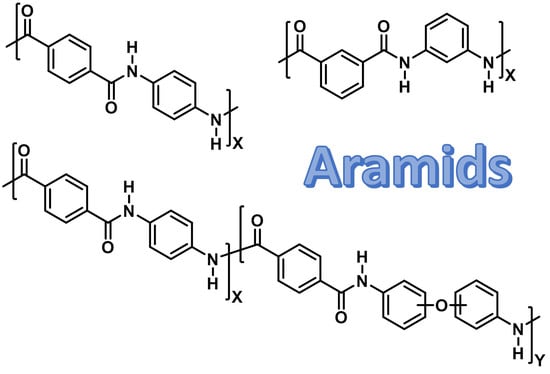
Functional Aromatic Polyamides
Abstract
:1. Introduction
2. Synthesis and Processing
2.1. Low and High Temperature Solution Synthetic Methods
2.2. Alternative Synthetic Methods
2.3. Processing
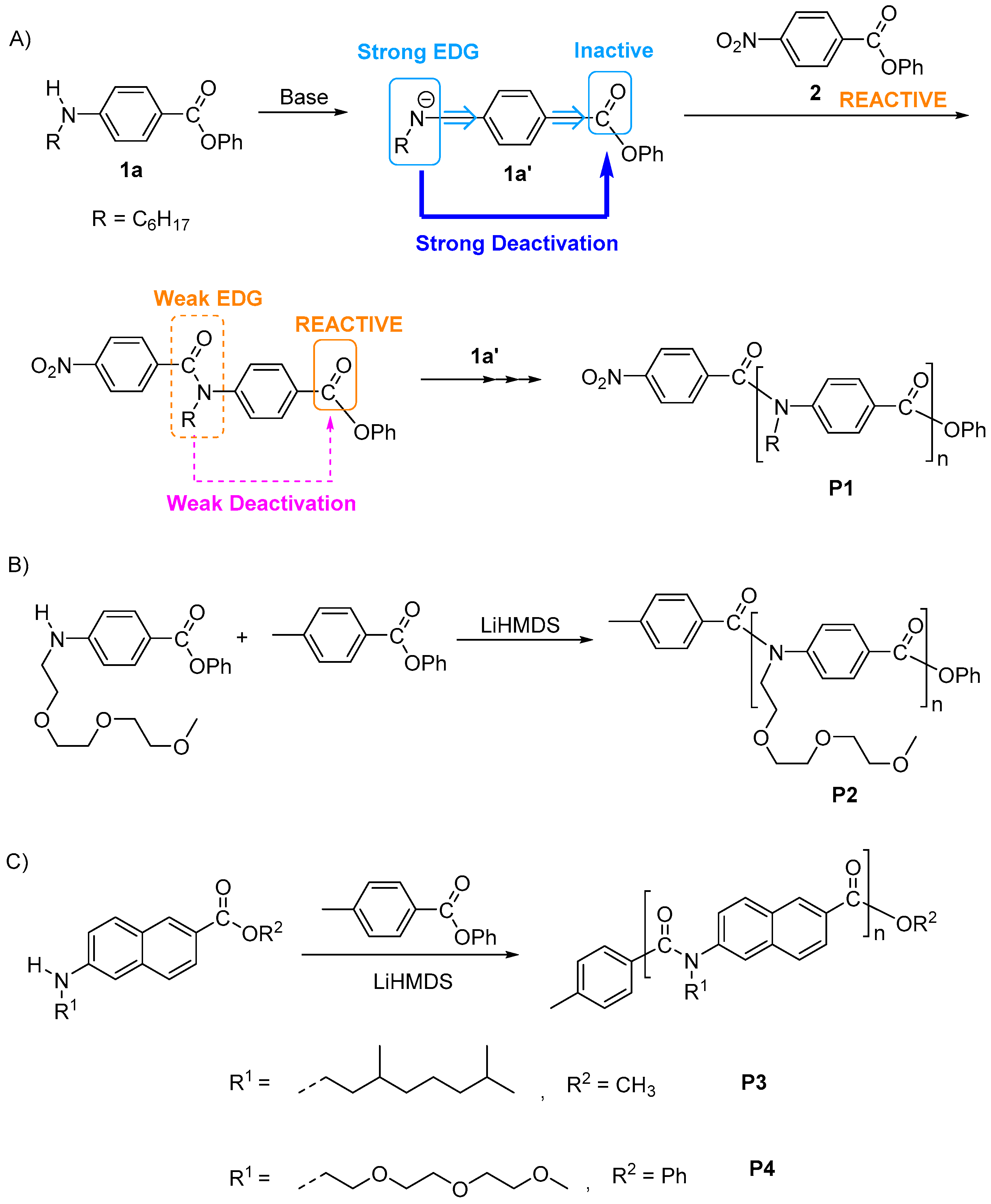
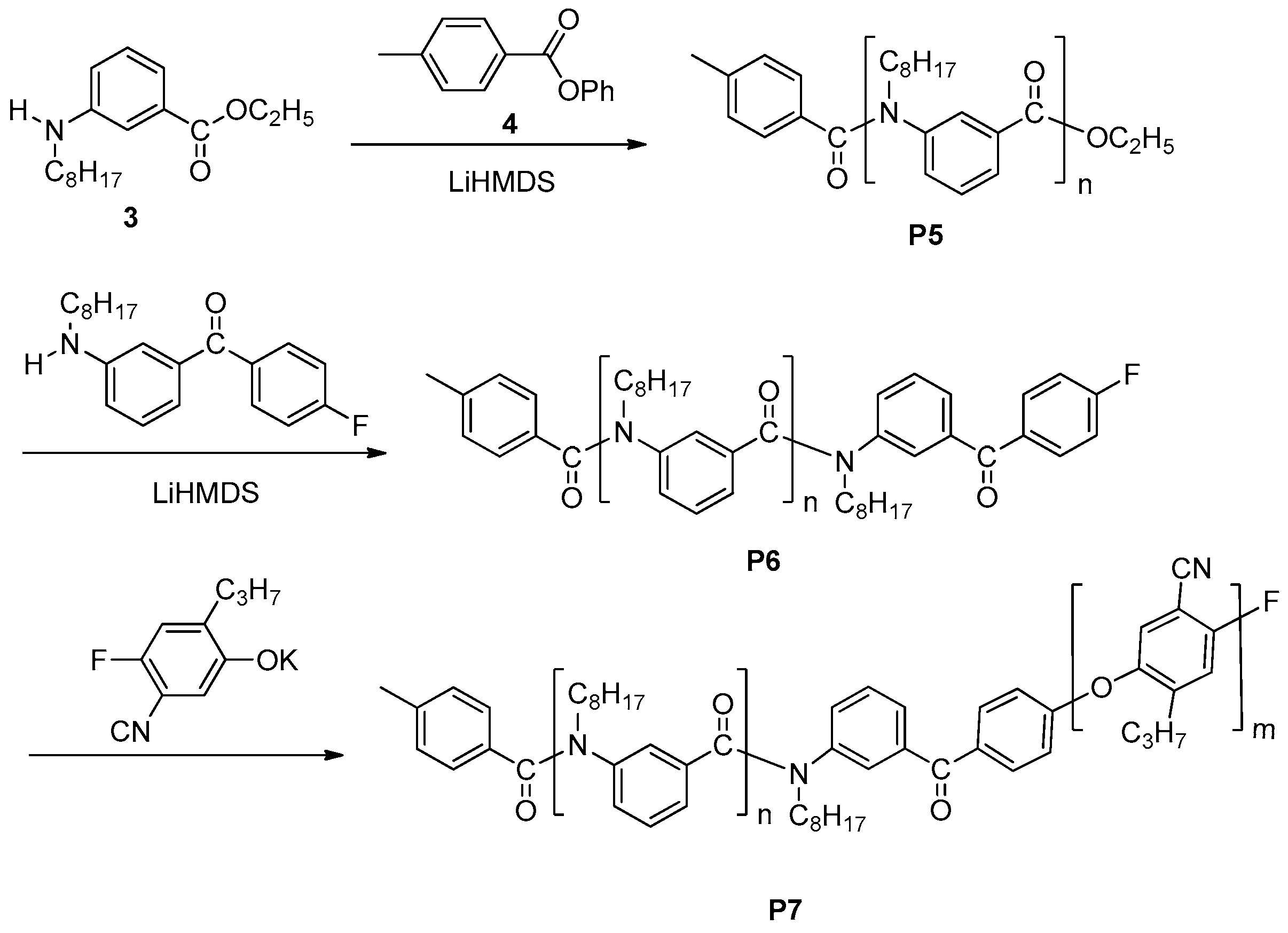
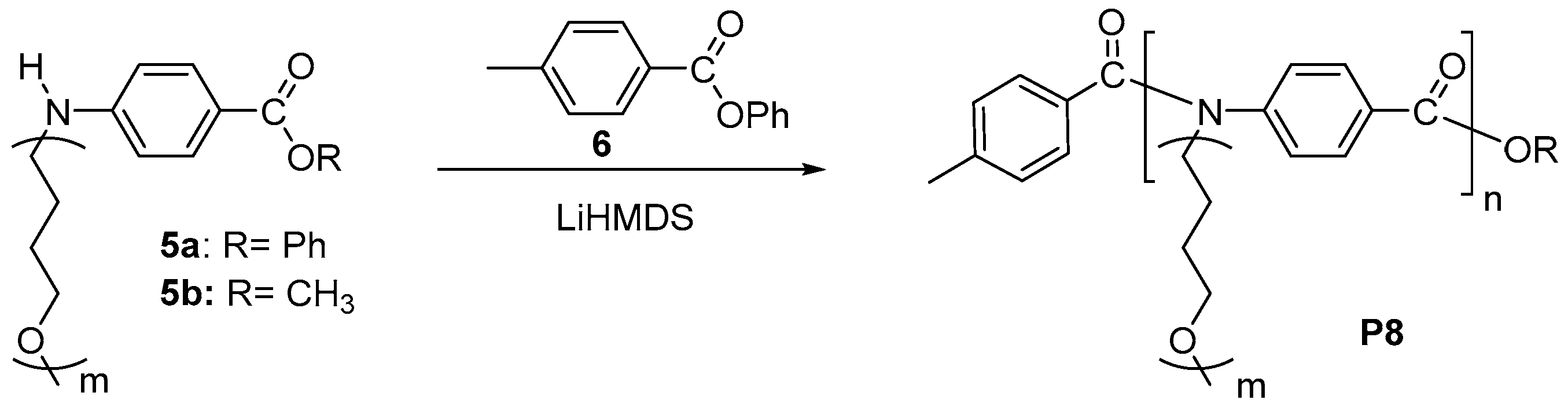
3. Synthesis of Functional Aromatic Polyamides with Controlled Structure
3.1. Chain-Growth Polycondensation
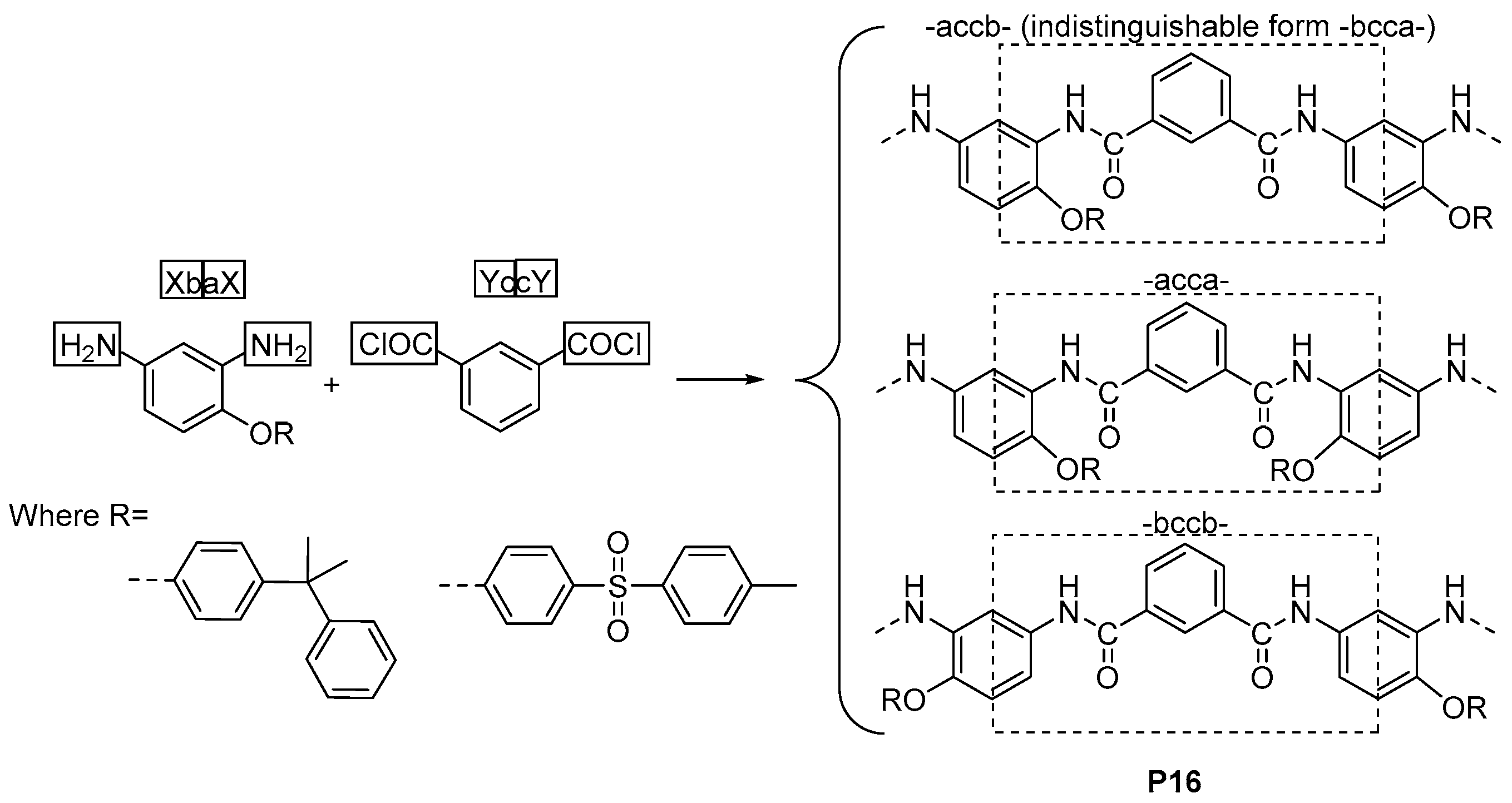
3.2. Constitutional Isomerism
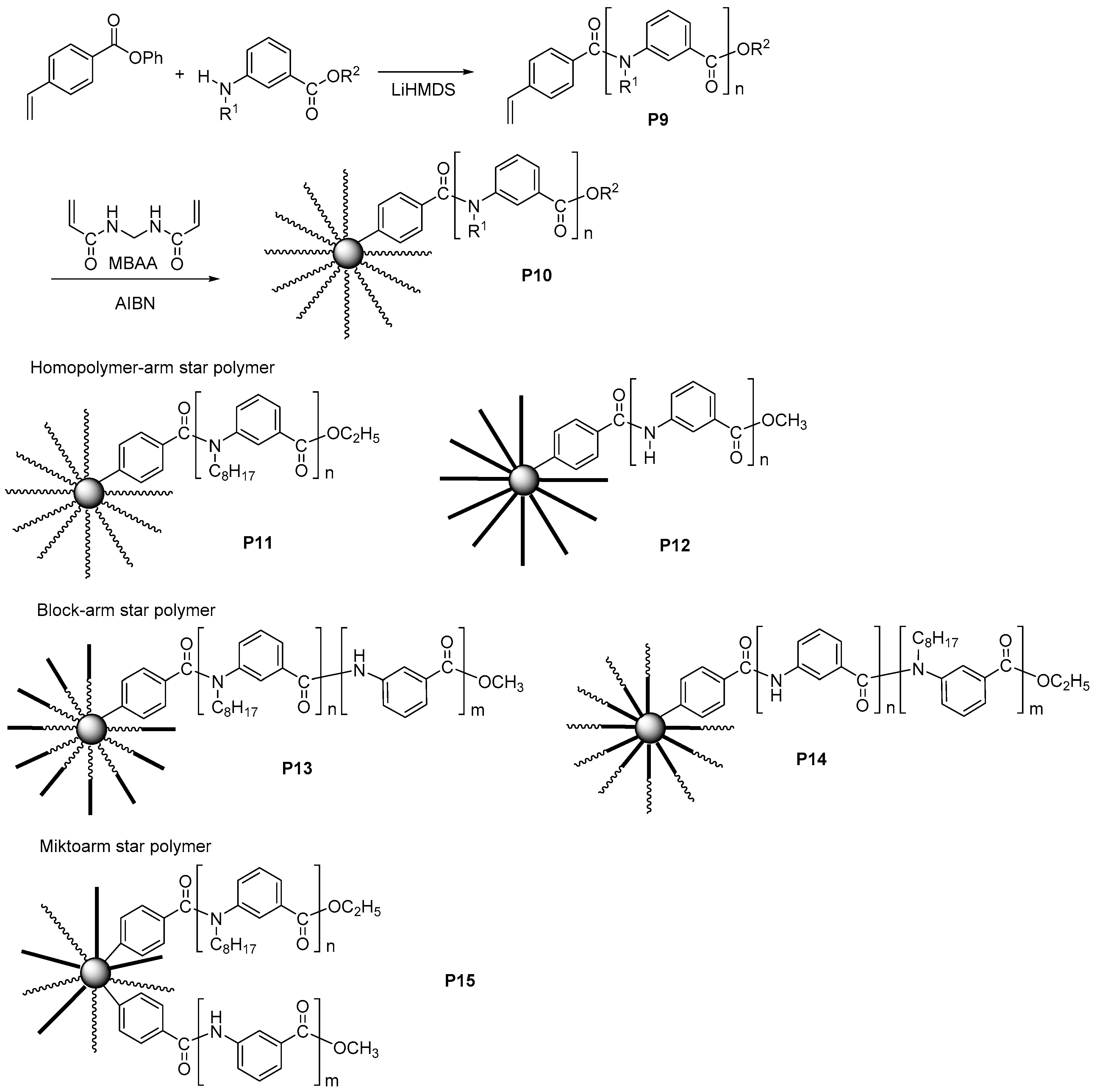
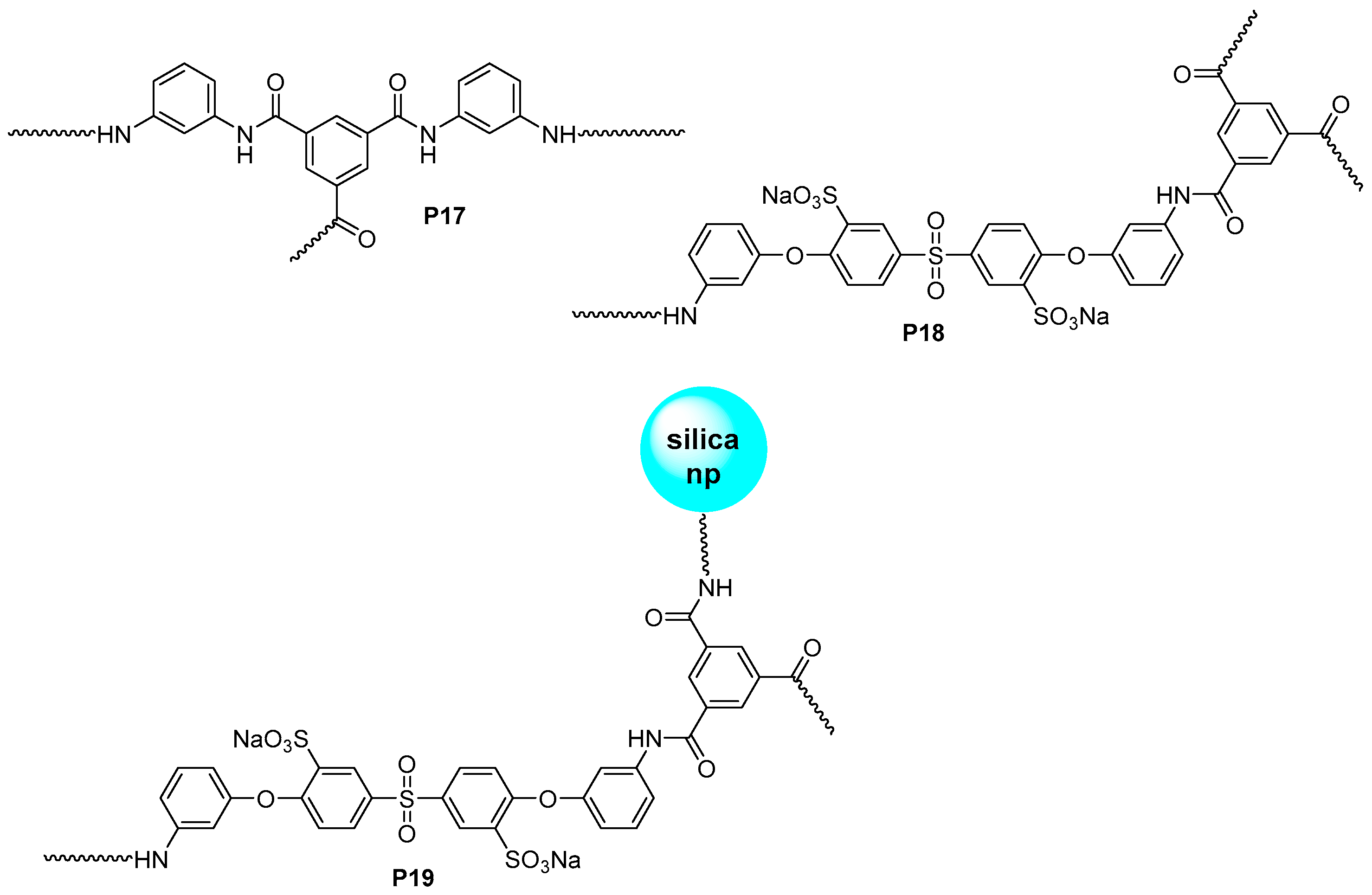
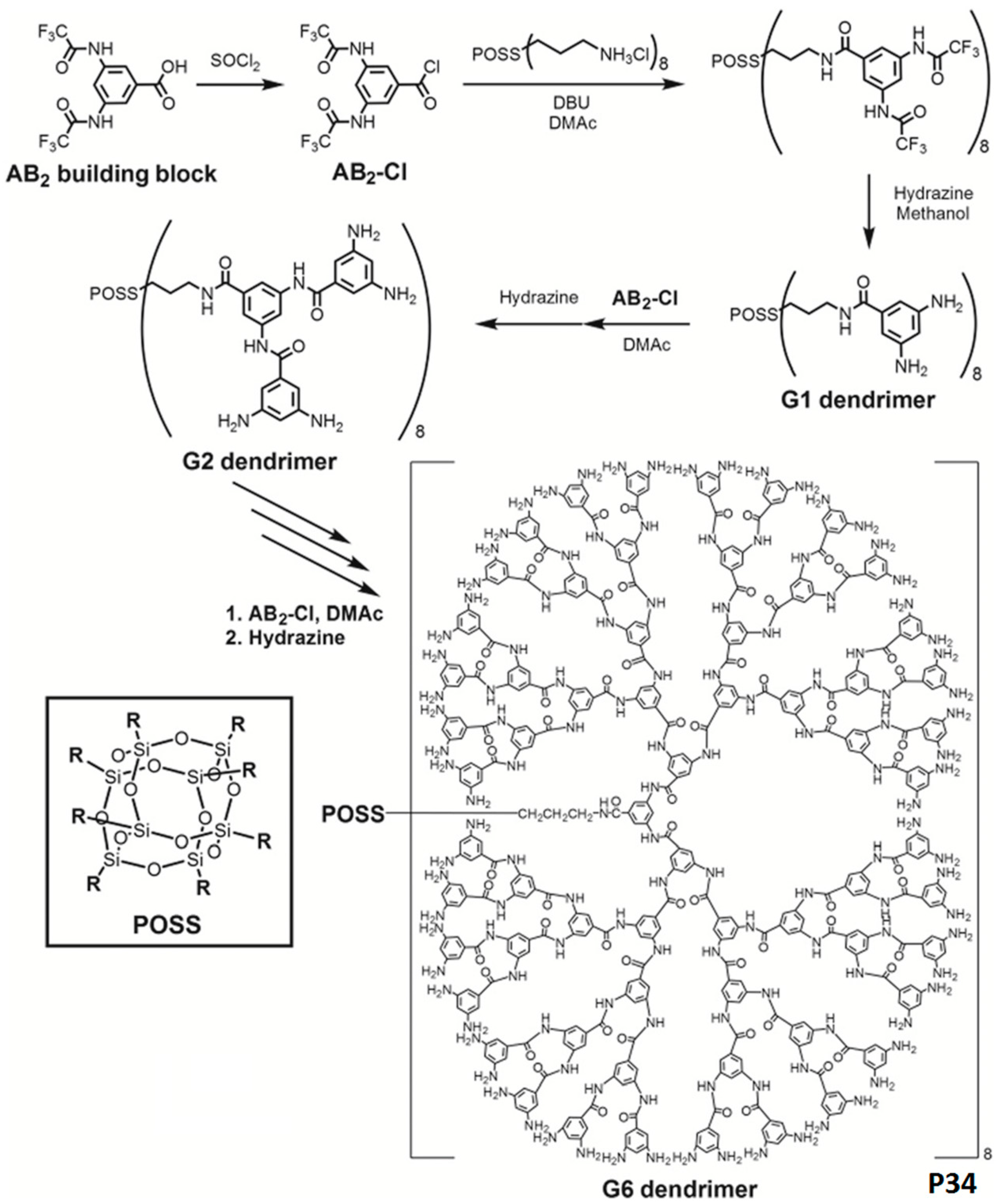
3.3. Aromatic Polyamides with Spherical-Like Structures
4. Aromatic Polyamides with Selected Properties and Applications
4.1. Polyamides with High Thermal Stability and Flame-Retardant Properties
4.2. Mechanical Properties
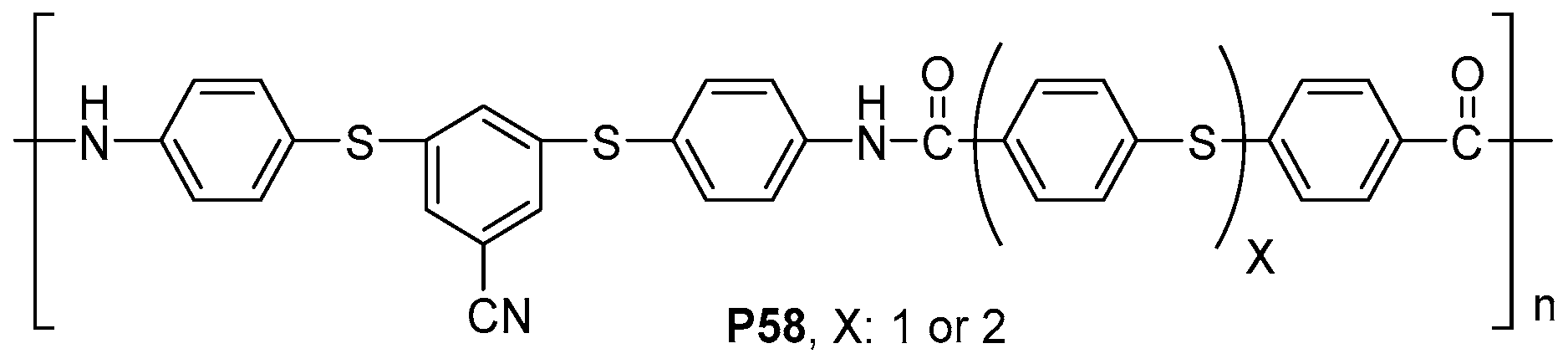
4.3. Sensing and Extraction Applications
4.4. Film, Membranes and Transport Properties
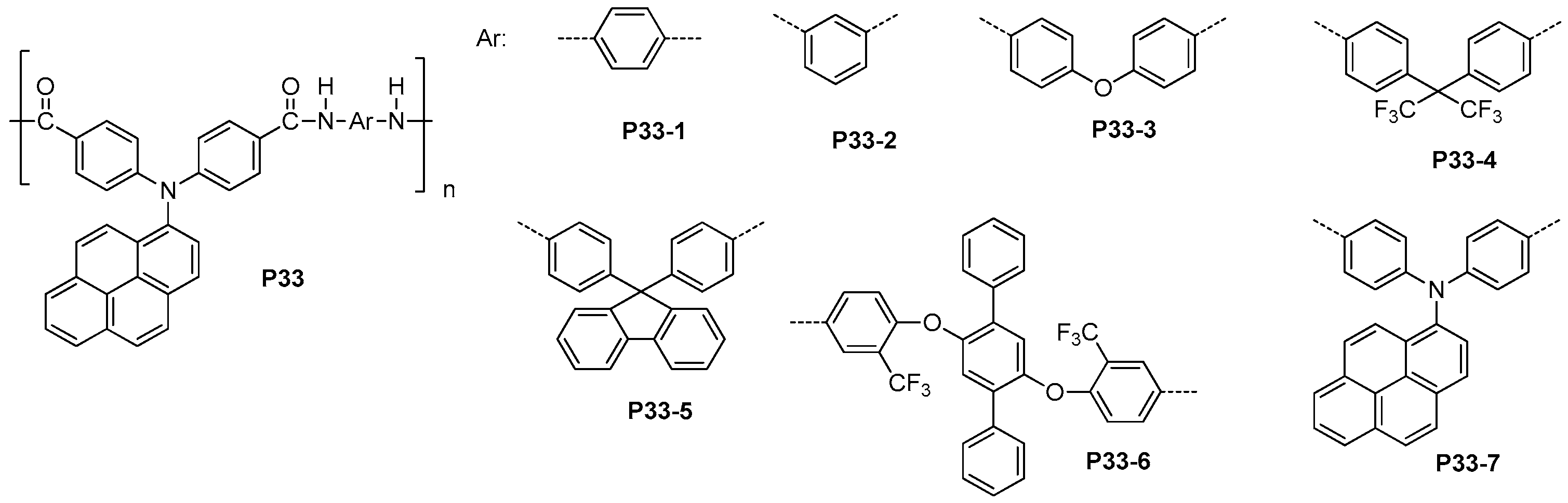
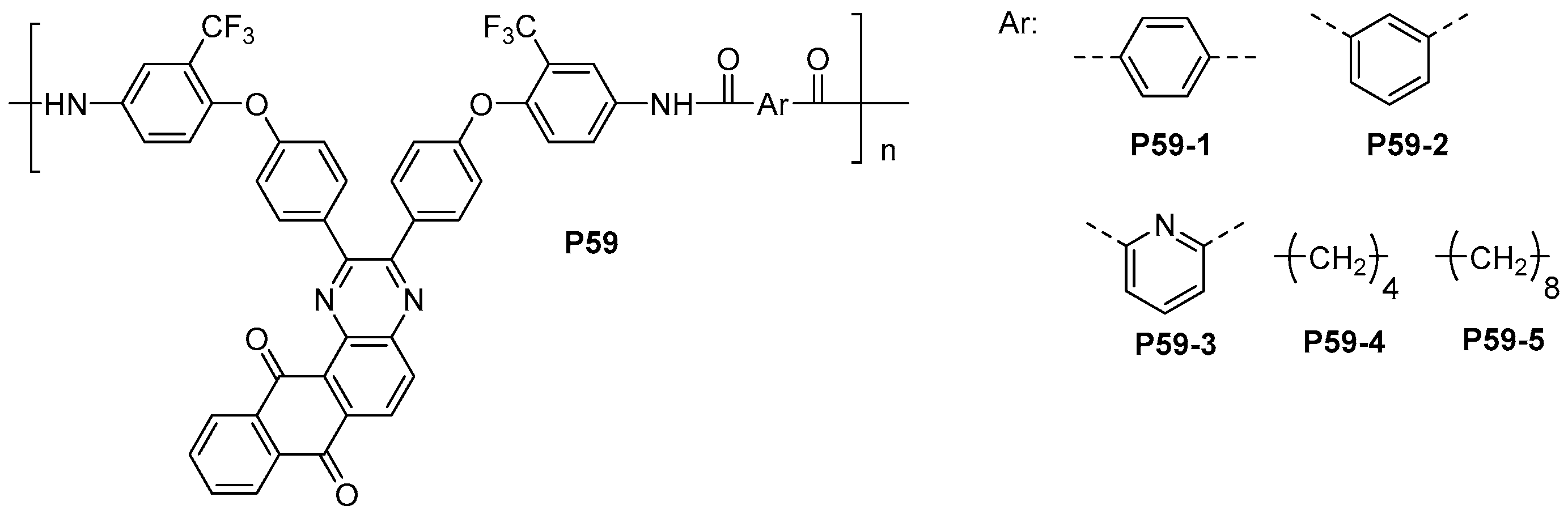
4.5. Optically Active and Fluorescent Polyamides
4.6. Electrochemical and Electrochromic Properties
4.7. Electrical and Magnetic Properties
4.8. Inherent Coloration
4.9. Aromatic Polyamides with Improved Solubility
4.10. Designing New Materials Based on Polyamides
5. Selected Polyamide Structures
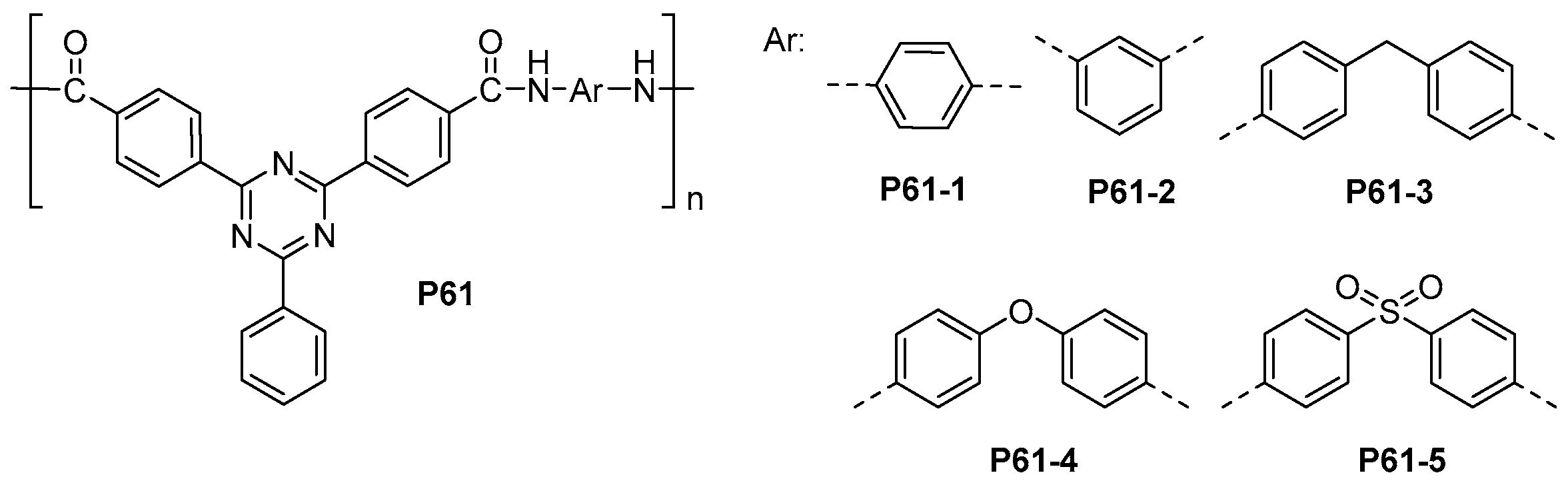
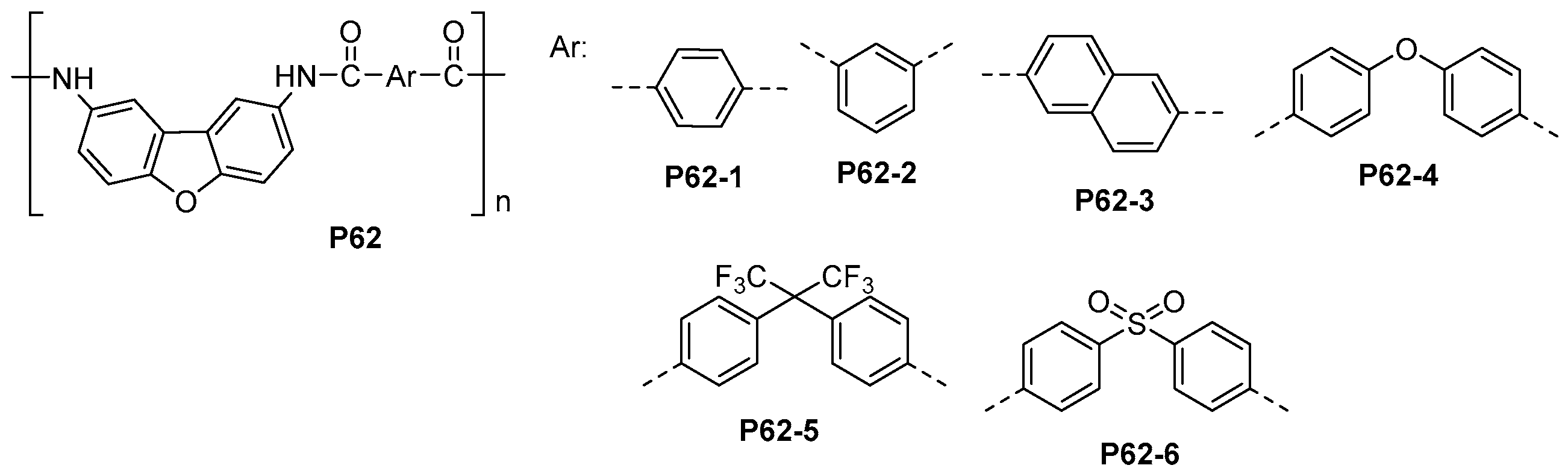
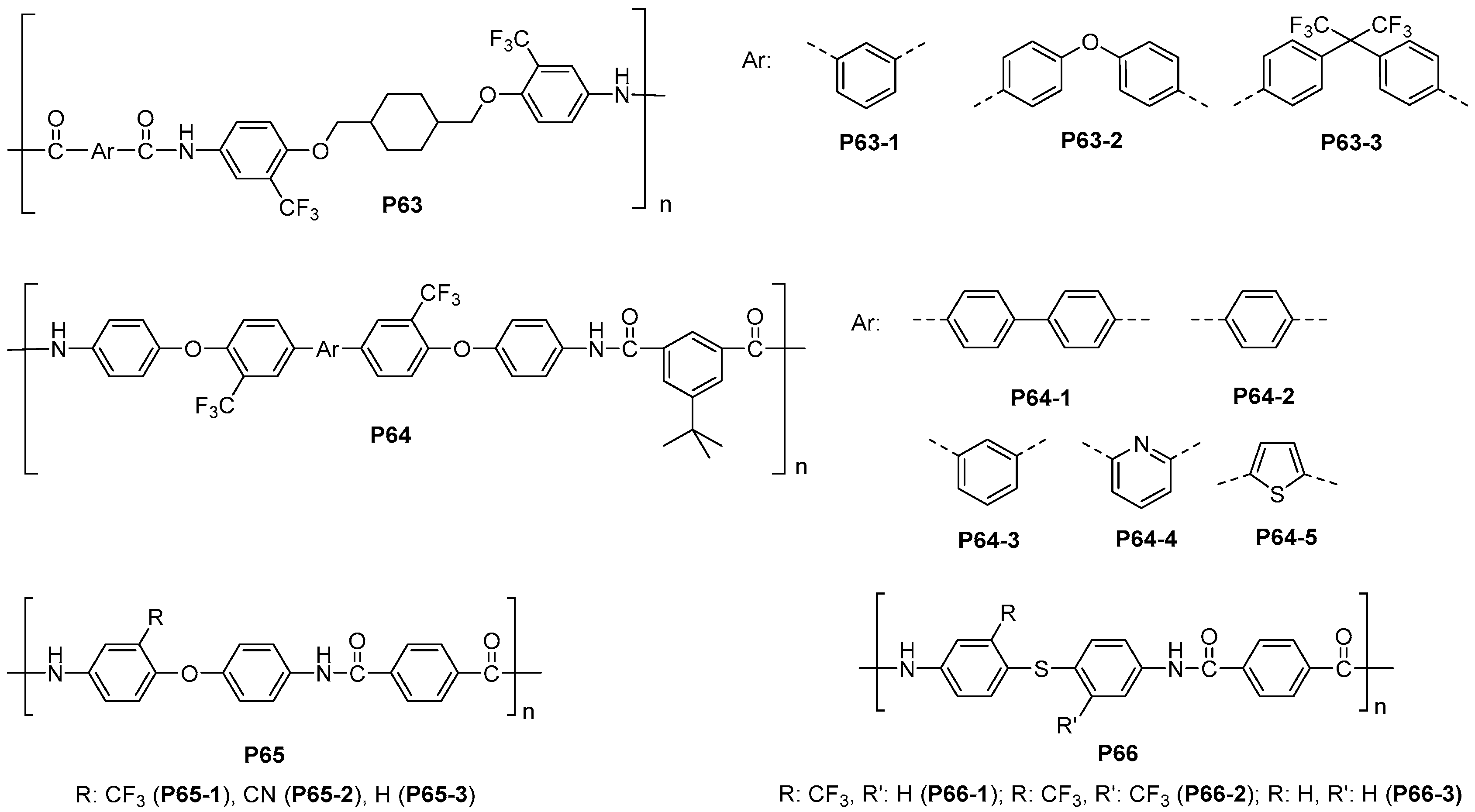
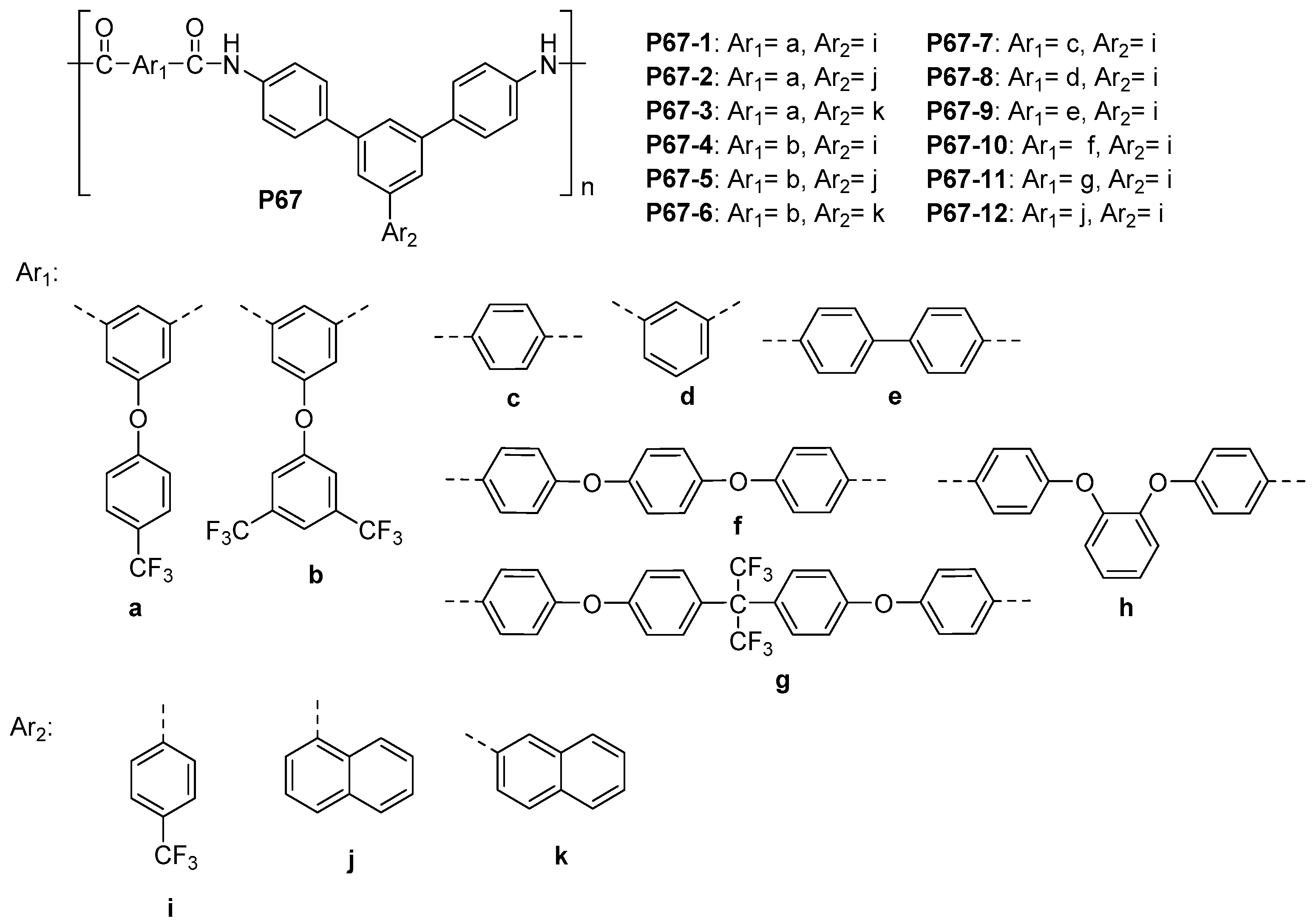
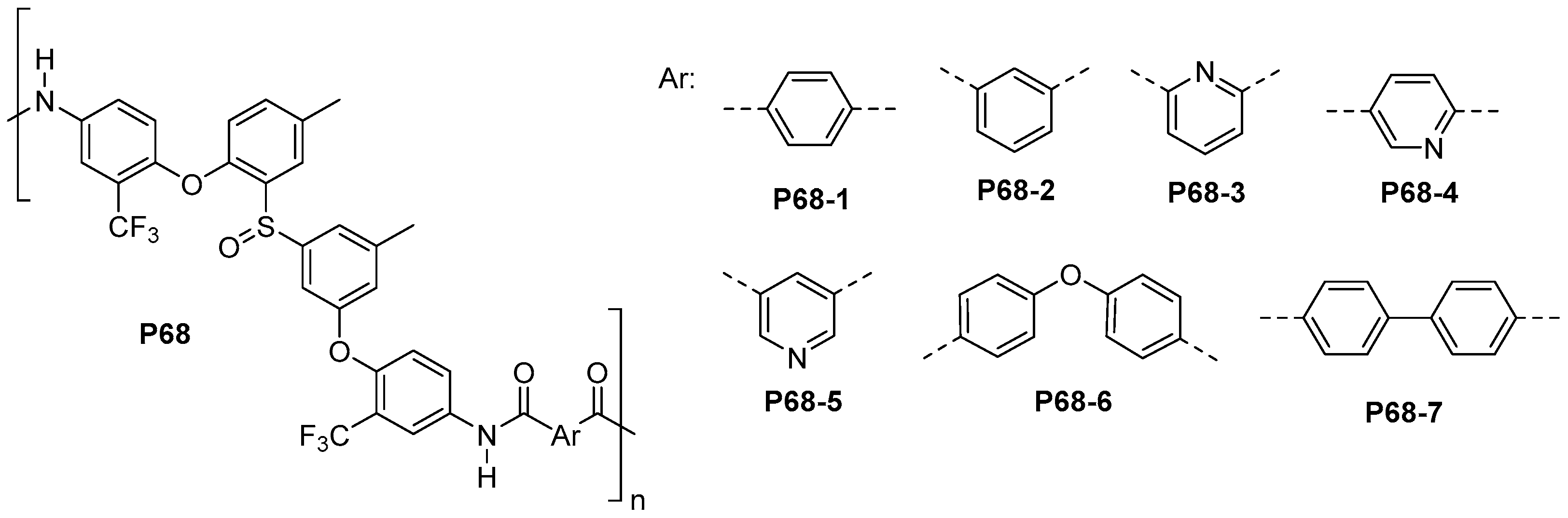
5.1. Polyamides with Heterocyclic Rings in the Main Chain
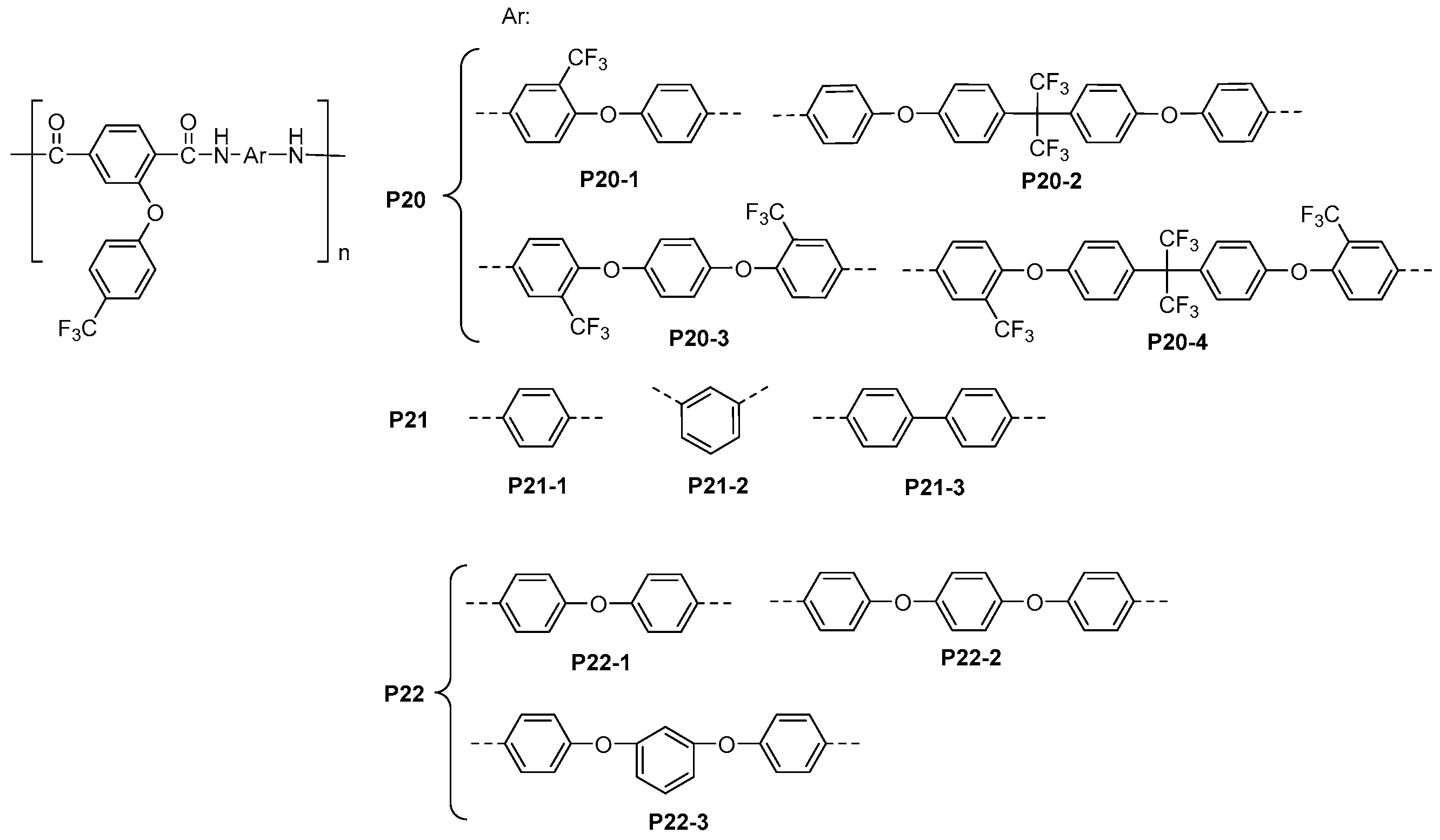
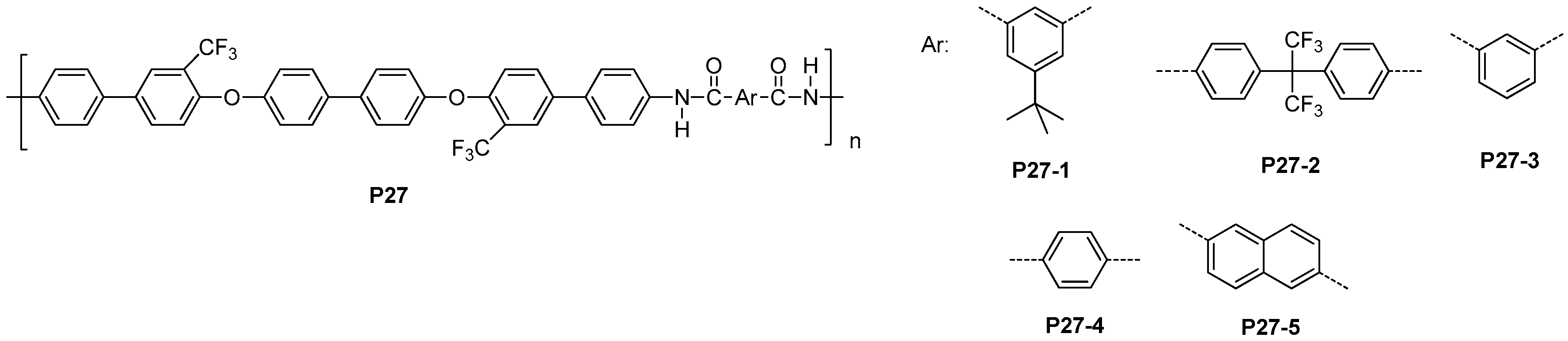
5.2. Fluorinated Polyamides
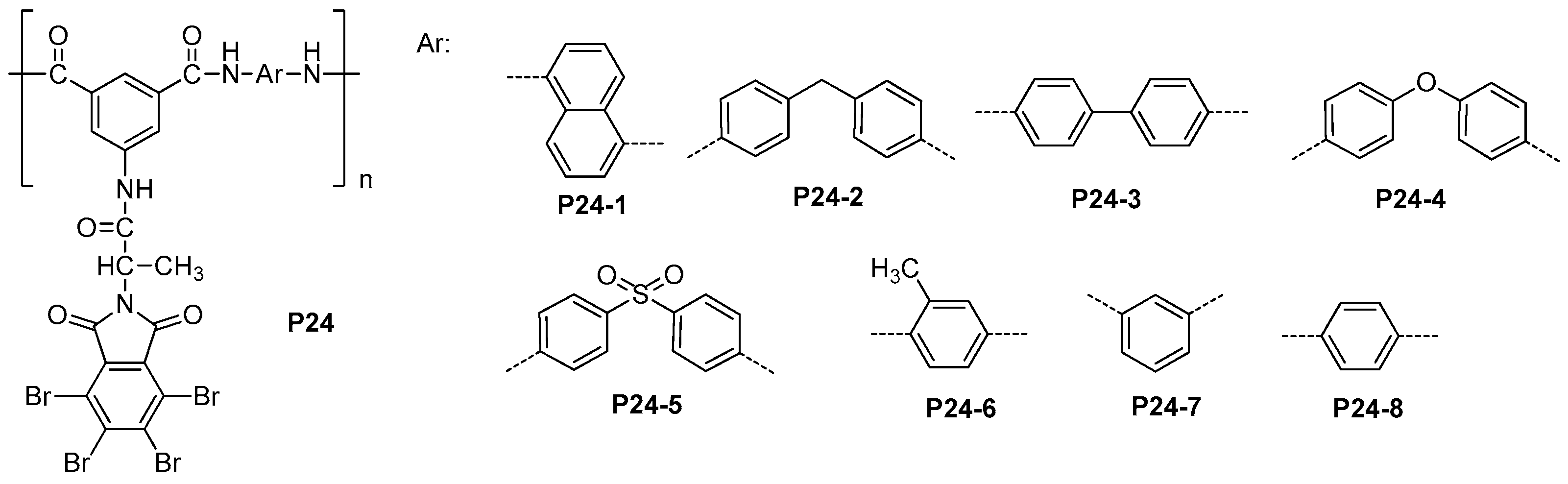
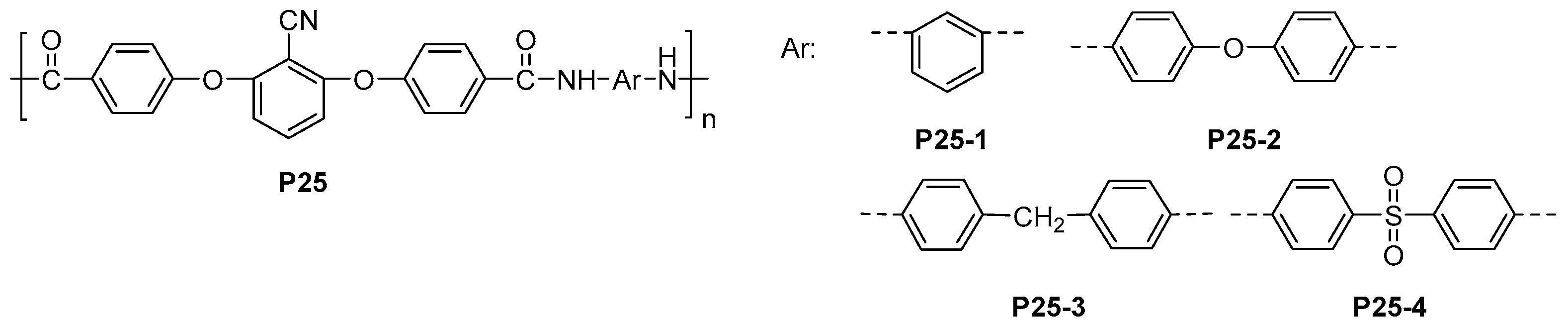
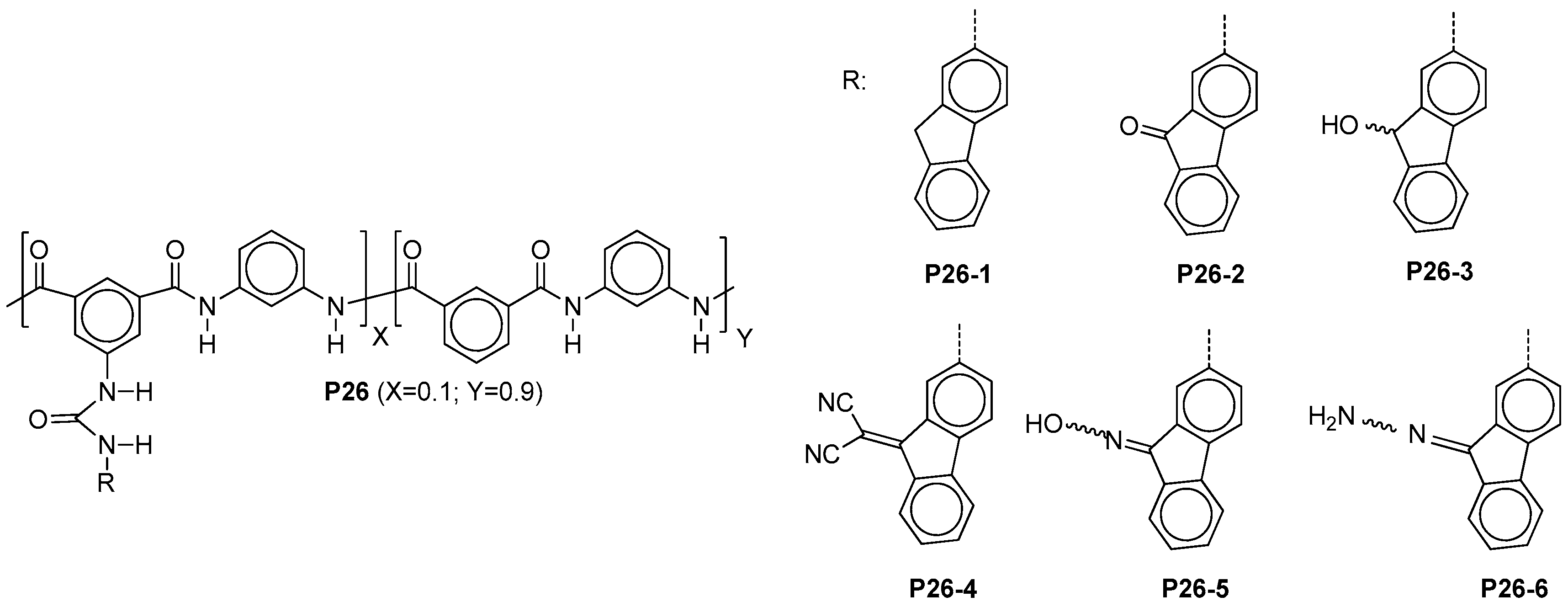
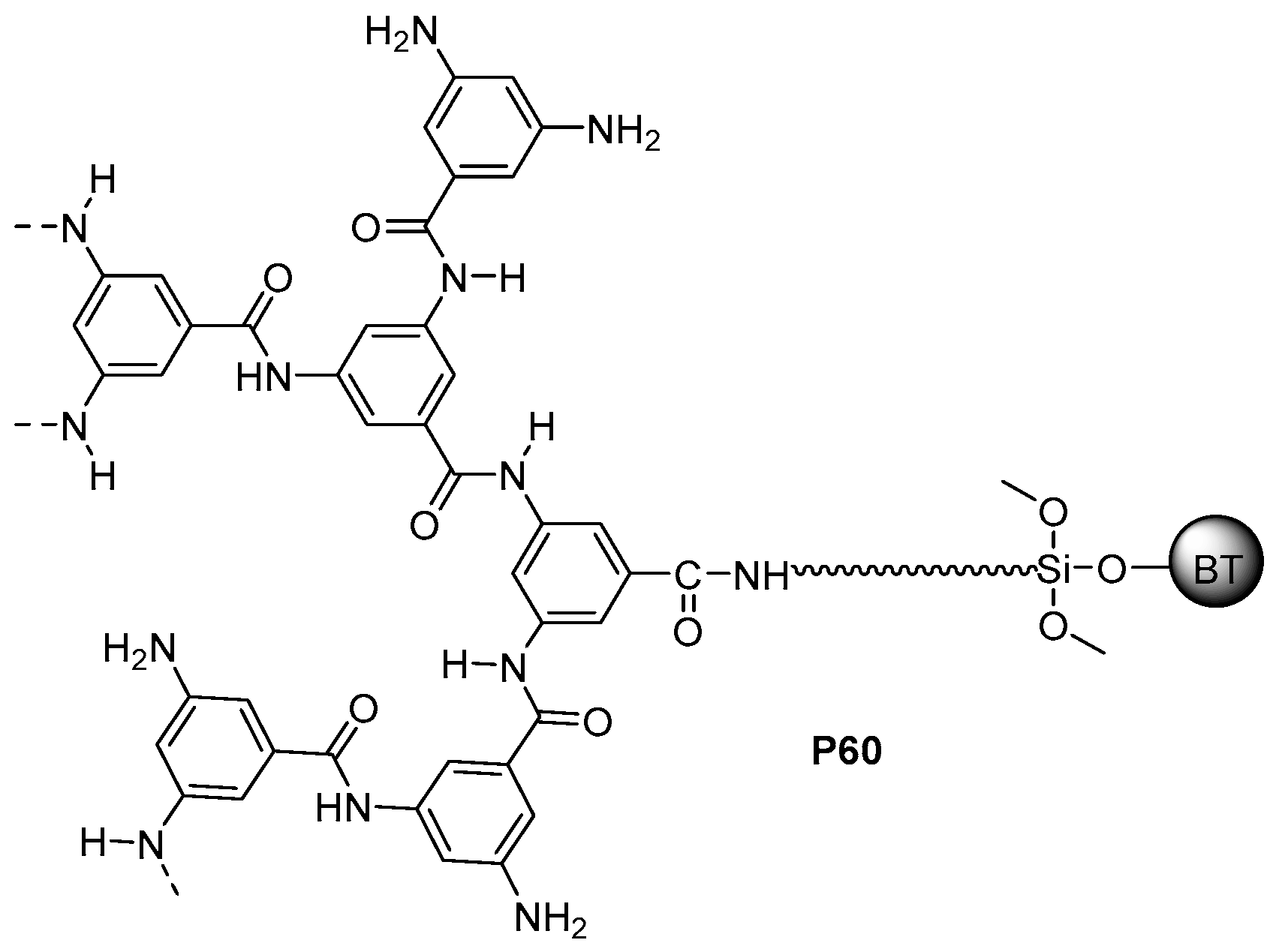
5.3. Miscellaneous
6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
Glossary
| DMA | N,N-dimethylacetamide |
| DMF | N,N-dimethylformamide |
| DMSO | dimethyl sulfoxide |
| DSC | differential scanning calorimetry |
| EDG | electron-donating group |
| EWG | electron-withdrawing group |
| ηinh | inherent viscosity |
| IPC | isophthaloyl dichloride |
| Mn, Mw | number-average and weight-average molecular masses |
| MPD | m-phenylenediamine |
| NMP | N-methyl-2-pyrrolidone |
| ODA | 3,4-diaminodiphenyl ether |
| ODA/PPTA | 3,4-diaminodiphenyl ether/poly(p-phenylene terephthalamide) |
| MPIA | poly(m-phenylene isophthalamide) |
| POSS | polyhedral oligomeric silsesquioxane |
| PPD | p-phenylenediamine |
| PPTA | poly(p-phenylene terephthalamide) |
| Tg | glass-transition temperature |
| Tm | melting temperature |
| TEG | tri(ethylene glycol) monomethyl ether |
| THF | tetrahydrofuran |
| TPA | triphenylamine |
| TPPA | N,N,N′,N′-tetraphenyl-1,4-phenylenediamine |
| TPC | terephthaloyl dichloride |
References
- Rules and Regulations under the Textile Fiber Products Identification Act in Title 16. Commercial Practices, Subchapter A, Chapter I, Part 303.7, Electronic Code of Federal Regulations (e-CFR). Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=d686e726b95f4e97353f2ee3103c7646&mc=true&node=pt16.1.303&rgn=div5#se16.1.303_17 (accessed on 29 May 2017).
- García, J.M.; García, F.C.; Serna, F.; de la Peña, J.L. High-performance aromatic polyamides. Prog. Polym. Sci. 2010, 35, 623–686. [Google Scholar] [CrossRef]
- Marchildon, K. Polyamides—Still strong after seventy years. Macromol. React. Eng. 2011, 5, 22–54. [Google Scholar] [CrossRef]
- Yamazaki, N.; Higasi, F.; Kawataba, J. Studies on reactions of the N-phosphonium salts of pyridines. XI. Preparation of polypeptides and polyamides by means of triaryl phosphites in pyridine. J. Polym. Sci. Polym. Chem. 1974, 12, 2149–2154. [Google Scholar] [CrossRef]
- Trigo-López, M.; Estevez, P.; San-José, N.; Gomez-Valdemoro, A.; García, F.C.; Serna, F.; de la Peña, J.L.; Garcia, J.M. Recent Patents on Aromatic Polyamides. Recent Pat. Mater. Sci. 2009, 2, 190–208. [Google Scholar] [CrossRef]
- Trigo-López, M.; Pablos, J.L.; García, F.C.; García, J.M. Aromatic Polyamides in Handbook of Thermoplastics, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gaymans, R.J. Polyamides: Synthetic Methods in Step-Growth Polymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Fink, J.K. High Performance Polymers; William Andrew Inc.: New York, NY, USA, 2008. [Google Scholar]
- Sekiguchi, H.; Coutin, B.; Polyamides, H.R. Handbook of Polymer Synthesis. Part A; Marcel-Dekker Inc.: New York, NY, USA, 1992. [Google Scholar]
- Vollbracht, L. Aromatic polyamides. In Comprehensive Polymer Science; Allen, G., Bevington, B., Eastmond, G.V., Ledwith, A., Russo, S., Sigwald, P., Eds.; Pergamon Press: Oxford, UK, 1989; Volume 5. [Google Scholar]
- Shoji, Y.; Zhang, C.; Higashihara, T.; Ueda, M. Synthesis of aramids by bulk polycondensation of aromatic dicarboxylic acids with 4,4′-oxydianiline. Polym. Chem. 2012, 3, 1978–1981. [Google Scholar] [CrossRef]
- Zhang, C.; Shoji, Y.; Higashihara, T.; Tsukuda, A.; Ochi, T.; Ueda, M. Synthesis of poly(m-phenyleneisophthalamide) by solid-state polycondensation of isophthalic acid with m-phenylenediamine. J. Polym. Chem. Part A Polym. Chem. 2011, 49, 4725–4726. [Google Scholar] [CrossRef]
- Kobashi, K.; Kobayashi, K.; Yasuda, H.; Arimachi, K.; Uchida, T.; Wakabayashi, K.; Yamazaki, S.; Kimura, K. Direct synthesis of wholly aromatic polyamides by using reaction-induced crystallization. Macromolecules 2009, 42, 6128–6135. [Google Scholar] [CrossRef]
- Cowie, J.M.G.; Arrighi, V. Polymers: Chemistry & Physics of Modern Materials, 3nd ed.; CRC Press: Boca Raton, FL, USA, 2007; p. 32. [Google Scholar]
- Yokozawa, T.; Yokoyama, A. Chain-growth polycondensation: The living polymerization process in polycondensation. Prog Polym. Sci. 2007, 3, 147–172. [Google Scholar] [CrossRef]
- Yokoyama, A.; Yokozawa, T. Converting step-growth to chain growth condensation polymerization. Macromolecules 2007, 40, 4093–4101. [Google Scholar] [CrossRef]
- Yokozawa, T.; Yoshihiro, O. Scope of controlled synthesis via chain-growth condensation polymerization: From aromatic polyamides to π-conjugated polymers. Chem. Commun. 2013, 49, 8281–8310. [Google Scholar] [CrossRef] [PubMed]
- Yokozawa, T.; Muroya, D.; Sugi, R.; Yokoyama, A. Convenient method of chain-growth polycondensation for well-defined aromatic polyamides. Macromol. Rapid Commun. 2005, 26, 979–981. [Google Scholar] [CrossRef]
- Yoshino, K.; Hachiman, K.; Yokoyama, A.; Yokozawa, T. Chain-growth condensation polymerization of 4-aminobenzoic acid esters bearing tri(ethylene glycol) side chain with lithium amide base. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 1357–1363. [Google Scholar] [CrossRef]
- Mikami, K.; Daikuhara, H.; Kasama, J.; Yokoyama, A.; Yokozawa, T. The Persistent Radical Effect: A Principle for Selective Radical Reactions and Living Radical Polymerizations. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3020–3029. [Google Scholar] [CrossRef]
- Yokoyama, A.; Masukawa, T.; Yamazaki, Y.; Yokozawa, T. Successive chain-growth condensation polymerization for the synthesis of well-defined diblock copolymers of aromatic polyamide and aromatic polyether. Macromol. Rapid. Commun. 2009, 30, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Sugi, R.; Tate, D.; Yokozawa, T. Synthesis of well-defined aromatic polyamide-graft-poly(tetrahydrofuran) by chain-growth condensation polymerization of macromonomer. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2725–2729. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 2006, 31, 1068–1132. [Google Scholar] [CrossRef]
- Ohishi, T.; Masukawa, T.; Fujii, S.; Yokoyama, A.; Yokozawa, T. Synthesis of core cross-linked star polymers consisting of well-defined aromatic polyamide arms. Macromolecules 2010, 43, 3206–3214. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, C.-W.; Liou, G.-S. Novel aromatic polyamides bearing pendant diphenylamino or carbozolyl groups. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 3302–3313. [Google Scholar] [CrossRef]
- Pino, P.; Lorenzi, G.P.; Suter, U.W.; Casartelli, P.; Steinman, I.; Bonner, F.J. Control of structure isomerism in polyamides. Macromolecules 1978, 11, 624–626. [Google Scholar] [CrossRef]
- Pal, R.R.; Patil, P.S.; Salunkhe, M.M.; Maldar, N.N.; Wadgaonkar, P.P. Synthesis, characterization and constitutional isomerism study of new aromatic polyamides containing pendant groups based on asymmetrically substituted meta-phenylene diamines. Eur. Polym. J. 2009, 45, 953–959. [Google Scholar] [CrossRef]
- Sadavarte, N.V.; Avadhani, C.V.; Wadgaonkar, P.P. Synthesis and characterization of new organosoluble aromatic polyamide and polyazomethines containing pendant pentadecyl chains. High Perform. Polym. 2011, 23, 494–505. [Google Scholar] [CrossRef]
- Damaceanu, M.D.; Rusu, R.-D.; Nicolescu, A.; Bruma, M.; Rusanov, A.L. Organosoluble asymmetric aromatic polyamides bearing pendant phenoxy groups. Polym. Int. 2011, 60, 1248–1258. [Google Scholar] [CrossRef]
- Ueda, M. Sequence control in one-step condensation polymerization. Prog. Polym. Sci. 1999, 24, 699–730. [Google Scholar] [CrossRef]
- Gentile, F.T.; Suter, U.W. Constitutional regularity in linear condensation polymers. In Comprehensive Polymer Science; Allens, F., Bevington, J.C., Eds.; Pergamon Press: Oxford, UK, 1989; Volume 5, pp. 97–115. [Google Scholar]
- Jikei, M.; Kakimoto, M. Dendritic aromatic polyamides and polyimides. J. Polym. Sci. A Polym. Chem. 2004, 42, 1293–1309. [Google Scholar] [CrossRef]
- Fréchet, J.M.J. Functional polymers and dendrimers-reactivity, molecular architecture, and interfacial energy. Science 1994, 263, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Scholl, M.; Kadlecova, Z.; Klok, H.-A. Dendritic and hyperbranched polyamides. Prog. Polym. Sci. 2009, 34, 24–61. [Google Scholar] [CrossRef]
- Matsumoto, K.; Nishi, K.; Ando, K.; Jikei, M. Synthesis and properties of aromatic polyamide dendrimers with polyhedral oligomeric silsesquioxane cores. Polym. Chem. 2015, 6, 4758–4765. [Google Scholar] [CrossRef]
- Tsushima, H.; Matsumoto, K.; Jikei, M. Solid-phase synthesis of aromatic polyamide dendrons. Polym. Adv. Technol. 2011, 22, 1292–1296. [Google Scholar] [CrossRef]
- Shabbir, S.; Zulfiqar, S.; Sarwar, M.I. Amine-terminated aromatic and semi-aromatic hyperbranched polyamides: Synthesis and characterization. J. Polym. Res. 2011, 18, 1919–1929. [Google Scholar] [CrossRef]
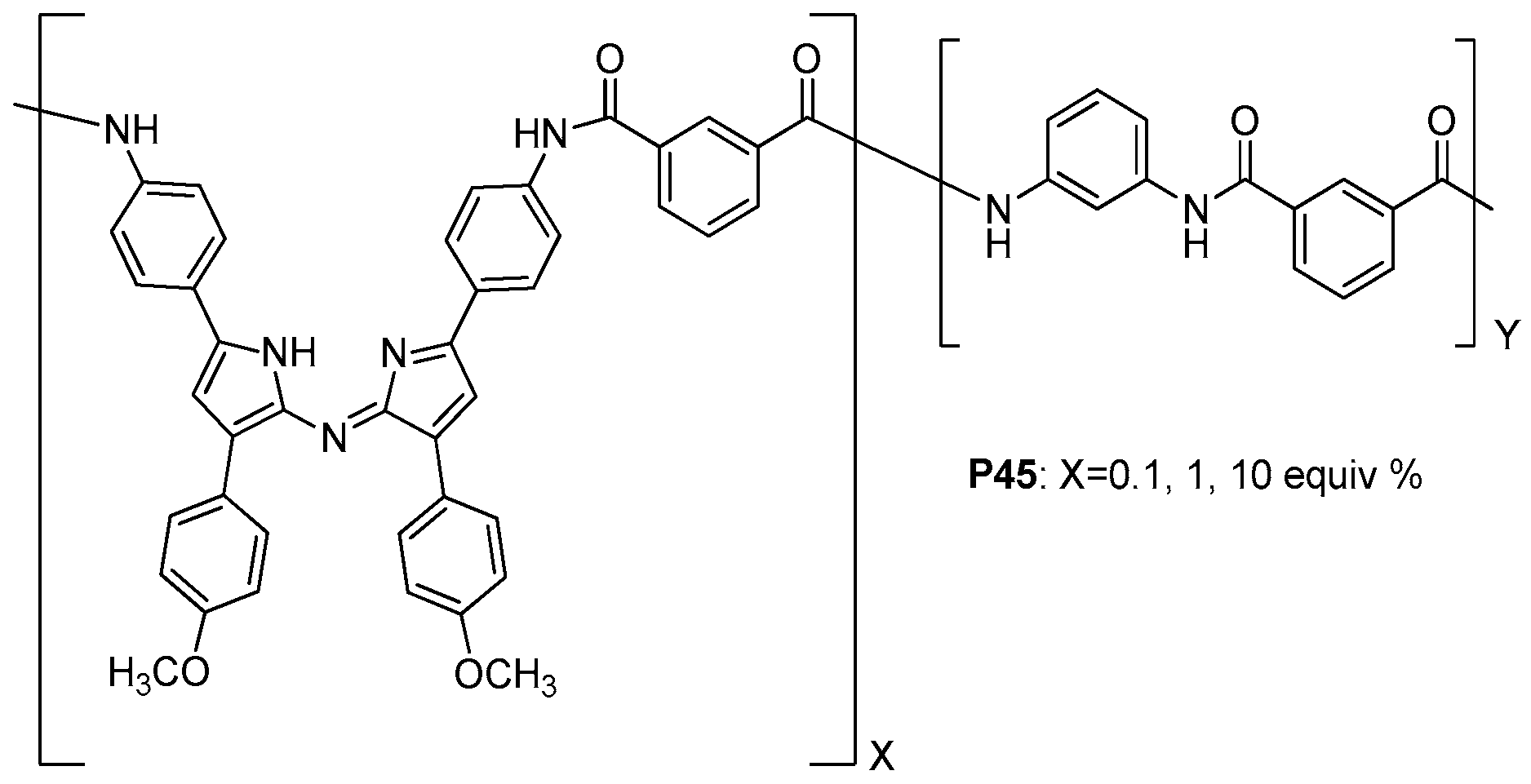
- Hu, X. Novel fluorescent porous hyperbranched aromatic polyamide containing 1,3,5-triphenylbenzene moieties: Synthesis and characterization. J. Appl. Polym. Sci. 2017, 134, 44505. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, S.G.; Chun, J.H.; Chun, B.-H.; Kim, S.H. Fabrication and characterization of the chlorine-tolerant disulfonated poly(arylene ether sulfone)/hyperbranched aromatic polyamide-grafted silica composite reverse osmosis membrane. Desalination Water Treat. 2012, 43, 221–229. [Google Scholar] [CrossRef]
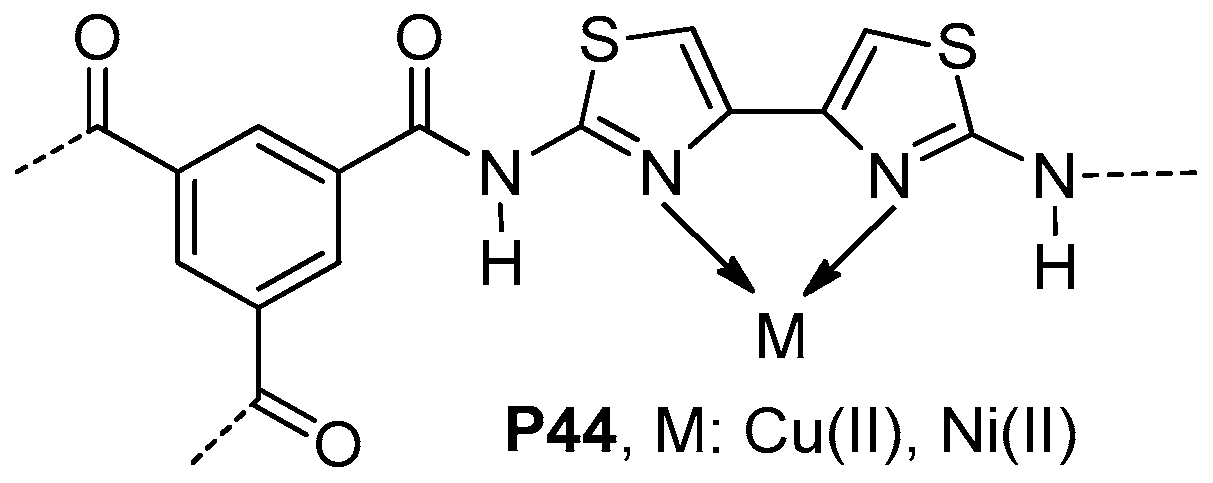
- Ding, N.; Lin, W.; Sun, W.; Shen, Z. A novel hyperbranched aromatic polyamide containing bithiazole: Synthesis, metal complexation and magnetic properties. Sci. China Chem. 2011, 54, 320–325. [Google Scholar] [CrossRef]
- Liou, G.-S.; Lin, H.-Y.; Yen, H.-J. Synthesis and characterization of electroactive hyperbranched aromatic polyamides based on A2B-type triphenylamine moieties. J. Mater. Chem. 2009, 19, 7666–7673. [Google Scholar] [CrossRef]
- Yu, J.; Huang, X.; Wang, L.; Peng, P.; Wu, C.; Wu, X.; Jiang, P. Preparation of hyperbranched aromatic polyamide grafted nanoparticles for thermal properties reinforcement of epoxy composites. Polym. Chem. 2011, 2, 1380–1388. [Google Scholar] [CrossRef]
- Qian, R.; Yu, J.; Xie, L.; Li, Y.; Jiang, P. Efficient thermal properties enhancement to hyperbranched aromatic polyamide grafted aluminum nitride in epoxy composites. Polym. Adv. Technol. 2013, 24, 348–356. [Google Scholar] [CrossRef]
- Jiang, J.-W.; Pei, X.L.; Sheng, S.R.; Wu, X.-Y.; Liu, X.L.; Song, C.-S. Novel soluble fluorine-containing polyamides derived from 2-(4-trifluoromethylphenoxy)terephthalic acid and trifluoromethyl-substituted aromatic bis(ether amine)s. Polym. Bull. 2011, 65, 263–274. [Google Scholar] [CrossRef]
- Ghaemy, M.; Masoumi, A.; Nasab, S.M.A.; Hassanzadeh, M. Synthesis and characterization of novel aromatic polyamides bearing CF3, quinoxaline-anthraquinone pendants: Study of photophysical and electrochemical properties by using nanocomposite electrode paste. J. Appl. Polym. Sci. 2013, 127, 3169–3177. [Google Scholar] [CrossRef]
- Shockravi, A.; Abouzari-Lotf, E.; Javadi, A.; Taheri, S. Synthesis and properties of novel fluorinated polyamides based on noncoplanar sulfoxide containing aromatic bis(ether amine). Polym. J. 2009, 41, 174–180. [Google Scholar] [CrossRef]
- Damaceanu, M.-D.; Rusu, R.-D.; Cristea, M.; Musteata, V.-E.; Bruma, M.; Wolinska-Grabczyk, A. Insights into the chain and local mobility of some aromatic polyamides and their influence on the physicochemical properties. Macromol. Chem. Phys. 2014, 215, 1573–1587. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, S.; Liu, Y.; Sheng, S.; Huang, Z.; Song, C. Synthesis and characterization of new soluble polyamides from 9,9-Bis[4-(chloroformylphenoxy)phenyl]xanthene and various aromatic diamines. Chin. J. Chem. 2010, 28, 102–110. [Google Scholar] [CrossRef]
- Patil, A.S.; Medhi, M.; Sadavarte, N.V.; Wadgaonkar, P.P.; Maldar, N.N. Synthesis and characterization of novel aromatic–aliphatic polyamides from bis-[(4-aminobenzyl)-4-benzamide] ether. Mater. Sci. Eng. B 2010, 168, 111–116. [Google Scholar] [CrossRef]
- Shabbir, S.; Zulfiqar, S.; Ahmad, Z.; Sarwar, M.I. Pyrimidine based carboxylic acid terminated aromatic and semiaromatic hyperbranched polyamide-esters: Synthesis and characterization. Tetrahedron 2010, 66, 7204–7212. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Synthesis of optically active and thermally stable polyamides with bulky aromatic side chain in an ionic liquid (tetrabutylammonium bromide). High Perform. Polym. 2010, 22, 567–580. [Google Scholar] [CrossRef]
- Mallakpour, S.; Taghavi, M. Kinetics and thermal degradation study of optically active and thermally stable aromatic polyamides with flame-retardancy properties. Polym. J. 2009, 41, 308–318. [Google Scholar] [CrossRef]
- Rafiee, Z.; Mallakpour, S. Synthesis and properties of novel brominated chiral polyamides derived from 5-[4-(2-tetrabromophthalimidylpropanoylamino) benzoylamino]isophthalic acid and aromatic diamines. Polym. Bull. 2016, 73, 1951–1964. [Google Scholar] [CrossRef]
- Yoshioka, Y. Control of the size and characteristic features of fluorine-containing aromatic polyamide particles. Colloid Polym. Sci. 2013, 291, 1641–1648. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Tashiro, K. Self-assembled aromatic polyamide nanofibers with trifluoromethyl groups via precipitation polymerization. Colloids Surf. A 2014, 447, 148–154. [Google Scholar] [CrossRef]
- Tan, J.; Wang, C.; Peng, W.; Li, G.; Jiang, J.-M. Synthesis, characterization, and properties of novel aromatic polyamides containing phthalazinone moiety. Polym. Bull. 2009, 62, 195–207. [Google Scholar] [CrossRef]
- Yu, Y.; Cai, M.; Zhang, Y. Study on synthesis of novel soluble aromatic polyamides with pendant cyano groups. Polym. Bull. 2010, 65, 309–318. [Google Scholar] [CrossRef]
- Bera, D.; Dasgupta, B.; Chatterjee, S.; Maji, S.; Banerjee, S. Synthesis, characterization, and properties of semifluorinated organo-soluble new aromatic polyamides. Polym. Adv. Technol. 2012, 23, 77–84. [Google Scholar] [CrossRef]
- Sheng, S.-R.; Ma, C.-X.; Jiang, J.-W.; Huang, Z.-Z.; Song, C.-S. Synthesis and properties of novel aromatic polyamides with xanthene cardo groups. J. Appl. Polym. Sci. 2010, 116, 1650–1659. [Google Scholar] [CrossRef]
- Zou, F.; Wen, H.; Yan, T.; Cai, M. Synthesis and properties of novel soluble aromatic polyamides containing 4-aryl-2,6-diphenylpyridine moieties and pendant fluorinated phenoxy groups. J. Polym. Res. 2016, 23, 225. [Google Scholar] [CrossRef]
- Li, K.; Luo, L.; Huang, J.; Wang, H.; Feng, Y.; Liu, X. Enhancing mechanical properties of aromatic polyamide fibers containing benzimidazole units via temporarily suppressing hydrogen bonding and crystallization. J. Appl. Polym. Sci. 2015, 132, 42482. [Google Scholar] [CrossRef]
- Abronin, I.A.; Bandurkin, A.V.; Volkova, L.V.; Shablygin, M.V. Features of hydrogen bonds in aromatic polyamides containing benzimidazole groups according to ab initio quantum chemical calculations. Fibre Chem. 2016, 47, 377–379. [Google Scholar] [CrossRef]
- San-José, N.; Gómez-Valdemoro, A.; Calderón, V.; de la Peña, J.L.; Serna, F.; García, F.C.; García, J.M. Polyamide model compound containing the urea group as selective colorimetric sensing probe towards aromatic diamines. Supramol. Chem. 2009, 21, 337–343. [Google Scholar] [CrossRef]
- Trigo-López, M.; Pablos, J.L.; Muñoz, A.; Ibeas, S.; Serna, F.; García, F.C.; García, J.M. Aromatic polyamides and acrylic polymers as solid sensory materials and smart coated fibres for high acidity colorimetric sensing. Polym. Chem. 2015, 6, 3110–3120. [Google Scholar] [CrossRef]
- Estévez, P.; El-Kaoutit, H.; García, F.C.; Serna, F.; De La Peña, J.L.; García, J.M. Chemical modification of the pendant structure of wholly aromatic polyamides: Toward functional high-performance materials with tuned chromogenic and fluorogenic behavior. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3823–3833. [Google Scholar] [CrossRef]
- Barrio-Manso, J.L.; Calvo, P.; García, F.C.; Pablos, J.L.; Torroba, T.; García, J.M. Functional fluorescent aramids: Aromatic polyamides containing a dipicolinic acid derivative as luminescent converters and sensory materials for the fluorescence detection and quantification of Cr(VI), Fe(III) and Cu(II). Polym. Chem. 2013, 4, 4256–4264. [Google Scholar] [CrossRef]
- Gómez-Valdemoro, A.; San-José, N.; García, F.C.; De La Peña, J.L.; Serna, F.; García, J.M. Novel aromatic polyamides with main chain and pendant 1,2,4-triazole moieties and their application to the extraction/elimination of mercury cations from aqueous media. Polym. Chem. 2010, 1, 1291–1301. [Google Scholar] [CrossRef]
- Yen, H.-J.; Lin, J.-H.; Su, Y.O.; Liou, G.-S. Novel triarylamine-based aromatic polyamides bearing secondary amines: Synthesis and redox potential inversion characteristics induced by pyridines. J. Mater. Chem. C 2016, 4, 10381–10385. [Google Scholar] [CrossRef]
- Bandyopadhyay, P.; Bera, D.; Banerjee, S. Synthesis, characterization and gas transport properties of semifluorinated new aromatic polyamides. Sep. Purif. Technol. 2013, 104, 138–149. [Google Scholar] [CrossRef]
- Bisoi, S.; Mandal, A.K.; Padmanabhan, V.; Banerjee, S. Aromatic polyamides containing trityl substituted triphenylamine: Gas transport properties and molecular dynamics simulations. J. Membr. Sci. 2017, 522, 77–90. [Google Scholar] [CrossRef]
- Smith, Z.P.; Hernández, G.; Gleason, K.L.; Anand, A.; Doherty, C.M.; Konstas, K.; Alvarez, C.; Hill, A.J.; Lozano, A.E.; Paul, D.R.; et al. Effect of polymer structure on gas transport properties of selected aromatic polyimides, polyamides and TR polymers. J. Membr. Sci. 2015, 493, 766–781. [Google Scholar] [CrossRef]
- Smirnova, N.N.; Fedotov, Y.A.; Tverskoi, V.A. Gas separation membranes based on interpolymer complexes of sulfonate containing aromatic polyamides. Polym. Sci. Ser. A 2010, 52, 413–418. [Google Scholar] [CrossRef]
- Bera, D.; Bandyopadhyay, P.; Ghosh, S.; Banerjee, S. Gas transport properties of aromatic polyamides containing adamantyl moiety. J. Membr. Sci. 2014, 453, 175–191. [Google Scholar] [CrossRef]
- Bera, D.; Bandyopadhyay, P.; Ghosh, S.; Banerjee, S.; Padmanabhan, V. Highly gas permeable aromatic polyamides containing adamantane substituted triphenylamine. J. Membr. Sci. 2015, 474, 20–31. [Google Scholar] [CrossRef]
- Plaza-Lozano, D.; Comesaña-Gándara, B.; de la Viuda, M.; Seong, J.G.; Palacio, L.; Prádanos, P.; de la Campa, J.G.; Cuadrado, P.; Lee, Y.M.; Hernández, A.; et al. New aromatic polyamides and polyimides having an adamantane bulky group. Mater. Today Commun. 2015, 5, 23–31. [Google Scholar] [CrossRef]
- Chang, K.-S.; Huang, Y.-H.; Lee, K.-R.; Tung, K.-L. Free volume and polymeric structure analyses of aromatic polyamide membranes: A molecular simulation and experimental study. J. Membr. Sci. 2010, 354, 93–100. [Google Scholar] [CrossRef]
- Lee, J.; Hill, A.; Kentish, S. Formation of a thick aromatic polyamide membrane by interfacial polymerization. Sep. Purif. Technol. 2013, 104, 276–283. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Z.; Wang, J.; Wang, S. Positively charged aromatic polyamide reverse osmosis membrane with high anti-fouling property prepared by polyethylenimine grafting. Desalination 2015, 365, 398–406. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Z.; Xu, J.; Wang, J.; Wang, S. Surface modification of commercial aromatic polyamide reverse osmosis membranes by graft polymerization of 3-allyl-5,5-dimethylhydantoin. J. Membr. Sci. 2010, 351, 222–233. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Wang, J.; Wang, S. Enhancing chlorine resistances and anti-biofouling properties of commercial aromatic polyamide reverse osmosis membranes by grafting 3-allyl-5,5-dimethylhydantoin and N,N′-Methylenebis(acrylamide). Desalination 2013, 309, 187–196. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, K.; Yan, F.; Shi, Y.; Yu, P.; Yu, S.; Li, S.; Gao, C. Enhancing the performance of aromatic polyamide reverse osmosis membrane by surface modification via covalent attachment of polyvinyl alcohol (PVA). J. Membr. Sci. 2016, 501, 209–219. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Wang, L.; Yu, S.; Gao, C. Improving fouling resistance and chlorine stability of aromatic polyamide thin-film composite RO membrane by surface grafting of polyvinyl alcohol (PVA). Desalination 2015, 367, 11–20. [Google Scholar] [CrossRef]
- Wu, D.; Liu, X.; Yu, S.; Liu, M.; Gao, C. Modification of aromatic polyamide thin-film composite reverse osmosis membranes by surface coating of thermo-responsive copolymers P(NIPAM-co-Am): Preparation and characterization. J. Membr. Sci. 2010, 352, 76–85. [Google Scholar] [CrossRef]
- Gao, Y.; De Jubera, A.M.S.; Mariñas, B.J.; Moore, J.S. Nanofiltration membranes with modified active layer using aromatic polyamide dendrimers. Adv. Funct. Mater. 2013, 23, 598–607. [Google Scholar] [CrossRef]
- Yan, H.; Miao, X.; Xu, J.; Pan, G.; Zhang, Y.; Shi, Y.; Guo, M.; Liu, Y. The porous structure of the fully-aromatic polyamide film in reverse osmosis membranes. J. Membr. Sci. 2015, 475, 504–510. [Google Scholar] [CrossRef]
- Wang, C.; Shen, B.; Zhou, Y.; Xu, C.; Chen, W.; Zhao, X.; Li, J. Sulfonated aromatic polyamides containing nitrile groups as proton exchange fuel cell membranes. Int. J. Hydrog. Energy 2015, 40, 6422–6429. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Preparation of thermally stable and optically active organosoluble aromatic polyamides containing l-leucine amino acid under green conditions. Polym. Bull. 2009, 63, 623–635. [Google Scholar] [CrossRef]
- Mallakpour, S.; Seyedjamali, H. Ionic liquid catalyzed synthesis of organosoluble wholly aromatic optically active polyamides. Polym. Bull. 2009, 62, 605–614. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M.A. Study of the ionic liquid mediated microwave heating for the synthesis of new thermally stable and optically active aromatic polyamides under green procedure. Macromol. Res. 2010, 18, 129–136. [Google Scholar] [CrossRef]
- Mallakpour, S.; Zadehnazari, A. Fast synthesis, using microwave induction heating in ionic liquid and characterization of optically active aromatic polyamides. J. Macromol. Sci. Pure 2009, 46, 783–789. [Google Scholar] [CrossRef]
- Mallakpour, S.; Rafiee, Z. Expeditious synthesis of novel aromatic polyamides from 5-[3-phenyl-2-(9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximido)-propanoylamino]isophthalic acid and various diamines using microwave-assisted polycondensation. React. Funct. Polym. 2009, 69, 252–258. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Pyrenylamine-functionalized aromatic polyamides as efficient blue-emitters and multicolored electrochromic materials. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 3475–3490. [Google Scholar] [CrossRef]
- Lee, B.; Byun, T.; Kim, S.D.; Kang, H.A.; Kim, S.Y.; Chung, I.S. Soluble para-linked aromatic polyamides with pendant groups. Macromol. Res. 2015, 23, 838–843. [Google Scholar] [CrossRef]
- Li, P.-H.; Wang, C.-Y.; Li, G.; Jiang, J.-M. Synthesis and characterization of novel polyamides derived from 1,4-bis((4-amino-2-(trifluoromethyl)phenoxy)methyl)cyclohexane and aromatic dicarboxylic acids. Polym. Bull. 2010, 64, 127–140. [Google Scholar] [CrossRef]
- Nechifor, M. Synthesis and properties of some aromatic polyamides with coumarin chromophores. React. Funct. Polym. 2009, 69, 27–35. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, C.-N. Solution-processable and electroactive aromatic polyamides with 3,5-bis(trifluoromethyl)triphenylamine moiety. Polym. Int. 2017, 66, 916–924. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chou, Y.-T. Synthesis and electrochromic properties of aromatic polyamides with pendant triphenylamine units. Macromol. Chem. Phys. 2014, 215, 958–970. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Peng, S.-C.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Synthesis and electro-optical properties of aromatic polyamides and polyimides bearing pendant 3,6 dimethoxycarbazole units. Eur. Polym. J. 2015, 73, 50–64. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Kung, Y.I.-C.; Hsiung, T.-J. Synthesis and properties of new aromatic polyamides with redox-active 2,4-dimethoxytriphenylamine moieties. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 3392–3401. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Jan, J.-S. Solution-processable transmissive-to-green switching electrochromic polyamides bearing 2,7-bis(diphenylamino)naphthalene units. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1409–1421. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, K.-H. A comparative study on the properties of aromatic polyamides with methyl- or trifluoromethyl-substituted triphenylamine groups. J. Fluor. Chem. 2016, 188, 33–42. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Kung, Y.-C.; Pan, H.-Y.; Kuo, C.-H. Electroactive aromatic polyamides and polyimides with adamantylphenoxy-substituted triphenylamine units. Eur. Polym. J. 2009, 45, 2234–2248. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wu, C.-N. Electrochemical and electrochromic studies of redox-active aromatic polyamides with 3,5-dimethyltriphenylamine units. J. Electroanal. Chem. 2016, 776, 139–147. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Kung, Y.-C.; Chang, Y.-M. Fluorescent and electrochromic aromatic polyamides with 4-tert-butyltriphenylamine chromophore. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2798–2809. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Cheng, S.-L. New electroactive and electrochromic aromatic polyamides with ether-linked bis(triphenylamine) units. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 496–510. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsiao, Y.-H.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Triphenylamine-based redox-active aramids with 1-piperidinyl substituent as an auxiliary donor: Enhanced electrochemical stability and electrochromic performance. React. Funct. Polym. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M. Facile fabrication of redox-active and electrochromic poly(amide-amine) films through electrochemical oxidative coupling of arylamino groups. Polym. Sci. Part A Polym. Chem. 2016, 54, 2476–2485. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Hsiao, Y.-H.; Kung, Y.-R. Highly redox-stable and electrochromic aramids with morpholinyl-substituted triphenylamine units. Polym. Sci. Part A Polym. Chem. 2016, 54, 1289–1298. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chiu, Y.-T. Electrosynthesis and electrochromic properties of poly(amide-triarylamine)s containing triptycene units. RSC Adv. 2015, 5, 90941–90951. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liu, N.-E.; Kung, Y.-R. Synthesis of electroactive and electrochromic poly(amide-imide)s containing diphenylpyrenylamine moieties. J. Polym. Res. 2015, 22, 9. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Teng, C.-Y.; Kung, Y.-R. Synthesis and characterization of novel electrochromic poly(amide-imide)s with N,N′-di(4-methoxyphenyl)-N,N′-diphenyl-p-phenylenediamine units. RSC Adv. 2015, 5, 93591–93606. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Liao, S.-H. Redox-stable and visible/near-infrared electrochromic aramids with main-chain triphenylamine and pendant 3,6-di-tert-butylcarbazole units. Polym. Chem. 2014, 5, 2473–2483. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, J.-W. Facile fabrication of electrochromic poly(amine-amide) and poly(amine-imide) films via carbazole-based oxidative coupling electropolymerization. Macromol. Chem. Phys. 2014, 215, 705–715. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Lin, J.-W.; Guo, W.; Kung, Y.-R.; Leu, C.-M.; Lee, T.-M. Synthesis and electrochromic properties of polyamides having pendant carbazole groups. Mater. Chem. Phys. 2013, 141, 665–673. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Chang, P.-C.; Kung, Y.-R.; Lee, T.-M. Novel organosoluble aromatic polyetheramides bearing triphenylamine moieties: Synthesis, electrochemistry, and electrochromism. J. Polym. Res. 2013, 20, 154. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Guo, W.; Kung, Y.-C.; Lee, Y.-J. Redox-active and electrochromic aromatic poly(amide-imide)s with 2,4-dimethoxytriphenylamine chromophores. J. Polym. Res. 2011, 18, 1353–1364. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Guo, W.; Lee, W.-F.; Kung, Y.-C.; Lee, Y.-J. Synthesis and characterization of electrochromic poly(amide-imide)s bearing methoxy-substituted triphenylamine units. Mater. Chem. Phys. 2011, 130, 1086–1093. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Kung, Y.-C.; Lee, Y.-J. Synthesis and characterization of electrochromic poly(amide-imide)s based on the diimide-diacid from 4,4′-diamino-4″-methoxytriphenylamine and trimellitic anhydride. Eur. Polym. J. 2010, 46, 1355–1366. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Liou, G.-S.; Wang, H.-M. Highly stable electrochromic polyamides based on N,N-Bis(4-aminophenyl)-N′,N′-bis(4-tert-butylphenyl)-1,4-phenylenediamine. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2330–2343. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Enhanced redox stability and electrochromic properties of aromatic polyamides based on N,N-Bis(4-carboxyphenyl)- N′,N′-bis(4-tert-butylphenyl)-1,4-phenylenediamine. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 337–351. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Enhancement of redox stability and electrochromic performance of aromatic polyamides by incorporation of (3,6-Dimethoxycarbazol-9-yl)-triphenylamine units. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 272–286. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H.; Liou, G.-S.; Sun, C.-H. Synthesis, photoluminescence, and electrochromism of polyamides containing (3,6-di-tert-butylcarbazol-9-yl)triphenylamine units. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 4775–4789. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Multicolor electrochromic poly(amide-imide)s with N,N-diphenyl- N′,N′-di-4-tert-butylphenyl-1,4-phenylenediamine moieties. Polym. Chem. 2010, 1, 1013–1023. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Soluble, redox-active, and blue-emitting poly(amide-hydrazide)s and poly(amide-1,3,4-oxadiazole)s containing pyrenylamine units. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4830–4840. [Google Scholar] [CrossRef]
- Kung, Y.-C.; Hsiao, S.-H. Fluorescent and electrochromic polyamides with pyrenylamine chromophore. J. Mater. Chem. 2010, 20, 5481–5492. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, Z.; Chao, D.; Chu, X.; Du, Y.; Zhao, X.; Wang, D.; Chen, C. Novel aromatic polyamides containing 2-diphenylamino-(9,9-dimethylamine) units as multicolored electrochromic and high-contrast electrofluorescent materials. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 213–222. [Google Scholar] [CrossRef]
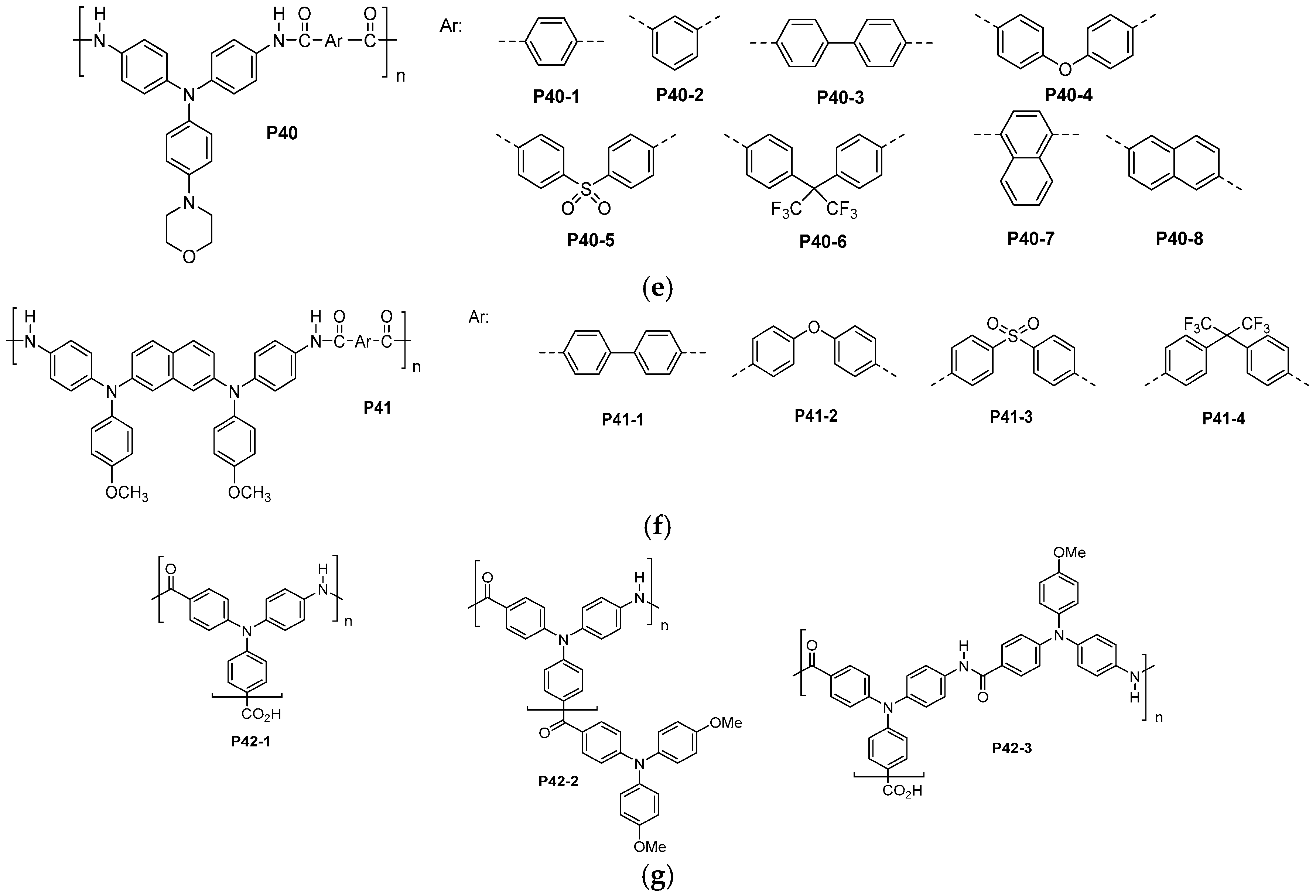
- He, L.; Chao, D.; Zhang, C.; Jia, X.; Liu, H.; Liu, X.; Wang, C. Novel electroactive aromatic polyamide with oligoanilines and azo groups in the backbone: Synthesis, characterization and dielectric properties. J. Polym. Res. 2012, 19, 9746. [Google Scholar] [CrossRef]
- Ravikumar, L.; Saravanan, R. Synthesis of soluble, curable, and thermally stable aromatic polyamides bearing thiourea and pendant 4-pyridylformylimino groups. Int. J. Polym. Mater. Polym. Biomater. 2012, 61, 1050–1064. [Google Scholar] [CrossRef]
- Chen, C.-J.; Hu, Y.-C.; Liou, G.-S. Linkage effect on the memory behavior of sulfonyl-containing aromatic polyether, polyester, polyamide, and polyimide. Chem. Commun. 2013, 49, 2536–2538. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Tsai, C.-L.; Hsiao, S.-H.; Liou, G.-S. Linkage and donor-acceptor effects on resistive switching memory devices of 4-(N-carbazolyl)triphenylamine-based polymers. RSC Adv. 2016, 6, 28815–28819. [Google Scholar] [CrossRef]
- Safabakhsh, B.; Khosravi, A.; Gharanjig, K.; Kowsari, E.; Khorassani, M.; Tafaghodi, S. Synthesis of a novel fluorescent coloured copolymer based on 4-butylthio-1,8-naphthalimide. Color Technol. 2012, 128, 218–222. [Google Scholar] [CrossRef]
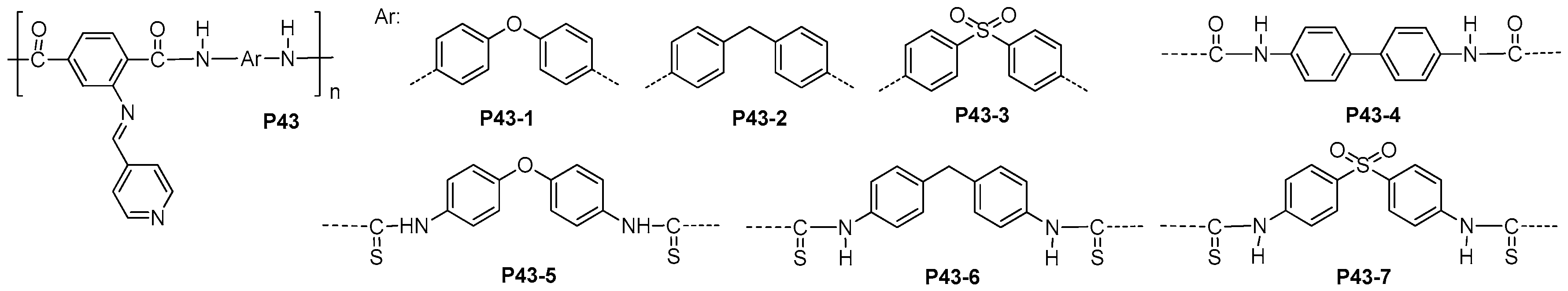
- Mohamed, N.A.; Sammour, M.H.; Elshafai, A.M. Novel self-dyed wholly aromatic polyamide-hydrazides covalently bonded with azo groups in their main chains: 1. Structure-Property relationships. Molecules 2012, 17, 13969–13988. [Google Scholar] [CrossRef] [PubMed]
- Trigo-López, M.; Miguel-Ortega, Á.; Vallejos, S.; Muñoz, A.; Izquierdo, D.; Colina, Á.; García, F.C.; García, J.M. Intrinsically colored wholly aromatic polyamides (aramids). Dyes Pigment. 2015, 122, 177–183. [Google Scholar] [CrossRef]
- Ohsedo, Y.; Oono, M.; Saruhashi, K.; Watanabe, H. A new water-soluble aromatic polyamide hydrogelator with thixotropic properties. RSC Adv. 2015, 5, 82772–82776. [Google Scholar] [CrossRef]
- Behniafar, H.; Khosravi-borna, S. Synthesis and characterization of novel aromatic polyamides derived from 2,2′-bis(p-phenoxyphenyl)-4,4′-diaminodiphenyl ether. Polym. Int. 2009, 58, 1299–1307. [Google Scholar] [CrossRef]
- Chen, J.-C.; Rajendran, K.; Huang, S.-W.; Chang, H.-W. Synthesis and characterization of aromatic polyamides derived from various derivatives of 4,4′-oxydianiline. J. Polym. Res. 2011, 18, 1693–1703. [Google Scholar] [CrossRef]
- Carja, I.-D.; Hamciuc, C.; Hamciuc, E.; Vlad-Bubulac, T.; Lisa, G. New highly thermostable aromatic polyamides with pendant phthalonitrile groups. Macromol. Res. 2012, 20, 1011–1020. [Google Scholar] [CrossRef]
- Nechifor, M. Novel chalcone-based aromatic polyamides: Synthesis, characterization, and properties. Des. Monomers Polym. 2016, 19, 161–171. [Google Scholar] [CrossRef]
- Palí Casanova, R.J.; Córdova Quiroz, V.; Zavala Loria, J.C.; Aguilar-Vega, M.; Loría-Bastarrachea, M. Structural rigidity of aromatic polyamides with bulky lateral substitutions. High Perform. Polym. 2009, 21, 315–339. [Google Scholar] [CrossRef]
- Fiori, S.; Monticelli, O.; Alzari, V.; Mariani, A. New sets of solubility parameters of linear and crosslinked aromatic polyamides. J. Appl. Polym. Sci. 2010, 115, 3155–3160. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Guo, W.; Tsai, T.-H.; Chiu, Y.-T. Synthesis of soluble and thermally stable triptycene-based poly(amide-imide)s. J. Polym. Res. 2014, 21, 391. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Chou, J.-S.; Guo, W.; Tsai, T.-H. Synthesis and characterization of novel organosoluble and thermally stable polyamides bearing triptycene in their backbones. J. Polym. Res. 2012, 19, 9902. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Fang, J.; Yin, J. Synthesis and characterization of novel aromatic polyamides via Yamazaki–Higashi phosphorylation method. J. Appl. Polym. Sci. 2012, 126, 244–252. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, H.; Jiang, X.; Yin, J. Multi-responsive wholly aromatic sulfonated polyamide ultra-sensitive to pH value. Sci. China Chem. 2012, 55, 2503–2506. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, S.; Li, D.; Zhang, M.; Zhang, G.; Yang, J. Facile synthesis of processable aromatic polyamides containing thioether units. Polym. Int. 2013, 62, 411–418. [Google Scholar] [CrossRef]
- Hassan, H.H.A.M.; Elhusseiny, A.F.; Elkony, Y.M.A.; Mansour, E.-S.M.E. Synthesis and characterization of thermally stable aromatic polyamides and poly(1,3,4-oxadiazole-amide)s nanoparticles containing pendant substituted bezamides. Chem. Cent. J. 2013, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Shabanian, M.; Kang, N.-J.; Wang, D.-Y.; Wagenknecht, U.; Heinrich, G. Synthesis, characterization and properties of novel aliphatic–aromatic polyamide/functional carbon nanotube nanocomposites via in situ polymerization. RSC Adv. 2013, 3, 20738–20745. [Google Scholar] [CrossRef]
- Kadokawa, J.-I.; Nishikawa, T.; Sasaki, Y.; Tanaka, Y.; Kato, T.; Kaneko, Y. Synthesis of aromatic polyamide having fluorescein moieties in the main-chain and its conversion into ionically cross-linked nanoparticles with Ca2+. Polym. J. 2009, 41, 893–897. [Google Scholar] [CrossRef]
- Chen, T.; Qiu, J.; Zhu, K.; Li, J.; Wang, J.; Li, S.; Wang, X. Achieving high performance electric field induced strain: A rational design of hyperbranched aromatic polyamide functionalized grapheme–polyurethane dielectric elastomer composites. J. Phys. Chem. B 2015, 119, 4521–4530. [Google Scholar] [CrossRef] [PubMed]
- Madeshwaran, S.R.; Kwon, J.K.; Cho, J.W. Functionalized multi-walled carbon nanotubes with hyperbranched aromatic polyamide for poly(methyl methacrylate) composites. Fibers Polym. 2013, 14, 182–187. [Google Scholar] [CrossRef]
- Yu, Y.; Rong, M.Z.; Zhang, M.Q. Grafting of hyperbranched aromatic polyamide onto silica nanoparticles. Polymer 2010, 51, 492–499. [Google Scholar] [CrossRef]
- Yoshioka, Y. Analysis and characterization of aromatic polyamide copolymer particles with trifluoromethyl and amino groups. Int. J. Polym. Anal. Charact. 2011, 16, 551–560. [Google Scholar] [CrossRef]
- Sánchez-Sánchez, A.; Suárez-García, F.; Martínez-Alonso, A.; Tascón, J.M.D. Aromatic polyamides as new precursors of nitrogen and oxygen-doped ordered mesoporous carbons. Carbon 2014, 70, 119–129. [Google Scholar] [CrossRef]
- Xie, L.; Huang, X.; Huang, Y.; Yang, K.; Jiang, P. Core-shell structured hyperbranched aromatic polyamide/BaTiO3 hybrid filler for poly(vinylidene fluoride-trifluoroethylenechlorofluoroethylene) nanocomposites with the dielectric constant comparable to that of percolative composites. ACS Appl. Mater. Interfaces 2013, 5, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Li, B.; Liu, J.; Wu, S.; Tan, H.; Pan, C.; Jian, X. Novel thermally stable and organosoluble aromatic polyamides with main chain phenyl-1,3,5-triazine moieties. Polym. Degrad. Stab. 2012, 97, 1807–1814. [Google Scholar] [CrossRef]
- Sheng, S.-R.; Pei, X.-L.; Huang, Z.-Z.; Liu, X.-L.; Song, C.-S. Novel soluble fluorinated aromatic polyamides derived from 2-(4-trifluoromethylphenoxy)terephthaloyl chloride with various aromatic diamines. Eur. Polym. J. 2009, 45, 230–236. [Google Scholar] [CrossRef]
- Li, X.; Jiang, J.-W.; Pan, Y.; Sheng, S.-R.; Liu, X.-L. New aromatic polyamides with 2,6-biphenylpyridyl moieties and 4-trifluoromethylphenyl pendant groups. High Perform. Polym. 2015, 27, 37–45. [Google Scholar] [CrossRef]
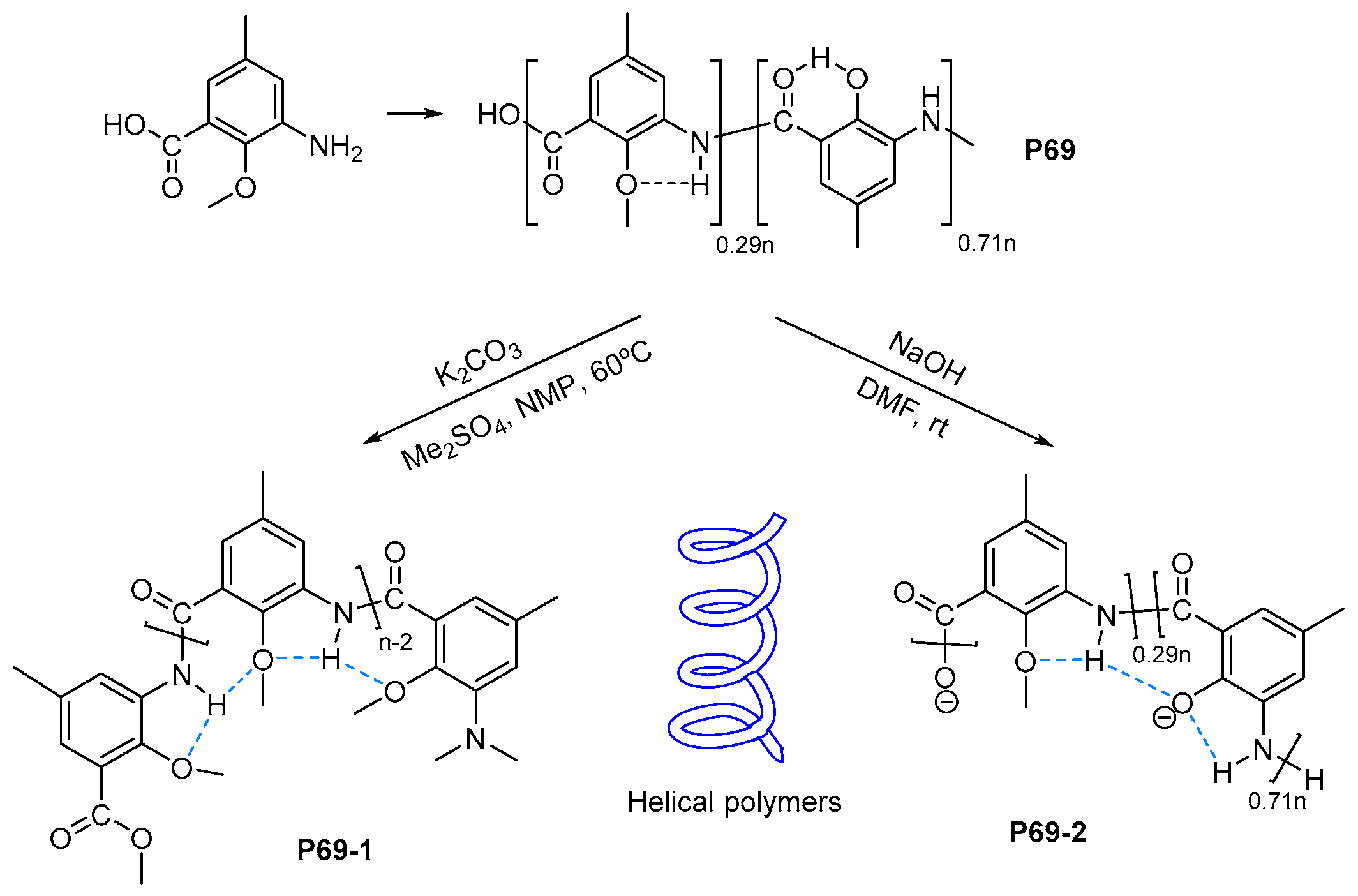
- Lu, Y.-X.; Shi, Z.-M.; Li, Z.-T.; Guan, Z. Helical polymers based on intramolecularly hydrogen-bonded aromatic polyamides. Chem. Commun. 2010, 46, 9019–9021. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Kline, M.; Chen, Z.; Luan, B.; Lv, M.; Zhang, W.; Lian, C.; Wang, Q.; Huang, Q.; Wei, X.; et al. Preparation and helical folding of aromatic polyamides. Chem. Commun. 2012, 48, 11112–11114. [Google Scholar] [CrossRef] [PubMed]
- Aiba, M.; Higashihara, T.; Ashizawa, M.; Otsuka, H.; Matsumoto, H. Triggered structural control of dynamic covalent aromatic polyamides: Effects of thermal reorganization behavior in solution and solid states. Macromolecules 2016, 49, 2153–2161. [Google Scholar] [CrossRef]
- Trigo-López, M.; Barrio-Manso, J.L.; Serna, F.; García, F.C.; García, J.M. Crosslinked Aromatic Polyamides: A Further Step in High-Performance Materials. Macromol. Chem. Phys. 2013, 214, 2223–2231. [Google Scholar] [CrossRef]
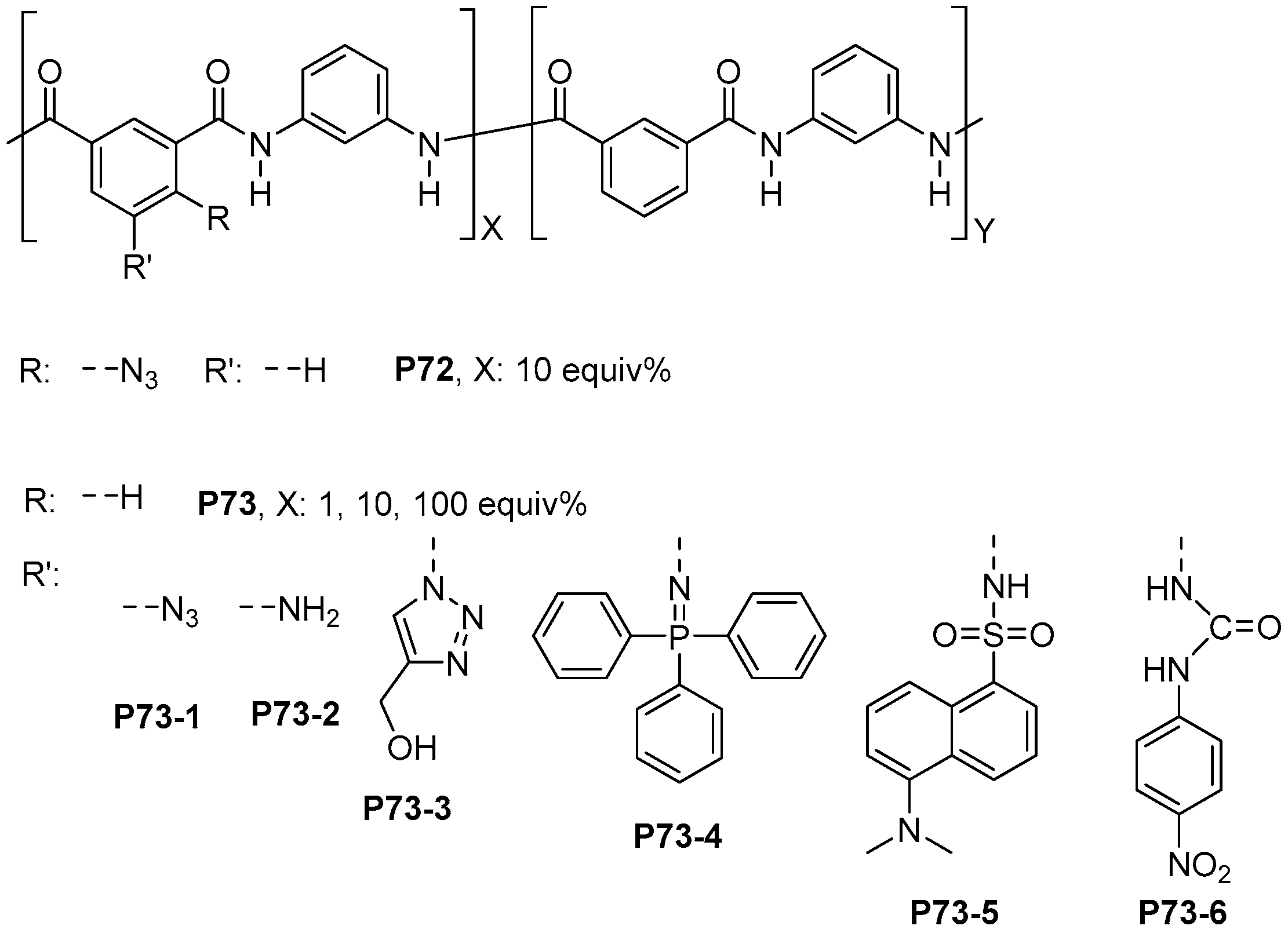
- Trigo-López, M.; Pablos, J.L.; García, F.C.; Serna, F.; García, J.M. Functional Aramids: Aromatic Polyamides with Reactive Azido and Amino Groups in the Pendant Structure. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1469–1477. [Google Scholar] [CrossRef]
- Karimi Zarchi, M.A.; Tayefi, M.; Tirgir, F.; Sabzalian, M.R. Synthesis and degradation study of novel polyamides derived from a biologically active aromatic diacid monomer 5-(2-thalimidoethanesulfonamido) isophthalic acid. J. Polym Res. 2012, 19, 9865–9872. [Google Scholar] [CrossRef]
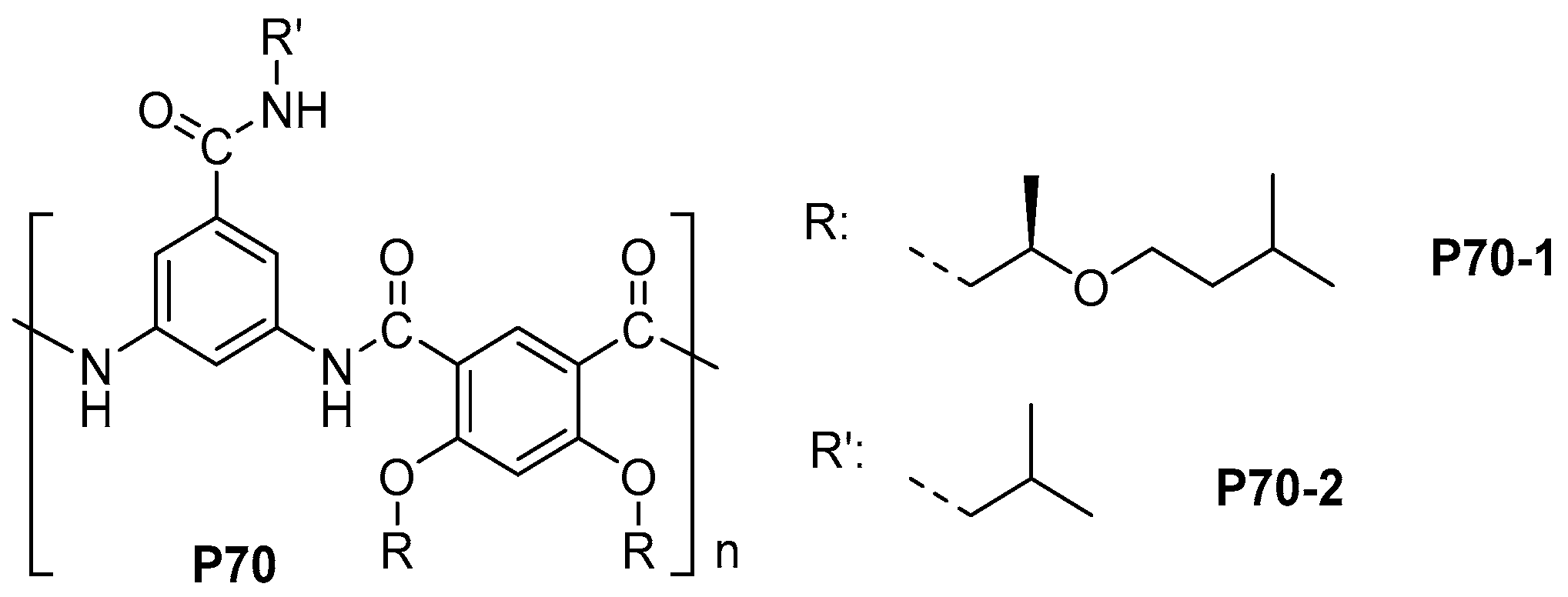
- Su, L.; Tao, L.; Wang, T.; Wang, Q. Phenylphosphine oxide-containing aromatic polyamide films with high atomic oxygen erosion resistance. Polym. Degrad. Stab. 2012, 97, 981–986. [Google Scholar] [CrossRef]
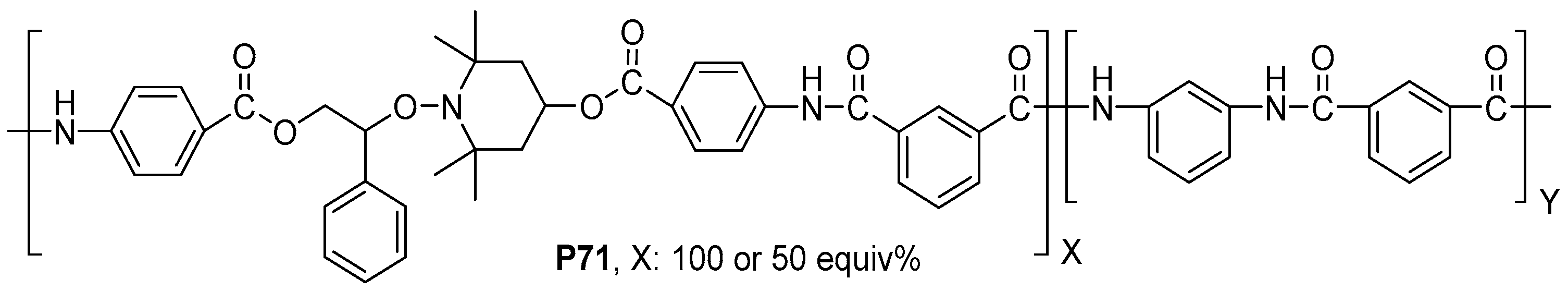
- Zhang, L.; Zhu, P.; Xu, H.; Zhong, Y.; Mao, Z. Novel Assemblies of Organo-Soluble Aromatic Polyamides Containing Copper(II) Coordination Complex Units in the Main Chain. J. Inorg. Organomet. Polym. 2013, 23, 546–552. [Google Scholar] [CrossRef]

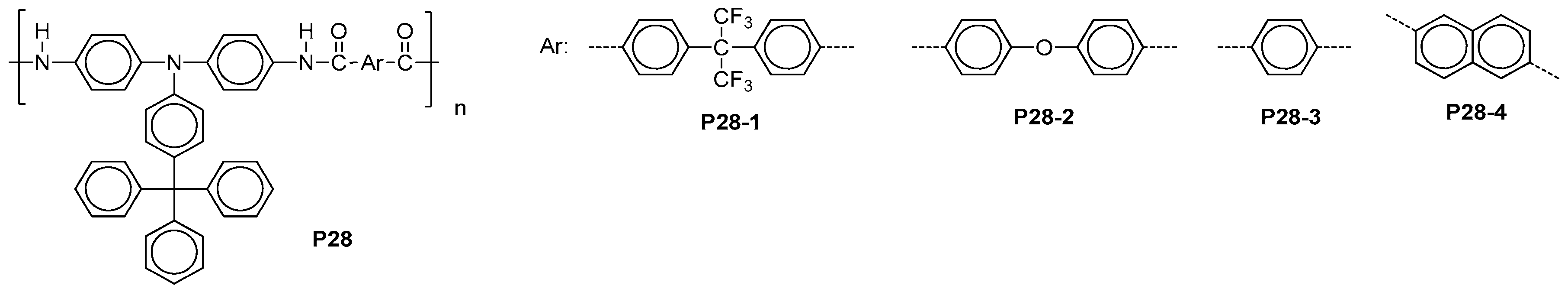
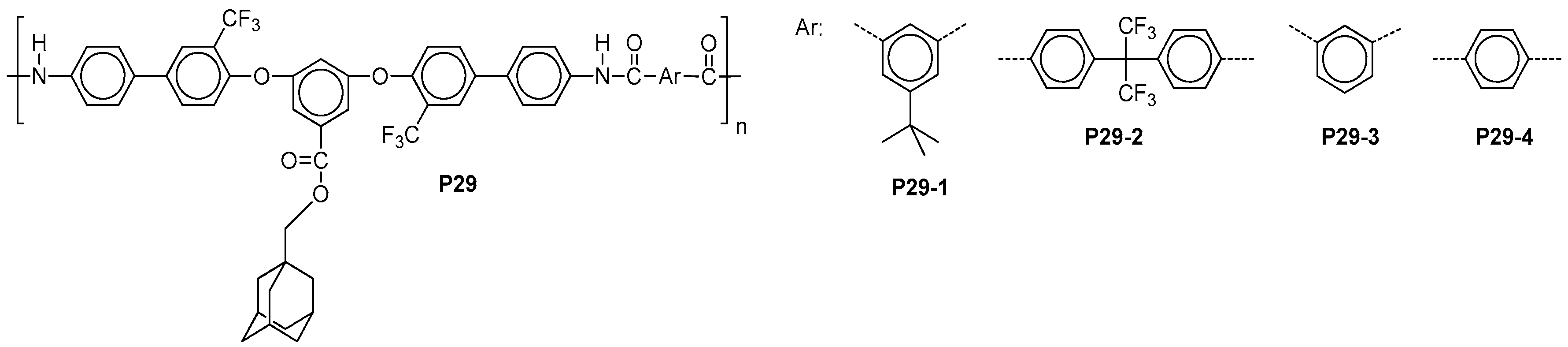
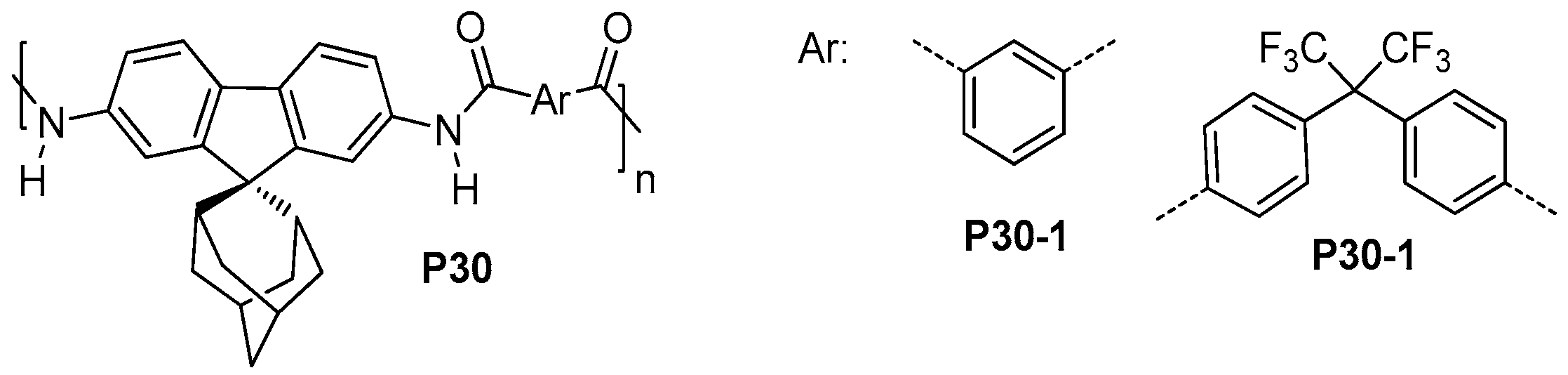
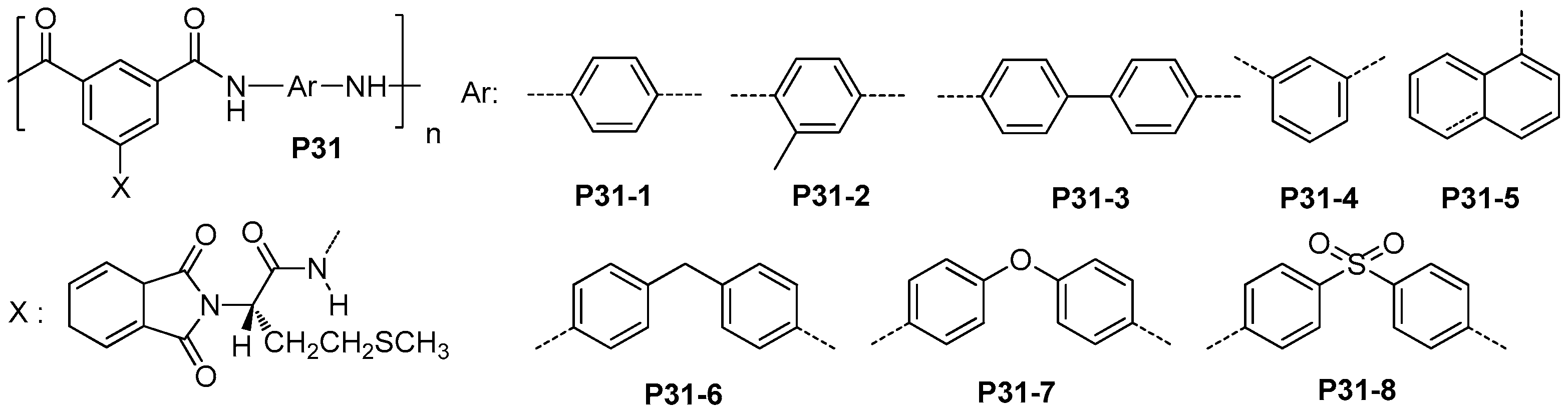
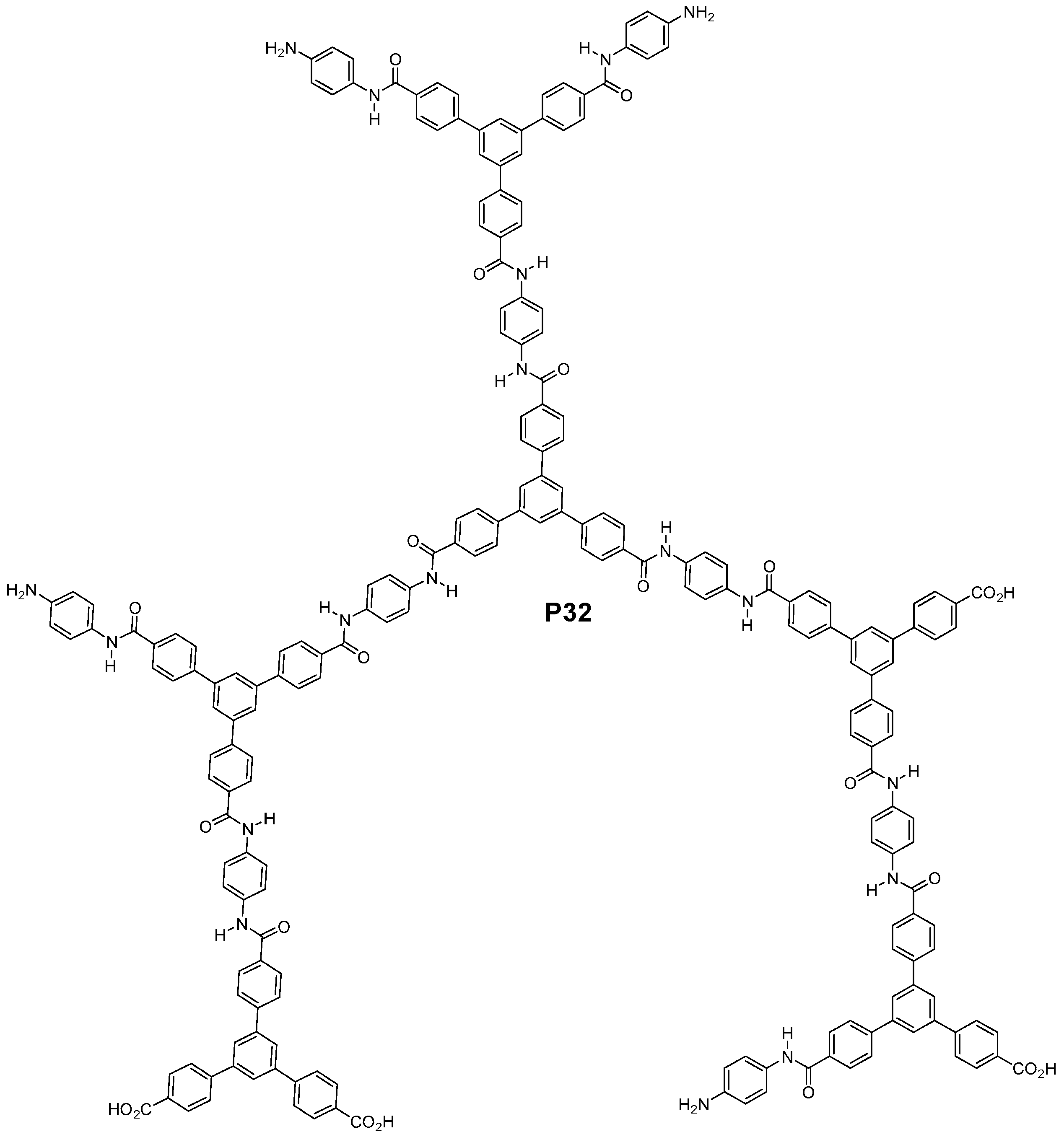
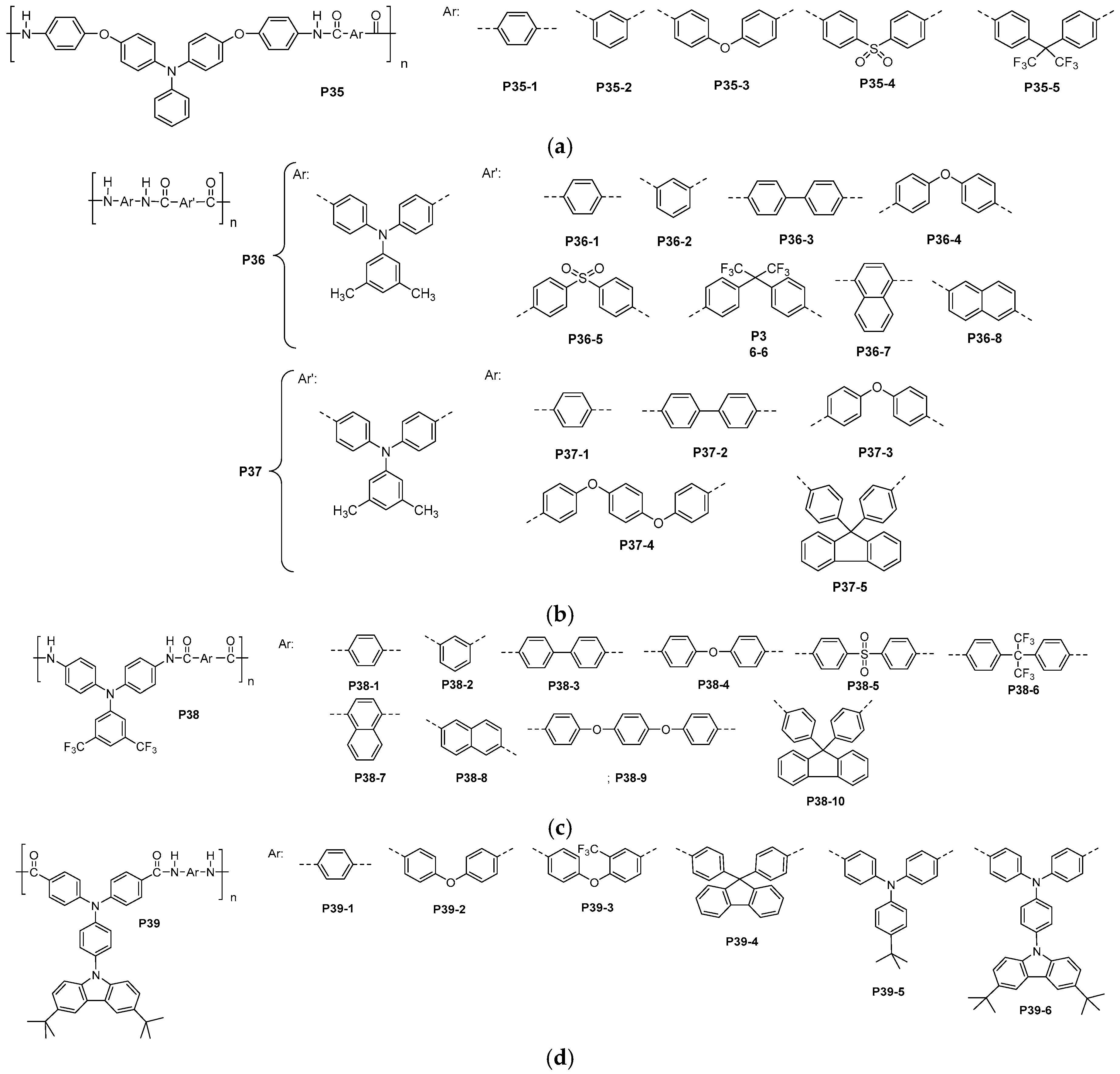
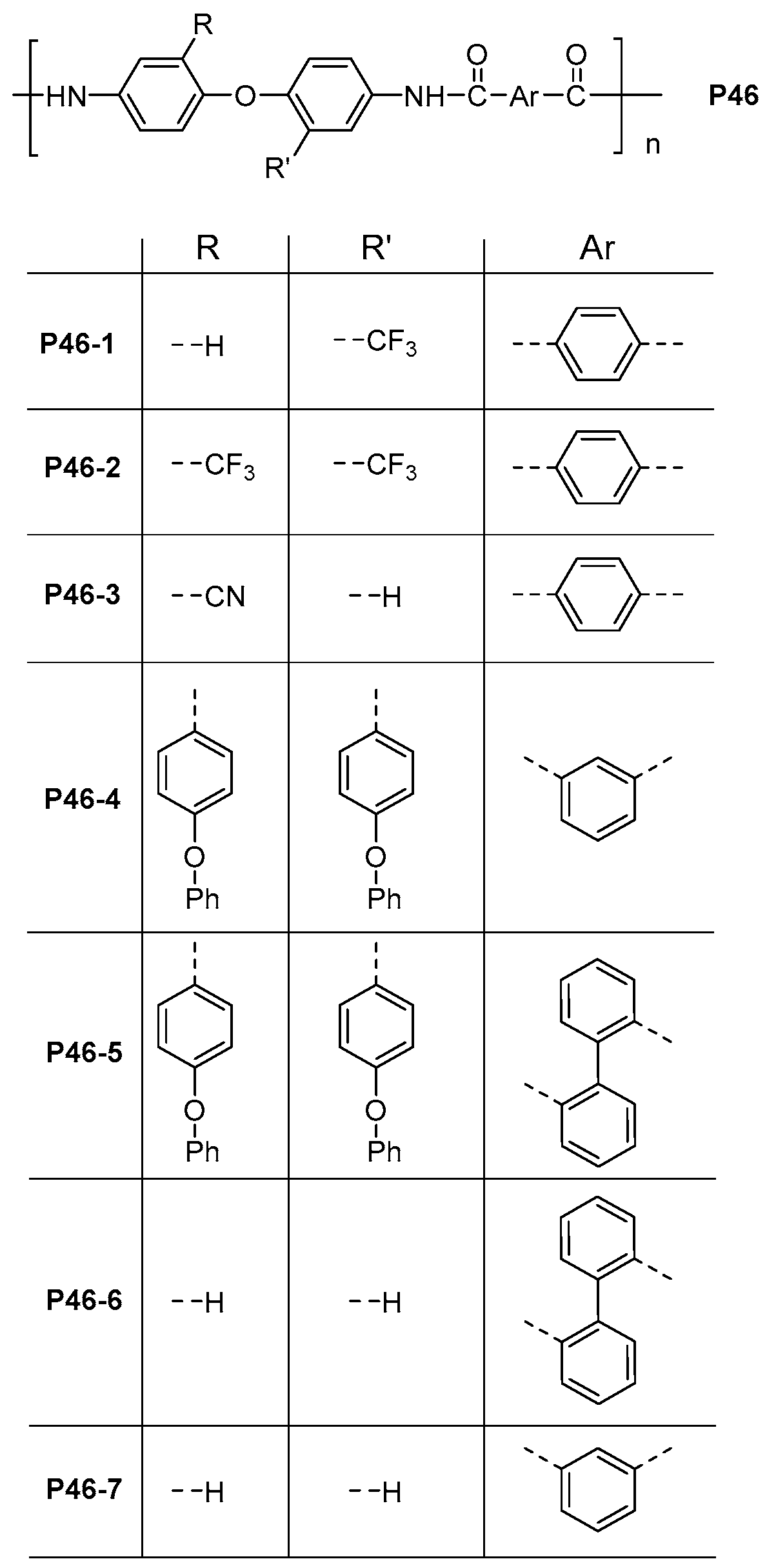
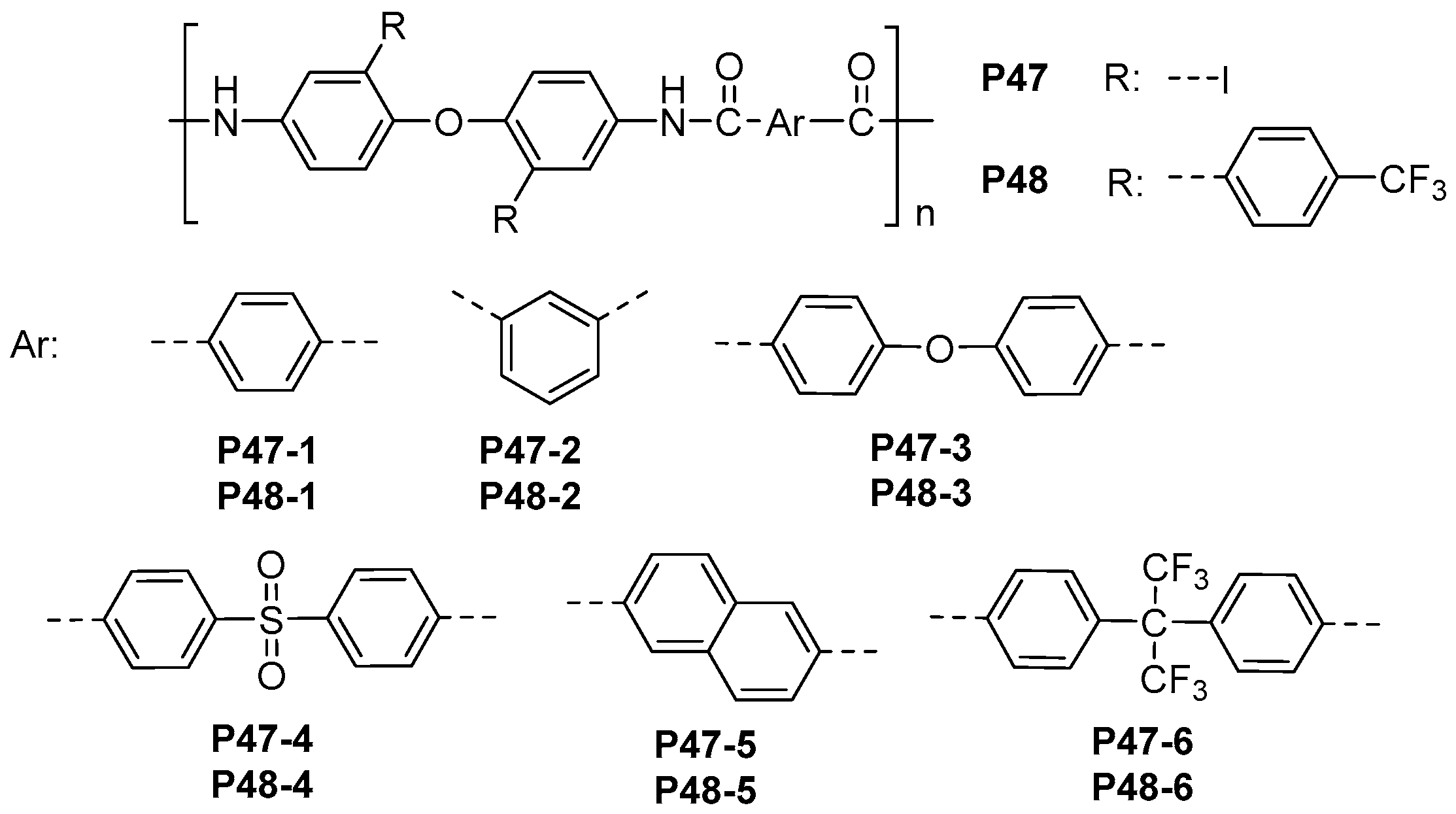
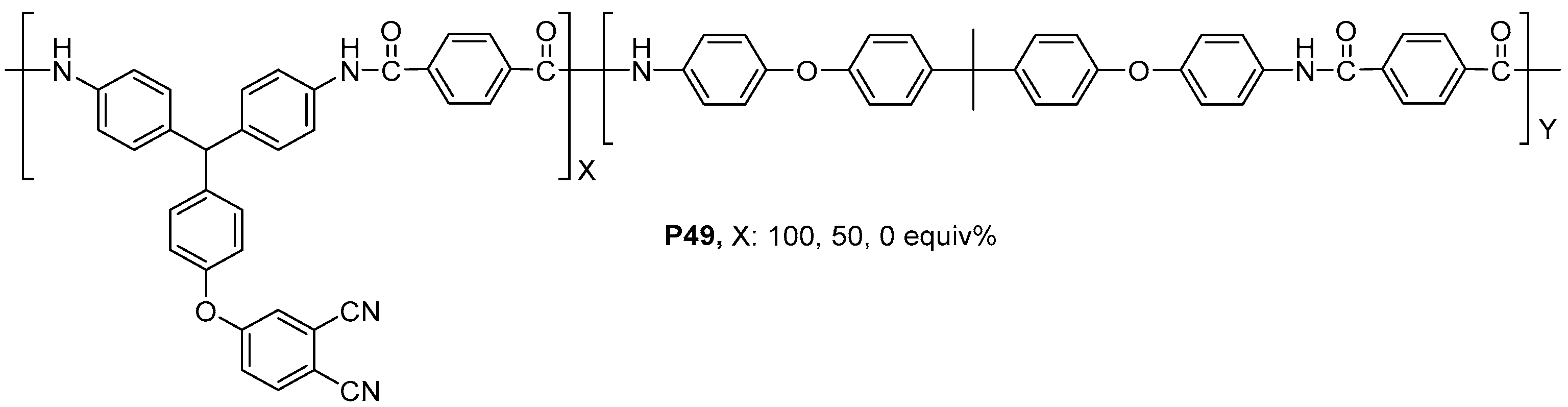
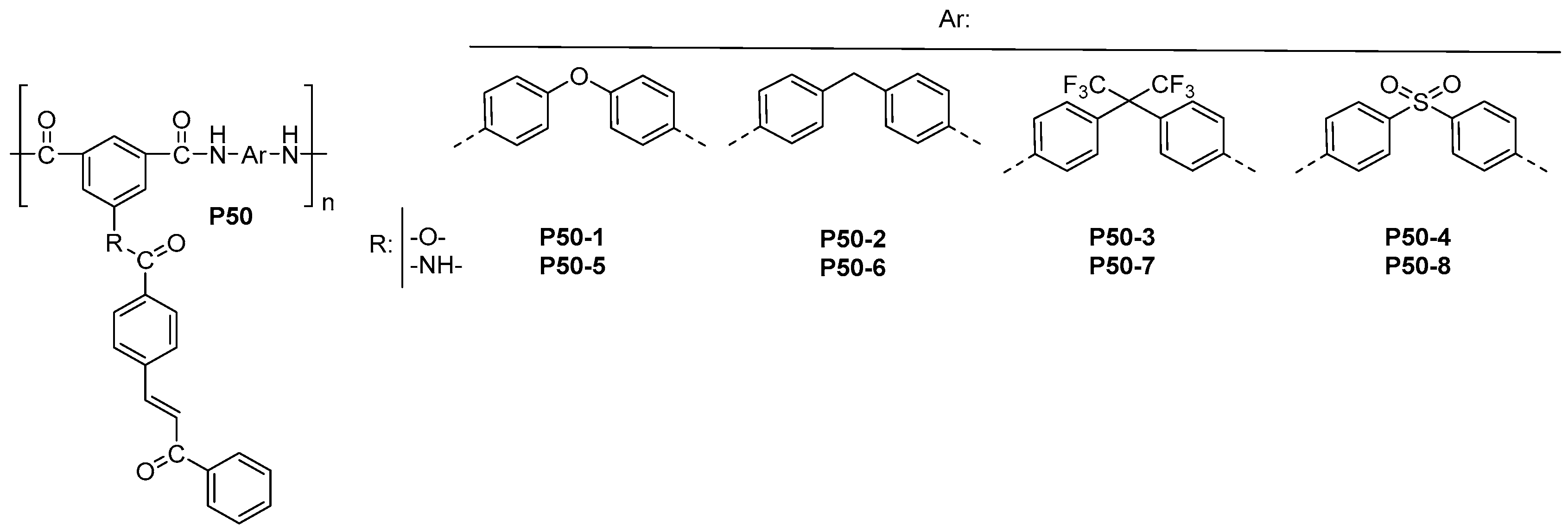
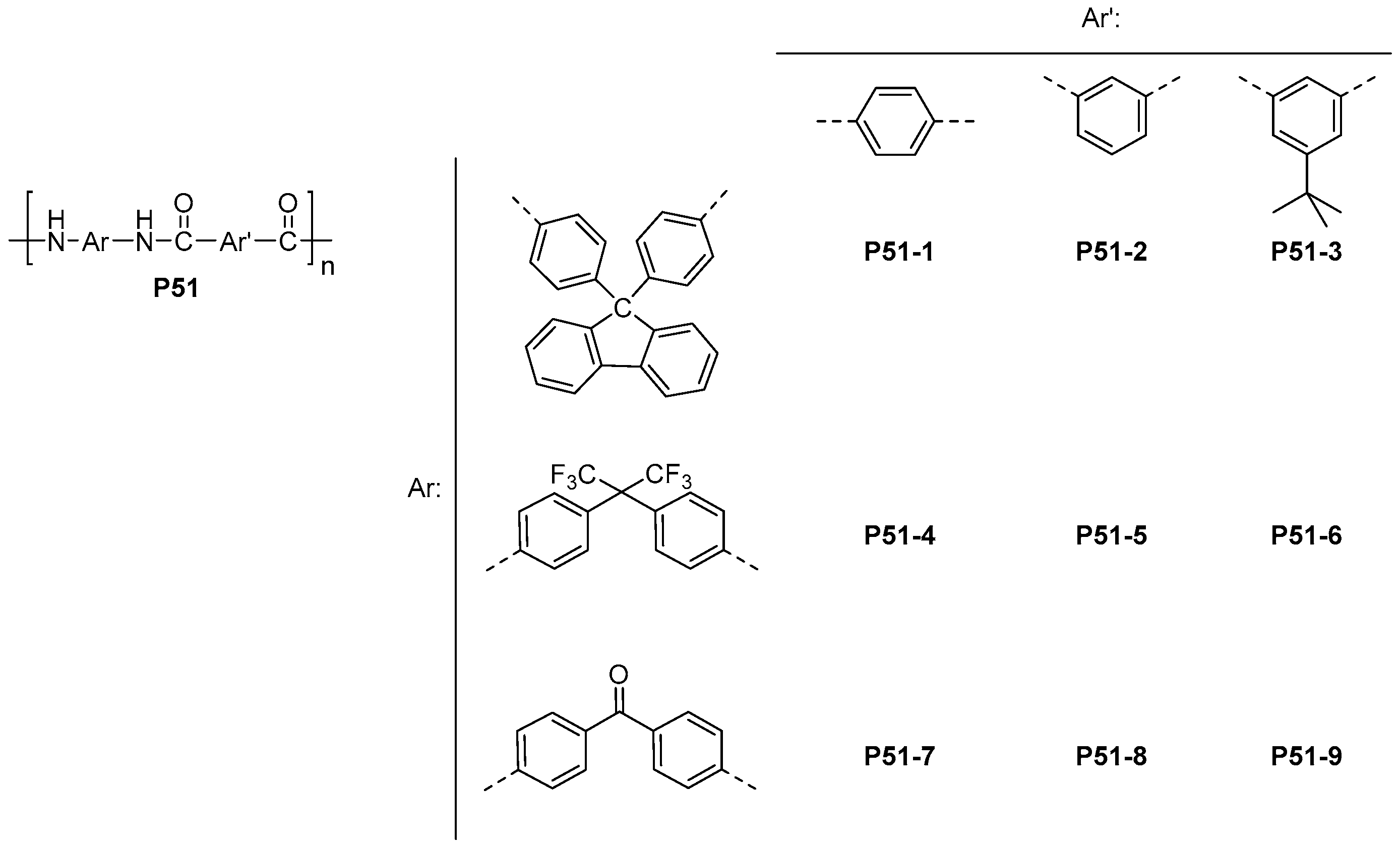

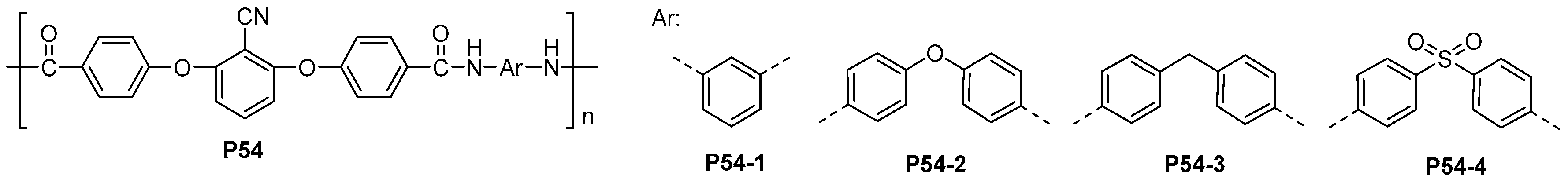
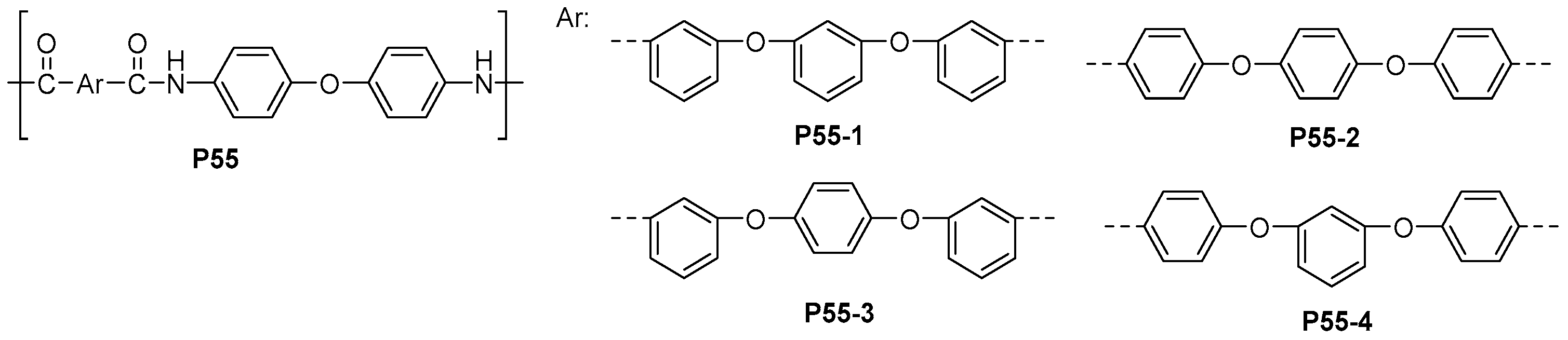
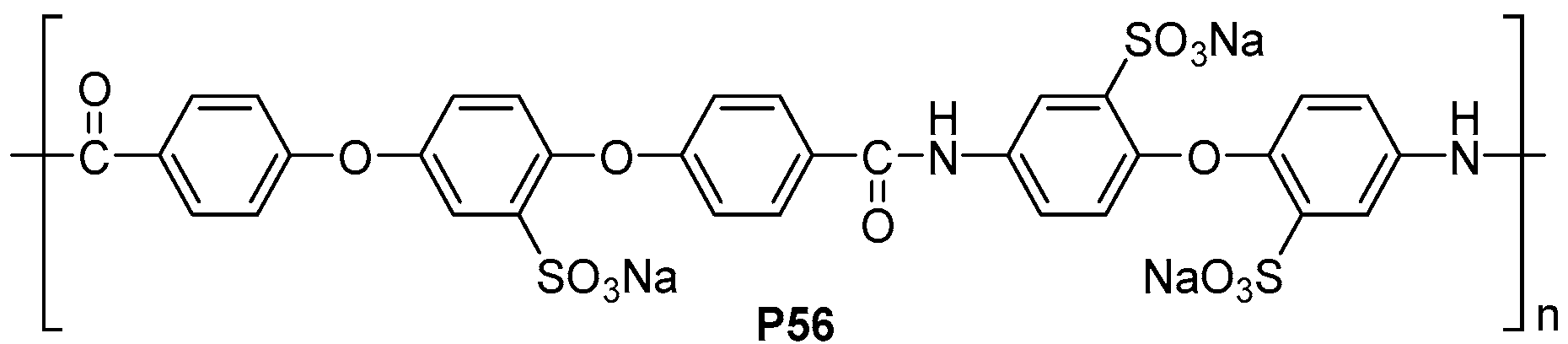

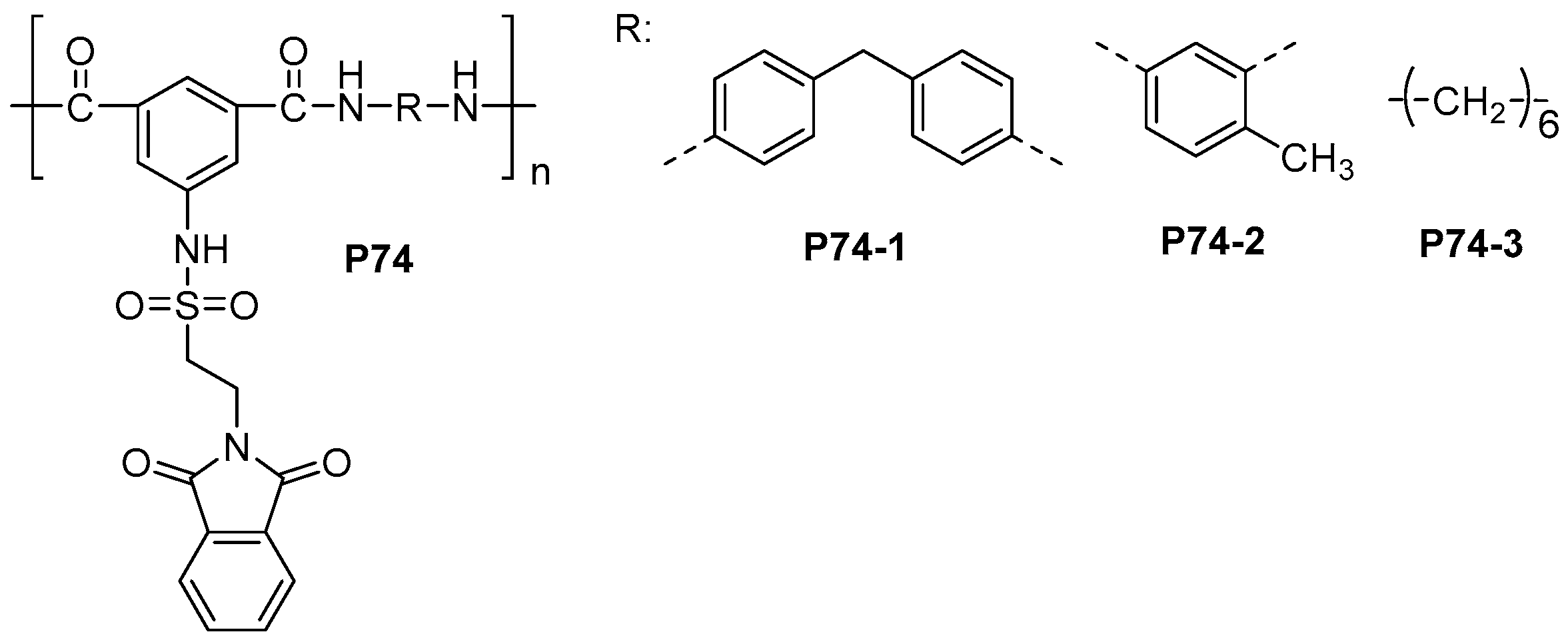
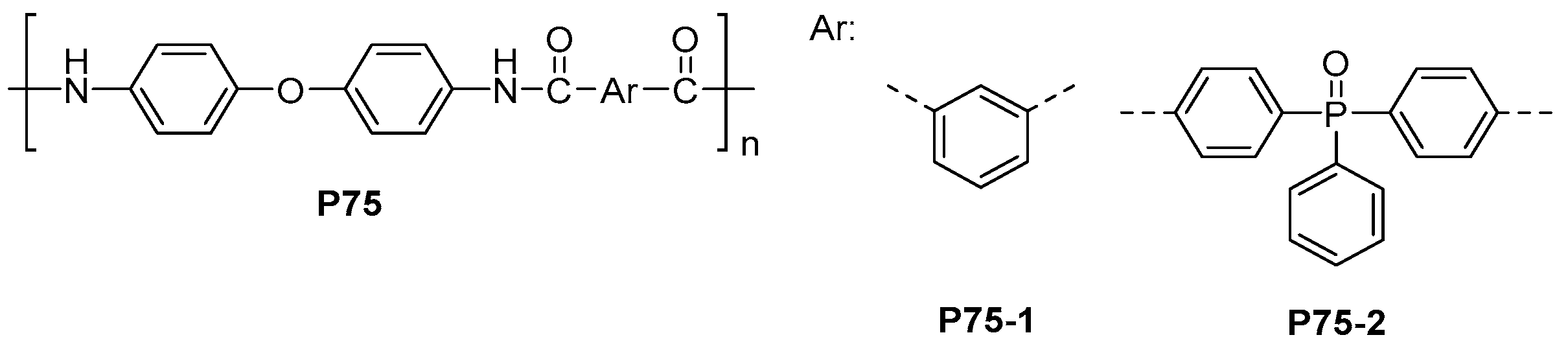
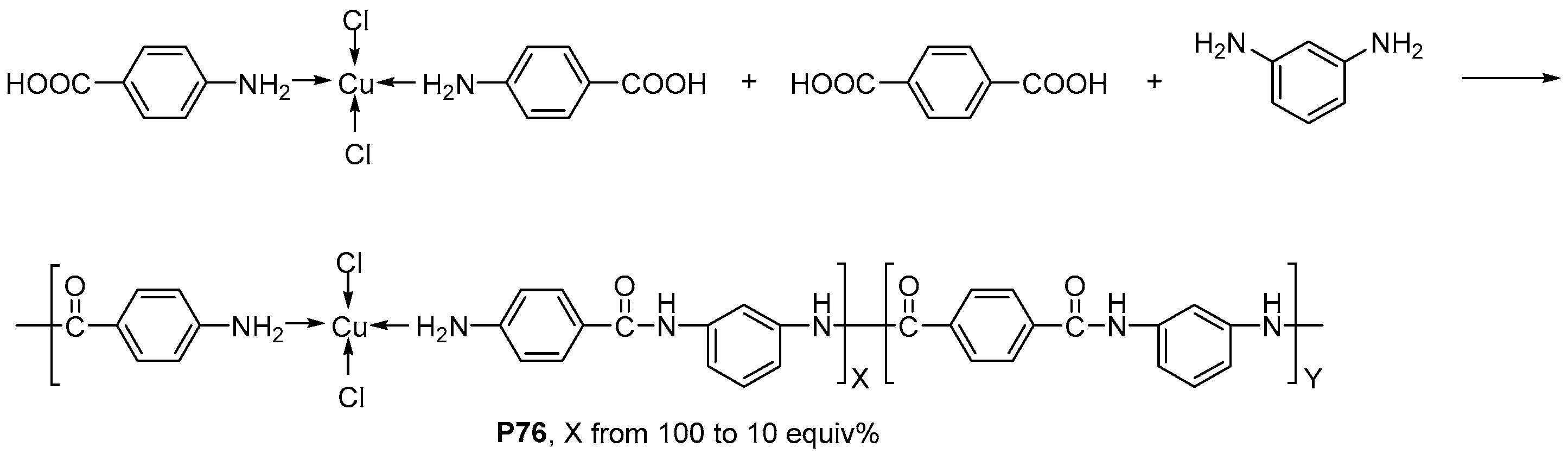
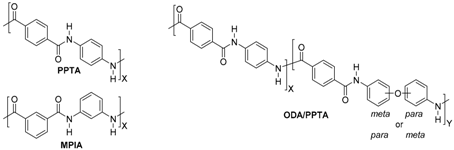 | ||||
|---|---|---|---|---|
| Aramid type | Brand names | Company | ||
| PPTA | MPIA | ODA/PPTA | ||
| X | Kevlar® | DuPont, Wilmington, DE, United States | ||
| X | Nomex® | |||
| X | Twaron® | Teijin, Arnhem, the Netherlands | ||
| X | Teijinconex® | |||
| X | Technora® | |||
| X | Heracron® | Kolon Industries, Seoul, Korea | ||
| X | Alkex® | Hyosung, Seoul, Korea | ||
| X | Meta Aramid Yarn & Thread® | SRO Group, Heatherbrae, New South Wales, Australia | ||
| X | Taparan® Para-aramid | Yantai Tayho Advanced Materials Co., Ltd, Yantai, Shandong, China | ||
| X | Newstar® Meta-Aramid | |||
| X | Arawin® | Toray Chemical Korea Inc., Seoul, Korea | ||
| X | Metaone | Huvis, Seoul, Korea | ||
| Reference | Tensile strength (MPa) | Young’s moduli (GPa) | Elongation at break (%) |
|---|---|---|---|
| [44] | 71–115 | 2.7–3.2 | 6–9 |
| [55] | 63.9–81.6 | – | 7.2–11.4 |
| [56] | 79–93 | 1.7–2.6 | 9–15 |
| [57] | Up to 88 | Up to 1.81 | Up to 25 |
| [58] | 82–106 | 2.0–2.8 | 10–25 |
| [59] | 86–109 | 2.15–2.63 | 13–22 |
| [60] | 72.5–87.3 | 2.35–2.87 | 5.3–9.5 |
| [61] | Up to 131 | Up to 3.5 | Up to 4.5 |
| Reference | Main electroactive group | Auxiliary EDG or EWG | Comments |
|---|---|---|---|
| [41] | triphenylamine, TPA | -- | hyperbranched |
| [115] | -- | ||
| [121] | 3,6-dimethoxycarbazole | ||
| [112,122] | 3,6-di-tert-butylcarbazole | ||
| [96,101,103] | methyl, trifluoromethyl | ||
| [104] | 4-tert-butyl | ||
| [99,116,117,118] | 2,4-dimethoxy | ||
| [102] | adamantylphenoxy | ||
| [105] | -- | ether-linked bis(triphenylamine) | |
| [106] | piperidinyl | ||
| [108] | morpholinyl | ||
| [97] | TPA (pendant) | -- | |
| [107,109,113] | TPA or carbazole | electrompolymerization | |
| [110,124,125] | diphenylpyrenylamine | ||
| [98] | carbazole | methoxy | |
| [114] | carbazole (pendant) | ||
| [119,120,123] | N,N,N′,N′-tetraphenyl-1,4-phenylenediamine, TPPA | tert-butyl | |
| [111] | methoxy | ||
| [100] | bis(diphenylamino)naphthalene | metoxy | |
| [126] | diphenylfluorenylamine | methyl | |
| [127] | aniline pentamer/azo group |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reglero Ruiz, J.A.; Trigo-López, M.; García, F.C.; García, J.M. Functional Aromatic Polyamides. Polymers 2017, 9, 414. https://doi.org/10.3390/polym9090414
Reglero Ruiz JA, Trigo-López M, García FC, García JM. Functional Aromatic Polyamides. Polymers. 2017; 9(9):414. https://doi.org/10.3390/polym9090414
Chicago/Turabian StyleReglero Ruiz, José A., Miriam Trigo-López, Félix C. García, and José M. García. 2017. "Functional Aromatic Polyamides" Polymers 9, no. 9: 414. https://doi.org/10.3390/polym9090414