1. Introduction
Dramatic increases in food production have been observed in the latter half of the twentieth century owing to the use of agro-chemicals, mechanization, irrigation, high yielding varieties, and post-harvest technology. The production of wheat in Pakistan has increased to ~25 m ton from 4.55 m ton in 1965 [
1,
2]. The pest attacks continue to incur losses to crop production owing to the diversity of pests and their resistance to prevailing control practices. The use of pesticides has increased from 15 to 20-fold over the last fifty years [
3]. Chemical herbicides have gained importance in crop production in the face of a shortage of labor and limited application of mechanical control [
4]. The mechanical control is known to contribute to soil erosion and its degradation [
5]. Herbicides have led to the emergence of resistant biotypes of weeds, making the herbicide compounds useless to control these weeds [
6]. Hence, the discovery of new compounds with novel modes of action is needed to replace these herbicides with more effective compounds to control such weeds. The discovery of such compounds, having herbicidal properties, has reduced over time. Further, the control of one type of weeds with herbicides has provided space to the proliferation of other weed species, which were less problematic for crop production in the past [
7]. They have caused losses of biodiversity in the environment. It has deprived the ecosystems of some of their vital functions. Herbicides have aggravated the loss of biodiversity by killing the susceptible species, restricting the growth of others and the degradation of natural resources [
8]. Poisoning, growth retardation, sterility, and deaths of wildlife owing to herbicide exposure have been reported by [
9]. The residues of herbicides, apart from polluting the natural resources and destroying life forms, may also accumulate in the edible portions of plants, which facilitate their entry to the food chain and bodies of humans. It causes poisoning and chronic diseases in human beings, leading to deaths [
10]. Human health disorders caused by herbicides include disorders of the nervous system, malformation of the embryo, loss of fertility, loss of immunity, kidney disorders, and liver disorders [
11].
Farmers pay only the costs of manufacturing and marketing of herbicides, which provides economic access to farmers to adopt chemical weed control. The additional costs incurred on the treatment of human illnesses, degradation of natural resources and environment, and loss of biodiversity also need to be paid by farmers, society, or governments. Hence, the scenario of economic, environmental, and biological costs of chemical weed control pushes the researchers towards finding out safer weed control techniques. The importance of biological control has dramatically increased in the present situation. It presents a safer, inexpensive, and easier solution to the above-discussed issues of other control practices. It relies on increasing the strength, population, and activities of the organisms, resulting in growth reduction of weeds [
12].
The past efforts in this area were focused on pathogens causing diseases in weeds [
13] and insects feeding on weeds [
14]. The success of insect biocontrol agents is limited by the existence of multiple hosts of insects in nature, which may cause the emergence of new pests of crops [
15]. The pathogens of weeds used for biocontrol wait for suitable environmental conditions to cause infections and diseases in weed plants [
13]. It may usually lead to delayed disease development, even after the weeds have caused economic losses of crops. Plant allelochemicals have also been investigated for biological weed control [
16]. Their efficacy for weed control is reduced owing to the soil reactions, biodegradation, and mobility. It reduces their bioavailability and phytotoxicity on weeds [
17]. These limitations of conventional biological weed control have discouraged researchers of this field, and the popularity of chemical weed control has increased dramatically.
The low success rate in conventional biological weed control has driven scientists to explore the characteristics of the rhizosphere inhabiting bacteria of weeds and crops for the development of novel weed biocontrol techniques. However, researchers have made efforts to explore the type of rhizobacteria, which produce substances inhibitory to the growth of weeds and are the least explored candidates for biological weed control. They release their secondary metabolites (phytotoxic in nature) in the rhizosphere, which is followed by their absorption in weeds. It results in a growth reduction of these weeds. The nature of this interaction between plants and microorganisms may be termed as plant-microbe allelopathy, and the bacteria responsible for these interactions may be called as allelopathic bacteria (AB) [
18]. The discovery of host specificity in such microbial interactions with plants by [
19] has opened ways for their potential application in crops for weed control. It reflects the properties of non-inhibition or even promotion of growth of crops among these rhizobacteria [
20]. Therefore, the present study was conducted to explore such bacteria from the rhizosphere of weeds and wheat growing in fields facing weed invasions chronically, characterize them for the biological weed control, and evaluate their effects on the growth of wheat and weeds species of wheat.
4. Discussion
The study of diverse forms of soil-inhabiting microorganisms and their activities may be helpful in resolving many agricultural, environmental, and ecological issues created by unsustainable farming practices. The invasion of weeds in crops reduces their yields, and farmers adopt unsustainable and unhealthy practices to reduce the losses of their crops. The harmful impacts of tillage and chemicals have been established. Therefore, the present study explored an alternative, inexpensive, sustainable, and environmentally and ecologically safe technique for weed control in crops. It was aimed at finding out the natural mechanisms of rhizobacteria, which function to limit the growth of weeds, alleviate the biotic stress of weeds on crops, and produce a vigorous crop stand. Strengthening such natural processes through augmentation, inoculation, or other processes is required for the development of biological weed control in crops. This may help us to resolve the above-mentioned issues created by conventional control practices [
11].
The rhizosphere inhabiting bacteria, which release phytotoxic metabolites in the rhizosphere and result in germination/growth reduction of weeds, are called allelopathic bacteria [
18]. The present study is the pioneering work executed in Pakistan, which is aimed at searching such rhizobacteria with their novel characteristics to develop biological weed control. The probability of the existence of such bacteria has been speculated in the rhizosphere of weeds and crops, which are growing together over many years or where the weed invasions occur more frequently [
28]. Therefore, we collected the samples of weeds and wheat from areas/fields across the District of Faisalabad, Pakistan, where the weed invasions were more frequent. The findings of this work support the above-mentioned finding of Schippers et al. [
28]. They also reported that growth inhibitory rhizobacteria grew, strengthened, and increased their activities in the agricultural crops, where a single crop is grown year after year. It resulted in the reduction of yields of crops. They reported the increase in cyanogenic bacteria and cyanide production in the rhizosphere of potatoes when this crop was continuously grown over a field for 3 years. Their findings increased the importance of crop rotation.
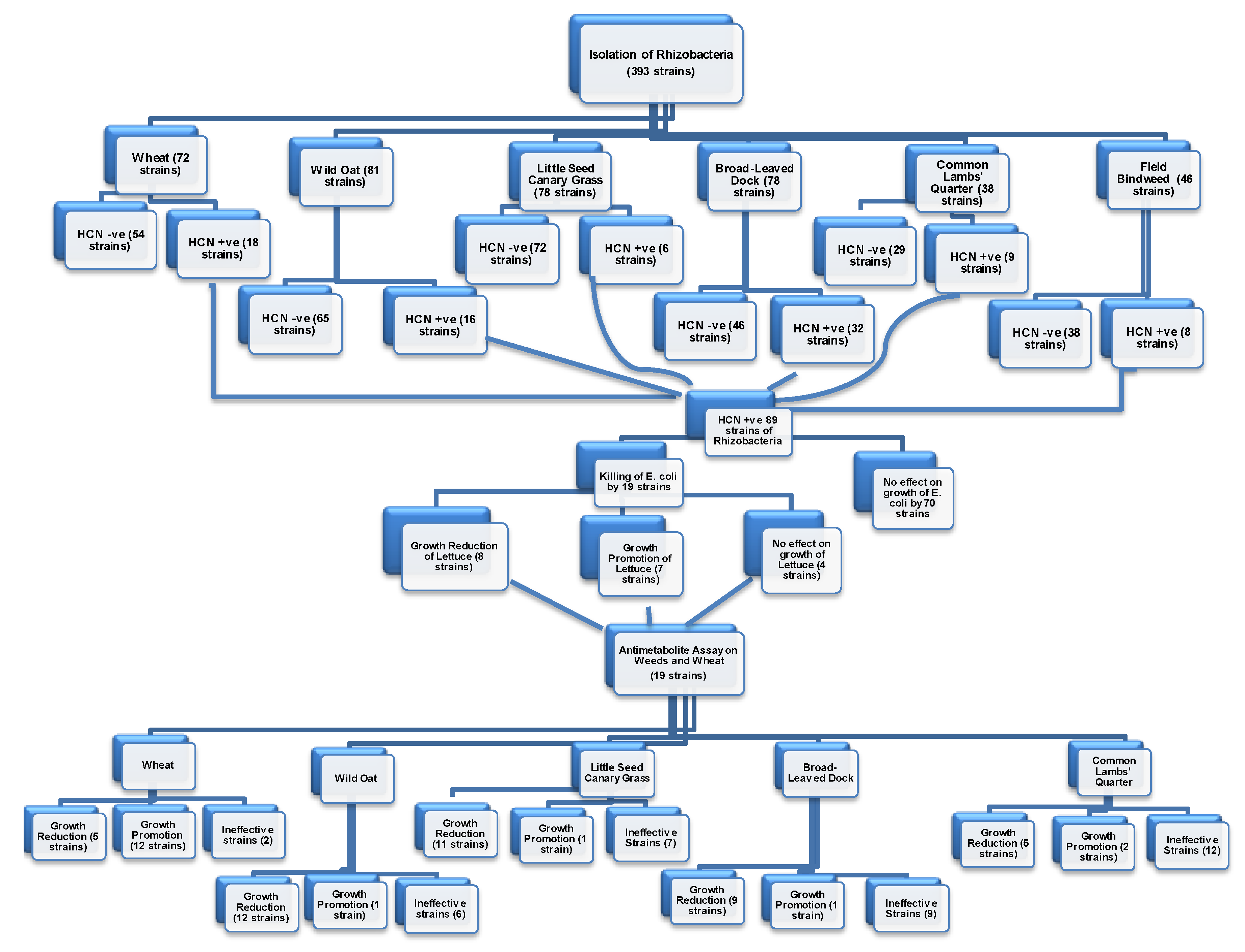
We isolated 393 strains of rhizobacteria from the rhizosphere of five weeds and wheat in this study. These strains were passed through a comprehensive screening process based on the production of phytotoxic metabolites in vitro, suppression of indicator bacteria and plants, in vivo suppression of weeds, and their effects on wheat crops. The protocols followed for these purposes obtained support from the findings of Bakker and Schipper [
22], Kremer and Souissi [
29], and Kremer [
24]. The first test conducted on these strains was the qualitative production of HCN. It was considered a major substance responsible for the growth inhibition of some plants by Kremer and Souissi [
29]. This study obtained 22.6% of strains (89) to have produced cyanide at various levels. The distribution of cyanogenic strains in different weeds and wheat was also variable. This was synonymous with the findings of Kremer and Souissi [
29]. The proportion of cyanogenic strains in their study (32%) was, however, higher than in our study. This difference might be due to differences in agro-ecological conditions and prevalent agricultural practices. Zeller et al. [
19] found that the sensitivity of different weeds and crops to cyanide was variable, and the cyanogenic bacteria might cause suppression of some weeds without imparting harmful effects on the accompanying crop in certain cases. They applied various levels of cyanide to five weeds and wheat and reported that this characteristic of rhizobacteria might be used for the selective suppression of three seeds (
C. jacea,
G. mollugo, and
H. murinum), invading the wheat crop without disturbing the growth of wheat.
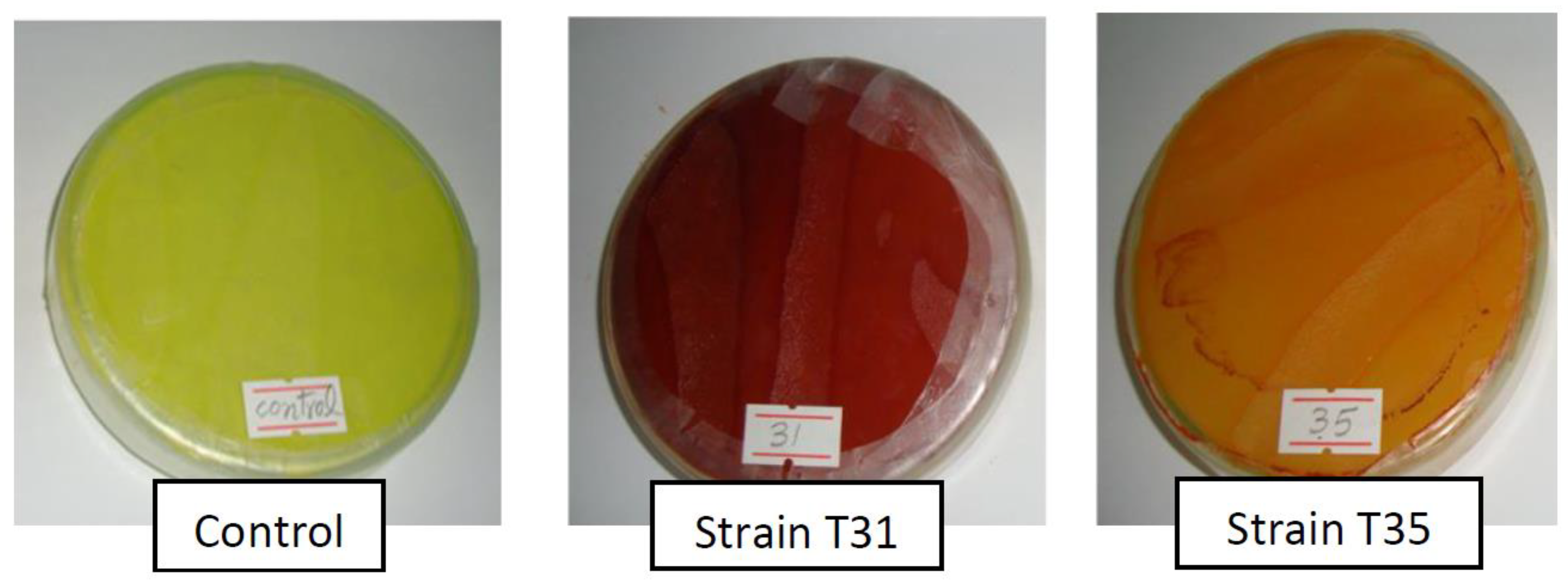
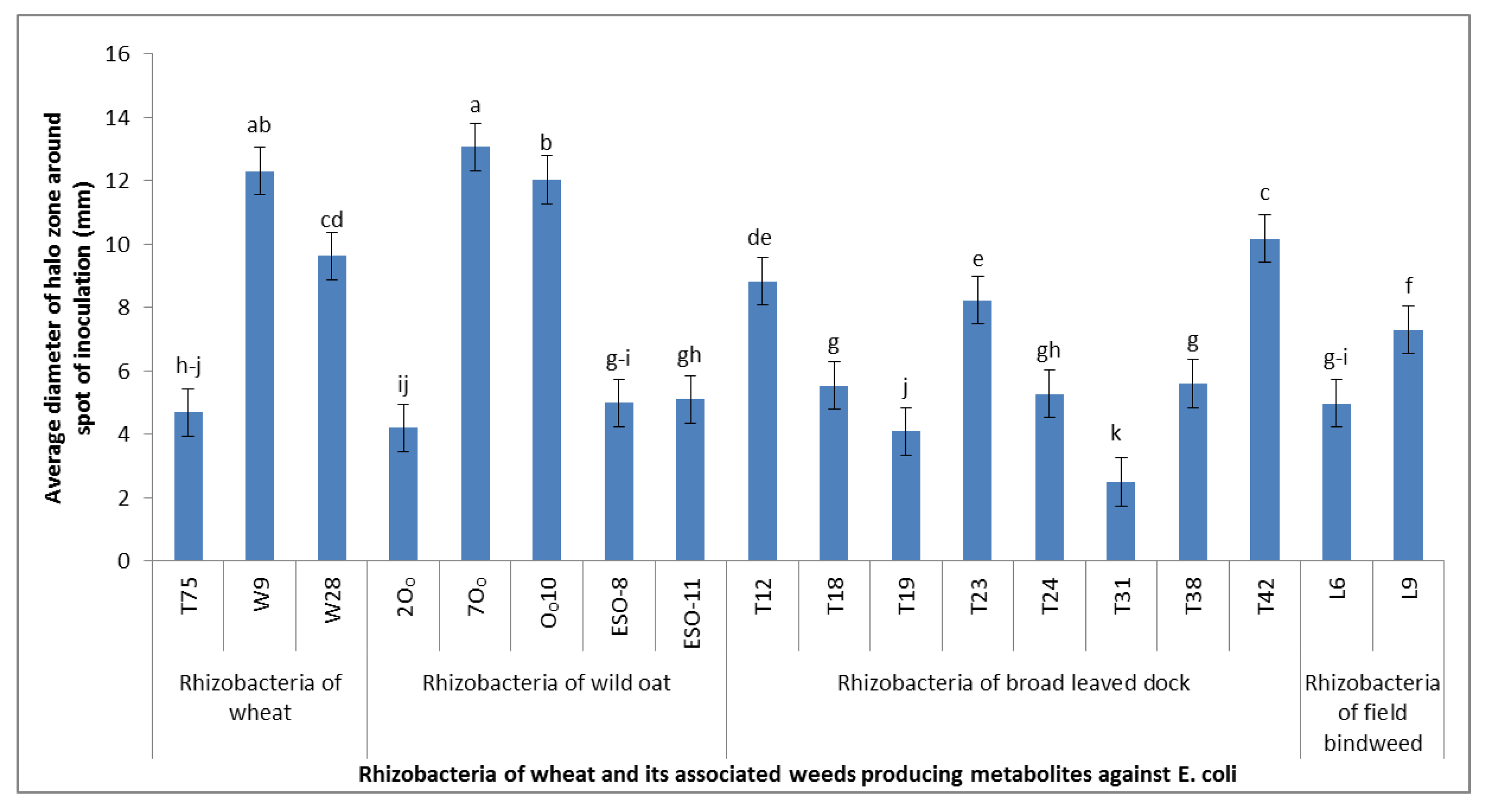
The cyanogenic strains of our study were further tested for the production of toxic metabolites using the indicator of sensitive bacteria (
E. coli strain K12). The relevance of this assay for the screening of rhizobacteria weed control agents was reported by Kremer et al. [
30]. We got 21.3% of the cyanogenic strains to suppress the growth of sensitive bacteria. As all the cyanogenic strains did not suppress the growth of sensitive bacteria in this study, one may speculate that the strains inhibiting the growth of bacteria may also have possessed the characteristics of production of some other toxic compounds along with cyanide. This assay indicated that the strains inhibiting the growth of sensitive bacteria might be producing multiple growth inhibitory compounds, collectively termed as antimetabolites, and could be more suitable for testing on weeds and wheat in the next screening studies.
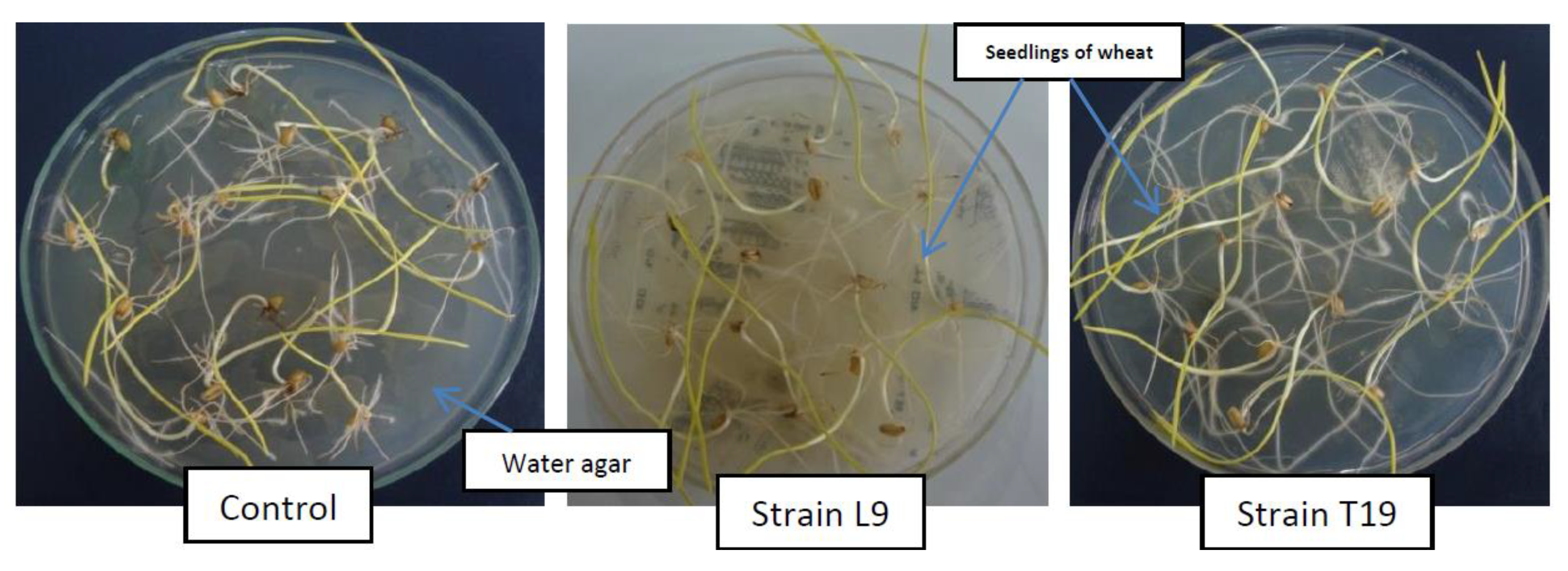
Nineteen strains, obtained from the above screening procedures, were tested on sensitive plant species, i.e., lettuce. The effects of these strains on the growth of the seedlings of lettuce were variable. Some strains inhibited, some promoted, and others remained ineffective. Hence, all the strains inhibiting the growth of
E. coli did not inhibit the growth of lettuce in our study. This finding agreed with Kremer et al. [
30]. Kremer and Kennedy [
31] also reported the growth reduction of lettuce by such rhizobacteria. As the strains tested on lettuce were all cyanogenic in nature, Zermane et al. [
32] also reported mixed effects of cyanogenic rhizobacteria on lettuce. The non-inhibition of lettuce by some strains may be due to the non-host interactions, where these strains needed to grow with their host in order to express their characteristics [
33]. There also exist differences in the metabolic functions of
E. coli and lettuce, the former being a prokaryote, and the latter being a eukaryotic plant species. There may also be the difference of compounds, causing antibiosis against bacteria and plants. The results obtained in our study reflected the release of diverse types of metabolites and their functions by these strains, which affected the growth of bacteria and plants. For similar reasons, we tested all the above-mentioned strains on weeds and wheat in the further screening process. This decision in our study has grounds in Souissi and Kremer [
34]. They reported the reduction in the growth of weeds by those strains of rhizobacteria, which did not reduce the growth of lettuce. In other words, the growth reduction of lettuce and weeds by rhizobacteria could not be correlated in their study.
Stability or consistency in the characteristics of strains of our study may be evident from the above studies. It increased our reliance on these strains for further studies regarding their effects on weeds and wheat. We found all type of effects of the strains on weeds and wheat, i.e., there were strains inhibitory to all the weeds and wheat, suppressive to one or more weeds and wheat, suppressive to one or more weeds but not to wheat, and suppressive to one or more weeds but promoted the growth of wheat. This array of responses by the strains of allelopathic bacteria has multiple applications if further studies on their characterization and response under natural conditions are carried out. These may be developed for application to control weeds and strengthen crop in poor agricultural systems (selective strains) and control weeds in non-agricultural systems (non-selective strains). The reasons for selectivity may be a difference of tolerance to toxic metabolites in weeds and crop, release of toxic metabolites by these strains only in the rhizosphere of their host plants, the difference in availability of substrates required for the production of toxic metabolites in the rhizosphere of weeds and wheat, a difference of survival, colonization, and establishment in the rhizosphere of weeds and wheat, and difference of mechanisms in the rhizosphere of host and non-host plants [
19,
20,
35]. The findings of our study became more evident when the strains were further characterized by the production of indole-3-acetic acid, exopolysaccharides, siderophores, catalases, chitinases, oxidases, and P solubilization. The most prominent strains were identified as pseudomonads. The effects of the five most efficient strains on weeds and wheat were tested under axenic conditions in Abbas et al. [
36]. The strains inhibiting one or more weeds and promoting wheat may be more successful for weed control under natural conditions. These may strengthen the weak crop plants, increase their competitive ability, and, hence, increase the scale of weed control by allelopathic bacteria. The non-selective strains inhibitory to wheat may be tested for their effects on other crops to explore opportunities for their application in other cropping systems. The efforts on augmentation of effects of allelopathic bacteria under natural conditions may be helpful to realize the dream of biological weed control. The strains of allelopathic bacteria obtained from this study can be further tested for their effects on weeds and wheat under field conditions. Further efforts may be required to improve their efficiency of weed control under natural conditions. Application methods of allelopathic bacteria may also be needed to be optimized. This will produce a bioherbicide for the control of weeds in an environmentally friendly and sustainable manner.