Confirmation and Characterization of the First Case of Acetolactate Synthase (ALS)-Inhibitor—Resistant Wild Buckwheat (Polygonum convolvulus L.) in the United States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Chlorsulfuron Dose-Response Assay
2.3. Response to Different ALS-Inhibitors
2.4. ALS Gene Sequencing
2.5. Statistical Analysis
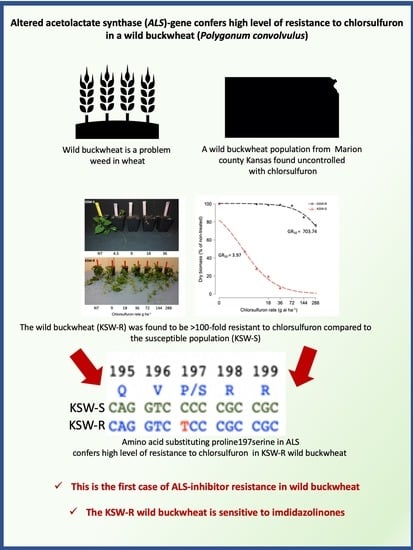
3. Results and Discussion
3.1. Chlorsulfuron Dose-Response
3.2. Response to Different ALS-Inhibitors
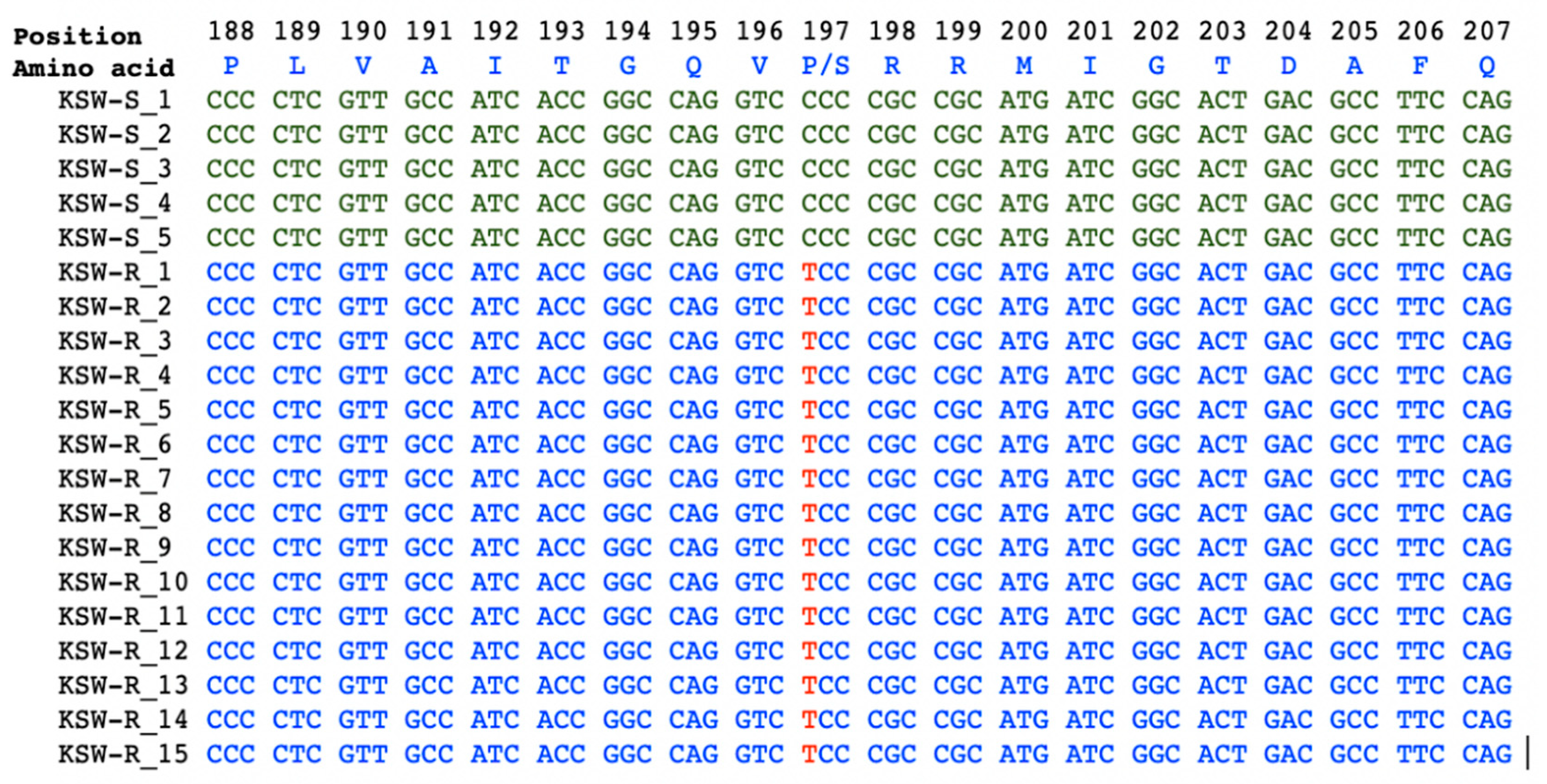
3.3. Molecular Basis of ALS-Inhibitor Resistance in KSW-R Wild Buckwheat
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forsberg, D.E.; Best, K.F. The emergence and plant development of wild buckwheat (Polygonum convolvulus). Can. J. Plant Sci. 1964, 44, 100–103. [Google Scholar] [CrossRef]
- Zollinger, R.K.; Peterson, D.; Moechnig, M.J. Biology and Management of Wild Buckwheat; Purdue University Extension: West Lafayette, IN, USA, 2006; p. 11. [Google Scholar]
- McCurdy, E.V.; Molberg, E.S. Effects of the continuous use of 2, 4-D and MCPA on spring wheat production and weed populations. Can. J. Plant Sci. 1974, 54, 241–245. [Google Scholar] [CrossRef]
- Ray, T.B. Site of action of chlorsulfuron: Inhibition of valine and isoleucine biosynthesis in plants. Plant Physiol. 1984, 75, 827–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Q.; Liu, W.; Zhang, Y.; Liu, K.K. Action mechanisms of acetolactate synthase-inhibiting herbicides. Pestic. Biochem. Physiol. 2007, 89, 89–96. [Google Scholar] [CrossRef]
- Tranel, P.J.; Wright, T.R. Resistance of weeds to ALS-inhibiting herbicides: What have we learned? Weed Sci. 2002, 50, 700–712. [Google Scholar] [CrossRef]
- Heap, I. International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 25 August 2020).
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Guo, J.; Riggins, C.W.; Hausman, N.E.; Hager, A.G.; Riechers, D.E.; Davis, A.S.; Tranel, P.J. Nontarget-Site Resistance to ALS Inhibitors in Waterhemp (Amaranthus tuberculatus). Weed Sci. 2015, 63, 399–407. [Google Scholar] [CrossRef] [Green Version]
- Nakka, S.; Thompson, C.R.; Peterson, D.E.; Jugulam, M. Target Site–Based and Non–Target Site Based Resistance to ALS Inhibitors in Palmer Amaranth (Amaranthus palmeri). Weed Sci. 2017, 65, 681–689. [Google Scholar] [CrossRef]
- Busi, R.; Vila-Aiub, M.M.; Powles, S.B. Genetic control of a cytochrome P450 metabolism-based herbicide resistance mechanism in Lolium rigidum. Hered. 2010, 106, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Yun, M.-S.; Yogo, Y.; Miura, R.; Yamasue, Y.; Fischer, A.J. Cytochrome P-450 monooxygenase activity in herbicide-resistant and -susceptible late watergrass (Echinochloa phyllopogon). Pestic. Biochem. Physiol. 2005, 83, 107–114. [Google Scholar] [CrossRef]
- Gardin, J.A.C.; Gouzy, J.; Carrère, S.; Délye, C. ALOMYbase, a resource to investigate non-target-site-based resistance to herbicides inhibiting acetolactate-synthase (ALS) in the major grass weed Alopecurus myosuroides (black-grass). BMC Genom. 2015, 16, 590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakka, S.; Jugulam, M.; Peterson, D.; Asif, M.; Mohammad, A. Herbicide resistance: Development of wheat production systems and current status of resistant weeds in wheat cropping systems. Crop. J. 2019, 7, 750–760. [Google Scholar] [CrossRef]
- Beckie, H.J.; Warwick, S.I.; Sauder, C.A. Acetolactate Synthase (ALS) Inhibitor-Resistant Wild Buckwheat (Polygonum convolvulus) in Alberta. Weed Technol. 2012, 26, 156–160. [Google Scholar] [CrossRef]
- Masoudi-Nejad, A.; Tonomura, K.; Kawashima, S.; Moriya, Y.; Suzuki, M.; Itoh, M.; Kanehisa, M.; Endo, T.; Goto, S. EGassembler: Online bioinformatics service for large-scale processing, clustering and assembling ESTs and genomic DNA fragments. Nucleic Acids Res. 2006, 34, W459–W462. [Google Scholar] [CrossRef] [Green Version]
- Artimo, P.; Jonnalagedda, M.; Arnold, K.; Baratin, D.; Csardi, G.; De Castro, E.; Duvaud, S.; Flegel, V.; Fortier, A.; Gasteiger, E.; et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012, 40, W597–W603. [Google Scholar] [CrossRef]
- Shyam, C.; Jhala, A.J.; Kruger, G.; Jugulam, M. Rapid metabolism increases the level of 2,4-D resistance at high temperature in common waterhemp (Amaranthus tuberculatus). Sci. Rep. 2019, 9, 16695. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J.C. Bioassay analysis using R. J. Stat. Softw. 2005, 12, 1–22. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013; Available online: http://www.R-project.org/ (accessed on 15 June 2020).
- Seefeldt, S.S.; Jensen, J.E.; Fuerst, E.P. Log-logistic analysis of herbicide dose-response relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. CRAN Repository. 2014. Available online: https://cran.r-project.org/web/packages/agricolae/index.html (accessed on 15 June 2020).
- Varanasi, V.K.; Godar, A.S.; Peterson, D.E.; Shoup, D.; Jugulam, M. A target-site point mutation in henbit (Lamium amplexicaule) confers high-level resistance to ALS-inhibitors. Weed Sci. 2016, 64, 231–239. [Google Scholar] [CrossRef]
- Yu, Q.; Nelson, J.K.; Zheng, M.Q.; Jackson, M.; Powles, S.B. Molecular characterisation of resistance to ALS-inhibiting herbicides in Hordeum leporinum biotypes. Pest Manag. Sci. 2007, 63, 918–927. [Google Scholar] [CrossRef]
- Beckie, H.J.; Tardif, F.J. Herbicide cross resistance in weeds. Crop. Prot. 2012, 35, 15–28. [Google Scholar] [CrossRef]
- Iwakami, S.; Kamidate, Y.; Yamaguchi, T.; Ishizaka, M.; Endo, M.; Suda, H.; Nagai, K.; Sunohara, Y.; Toki, S.; Uchino, A.; et al. CYP 81A P450s are involved in concomitant cross-resistance to acetolactate synthase and acetyl-CoA carboxylase herbicides in Echinochloa phyllopogon. New Phytol. 2018, 221, 2112–2122. [Google Scholar] [CrossRef] [PubMed]
- Tranel, P.J.; Wright, T.R.; Heap, I.M. Mutations in Herbicide-Resistant Weeds to ALS Inhibitors. Available online: http://www.weedscience.com (accessed on 25 August 2020).
- A Gaines, T.; O Duke, S.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; E Dayan, F. Mechanisms of evolved herbicide resistance. J. Boil. Chem. 2020. [Google Scholar] [CrossRef]
- Park, K.W.; A Mallory-Smith, C. Physiological and molecular basis for ALS inhibitor resistance in Bromus tectorum biotypes. Weed Res. 2004, 44, 71–77. [Google Scholar] [CrossRef]
- Singh, S.; Singh, V.; Salas-Perez, R.A.; Bagavathiannan, M.; Lawton-Rauh, A.L.; Burgos, N.R. Target-site mutation accumulation among ALS inhibitor-resistant Palmer amaranth. Pest Manag. Sci. 2018, 8, 350. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, X.Q.; Hashem, A.; Walsh, M.J.; Powles, S.B. ALS gene proline (197) mutations confer ALS herbicide resistance in eight separated wild radish (Raphanus raphanistrum) populations. Weed Sci. 2003, 51, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Eberlein, C.V.; Guttieri, M.J.; Berger, P.H.; Fellman, J.K.; Mallory-Smith, C.A.; Thill, D.C.; Baerg, R.J.; Belknap, W.R. Physiological consequences of mutation for ALS-inhibitor resistance. Weed Sci. 1999, 47, 383–392. [Google Scholar] [CrossRef]
- Jugulam, M.; Shyam, C. Non-Target-Site Resistance to Herbicides: Recent Developments. Plants 2019, 8, 417. [Google Scholar] [CrossRef] [Green Version]
- Rey-Caballero, J.; Menéndez, J.; Osuna, M.D.; Salas, M.; Torra, J. Target-site and non-target-site resistance mechanisms to ALS inhibiting herbicides in Papaver rhoeas. Pestic. Biochem. Physiol. 2017, 138, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Iwakami, S.; Endo, M.; Saika, H.; Okuno, J.; Nakamura, N.; Yokoyama, M.; Watanabe, H.; Toki, S.; Uchino, A.; Inamura, T. Cytochrome P450 CYP81A12 and CYP81A21 are associated with resistance to two acetolactate synthase inhibitors in Echinochloa phyllopogon. Plant Physiol. 2014, 165, 618–629. [Google Scholar] [CrossRef] [Green Version]
- Shergill, L.S.; Bish, M.D.; Jugulam, M.; Bradley, K.W. Molecular and physiological characterization of six-way resistance in an Amaranthus tuberculatus var. rudis biotype from Missouri. Pest Manag. Sci. 2018, 74, 2688–2698. [Google Scholar] [CrossRef] [PubMed]


| Herbicide | Trade Name | Chemical Family | Manufacturer | Field Rate |
|---|---|---|---|---|
| Glean | Chlorsulfuron | Sulfonylurea | DuPont Wilmington, DE http://cropprotection.dupont.com | 18 g ai ha−1 |
| Permit | Halosulfuron | Sulfonylurea | Gowan Company, Yuma, AZ www.gowanco.com | 36 g ai ha−1 |
| Harmony | Thifensulfuron | Sulfonylurea | DuPont Wilmington, DE http://cropprotection.dupont.com | 36 g ai ha−1 |
| Accent | Nicosulfuron | Sulfonylurea | DuPont Wilmington, DE http://cropprotection.dupont.com | 36 g ai ha−1 |
| Pursuit | Imazethapyr | Imidazolinone | BASF Corporation, Research Triangle Park, NC, USA | 72 g ai ha−1 |
| Genotype | GR50 (SE) | b (SE) | d (SE) |
|---|---|---|---|
| KSW-R | 703.74 (333.11) | 1.24 (0.55) | 100.46 (3.0) |
| KSW-S | 3.97 (0.79) | 1.06 (0.21) | 100.01 (4.80) |
| Trade Name | Field Rate | % Survival a | |
|---|---|---|---|
| KSW-R | KSW-S | ||
| Chlorsulfuron | 18 g ai ha−1 | 100% | 0% |
| Halosulfuron | 36 g ai ha−1 | 100% | 0% |
| Thifensulfuron | 36 g ai ha−1 | 90% | 0% |
| Nicosulfuron | 36 g ai ha−1 | 90% | 0% |
| Imazethapyr | 72 g ai ha−1 | 0% | 0% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pandian, B.A.; Friesen, A.; Laforest, M.; Peterson, D.E.; Vara Prasad, P.V.; Jugulam, M. Confirmation and Characterization of the First Case of Acetolactate Synthase (ALS)-Inhibitor—Resistant Wild Buckwheat (Polygonum convolvulus L.) in the United States. Agronomy 2020, 10, 1496. https://doi.org/10.3390/agronomy10101496
Pandian BA, Friesen A, Laforest M, Peterson DE, Vara Prasad PV, Jugulam M. Confirmation and Characterization of the First Case of Acetolactate Synthase (ALS)-Inhibitor—Resistant Wild Buckwheat (Polygonum convolvulus L.) in the United States. Agronomy. 2020; 10(10):1496. https://doi.org/10.3390/agronomy10101496
Chicago/Turabian StylePandian, Balaji Aravindhan, Abigail Friesen, Martin Laforest, Dallas E. Peterson, P. V. Vara Prasad, and Mithila Jugulam. 2020. "Confirmation and Characterization of the First Case of Acetolactate Synthase (ALS)-Inhibitor—Resistant Wild Buckwheat (Polygonum convolvulus L.) in the United States" Agronomy 10, no. 10: 1496. https://doi.org/10.3390/agronomy10101496