun, Watanachai Lontom, and Patcharin Songsri. 2021. "Physiological and Agronomic Traits of Certain Sugarcane Genotypes Grown under Field Conditions as Influenced by Early Drought Stress" Agronomy 11, no. 11: 2319. https://doi.org/10.3390/agronomy11112319







