Temporal Changes Analysis of Soil Properties Associated with Ganoderma boninense Pat. Infection in Oil Palm Seedlings in a Controlled Environment
Abstract
:1. Introduction
2. Materials and Methods
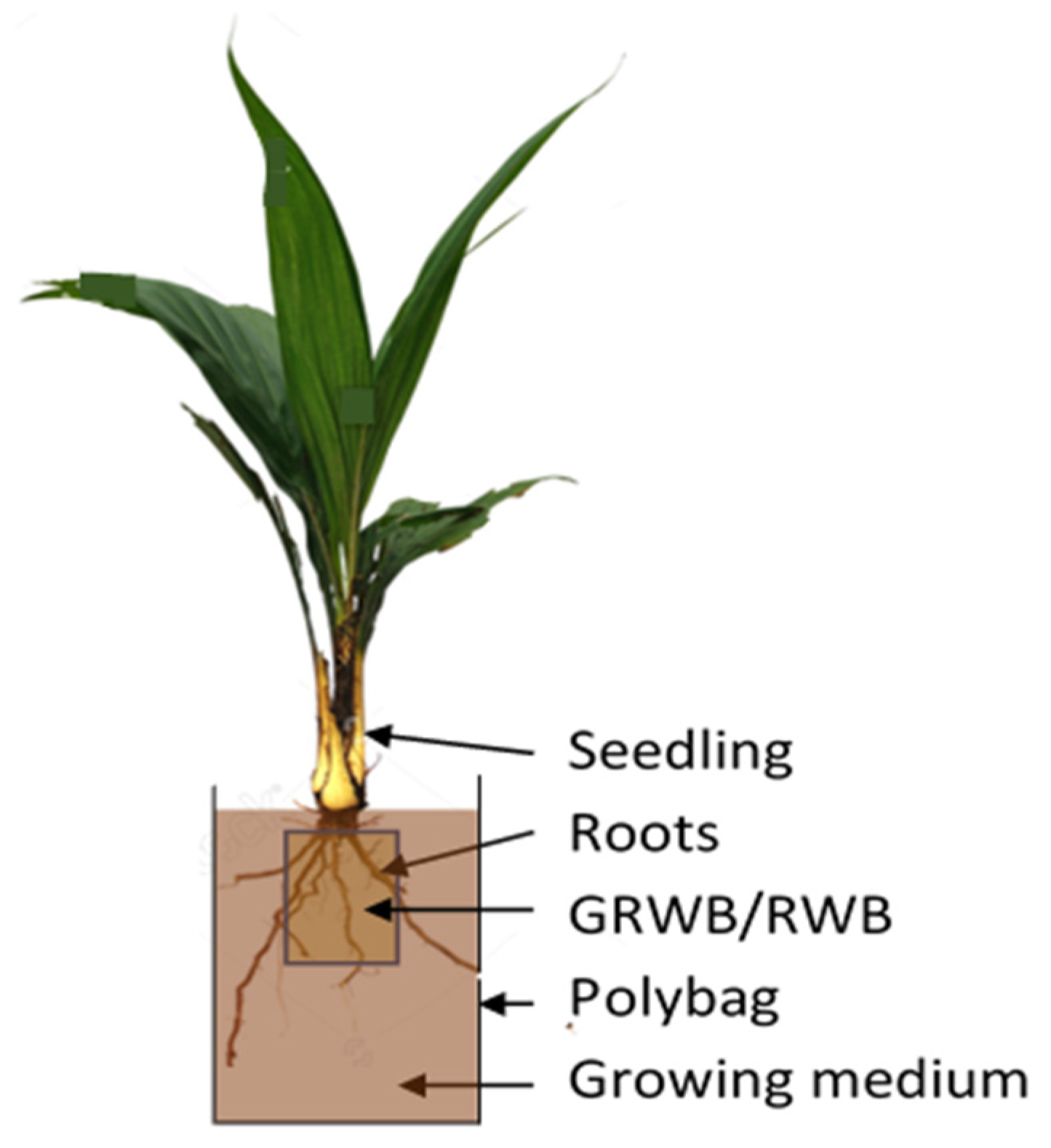
2.1. Study Area
2.2. Physical Growth Measurement
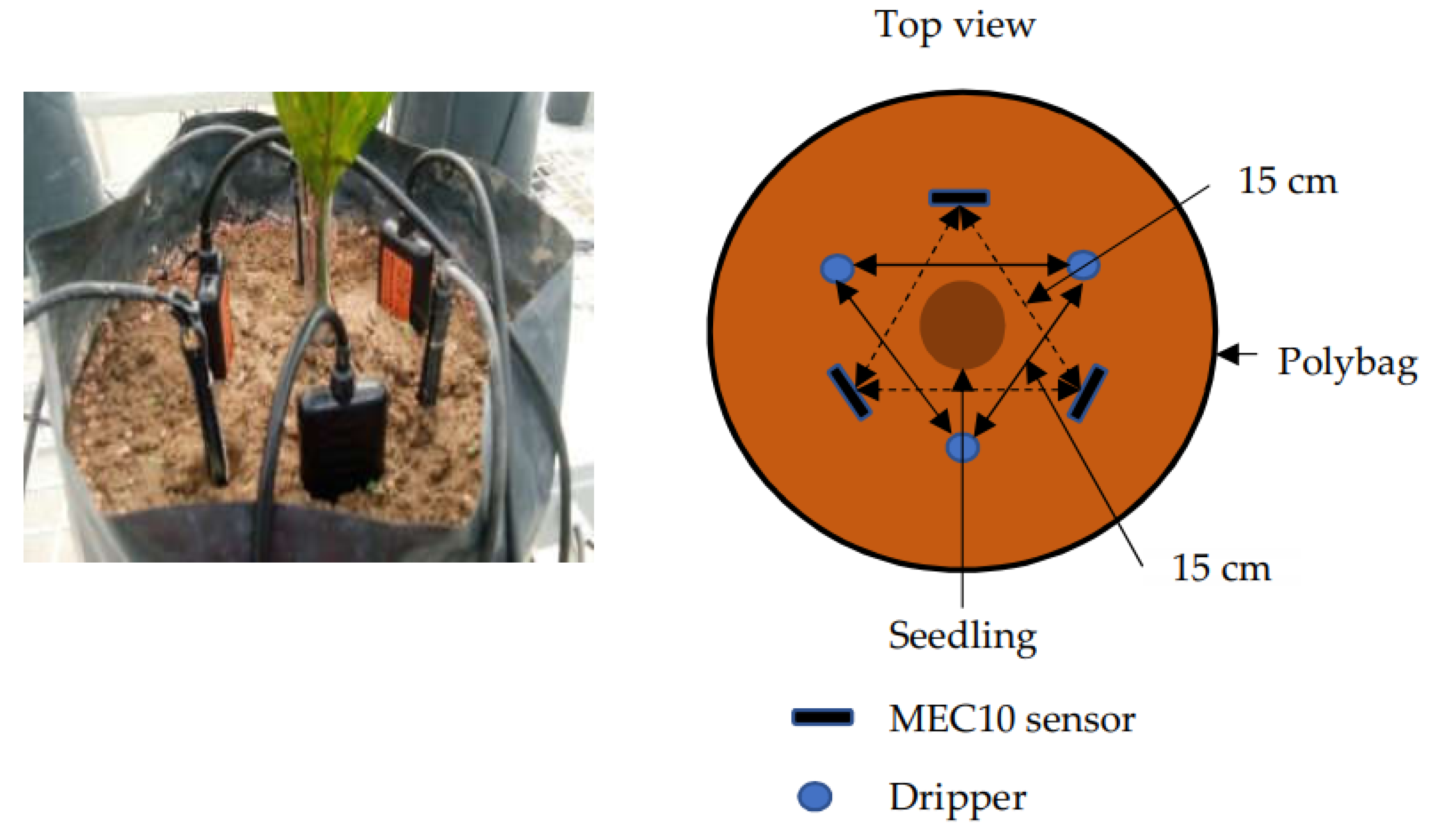
2.3. MEC10 Soil Sensor
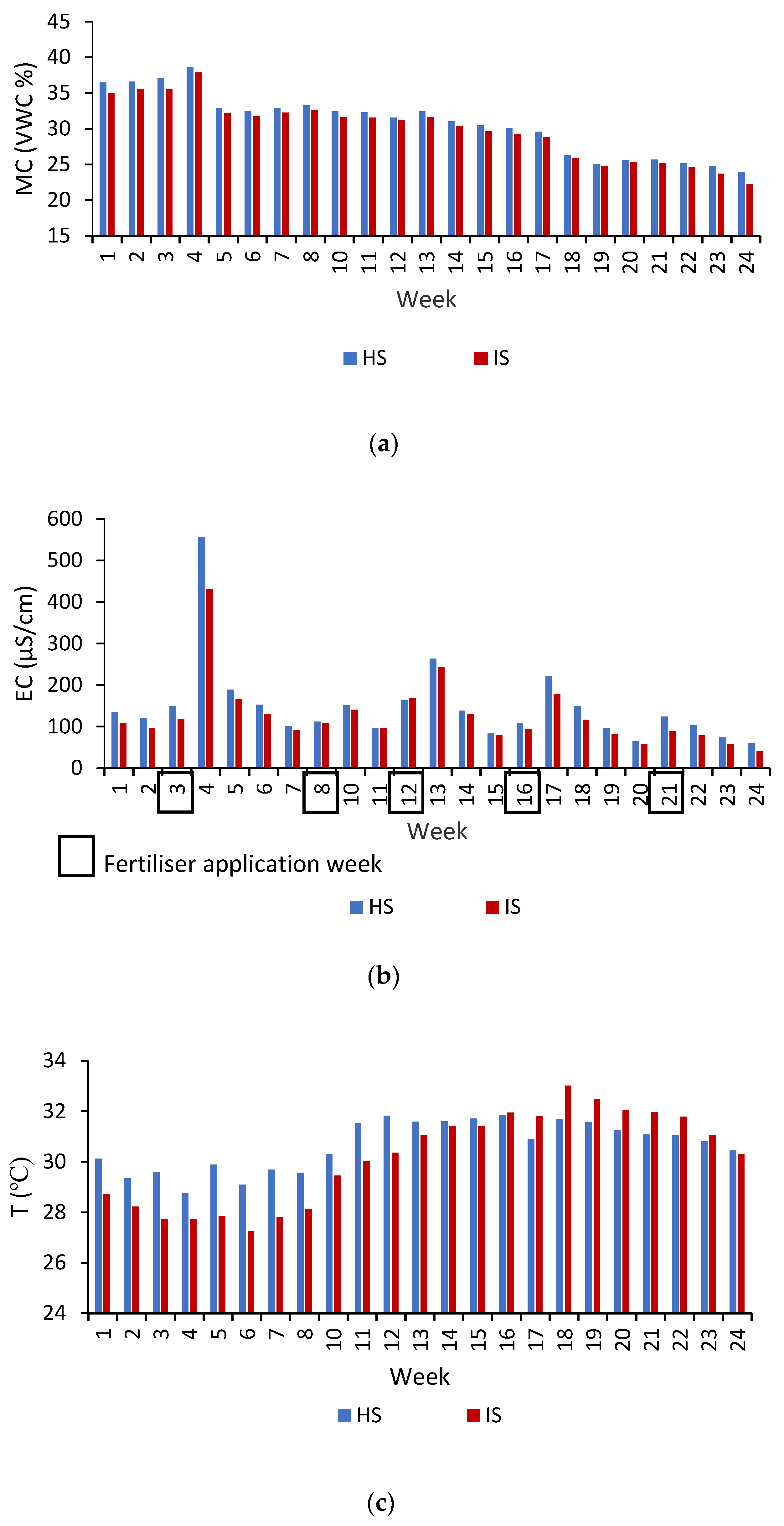
2.4. Data Collection
2.5. Data Analysis
3. Results and Discussion
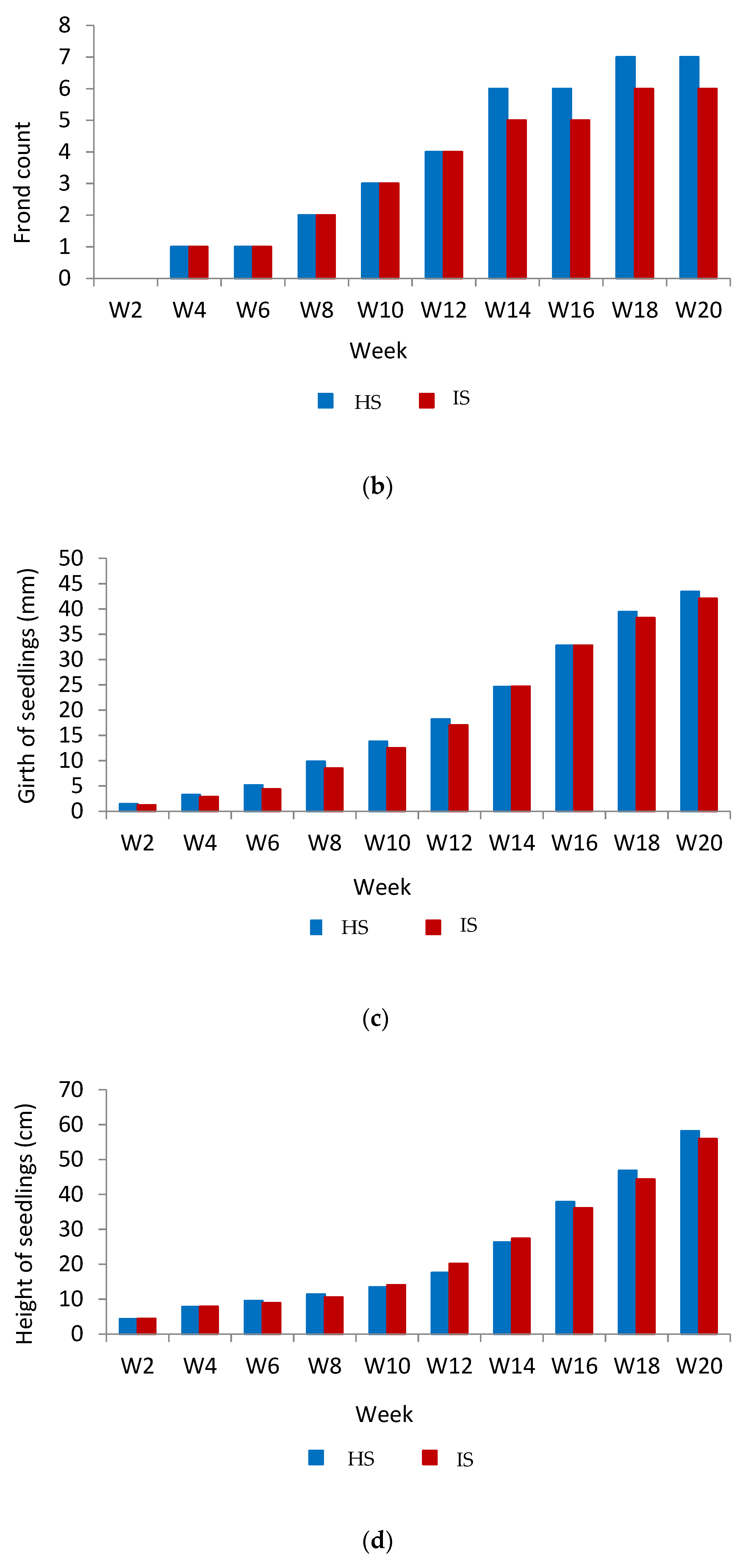
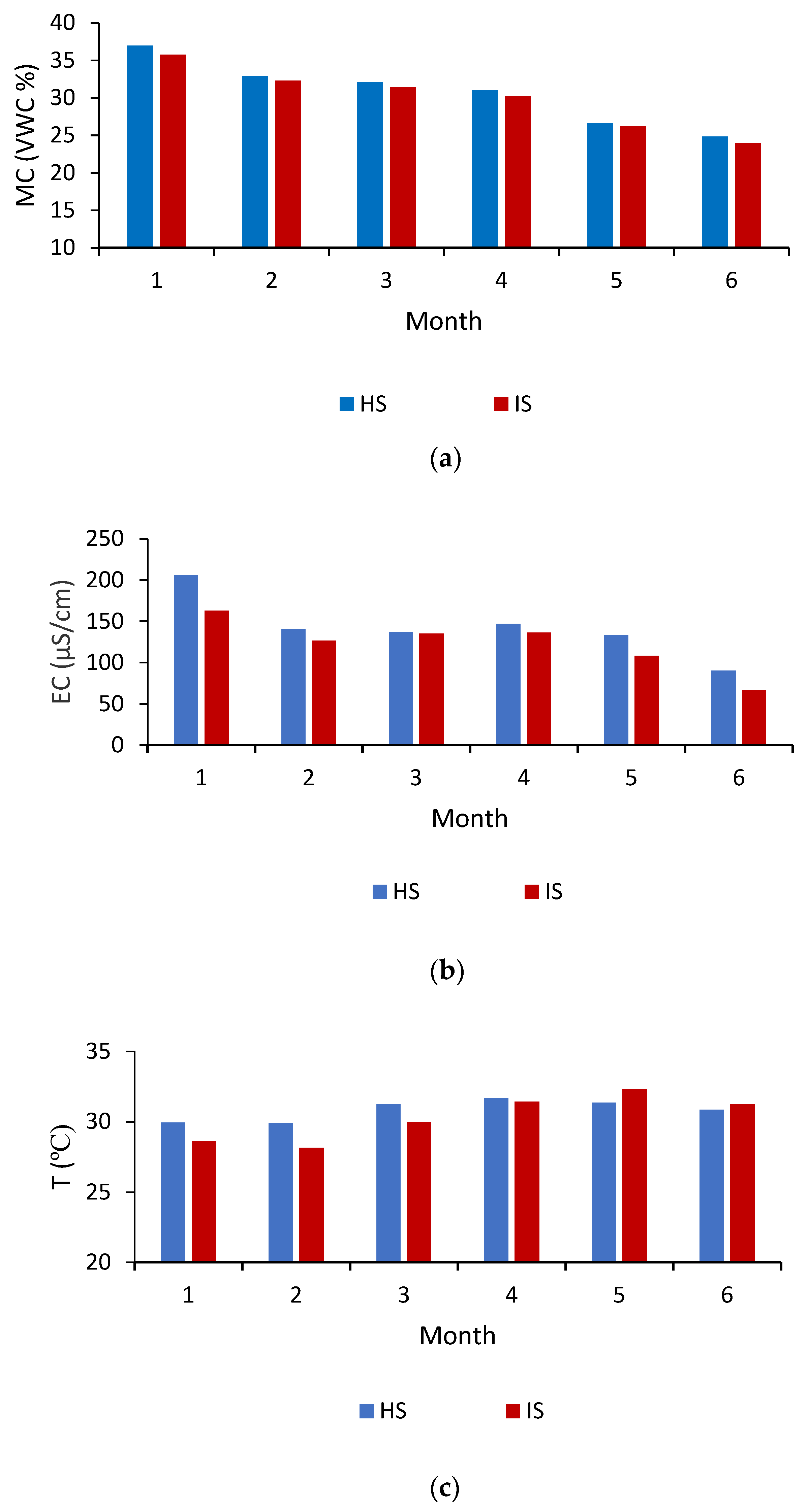
3.1. Analysis of Physical Properties
3.2. Monthly Analysis
3.3. Weekly Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Idris, S.; Arifin, D.; Swinburne, R.; Watt, A.A. The Identity of Ganoderma Species Responsible for BSR Disease of Oil Palm in Malaysia Morphological Characteristics; Malaysian Palm Oil Board: Kuala Lumpur, Malaysia, 2000; Volume 102, p. 77a.
- Chong, K.P.; Dayou, J.; Alexander, A. Pathogenic nature of Ganoderma boninense and basal stem rot disease. In Detection and Control of Ganoderma Boninense in Oil Palm Crop; Springer: Berlin/Heidelberg, Germany, 2017; pp. 5–12. [Google Scholar]
- Toh Choon, R.L.; Sariah, M.; Siti Mariam, M.N. Ergosterol from the soilborne fungus Ganoderma boninense. J. Basic Microbiol. 2012, 52, 608–612. [Google Scholar] [CrossRef]
- Sanderson, F.R. An insight into spore dispersal of Ganoderma boninense on oil palm. Mycopathologia 2005, 159, 139–141. [Google Scholar] [CrossRef]
- Turner, P.D. Oil Palm Diseases and Disorder; The Incorporated Society of Planters: Kuala Lumpur, Malaysia, 1981. [Google Scholar]
- Rees, R.W.; Flood, J.; Hasan, Y.; Potter, U.; Cooper, R.M. Basal stem rot of oil palm (Elaeis guineensis); mode of root infection and lower stem invasion by Ganoderma boninense. Plant Pathol. 2009, 58, 982–989. [Google Scholar] [CrossRef]
- Alexander, A.; Sipaut, C.S.; Dayou, J.; Chong, K.P. Oil palm roots colonisation by Ganoderma boninense: An insight study using scanning electron microscopy. J. Oil Palm Res. 2017, 29, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, P.; Muharam, F.M.; Ahmad, K.; Mansor, S.; Abu Seman, I. Early detection of Ganoderma basal stem rot of oil palms using artificial neural network spectral analysis. Plant Dis. 2017, 101, 1009–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastburn, D.M.; Butler, E.E. Effects of soil moisture and temperature on the saprophytic ability of Trichoderma harzianum. Mycologia 1991, 83, 257–263. [Google Scholar] [CrossRef]
- Garrett, S.D. Soil conditions and the root-infecting fungi. Biol. Rev. 1938, 13, 159–184. [Google Scholar] [CrossRef]
- Gill, J.S.; Sivasithamparam, K.; Smettem, K.R.J. Effect of soil moisture at different temperatures on Rhizoctonia root rot of wheat seedlings. Plant Soil 2001, 231, 91–96. [Google Scholar] [CrossRef]
- Chang, T. Effect of soil moisture content on the survival of Ganoderma species and other wood-inhabiting fungi. Plant Dis. 2003, 87, 1201–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, J.; Verma, J.P.; Yadav, S.K.; Tiwari, K.N. Effect of salt concentration and pH on soil inhabiting fungus Penicillium citrinum Thom. for solubilization of tricalcium phosphate. Microbiol. J. 2011, 1, 25–32. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Nawawi, A.; Ho, Y.W. Effect of temperature and pH on growth pattern of Ganoderma boninense from oil palm in Peninsular Malaysia. Pertanika 1990, 13, 303–307. [Google Scholar]
- Saremi, H.; Burgess, L.W. Effect of soil temperature on distribution and population dynamics of Fusarium species. J. Agric. Sci. Technol. 2000, 2, 119–125. [Google Scholar]
- Rees, R.W.; Flood, J.; Hasan, Y.; Cooper, R.M. Effects of inoculum potential, shading and soil temperature on root infection of oil palm seedlings by the basal stem rot pathogen Ganoderma boninense. Plant Pathol. 2007, 56, 862–870. [Google Scholar] [CrossRef]
- Liddell, C.M. Measurement and Control of Soil Temperature and Water Potential. In Methods for Research on Soilborne Phytopathogenic Fungi; Singleton, L.L., Mihail, S.D., Ruch, C.M., Eds.; The American Phytopathological Society: St. Paul, Minnesota, 1992; pp. 187–203. [Google Scholar]
- Gurmit, S. Ganoderma-the scourge of oil palm in the coastal area. In Proceedings of the Ganoderma Workshop, Bangi, Selangor, Malaysia; Palm Oil Research Institute of Malaysia: Kuala Lumpur, Malaysia, 1990; pp. 7–35. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical conductivity and total dissolved solids. Methods Soil Anal. Part 3 Chem. Methods 1996, 5, 417–435. [Google Scholar]
- Shahid, S.A.; Zaman, M.; Heng, L. Introduction to soil salinity, sodicity and diagnostics techniques. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 1–42. [Google Scholar]
- Corwin, D.L.; Yemoto, K. Salinity: Electrical conductivity and total dissolved solids. Soil Sci. Soc. Am. J. 2000, 84, 1442–1461. [Google Scholar] [CrossRef]
- Goh, Y.K.; Choon, K.L.; Cheng, C.R.; Tan, S.Y.; Cheah, L.W.; Ah Tung, P.G.; Goh, K.J. Effects of chemical properties of different soils on ganoderma disease in oil palm (Elaeis guineensis). Oil Palm Bull. 2017, 75, 17–26. [Google Scholar]
- Bivi, M.S.H.R.; Paiko, A.S.; Khairulmazmi, A.; Akhtar, M.S.; Idris, A.S. Control of basal stem rot disease in oil palm by supplementation of calcium, copper, and salicylic acid. Plant Pathol. J. 2016, 32, 396. [Google Scholar] [CrossRef] [Green Version]
- Baharuddin, M.F.T.; Masirin, M.I.M.; Hazreek, Z.A.M.; Azman, M.A.A.; Madun, A. Resistivity-Chemistry Integrated Approaches for Investigating Groundwater Salinity of Water Supply and Agricultural Activity at Island Coastal Area. J. Phys. Conf. Ser. 2018, 995, 012093. [Google Scholar] [CrossRef]
- Ariffin, D.; Idris, A.S.; Singh, G. Status of Ganoderma in oil palm. In Ganoderma Diseases of Perennial Crops; Flood, J., Bridge, P.D., Holderness, M., Eds.; CABI Publishing: Wallingford, UK, 2000. [Google Scholar]
- Singh, G. Ganoderma-the scourge of oil palms in the coastal areas. Planter 1991, 67, 421–444. [Google Scholar]
- Naher, L.; Yusuf, U.K.; Ismail, A.; Tan, S.G.; Mondal, M.M.A. Ecological status of Ganoderma and basal stem rot disease of oil palms (Elaeis guineensis Jacq.). Aust. J. Crop Sci. 2013, 7, 1723–1727. [Google Scholar]
- Kubisch, P.; Leuschner, C.; Coners, H.; Gruber, A.; Hertel, D. Fine root abundance and dynamics of stone pine (Pinus cembra) at the alpine treeline is not impaired by self-shading. Front. Plant Sci. 2017, 8, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bote, A.D.; Struik, P.C. Effects of shade on growth, production and quality of coffee (Coffea arabica) in Ethiopia. J. Hortic. For. 2011, 3, 336–341. [Google Scholar]
- Alexander, A.; Dayou, J.; Abdullah, S.; Phin, C.K. Differential Expression and Profile of Oil Palm Root-sterols Composition Related to Ganoderma boninense Infection. ASM Sci. J. 2019, 12, 40–47. [Google Scholar]
- As’wad, A.M.; Sariah, M.; Paterson, R.R.M.; Abidin, M.Z.; Lima, N. Ergosterol analyses of oil palm seedlings and plants infected with Ganoderma. Crop Prot. 2011, 30, 1438–1442. [Google Scholar] [CrossRef] [Green Version]
- Idris, A.S.; Kushairi, D.; Ariffin, D.; Basri, M.W. Technique for inoculation of oil palm germinated seeds with Ganoderma. MPOB Inf. Ser. 2006, 314, 1–4. [Google Scholar]
- Breton, F.; Hasan, Y.; Lubis, Z.; De Franqueville, H. Characterization of parameters for the development of an early screening test for basal stem rot tolerance in oil palm progenies. J. Oil Palm Res. 2006, 24–36. Available online: http://palmoilis.mpob.gov.my/publications/jopr2006sp-ms24.pdf (accessed on 31 October 2021).
- Santoso, H.; Gunawan, T.; Jatmiko, R.H.; Darmosarkoro, W.; Minasny, B. Mapping and identifying basal stem rot disease in oil palms in North Sumatra with QuickBird imagery. Precis. Agric. 2011, 12, 233–248. [Google Scholar] [CrossRef]
- Utomo, C.; Niepold, F. The development of diagnostic tools of Ganoderma in oil palm. Phytopathology 2000, 148, 507–514. [Google Scholar] [CrossRef]
- Naidu, Y.; Siddiqui, Y.; Rafii, M.Y.; Saud, H.M.; Idris, A.S. Inoculation of oil palm seedlings in Malaysia with white-rot hymenomycetes: Assessment of pathogenicity and vegetative growth. Crop Prot. 2018, 110, 146–154. [Google Scholar] [CrossRef]
- Rakib, M.R.M.; Borhan, A.H.; Jawahir, A.N. The relationship between SPAD chlorophyll and disease severity index in Ganoderma-infected oil palm seedlings. J. Bangladesh Agric. Univ. 2019, 17, 355–358. [Google Scholar] [CrossRef] [Green Version]
- Intara, Y.I.; Nusantara, A.D.; Supanjani, S.; Caniago, Z.; Ekawita, R. Oil palm roots architecture in response to soil humidity. Int. J. Oil Palm 2018, 1, 79–89. [Google Scholar]
- Goh, K.M.; Dickinson, M.; Alderson, P.; Yap, L.V.; Supramaniam, C.V. Development of an in planta infection system for the early detection of Ganoderma spp. in oil palm. J. Plant Pathol. 2016, 98, 255–264. [Google Scholar]
- Sapak, Z.; Meon, S.; Ahmad, Z.A.M. Effect of endophytic bacteria on growth and suppression of Ganoderma infection in oil palm. Int. J. Agric. Biol. 2008, 10, 127–132. [Google Scholar]
- Govender, N.T.; Mahmood, M.; Seman, I.A.; Wong, M.Y. The phenylpropanoid pathway and lignin in defense against ganoderma boninense colonized root tissues in oil palm (Elaeis guineensis Jacq.). Front. Plant Sci. 2017, 8, 1395. [Google Scholar] [CrossRef] [Green Version]
- Uchida, R. Essential nutrients for plant growth: Nutrient functions and deficiency symptoms. Plant Nutr. Manag. Hawaiis Soils 2000, 4, 31–55. [Google Scholar]
- Vetterlein, D.; Doussan, C. Root age distribution: How does it matter in plant processes? A focus on water uptake. Plant Soil 2016, 407, 145–160. [Google Scholar] [CrossRef]
- Tachibana, Y.; Ohta, Y. Root surface area, as a parameter in relation to water and nutrient uptake by cucumber plant. Soil Sci. Plant Nutr. 1983, 29, 387–392. [Google Scholar] [CrossRef]
- Bouillet, J.P.; Laclau, J.P.; Arnaud, M.; M’Bou, A.T.; Saint-André, L.; Jourdan, C. Changes with age in the spatial distribution of roots of Eucalyptus clone in Congo: Impact on water and nutrient uptake. For. Ecol. Manag. 2002, 171, 43–57. [Google Scholar] [CrossRef]
- Gao, S.; Pan, W.L.; Koenig, R.T. Integrated root system age in relation to plant nutrient uptake activity. Agron. J. 1998, 90, 505–510. [Google Scholar] [CrossRef]
- Lakshmi, V.; Jackson, T.J.; Zehrfuhs, D. Soil moisture–temperature relationships: Results from two field experiments. Hydrol. Process. 2003, 17, 3041–3057. [Google Scholar] [CrossRef]
- Talley, P.J.; Blank, L.M. Some factors influencing the utilization of inorganic nitrogen by the root rot fungus. Plant Physiol. 1942, 17, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicholas, D.J.D. Utilization of inorganic nitrogen compounds and amino acids by fungi. Fungi 1965, 1, 349–376. [Google Scholar]
- Lussenhop, J. Mechanisms of microarthropod-microbial interactions in soil. Adv. Ecol. Res. 1992, 23, 1–33. [Google Scholar]
- Larsen, J.; Jaramillo-López, P.; Nájera-Rincon, M.; González-Esquivel, C.E. Biotic interactions in the rhizosphere in relation to plant and soil nutrient dynamics. J. Soil Sci. Plant Nutr. 2015, 15, 449–463. [Google Scholar] [CrossRef] [Green Version]



| Parameter | Condition | Mean | p-Value |
|---|---|---|---|
| MC | HS | 30.74 | 0.0010 * |
| IS | 29.96 | ||
| EC | HS | 142.34 | 0.0195 * |
| IS | 122.55 | ||
| T | HS | 30.82 | 0.2884 |
| IS | 30.28 |
| Parameter | Condition | Mean | p-Value |
|---|---|---|---|
| MC | HS | 30.72 | <0.0001 * |
| IS | 29.93 | ||
| EC | HS | 148.18 | 0.0004 * |
| IS | 125.99 | ||
| T | HS | 30.66 | 0.0338 * |
| IS | 30.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aziz, M.H.A.; Khairunniza-Bejo, S.; Wayayok, A.; Hashim, F.; Kondo, N.; Azmi, A.N.N. Temporal Changes Analysis of Soil Properties Associated with Ganoderma boninense Pat. Infection in Oil Palm Seedlings in a Controlled Environment. Agronomy 2021, 11, 2279. https://doi.org/10.3390/agronomy11112279
Aziz MHA, Khairunniza-Bejo S, Wayayok A, Hashim F, Kondo N, Azmi ANN. Temporal Changes Analysis of Soil Properties Associated with Ganoderma boninense Pat. Infection in Oil Palm Seedlings in a Controlled Environment. Agronomy. 2021; 11(11):2279. https://doi.org/10.3390/agronomy11112279
Chicago/Turabian StyleAziz, Mohd H. A., Siti Khairunniza-Bejo, Aimrun Wayayok, Fazirulhisyam Hashim, Naoshi Kondo, and Aiman N. N. Azmi. 2021. "Temporal Changes Analysis of Soil Properties Associated with Ganoderma boninense Pat. Infection in Oil Palm Seedlings in a Controlled Environment" Agronomy 11, no. 11: 2279. https://doi.org/10.3390/agronomy11112279






