Estimating Organ Contribution to Grain Filling and Potential for Source Upregulation in Wheat Cultivars with a Contrasting Source–Sink Balance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Design
2.2. Source Sink Manipulation Treatments
2.3. Crop Measurements
2.4. Statistical Analysis
3. Results
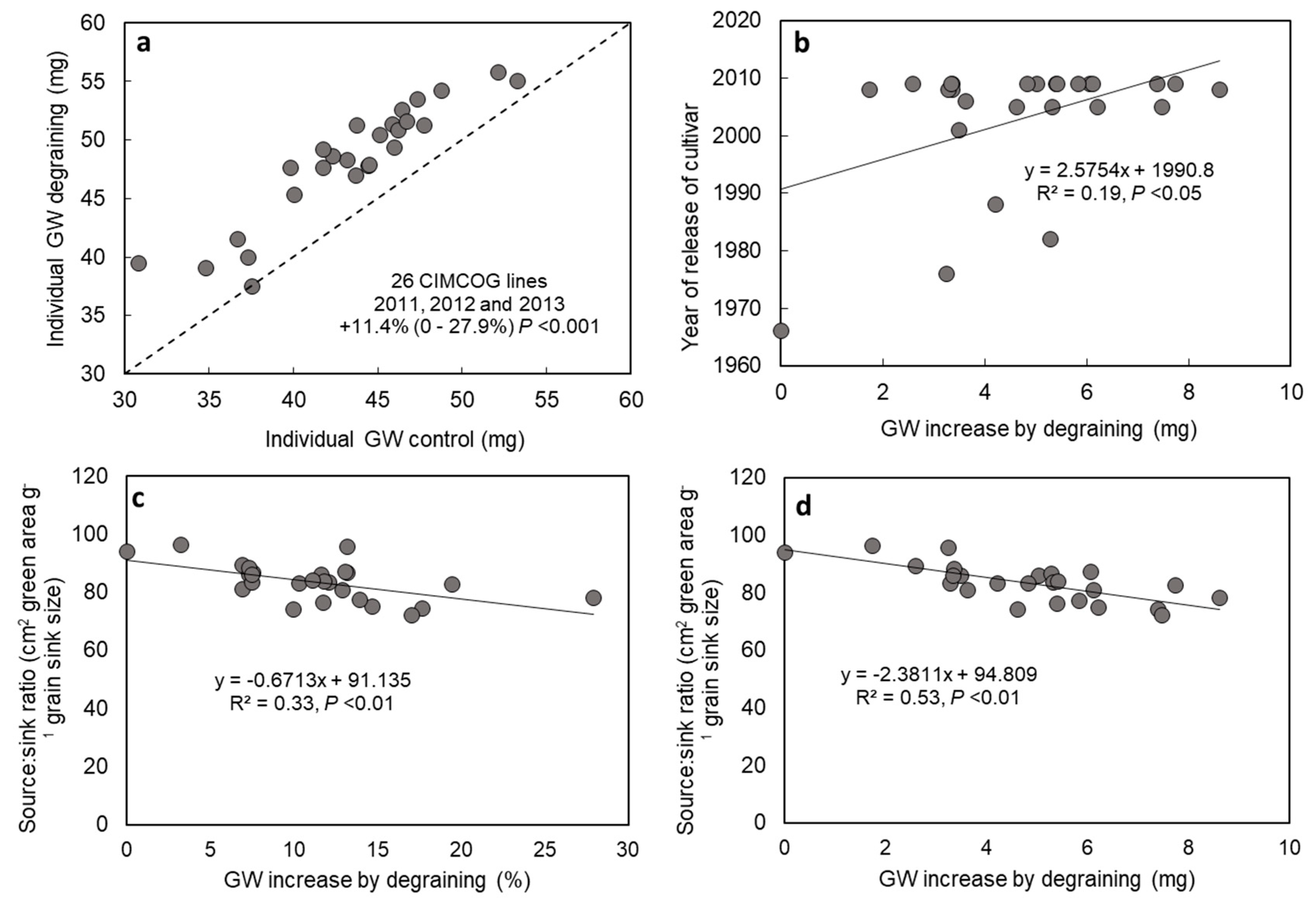
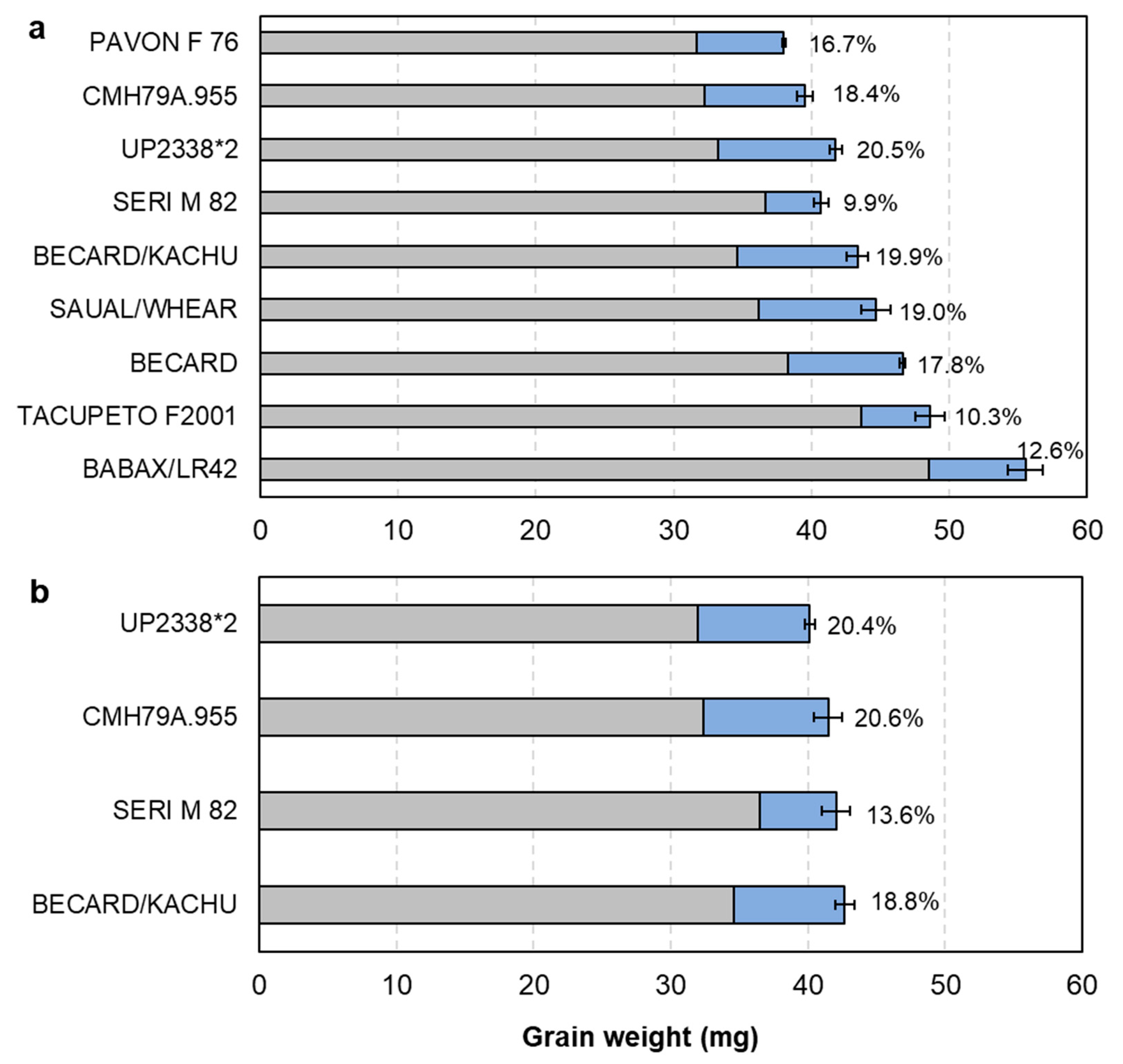
3.1. Source–Sink Balance in Spring Wheat CIMMYT Elite Cultivars (De-Graining Treatments)
3.2. Organ Contribution to Grain Growth and Post-Anthesis Photosynthetic Capacity
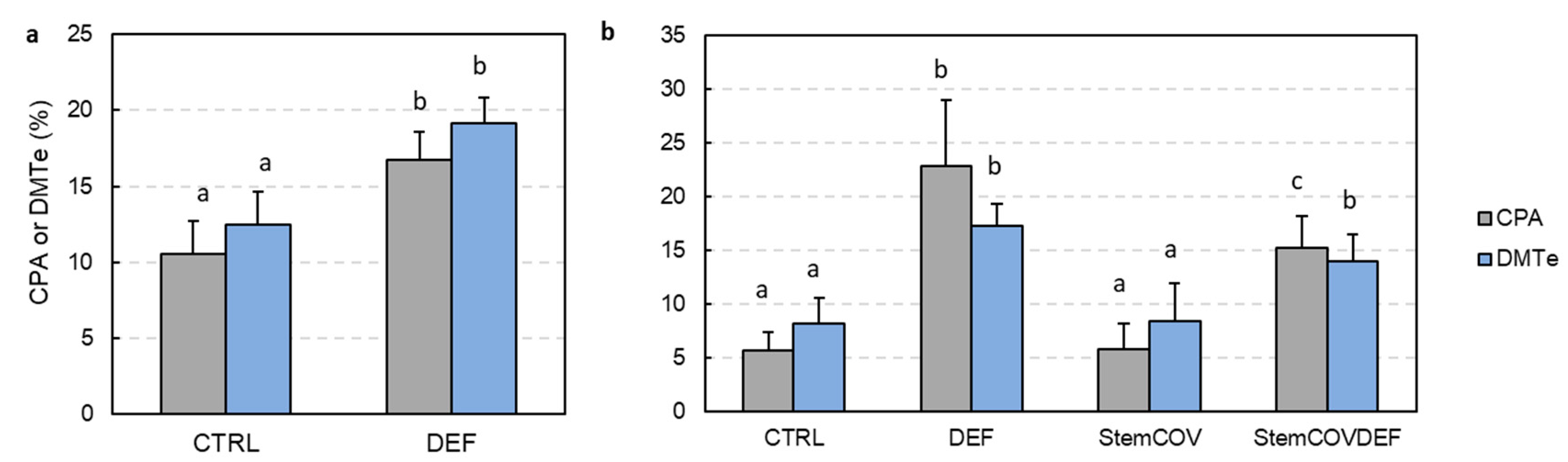
3.3. Upregulation of Photosynthesis and Stem Pre-Anthesis Reserve Remobilization
4. Discussion
4.1. Evidence for Changes in Source–Sink Balance with Plant Breeding
4.2. Contribution of Leaf Laminae and Stem Photosynthesis to Grain Growth during Grain Filling
4.3. Upregulation of Stem Pre-Anthesis Reserves and Photosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Slafer, G.A.; Savin, R. Source—sink relationships and grain mass at different positions within the spike in wheat. Field Crop. Res. 1994, 37, 39–49. [Google Scholar] [CrossRef]
- Aisawi, K.A.B.; Reynolds, M.P.; Singh, R.P.; Foulkes, M.J. The Physiological Basis of the Genetic Progress in Yield Potential of CIMMYT Spring Wheat Cultivars from 1966 to 2009. Crop Sci. 2015, 55, 1749. [Google Scholar] [CrossRef]
- Borrás, L.; Slafer, G.A.; Otegui, M.E. Seed dry weight response to source–sink manipulations in wheat, maize and soybean: A quantitative reappraisal. Field Crop. Res. 2004, 86, 131–146. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Pellegrineschi, A.; Skovmand, B. Sink-limitation to yield and biomass: A summary of some investigations in spring wheat. Ann. Appl. Biol. 2005, 146, 39–49. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Calderini, D.F.; Condon, A.G.; Rajaram, S. Physiological basis of yield gains in wheat associated with the LR19 translocation from Agropyron elongatum. Euphytica 2001, 119, 139–144. [Google Scholar] [CrossRef]
- Gaju, O.; Reynolds, M.P.; Sparkes, D.L.; Mayes, S.; Ribas-Vargas, G.; Crossa, J.; Foulkes, M.J. Relationships between physiological traits, grain number and yield potential in a wheat DH population of large spike phenotype. Field Crop. Res. 2014, 164, 126–135. [Google Scholar] [CrossRef]
- Ahmadi, A.; Joudi, M.; Janmohammadi, M. Late defoliation and wheat yield: Little evidence of post-anthesis source limitation. Field Crop. Res. 2009, 113, 90–93. [Google Scholar] [CrossRef]
- Acreche, M.M.; Slafer, G.A. Grain weight, radiation interception and use efficiency as affected by sink-strength in Mediterranean wheats released from 1940 to 2005. Field Crop. Res. 2009, 110, 98–105. [Google Scholar] [CrossRef]
- Álvaro, F.; Royo, C.; García del Moral, L.F.; Villegas, D. Grain Filling and Dry Matter Translocation Responses to Source–Sink Modifications in a Historical Series of Durum Wheat. Crop Sci. 2008, 48, 1523. [Google Scholar] [CrossRef] [Green Version]
- Shearman, V.J.; Sylvester-Bradley, R.; Scott, R.K.; Foulkes, M.J. Physiological processes associated with wheat yield progress in the UK. Crop Sci. 2005, 45, 175–185. [Google Scholar] [CrossRef]
- Lopes, M.S.; Reynolds, M.P.; Manes, Y.; Singh, R.P.; Crossa, J.; Braun, H.J. Genetic Yield Gains and Changes in Associated Traits of CIMMYT Spring Bread Wheat in a “Historic” Set Representing 30 Years of Breeding. Crop Sci. 2012, 52, 1123. [Google Scholar] [CrossRef]
- Bonnett, G.D.; Incoll, L.D. Effects on the Stem of Winter Barley of Manipulating the Source and Sink during Grain-filling: II. Changes in the composition of water soluble carbohydrate of internodes. J. Exp. Bot. 1993, 44, 83–91. [Google Scholar] [CrossRef]
- Calderini, D.F.; Reynolds, M.P. Changes in grain weight as a consequence of de-graining treatments at pre- and post-anthesis in synthetic hexaploid lines of wheat (Triticum durum x T. tauschii). Funct. Plant Biol. 2000, 27, 183–191. [Google Scholar] [CrossRef]
- Reynolds, M.; Langridge, P. Physiological breeding. Curr. Opin. Plant Biol. 2016, 31, 162–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bingham, I.J.; Blake, J.; Foulkes, M.J.; Spink, J. Is barley yield in the UK sink limited? Field Crop. Res. 2007, 101, 198–211. [Google Scholar] [CrossRef]
- Rivera-Amado, C.; Trujillo-Negrellos, E.; Molero, G.; Reynolds, M.P.; Sylvester-Bradley, R.; Foulkes, M.J. Optimizing dry-matter partitioning for increased spike growth, grain number and harvest index in spring wheat. Field Crop. Res. 2019. [Google Scholar] [CrossRef]
- Araus, J.L.; Brown, H.R.; Febrero, A.; Bort, J.; Serret, M.D. Ear photosynthesis, carbon isotope discrimination and the contribution of respiratory CO2 to differences in grain mass in durum wheat. Plant Cell Environ. 1993, 16, 383–392. [Google Scholar] [CrossRef]
- Evans, L.T.; Rawson, H.M. Photosynthesis and Respiration by the Flag Leaf and Components of the Ear During Grain Development In Wheat. Aust. J. Biol. Sci. 1970, 23, 245–254. [Google Scholar] [CrossRef]
- Kriedemann, P. The photosynthetic activity of the wheat ear. Ann. Bot. 1966, 30, 349–363. [Google Scholar] [CrossRef]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Graciano, C.; López, J.R.; Tambussi, E.A. The contribution of ear photosynthesis to grain filling in bread wheat (Triticum aestivum L.). Field Crop. Res. 2010, 119, 48–58. [Google Scholar] [CrossRef]
- Sanchez-Bragado, R.; Molero, G.; Reynolds, M.P.; Araus, J.L. Relative contribution of shoot and ear photosynthesis to grain filling in wheat under good agronomical conditions assessed by differential organ 13C. J. Exp. Bot. 2014, 65, 5401–5413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molero, G.; Reynolds, M.P. Spike photosynthesis measured at high throughput indicates genetic variation independent of flag leaf photosynthesis. Field Crop. Res. 2020, 255, 107866. [Google Scholar] [CrossRef]
- Simkin, A.J.; Faralli, M.; Ramamoorthy, S.; Lawson, T. Photosynthesis in non-foliar tissues: Implications for yield. Plant J. 2020, 101, 1001–1015. [Google Scholar] [CrossRef] [PubMed]
- Yiotis, C.; Psaras, G.K.; Manetas, Y. Seasonal photosynthetic changes in the green-stemmed Mediterranean shrub Calicotome villosa: A comparison with leaves. Photosynthetica 2008, 46. [Google Scholar] [CrossRef]
- Osmond, C.B.; Smith, S.D.; Gui-Ying, B.; Sharkey, T.D. Stem photosynthesis in a desert ephemeral, Eriogonum inflatum. Oecologia 1987, 72, 542–549. [Google Scholar] [CrossRef]
- Araus, J.L.; Tapia, L. Photosynthetic Gas Exchange Characteristics of Wheat Flag Leaf Blades and Sheaths during Grain Filling: The Case of a Spring Crop Grown under Mediterranean Climate Conditions. PLANT Physiol. 1987, 85, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Qin, F.; Du, F.; Xu, R.; Xu, Q.; Tian, C.; Li, F.; Wang, F. Photosynthesis in the different parts of a wheat plant. J. Food Agric. Environ. 2009, 7, 339–404. [Google Scholar]
- Serrago, R.A.; Alzueta, I.; Savin, R.; Slafer, G.A. Understanding grain yield responses to source–sink ratios during grain filling in wheat and barley under contrasting environments. Field Crop. Res. 2013, 150, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Maydup, M.L.; Antonietta, M.; Guiamet, J.J.; Tambussi, E.A. The contribution of green parts of the ear to grain filling in old and modern cultivars of bread wheat (Triticum aestivum L.): Evidence for genetic gains over the past century. Field Crop. Res. 2012, 134, 208–215. [Google Scholar] [CrossRef]
- Sayre, K.D.; Rajaram, S.; Fischer, R.A. Yield Potential Progress in Short Bread Wheats in Northwest Mexico. Crop Sci. 1997, 37, 36–42. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Slafer, G.A.; Davies, W.J.; Berry, P.M.; Sylvester-Bradley, R.; Martre, P.; Calderini, D.F.; Griffiths, S.; Reynolds, M.P. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. J. Exp. Bot. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pask, A.J.D.; Pietragalla, J.; Mullan, D.M.; Reynolds, M.P. Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: Mexico City, Mexico, 2012. [Google Scholar]
- Alvarado, G.; Rodríguez, F.M.; Pacheco, A.; Burgueño, J.; Crossa, J.; Vargas, M.; Pérez-Rodríguez, P.; Lopez-Cruz, M.A. META-R: A software to analyze data from multi-environment plant breeding trials. Crop J. 2020. [Google Scholar] [CrossRef]
- Aggarwal, P.K.; Fischer, R.A.; Liboon, S.P. Source–sink relations and effects of post-anthesis canopy defoliation in wheat at low latitudes. J. Agric. Sci. 1990, 114, 93–99. [Google Scholar] [CrossRef]
- Carr, D.J.; Wardlaw, I.F. Supply of photosynthetic assimilates to grain from flag leaf and ear of wheat. Aust. J. Biol. Sci. 1965, 18, 711. [Google Scholar]
- Evans, L.T.; Wardlaw, I.F.; Fischer, R.A. Wheat. In Crop Physiology: Some Case Histories; Evans, L.T., Ed.; Cambridge University Press: Cambridge, UK, 1975; pp. 101–149. [Google Scholar]
- Merah, O.; Monneveux, P. Contribution of Different Organs to Grain Filling in Durum Wheat under Mediterranean Conditions I. Contribution of Post-Anthesis Photosynthesis and Remobilization. J. Agron. Crop Sci. 2015, 201, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Thorne, G.N. Distribution between parts of the main shoot and the tillers of photosynthate produced before and after anthesis in the top three leaves of main shoots of Hobbit and Maris Huntsman winter wheat. Ann. Appl. Biol. 1982, 101, 553–559. [Google Scholar] [CrossRef]
- Kruk, B.C.; Calderini, D.F.; Slafer, G.A. Grain weight in wheat cultivars released from 1920 to 1990 as affected by post-anthesis defoliation. J. Agric. Sci. 1997, 128, 273–281. [Google Scholar] [CrossRef]
- Gebbing, T. The enclosed and exposed part of the peduncle of wheat (Triticum aestivum)—Spatial separation of fructan storage. New Phytol. 2003, 159, 245–252. [Google Scholar] [CrossRef]
- Kong, L.; Wang, F.; Feng, B.; Li, S.; Si, J.; Zhang, B. The structural and photosynthetic characteristics of the exposed peduncle of wheat (Triticum aestivum L.): An important photosynthate source for grain-filling. BMC Plant Biol. 2010, 10, 141. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wei, A.L.; Zheng, D.M. Photosynthetic Characteristics of Non-Leaf Organs of Winter Wheat Cultivars Differing in Ear Type and their Relationship with Grain Mass Per Ear. Photosynthetica 2001, 39, 239–244. [Google Scholar] [CrossRef]
- Schnyder, H. The role of carbohydrate storage and redistribution in the source—Sink relations of wheat and barley during grain filling—A review. New Phytol. 1993, 123, 233–245. [Google Scholar] [CrossRef]
- Parry, M.A.J.; Reynolds, M.; Salvucci, M.E.; Raines, C.; Andralojc, P.J.; Zhu, X.-G.; Price, G.D.; Condon, A.G.; Furbank, R.T. Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J. Exp. Bot. 2011, 62, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.X.; Midmore, D.J.; Radford, B.J.; Yule, D.F. Effect of timing of defoliation on wheat (Triticum aestivum) in central Queensland. Field Crop. Res. 2004, 88, 211–226. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Reyes, F.; Rodes, R.; Perez, I.; Dorado, M. Effect of Source-to-sink Ratio on Partitioning of Dry Matter and14C-photoassimilates in Wheat during Grain Filling. Ann. Bot. 1999, 83, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Bidinger, F.; Musgrave, R.B.; Fischer, R.A. Contribution of stored pre-anthesis assimilate to grain yield in wheat and barley. Nature 1977, 270, 431–433. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, M.J.; Scott, R.K.; Sylvester-Bradley, R. The ability of wheat cultivars to withstand drought in UK conditions: Formation of grain yield. J. Agric. Sci. 2002, 138, 153–169. [Google Scholar] [CrossRef]
- Gebbing, T.; Schnyder, H.; Kuhbauch, W. The utilization of pre-anthesis reserves in grain filling of wheat. Assessment by steady-state 13CO2/12CO2 labelling. Plant Cell Environ. 1999, 22, 851–858. [Google Scholar] [CrossRef]
- Wardlaw, I.F.; Porter, H.K. The Redistribution of Stem Sugars in Wheat during Grain Development. Aust. J. Biol. Sci. 1967, 20, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, J.N.; Biscoe, P.V.; Hunter, B. Effects of drought on grain growth. Nature 1976, 264, 541–542. [Google Scholar] [CrossRef]
- Gebbing, T.; Schnyder, H. Pre-Anthesis Reserve Utilization for Protein and Carbohydrate Synthesis in Grains of Wheat. Plant Physiol. 1999, 121, 871–878. [Google Scholar] [CrossRef] [Green Version]
- Yáñez, A.; Tapia, G.; Guerra, F.; del Pozo, A. Stem carbohydrate dynamics and expression of genes involved in fructan accumulation and remobilization during grain growth in wheat (Triticum aestivum L.) genotypes with contrasting tolerance to water stress. PLoS ONE 2017, 12, e0177667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Sun, Y.; Zhang, S.; Palta, J.A.; Deng, X. The Proportion of Superior Grains and the Sink Strength are the Main Yield Contributors in Modern Winter Wheat Varieties Grown in the Loess Plateau of China. Agronomy 2019, 9, 612. [Google Scholar] [CrossRef] [Green Version]
- Beed, F.D.; Paveley, N.D.; Sylvester-Bradley, R. Predictability of wheat growth and yield in light-limited conditions. J. Agric. Sci. 2007, 145, 63. [Google Scholar] [CrossRef]
- Savin, R.; Slafer, G.A. Shading effects on the yield of an Argentinian wheat cultivar. J. Agric. Sci. 1991, 116, 1–7. [Google Scholar] [CrossRef] [Green Version]



| Yield | TGW | HI | Biomass | GM2 | SM2 | GPS | DTA | DTM | PH | |
|---|---|---|---|---|---|---|---|---|---|---|
| g m−2 | g | Idx | g m−2 | # m−2 | # m−2 | # | DAE | DAE | cm | |
| Heritability | 0.74 | 0.98 | 0.84 | 0.74 | 0.93 | 0.91 | 0.86 | 0.90 | 0.90 | 0.95 |
| Min | 664 | 29.9 | 0.44 | 1258 | 13,103 | 240 | 44 | 77 | 123 | 80 |
| Max | 710 | 50.6 | 0.50 | 1517 | 22,780 | 395 | 70 | 96 | 133 | 116 |
| Grand Mean | 670 | 42.4 | 0.47 | 1411 | 15,864 | 304 | 53 | 88 | 128 | 105 |
| SED gen (125) | 17.9 | 1.01 | 0.008 | 38.9 | 544 | 12.9 | 2.48 | 0.640 | 1.05 | 1.77 |
| CV (%) | 5.4 | 4.8 | 3.3 | 5.6 | 6.9 | 8.4 | 9.4 | 1.5 | 1.6 | 3.4 |
| Gen sig. | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Env sig. | <0.05 | 0.452 | <0.001 | 0.102 | 0.863 | 1.00 | 0.091 | 0.421 | <0.05 | 0.054 |
| GenxEnv sig. | <0.01 | 0.408 | 0.089 | <0.05 | <0.01 | <0.01 | 0.066 | <0.001 | <0.05 | 0.054 |
| GPS | GW | DMTe | CPA | ||
|---|---|---|---|---|---|
| # | mg | % | % | ||
| 2013 | |||||
| Subset 1 (9 cultivars) | |||||
| P-value Gen | CTRL−DEF | <0.001 | <0.001 | <0.001 | <0.001 |
| P-value Treat | CTRL−DEF | 0.620 | <0.001 | 0.965 | 0.844 |
| P-value GxT | CTRL−DEF | 0.995 | <0.01 | 0.150 | 0.439 |
| SED Gen (df) | CTRL−DEF | 1.57 (34) | 0.640 (34) | 3.79 (26) | 3.47 (24) |
| Subset 2 (4 cultivars) | |||||
| P-value Gen | CTRL−DEF | <0.001 | <0.001 | <0.01 | <0.001 |
| P-value Treat | CTRL−DEF | 0.975 | <0.001 | 0.202 | 0.344 |
| P-value GxT | CTRL−DEF | 0.928 | <0.01 | 0.148 | 0.297 |
| SED Gen (df) | CTRL−DEF | 1.58 (14) | 0.571 (14) | 4.1 (11) | 3.91 (11) |
| 3 cultivars | |||||
| P-value Gen | CTRL−StemCOV | <0.001 | <0.001 | <0.01 | <0.01 |
| P-value Treat | CTRL−StemCOV | 0.804 | <0.001 | 0.777 | 0.643 |
| P-value GxT | CTRL−StemCOV | 0.254 | 0.425 | 0.968 | 0.849 |
| SED Gen (df) | CTRL−StemCOV | 2.62 (10) | 0.480 (9) | 5.62 (10) | 5.19 (9) |
| 2 cultivars | |||||
| P-value Gen | CTRL−StemCOVDEF | <0.001 | <0.001 | <0.01 | <0.01 |
| P-value Treat | CTRL−StemCOVDEF | 0.336 | 0.057 | 0.589 | 0.561 |
| P-value GxT | CTRL−StemCOVDEF | 0.771 | 0.111 | 0.936 | 0.421 |
| SED Gen (df) | CTRL−StemCOVDEF | 2.6 (6) | 0.895 (6) | 4.03 (6) | 7.05 (6) |
| 2014 | |||||
| Subset 2 (4 cultivars) | |||||
| P-value Gen | ALL TREATS | <0.001 | <0.01 | <0.01 | <0.01 |
| P-value Treat | ALL TREATS | 0.097 | <0.001 | <0.05 | <0.001 |
| P-value GxT | ALL TREATS | 0.609 | 0.360 | 0.991 | 0.237 |
| SED Gen (df) | ALL TREATS | 1.34 (26) | 0.866 (28) | 3.55 (30) | 4.41 (28) |
| Gross Photosynthesis | Organ Photosynthesis | |||
|---|---|---|---|---|
| Units | (µmol CO2 m−2 s−1) | (µmol CO2 Organ−1 s−1) | ||
| GS65+20d | GS65+20d | |||
| Leaf | Spike | Leaf | Spike | |
| BECARD/K | ||||
| CTRL | 28.1 | 14.55 | 0.075 | 0.040 |
| DEF | − | 14.45 | − | 0.041 |
| StemCOV | 27.9 | 16.11 | 0.075 | 0.046 |
| StemCOVDEF | − | 10.75 | − | 0.031 |
| Mean | 28.0 | 13.96 | 0.075 | 0.039 |
| CMH79A.955 | ||||
| CTRL | 22.4 | 14.62 | 0.093 | 0.068 |
| DEF | − | 7.59 | − | 0.033 |
| StemCOV | 25.5 | 11.43 | 0.120 | 0.054 |
| StemCOVDEF | − | 9.27 | − | 0.043 |
| Mean | 24.0 | 10.7 | 0.107 | 0.049 |
| Overall mean | 26.0 | 12.3 | 0.091 | 0.044 |
| CTRL | 25.3 | 14.6 | 0.084 | 0.054 |
| DEF | − | 11.0 | − | 0.037 |
| StemCOV | 26.7 | 13.8 | 0.098 | 0.050 |
| StemCOVDEF | − | 10.0 | − | 0.037 |
| P-value Gen (Df) | 0.279 (2) | 0.962 (2) | 0.702 (1) | 0.038 (2) |
| P-value Treat (Df) | 0.477 (3) | 0.106 (10) | 0.353 (1) | 0.711 (9) |
| P-value GxT (Df) | 0.595 (8) | 0.097 (17) | 0.589 (7) | 0.711 (9) |
| Gross Photosynthesis | Organ Photosynthesis | |||||||
|---|---|---|---|---|---|---|---|---|
| Units | (µmol CO2 m−2 s−1) | (µmol CO2 Organ−1 s−1) | ||||||
| GS65+15d | GS65+25d | GS65+15d | GS65+25d | |||||
| Leaf | Spike | Leaf | Spike | Leaf | Spike | Leaf | Spike | |
| BECARD/K | ||||||||
| CTRL | 27.3 | 15.7 | 20.7 | 10.2 | 0.097 | 0.049 | 0.073 | 0.029 |
| DEF | − | 16.1 | − | 12.2 | − | 0.050 | − | 0.034 |
| StemCOV | 29.1 | 15.5 | 24.4 | 12.2 | 0.104 | 0.048 | 0.090 | 0.039 |
| StemCOVDEF | − | 12.6 | − | 10.5 | − | 0.050 | − | 0.033 |
| Mean | 28.2 | 15.0 | 22.5 | 11.3 | 0.101 | 0.049 | 0.081 | 0.034 |
| SERI M82 | ||||||||
| CTRL | 28.2 | 9.6 | 28.1 | 11.0 | 0.117 | 0.031 | 0.112 | 0.039 |
| DEF | − | 9.1 | − | 11.4 | − | 0.029 | − | 0.042 |
| StemCOV | 26.4 | 14.6 | 23.8 | 9.1 | 0.110 | 0.048 | 0.095 | 0.033 |
| StemCOVDEF | − | 10.3 | − | 11.2 | − | 0.033 | − | 0.040 |
| Mean | 27.3 | 10.9 | 26.0 | 10.7 | 0.1137 | 0.035 | 0.104 | 0.039 |
| UP2338*2 | ||||||||
| CTRL | − | − | 22.4 | 11.5 | − | − | 0.07 | 0.034 |
| DEF | − | − | − | 9.6 | − | − | − | 0.027 |
| StemCOV | − | − | 21.7 | 10.8 | − | − | 0.074 | 0.031 |
| StemCOVDEF | − | − | − | 12.4 | − | − | − | 0.032 |
| Mean | − | 22.1 | 11.1 | 0.072 | 0.031 | |||
| Overall mean | 27.74 | 12.94 | 23.5 | 11.01 | 0.107 | 0.042 | 0.086 | 0.035 |
| P-value Gen (Df) | 0.56 (6) | <0.01 (13) | 0.289 (10) | 0.76 (22) | 0.138 (6) | <0.001 (14) | 0.062 (10) | <0.05 (22) |
| P-value Treat (Df) | 0.969 (1) | 0.134 (3) | 0.836 (1) | 0.901 (3) | 1 (1) | 0.263 (3) | 0.912 (1) | 0.983 (3) |
| P-value GxT (Df) | 0.268 (6) | 0.159 (13) | 0.330 (10) | 0.287 (22) | 0.371 (6) | 0.121 (14) | 0.404 (10) | 0.194 (22) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivera-Amado, C.; Molero, G.; Trujillo-Negrellos, E.; Reynolds, M.; Foulkes, J. Estimating Organ Contribution to Grain Filling and Potential for Source Upregulation in Wheat Cultivars with a Contrasting Source–Sink Balance. Agronomy 2020, 10, 1527. https://doi.org/10.3390/agronomy10101527
Rivera-Amado C, Molero G, Trujillo-Negrellos E, Reynolds M, Foulkes J. Estimating Organ Contribution to Grain Filling and Potential for Source Upregulation in Wheat Cultivars with a Contrasting Source–Sink Balance. Agronomy. 2020; 10(10):1527. https://doi.org/10.3390/agronomy10101527
Chicago/Turabian StyleRivera-Amado, Carolina, Gemma Molero, Eliseo Trujillo-Negrellos, Matthew Reynolds, and John Foulkes. 2020. "Estimating Organ Contribution to Grain Filling and Potential for Source Upregulation in Wheat Cultivars with a Contrasting Source–Sink Balance" Agronomy 10, no. 10: 1527. https://doi.org/10.3390/agronomy10101527






