Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review
Abstract
:1. Introduction
2. Epoxy as Matrix
3. Graphene as Reinforcement
4. Fracture Toughness
5. Structure and Fracture Toughness
6. Surface Area and Fracture Toughness
7. Weight Fraction and Fracture Toughness
8. Dispersion State and Fracture Toughness
9. Functionalization and Fracture Toughness
10. Crosslink Density and Fracture Toughness
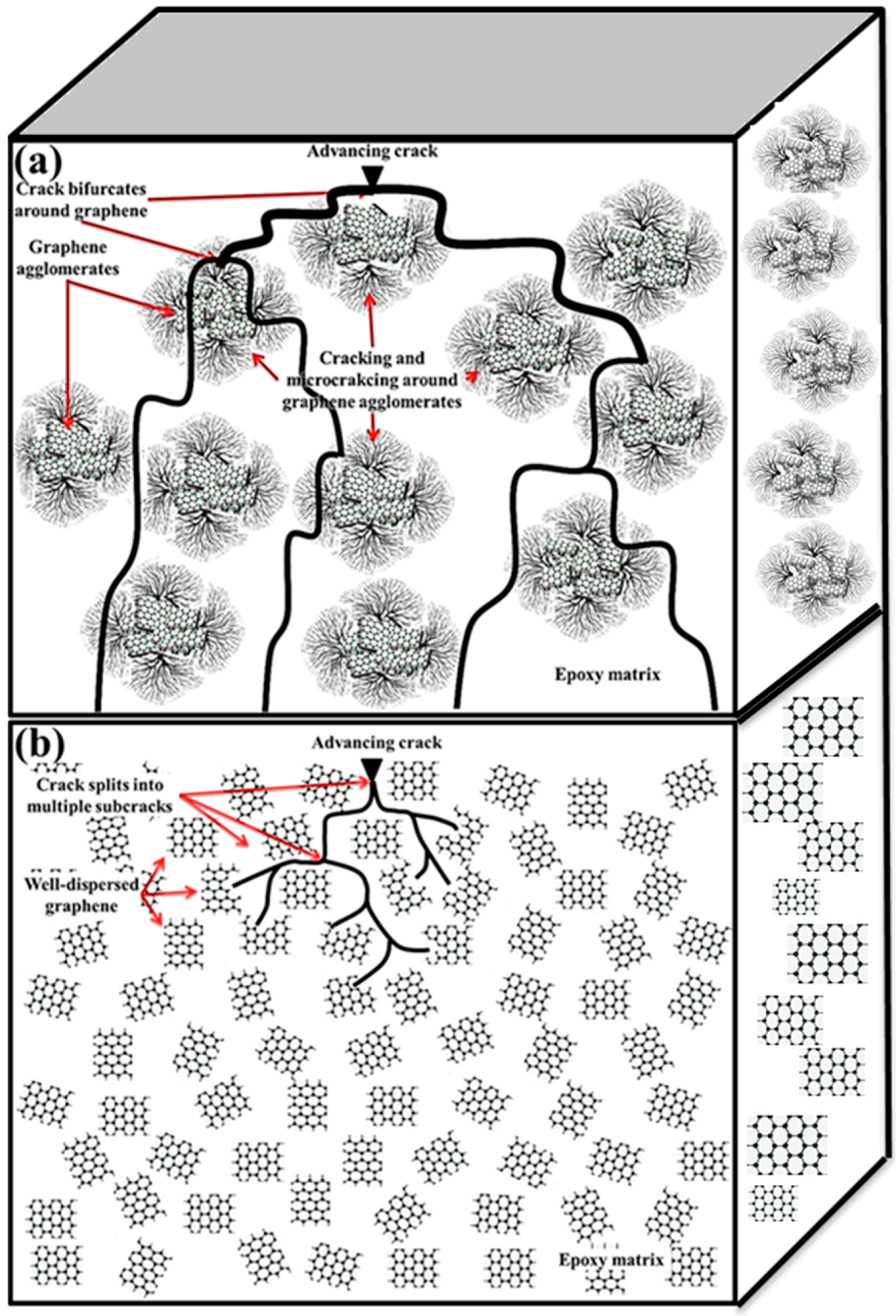
11. Fracture Patterns
12. Other Mechanical Properties
13. Thermal Properties
14. Electrical Properties
15. Conclusions
- Epoxy is an excellent matrix for graphene composites because of its efficient properties such as enhancement in composite mechanical properties, processing flexibility, and acceptable cost [2].
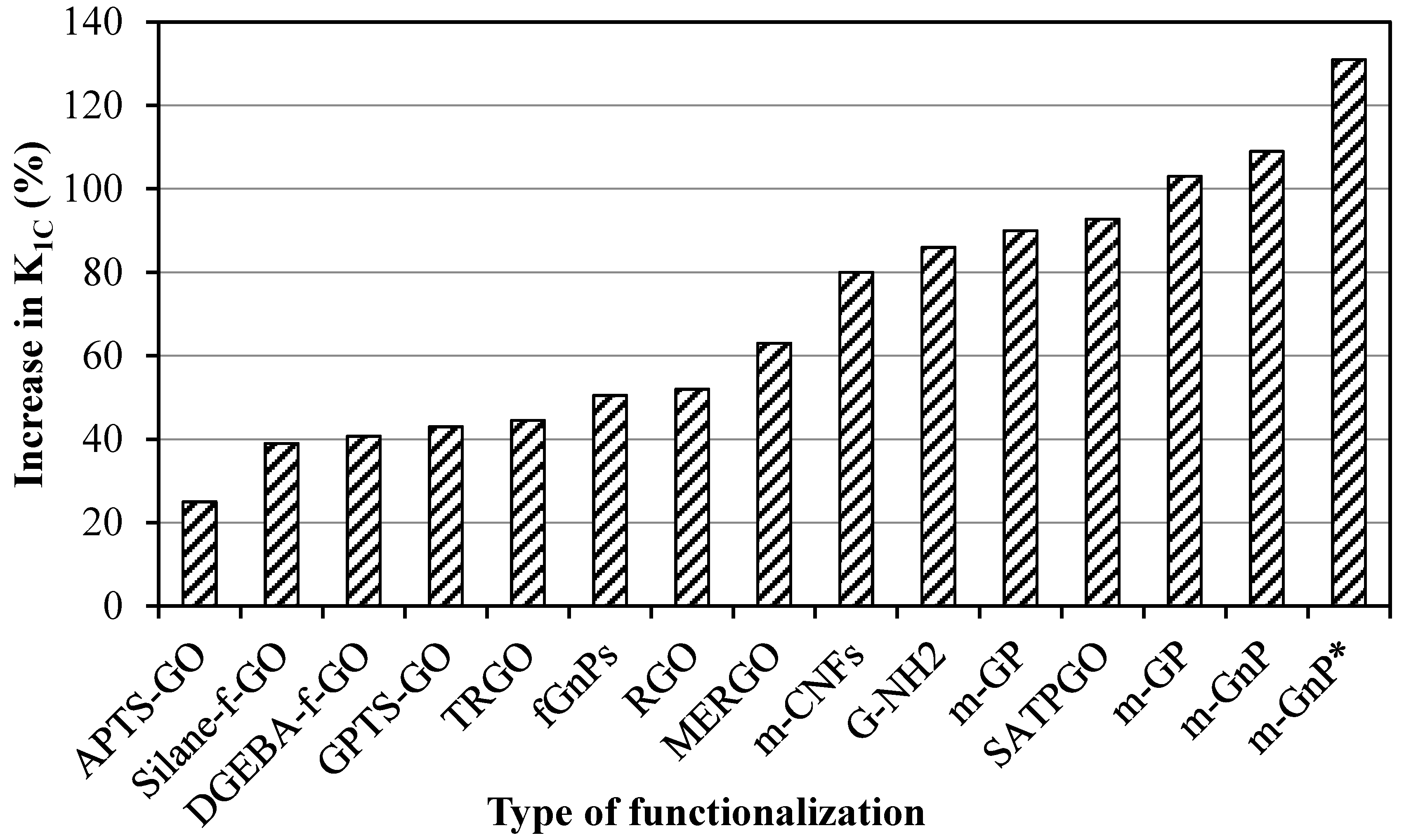
- Graphene can significantly enhance the fracture toughness of epoxy nanocomposites—i.e., up to 131% [59]. When epoxy is reinforced with graphene, the carbonaceous sheets shackle the crack and restrict its advancement. This obstruction and deflection of the crack by the graphene at the interface is the foremost mechanism of raising the fracture toughness of nanocomposites.
- The graphene sheets with smaller length, width, and thickness are more efficient in improving the fracture toughness than those with larger dimensions [57]. Large graphene sheets have a high stress concentration factor, because of which crack generation becomes easy in the epoxy matrix [118,119]. The cracks deteriorate the efficiency of graphene in enhancing the fracture toughness of epoxy/graphene nanocomposites.
- Uniformly dispersed graphene improves fracture toughness significantly as compared to the poorly dispersed graphene [72]. It is evident from the published literature that the fracture toughness dropped when graphene weight fraction was increased beyond 1 wt %. The decrease in fracture toughness with higher weight fraction of graphene can be correlated with the dispersion state of graphene. As graphene weight fraction increases beyond 1 wt %, the dispersion state becomes inferior.
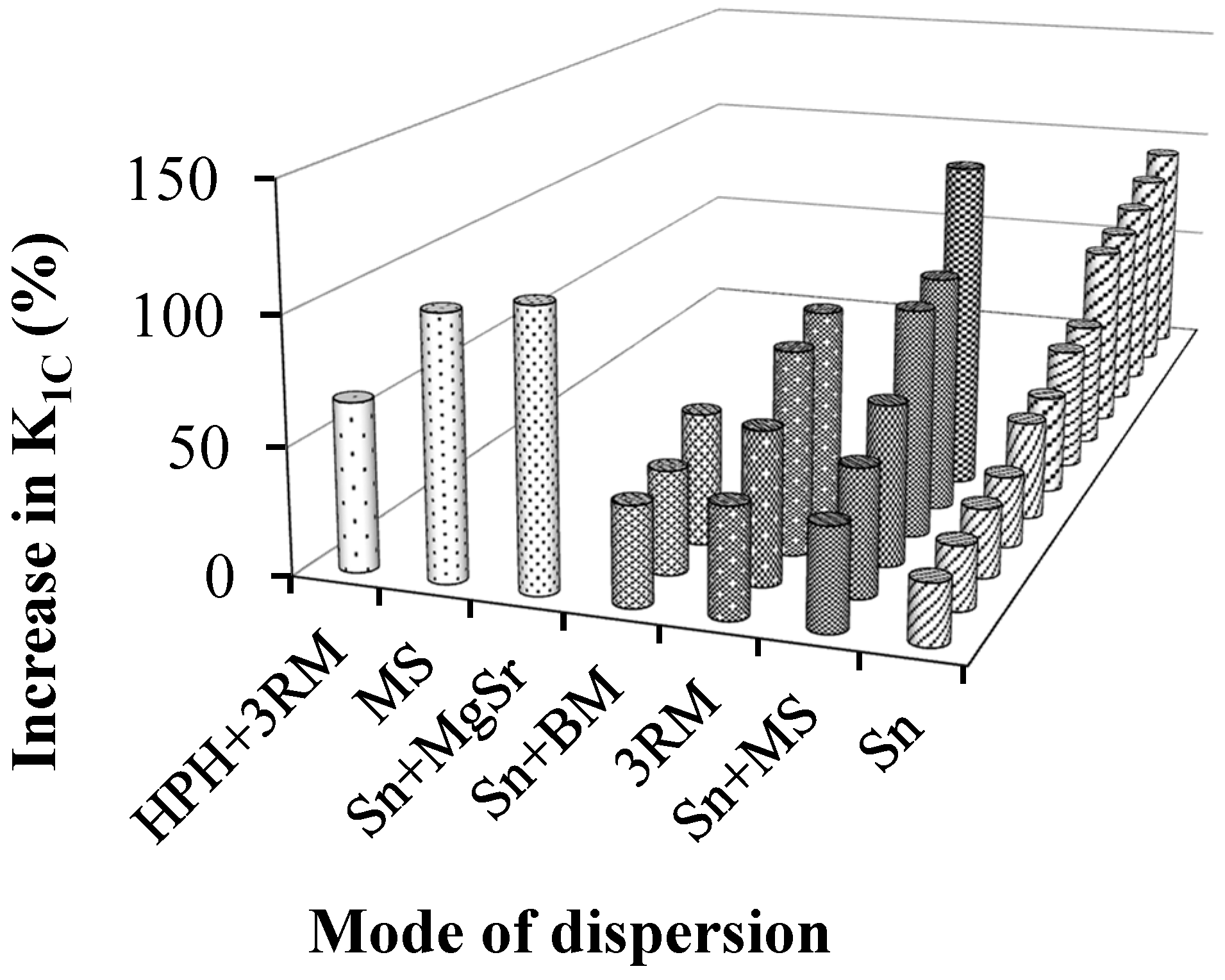
- Three roll milling or calendering process is an efficient way of dispersing the reinforcement into a polymer matrix, as it involves high shear forces [244,245,246,247,248]. However, the maximum enhancement in fracture toughness was achieved with a combination of sonication and mechanical stirring [59].
- In thermosetting materials such as epoxy, high crosslink density is desirable for improved mechanical properties. However, fracture toughness is dropped with high crosslinking [57].
- The literature has proved the absence of consensus of graphene’s role in improving the mechanical properties of nanocomposites [150,151,152,153,154]. Generally, graphene acts as panacea and raises the mechanical properties [116,155,156,157,158]. On the contrary, it acts as placebo and shows no effect on mechanical properties. Even worse, it is inimical and razes the mechanical properties [160,161,162,163,164]. The main factors that dictate graphene’s influence on the mechanical properties of epoxy nanocomposites include topographical features, morphology, weight fraction, dispersion state, surface modifications, and interfacial interactions.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 3DGN | Three dimensional graphene network |
| 3RM | Three roll milling |
| A | Aramid fibers |
| APTS-GO | Amino-functionalized graphene oxide (GO) |
| ATGO | 3-Aminopropyltriethoxysilane functionalized silica nanoparticles attached GO |
| ATP | Attapulgite |
| ATS | 3-amino functionalized silica nanoparticles |
| BM | Ball milling |
| CM | Centrifugal mixing |
| CNF | Carbon nanofiber |
| CNFs | Vapor grown carbon nanofibers |
| CNTs | Carbon nanotubes |
| DDS | Diaminodiphenylsulfone |
| DGEBA-f-GO | Diglycidyl ether of bisphenol-A functionalized GO |
| DRA | Discontinuously reinforced aluminum |
| DRTi | Discontinously reinforced titanium |
| EGNPs | Amine functionalized expanded graphene nanoplatelets |
| EMCs | Epoxy matrix composites |
| fGnPs | Polybenzimidazole functionalized graphene platelets (GnPs) |
| GF | Graphene foam |
| G-NH2 | Amino-functionalized GNPs |
| GnPs | Graphene platelets |
| GNPs* | Graphite nanoplatelets |
| GNs | Amine functionalized graphene sheets |
| GNSs | Graphene nanosheets |
| GO | Graphene oxide |
| GP | Graphite particles |
| GPLs | Graphene nanoplatelets |
| GPTS-GO | Epoxy functionalized GO |
| G-Si | Silane modified GNPs |
| HPH + 3RM | High pressure homogenizer + three roll milling |
| HSM | High speed mixing |
| m-clay | Surface modified nano clay |
| m-CNFs | Triazole functionalized carbon nanofibers |
| MERGO | Microwave exfoliated reduced graphene oxide |
| m-GnP | Surface modified GnP |
| m-GnP* | Surfactant modified graphene platelets |
| m-GP | Surface modified graphene platelets |
| MgSr | Magnetic stirring |
| MLG | Multi-layer graphene |
| MS | Mechanical stirring |
| MS + USn | Mechanical stirring + Ultrasonication |
| MWCNTs | Multi-walled carbon nanotubes |
| MWNTs | Multi-walled carbon nanotubes |
| ND | Nanodiamond |
| P | Polyacrylonitrile (PAN) fibers |
| p-CNFs | Pristine carbon nanofibers |
| PEA | Polyetheramine |
| phr | Per hundred parts of resin |
| PMCs | Polymer matrix composites |
| Q/I | Quasi-isotropic |
| RGO | Thermally reduced graphene oxide |
| SA | Surface area |
| SATPGO | 3-Aminopropyltriethoxysilane modified silica nanoparticles attached graphene oxide |
| SCFs | Short carbon fibers |
| ShM | Shear mixing |
| Silane-f-GO | Silane functionalized GO |
| SM | Speed mixing |
| Sn | Sonication |
| Sn + BM | Sonication + Ball milling |
| Sn + MgSr | Sonication + Magnetic stirring |
| Sn + MS | Sonication + Mechanical stirring |
| SnP | Silver nanoparticles |
| SnW | Silver nanowires |
| SWCNTs | Single-walled carbon nanotubes |
| SWNTs | Single-walled carbon nanotubes |
| TEM | Transmission electron microscopy |
| TPE | Two phase extraction |
| UG | Unmodified graphene nanoplatelets |
| U-GnP | Unmodified graphene platelets |
| USn | Ultrasonication |
References
- Carlson, R.L.; Kardomateas, G.A.; Craig, J.I. Mechanics of Failure Mechanisms in Structures, 1st ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Miracle, D.B.; Donaldson, S.L. (Eds.) ASM Handbook Volume 21: Composites; ASM International: Materials Park, OH, USA, 2001.
- Yao, X.F.; Zhou, D.; Yeh, H.Y. Macro/microscopic fracture characterizations of SiO2/epoxy nanocomposites. Aerosp. Sci. Technol. 2008, 12, 223–230. [Google Scholar] [CrossRef]
- Wetzel, B.; Rosso, P.; Haupert, F.; Friedrich, K. Epoxy nanocomposites—Fracture and toughening mechanisms. Eng. Fract. Mech. 2006, 73, 2375–2398. [Google Scholar] [CrossRef]
- Naous, W.; Yu, X.Y.; Zhang, Q.X.; Naito, K.; Kagawa, Y. Morphology, tensile properties, and fracture toughness of epoxy/Al2O3 nanocomposites. J. Polym. Sci. Part B 2006, 44, 1466–1473. [Google Scholar] [CrossRef]
- Kim, B.C.; Park, S.W.; Lee, D.G. Fracture toughness of the nano-particle reinforced epoxy composite. Compos. Struct. 2008, 86, 69–77. [Google Scholar] [CrossRef]
- Wang, K.; Chen, L.; Wu, J.; Toh, M.L.; He, C.; Yee, A.F. Epoxy nanocomposites with highly exfoliated clay: Mechanical properties and fracture mechanisms. Macromolecules 2005, 38, 788–800. [Google Scholar] [CrossRef]
- Liu, W.; Hoa, S.V.; Pugh, M. Fracture toughness and water uptake of high-performance epoxy/nanoclay nanocomposites. Compos. Sci. Technol. 2005, 65, 2364–2373. [Google Scholar] [CrossRef]
- Gojny, F.H.; Wichmann, M.H.G.; Köpke, U.; Fiedler, B.; Schulte, K. Carbon nanotube-reinforced epoxy-composites: Enhanced stiffness and fracture toughness at low nanotube content. Compos. Sci. Technol. 2004, 64, 2363–2371. [Google Scholar] [CrossRef]
- Yu, N.; Zhang, Z.H.; He, S.Y. Fracture toughness and fatigue life of MWCNT/epoxy composites. Mater. Sci. Eng. A 2008, 494, 380–384. [Google Scholar] [CrossRef]
- Srikanth, I.; Kumar, S.; Kumar, A.; Ghosal, P.; Subrahmanyam, C. Effect of amino functionalized MWCNT on the crosslink density, fracture toughness of epoxy and mechanical properties of carbon-epoxy composites. Compos. Part. A Appl. Sci. Manuf. 2012, 43, 2083–2086. [Google Scholar] [CrossRef]
- Mathews, M.J.; Swanson, S.R. Characterization of the interlaminar fracture toughness of a laminated carbon/epoxy composite. Compos. Sci. Technol. 2007, 67, 1489–1498. [Google Scholar] [CrossRef]
- Arai, M.; Noro, Y.; Sugimoto, K.I.; Endo, M. Mode-I and mode II interlaminar fracture toughness of CFRP laminates toughened by carbon nanofiber interlayer. Compos. Sci. Technol. 2008, 68, 516–525. [Google Scholar] [CrossRef]
- Wong, D.W.Y.; Lin, L.; McGrail, P.T.; Peijs, T.; Hogg, P.J. Improved fracture toughness of carbon fibre/epoxy composite laminates using dissolvable thermoplastic fibres. Compos. Part. A 2010, 41, 759–767. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Truong, Q.-T.; Lee, D.S. Multi-step microwave reduction of graphite oxide and its use in the formation of electrically conductive graphene/epoxy composites. Compos. Part B 2014, 64, 187–193. [Google Scholar] [CrossRef]
- Tian, M.; Qu, L.; Zhang, X.; Zhang, K.; Zhu, S.; Guo, X.; Han, G.; Tang, X.; Sun, Y. Enhanced mechanical and thermal properties of regenerated cellulose/graphene composite fibers. Carbohydr. Polym. 2014, 111, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Shan, M.; Li, Y.; Li, B.; Niu, J.; Zhou, B.; Qian, X. Organosilane-functionalized graphene oxide for enhanced antifouling and mechanical properties of polyvinylidene fluoride ultrafiltration membranes. J. Membr. Sci. 2014, 458, 1–13. [Google Scholar] [CrossRef]
- Bkakri, R.; Sayari, A.; Shalaan, E.; Wageh, S.; Al-Ghamdi, A.A.; Bouazizi, A. Effects of the graphene doping level on the optical and electrical properties of ITO/P3HT:Graphene/Au organic solar cells. Superlattices Microstruct. 2014, 76, 461–471. [Google Scholar] [CrossRef]
- Lian, Y.; He, F.; Wang, H.; Tong, F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of staphylococcus aureus. Biosens. Bioelectron. 2014, 65, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Abdin, Z.; Alim, M.A.; Saidur, R.; Islam, M.R.; Rashmi, W.; Mekhilef, S.; Wadi, A. Solar energy harvesting with the application of nanotechnology. Renew. Sustain. Energy Rev. 2013, 26, 837–852. [Google Scholar] [CrossRef]
- Sun, W.; Hu, R.; Liu, H.; Zeng, M.; Yang, L.; Wang, H.; Zhu, M. Embedding nano-silicon in graphene nanosheets by plasma assisted milling for high capacity anode materials in lithium ion batteries. J. Power Sources 2014, 268, 610–618. [Google Scholar] [CrossRef]
- Azeez, A.A.; Rhee, K.Y.; Park, S.J.; Hui, D. Epoxy clay nanocomposites—Processing, properties and applications: A review. Compos. Part. B 2013, 45, 308–320. [Google Scholar] [CrossRef]
- Aziz, A.; Lim, H.N.; Girei, S.H.; Yaacob, M.H.; Mahdi, M.A.; Huang, N.M.; Pandikumar, A. Silver/graphene nanocomposite-modified optical fiber sensor platform for ethanol detection in water medium. Sens. Actuators B Chem. 2015, 206, 119–125. [Google Scholar]
- Agnihotri, N.; Chowdhury, A.D.; De, A. Non-enzymatic electrochemical detection of cholesterol using β-cyclodextrin functionalized graphene. Biosens. Bioelectron. 2015, 63, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Galpaya, D.; Wang, M.; Liu, M.; Motta, N.; Waclawik, E.; Yan, C. Recent Advances in fabrication and characterization of graphene-polymer nanocomposites. Sci. Res. 2012, 2012, 30–49. [Google Scholar] [CrossRef]
- Shahil, K.M.F.; Balandin, A.A. Thermal properties of graphene and multilayer graphene: Applications in thermal interface materials. Solid State Commun. 2012, 152, 1331–1340. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Sanjinés, R.; Abad, M.D.; Vâju, C.; Smajda, R.; Mionić, M.; Magrez, A. Electrical properties and applications of carbon based nanocomposite materials: An overview. Surf. Coat. Technol. 2011, 206, 727–733. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer (Guildf) 2011, 52, 5–25. [Google Scholar] [CrossRef]
- Qin, F.; Brosseau, C. A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J. Appl. Phys. 2012. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Comprehensive review on synthesis and adsorption behaviors of graphene-based materials. Carbon Lett. 2012, 13, 73–87. [Google Scholar] [CrossRef]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene-based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Van Rooyen, L.J.; Karger-Kocsis, J.J.; Kock, L.D.; David Kock, L. Improving the helium gas barrier properties of epoxy coatings through the incorporation of graphene nanoplatelets and the influence of preparation techniques. J. Appl. Polym. Sci. 2015, 42584, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Abdala, A.A.; Macosko, C.W. Graphene/polymer nanocomposites. Macromolecules 2010, 43, 6515–6530. [Google Scholar] [CrossRef]
- Dhand, V.; Rhee, K.Y.; Kim, H.J.; Jung, D.H. A comprehensive review of graphene nanocomposites: research status and trends. J. Nanomater. 2015, 2013, 1–15. [Google Scholar] [CrossRef]
- Santamaria, A.; Muñoz, M.E.; Fernández, M.; Landa, M. Electrically conductive adhesives with a focus on adhesives that contain carbon nanotubes. J. Appl. Polym. Sci. 2013, 129, 1643–1652. [Google Scholar] [CrossRef]
- Yang, M.-Q.; Xu, Y.-J. Selective photoredox using graphene-based composite photocatalysts. Phys. Chem. Chem. Phys. 2013, 15, 19102–19118. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Guo, Z.X. Graphene-based materials: Synthesis and gas sorption, storage and separation. Prog. Mater. Sci. 2014. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Shan, M.; Guo, Q.; Wang, Z.; Qian, X. Nano-structure and property transformations of carbon systems under γ-ray irradiation: A review. RSC Adv. 2013. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Zaman, I.; Manshoor, B.; Khalid, A.; Araby, S. From clay to graphene for polymer nanocomposites—A survey. J. Polym. Res. 2014, 21, 429. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Sun, H.; Li, H.; Peng, H. Developing polymer composite materials: Carbon nanotubes or graphene? Adv. Mater. 2013, 25, 5153–5176. [Google Scholar] [CrossRef] [PubMed]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H.; Bose, S.; Lee, J.H. Recent advances in graphene-based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Rasheed, A.; Khalid, F.A. Fabrication and properties of CNTs reinforced polymeric matrix nanocomposites for sports applications. IOP Conf. Ser. Mater. Sci. Eng. 2014. [Google Scholar] [CrossRef]
- Yue, L.; Pircheraghi, G.; Monemian, S.A.; Manas-Zloczower, I. Epoxy composites with carbon nanotubes and graphene nanoplatelets—Dispersion and synergy effects. Carbon 2014, 78, 268–278. [Google Scholar] [CrossRef]
- Jean-Pierre, P.; Roberto, W. Epoxy Polymers New Materials and Innovations; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Sanjay, M. Composites Manufacturing Materials, Product, and Process Engineering; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Valery, V.; Evgeny, M. Mechanics and Analysis of Composite Materials; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Atif, R.; Inam, F. Influence of macro-topography on damage tolerance and fracture toughness of monolithic epoxy for tribological applications. World J. Eng. Technol. 2016, 4, 335–360. [Google Scholar] [CrossRef]
- Wongbong, C.; Jo-Won, L. Graphene Synthesis and Applications; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Warner, J.H.; Fransizka, S.; Mark, R.; Bachmatiuk, A. Graphene: Fundamentals and Emergent Applications; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Mikhail, K.; Iosifovich, K.M. Graphene: Carbon in Two Dimensions; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Wolf, E.L. Graphene: A New Paradigm in Condensed Matter and Device Physics; OUP: Oxford, UK, 2013. [Google Scholar]
- Quintana, M.; Spyrou, K.; Grzelczak, M.; Browne, W.R.; Rudolf, P.; Prato, M. Functionalization of graphene. ACS Nano 2010, 4, 3527–3533. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jin, J.; Song, M. An investigation of the mechanism of graphene toughening epoxy. Carbon 2013, 65, 324–333. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Kan, C.-M.; Lin, X.; Shen, X.; Kim, J.-K. Effects of processing and material parameters on synthesis of monolayer ultralarge graphene oxide sheets. Carbon 2014, 77, 244–254. [Google Scholar] [CrossRef]
- Ma, J.; Meng, Q.; Zaman, I.; Zhu, S.; Michelmore, A.; Kawashima, N.; Wang, C. H.; Kuan, H.-C. Development of polymer composites using modified, high-structural integrity graphene platelets. Compos. Sci. Technol. 2014, 91, 82–90. [Google Scholar] [CrossRef]
- Loomis, J.; Panchapakesan, B. Dimensional dependence of photomechanical response in carbon nanostructure composites: A case for carbon-based mixed-dimensional systems. Nanotechnology 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Dieter, G.E. Mechanical Metallurgy, SI Metric ed.; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]
- Wan, Y.-J.; Tang, L.-C.; Gong, L.-X.; Yan, D.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Bindu Sharmila, T.K.; Nair, A.B.; Abraham, B.T.; Beegum, P.M.S.; Thachil, E.T. Microwave exfoliated reduced graphene oxide epoxy nanocomposites for high performance applications. Polymer (Guildf) 2014, 55, 3614–3627. [Google Scholar]
- Zhang, Y.; Wang, Y.; Yu, J.; Chen, L.; Zhu, J.; Hu, Z. Tuning the interface of graphene platelets/epoxy composites by the covalent grafting of polybenzimidazole. Polymer (Guildf) 2014, 55, 4990–5000. [Google Scholar] [CrossRef]
- Ahmadi-Moghadam, B.; Sharafimasooleh, M.; Shadlou, S.; Taheri, F. Effect of functionalization of graphene nanoplatelets on the mechanical response of graphene/ epoxy composites. Mater. Des. 2014, 66, 142–149. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Sato, N.; Tölle, F.; Mülhaupt, R.; Fiedler, B.; Schulte, K. Fracture toughness and failure mechanism of graphene-based epoxy composites. Compos. Sci. Technol. 2014, 97, 90–99. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Zaman, I.; Manshoor, B.; Khalid, A.; Meng, Q.; Araby, S. Interface modification of clay and graphene platelets reinforced epoxy nanocomposites: A comparative study. J. Mater. Sci. 2014, 49, 5856–5865. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Lee, J.H. Effects of surface-modified silica nanoparticles attached graphene oxide using isocyanate-terminated flexible polymer chains on the mechanical properties of epoxy composites. J. Mater. Chem. A 2014. [Google Scholar] [CrossRef]
- Shokrieh, M.M.; Ghoreishi, S.M.; Esmkhani, M.; Zhao, Z. Effects of graphene nanoplatelets and graphene nanosheets on fracture toughness of epoxy nanocomposites. Fatigue Fract. Eng. Mater. Struct. 2014, 37, 1116–1123. [Google Scholar]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Seidel, C.; Schulte, K. Preparation and characterization of graphite nano-platelet (GNP)/epoxy nano-composite: Mechanical, electrical and thermal properties. Eur. Polym. J. 2013, 49, 3878–3888. [Google Scholar] [CrossRef]
- Li, Z.; Wang, R.; Young, R.J.; Deng, L.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. Control of the functionality of graphene oxide for its application in epoxy nanocomposites. Polymer (Guildf) 2013, 54, 6437–6446. [Google Scholar] [CrossRef]
- Shadlou, S.; Alishahi, E.; Ayatollahi, M.R. Fracture behavior of epoxy nanocomposites reinforced with different carbon nano-reinforcements. Compos. Struct. 2013, 95, 577–581. [Google Scholar] [CrossRef]
- Jiang, T.; Kuila, T.; Kim, N.H.; Ku, B.-C.; Lee, J.H. Enhanced mechanical properties of silanized silica nanoparticle attached graphene oxide/epoxy composites. Compos. Sci. Technol. 2013, 79, 115–125. [Google Scholar] [CrossRef]
- Liu, W.; Kong, J.; Toh, W.E.; Zhou, R.; Ding, G.; Huang, S.; Dong, Y.; Lu, X. Toughening of epoxies by covalently anchoring triazole-functionalized stacked-cup carbon nanofibers. Compos. Sci. Technol. 2013, 85, 1–9. [Google Scholar] [CrossRef]
- Wang, R.; Li, Z.; Liu, W.; Jiao, W.; Hao, L.; Yang, F. Attapulgite–graphene oxide hybrids as thermal and mechanical reinforcements for epoxy composites. Compos. Sci. Technol. 2013, 87, 29–35. [Google Scholar] [CrossRef]
- Alishahi, E.; Shadlou, S.; Doagou-R, S.; Ayatollahi, M.R. Effects of carbon nanoreinforcements of different shapes on the mechanical properties of epoxy-based nanocomposites. Macromol. Mater. Eng. 2013, 298, 670–678. [Google Scholar] [CrossRef]
- Ma, J.; Meng, Q.; Michelmore, A.; Kawashima, N.; Izzuddin, Z.; Bengtsson, C.; Kuan, H.-C. Covalently bonded interfaces for polymer/graphene composites. J. Mater. Chem. A 2013. [Google Scholar] [CrossRef]
- Feng, H.; Wang, X.; Wu, D. Fabrication of spirocyclic phosphazene epoxy-based nanocomposites with graphene via exfoliation of graphite platelets and thermal curing for enhancement of mechanical and conductive properties. Ind. Eng. Chem. Res. 2013, 52, 10160–10171. [Google Scholar] [CrossRef]
- Chatterjee, S.; Nafezarefi, F.; Tai, N.H.; Schlagenhauf, L.; Nüesch, F.A.; Chu, B.T.T. Size and synergy effects of nanofiller hybrids including graphene nanoplatelets and carbon nanotubes in mechanical properties of epoxy composites. Carbon 2012, 50, 5380–5386. [Google Scholar] [CrossRef]
- Chatterjee, S.; Wang, J.W.; Kuo, W.S.; Tai, N.H.; Salzmann, C.; Li, W.L.; Hollertz, R.; Nüesch, F.A.; Chu, B.T.T. Mechanical reinforcement and thermal conductivity in expanded graphene nanoplatelets reinforced epoxy composites. Chem. Phys. Lett. 2012, 531, 6–10. [Google Scholar] [CrossRef]
- Zaman, I.; Phan, T.T.; Kuan, H.-C.; Meng, Q.; Bao La, L.T.; Luong, L.; Youssf, O.; Ma, J. Epoxy/graphene platelets nanocomposites with two levels of interface strength. Polymer (Guildf) 2011, 52, 1603–1611. [Google Scholar] [CrossRef]
- Rana, S.; Alagirusamy, R.; Joshi, M. Development of carbon nanofibre incorporated three phase carbon/epoxy composites with enhanced mechanical, electrical and thermal properties. Compos. Part. A Appl. Sci. Manuf. 2011, 42, 439–445. [Google Scholar] [CrossRef]
- Bortz, D.R.; Merino, C.; Martin-Gullon, I. Carbon nanofibers enhance the fracture toughness and fatigue performance of a structural epoxy system. Compos. Sci. Technol. 2011, 71, 31–38. [Google Scholar] [CrossRef]
- Zhang, G.; Karger-Kocsis, J.; Zou, J. Synergetic effect of carbon nanofibers and short carbon fibers on the mechanical and fracture properties of epoxy resin. Carbon 2010, 48, 4289–4300. [Google Scholar] [CrossRef]
- Fang, M.; Zhang, Z.; Li, J.; Zhang, H.; Lu, H.; Yang, Y. Constructing hierarchically structured interphases for strong and tough epoxy nanocomposites by amine-rich graphene surfaces. J. Mater. Chem. 2010. [Google Scholar] [CrossRef]
- Jana, S.; Zhong, W.-H. Graphite particles with a “puffed” structure and enhancement in mechanical performance of their epoxy composites. Mater. Sci. Eng. A 2009, 525, 138–146. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Srivastava, I.; Wang, Z.; Song, H.; Yu, Z.-Z.; Koratkar, N. Fracture and fatigue in graphene nanocomposites. Small 2010, 6, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, H.; Medlin, D. ASM Handbook, Volume 8: Mechanical Testing and Evaluation; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Griffith, A.A. The Phenomena of rupture and flow in solids. Philos. Trans. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1921, 221, 163–198. [Google Scholar] [CrossRef]
- Zhang, W.; Srivastava, I.; Zhu, Y.F.; Picu, C.R.; Koratkar, N.A. Heterogeneity in epoxy nanocomposites initiates crazing: Significant improvements in fatigue resistance and toughening. Small 2009, 5, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- ASM Handbook Volume 19: Fatigue and Fracture; ASM International: Materials Park, OH, USA, 1996.
- Saharudin, M.S.; Atif, R.; Shyha, I.; Inam, F. The degradation of mechanical properties in polymer nano-composites exposed to liquid media—A review. RSC Adv. 2016, 6, 1076–1089. [Google Scholar] [CrossRef]
- Atif, R.; Shyha, I.; Inam, F. The degradation of mechanical properties due to stress concentration caused by retained acetone in epoxy nanocomposites. RSC Adv. 2016, 6, 34188–34197. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, W.; Guo, S.; Zhu, S.; Li, Q.; Li, X.; et al. Synthesis of well-aligned millimeter-sized tetragon-shaped graphene domains by tuning the copper substrate orientation. Carbon 2015, 93, 945–952. [Google Scholar] [CrossRef]
- Bhushan, B. Springer Handbook of Nanotechnology, 3rd ed.; Springer: Berlin, Germany, 2010. [Google Scholar]
- Faber, K.T.; Evans, A.G. Crack deflection processes—I. Theory. Acta Metall. 1983, 31, 565–576. [Google Scholar] [CrossRef]
- Faber, K.T.; Evans, A.G. Crack deflection processes—II. Experiment. Acta Metall. 1983, 31, 577–584. [Google Scholar] [CrossRef]
- Xie, F. A facile strategy for the reduction of graphene oxide and its effect on thermal conductivity of epoxy-based composites. Express Polym. Lett. 2016, 10, 470–478. [Google Scholar] [CrossRef]
- Atif, R.; Inam, F. The dissimilarities between graphene and frame-like structures. Graphene 2016, 1, 55–72. [Google Scholar] [CrossRef]
- Fan, B.-B.; Guo, H.-H.; Zhang, R.; Jia, Y.; Shi, C.-Y. Structural evolution during the oxidation process of graphite. Chin. Phys. Lett. 2014. [Google Scholar] [CrossRef]
- Xu, Z.; Xue, K. Engineering graphene by oxidation: A first-principles study. Nanotechnology 2010. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.-S.; Tai, N.-H.; Chang, T.-W. Deformation and fracture in graphene nanosheets. Compos. Part A Appl. Sci. Manuf. 2013, 51, 56–61. [Google Scholar] [CrossRef]
- Palmeri, M.J.; Putz, K.W.; Brinson, L.C. Sacrificial bonds in stacked-cup carbon nanofibers: Biomimetic toughening mechanisms for composite systems. ACS Nano 2010, 4, 4256–4264. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Zou, X.; Zhu, X.; Seo, J.W.; Cole, J.M.; Bondino, F.; Magnano, E.; Nair, S. K.; Su, H. Ultrafast carrier phonon dynamics in NaOH-reacted graphite oxide film. Appl. Phys. Lett. 2012. [Google Scholar] [CrossRef]
- Shojaee, S.A.; Zandiatashbar, A.; Koratkar, N.; Lucca, D.A. Raman spectroscopic imaging of graphene dispersion in polymer composites. Carbon 2013, 62, 510–513. [Google Scholar] [CrossRef]
- Tamburrano, A.; Sarasini, F.; de Bellis, G.; D’Aloia, A.G.; Sarto, M.S. The piezoresistive effect in graphene-based polymeric composites. Nanotechnology 2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, F.; Shan, C.; Han, D.; Zhang, Q.; Niu, L.; Ivaska, A. Covalent functionalization of chemically converted graphene sheets via silane and its reinforcement. J. Mater. Chem. 2009, 19, 4632–4638. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterisation of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Samanman, S.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. Highly-sensitive label-free electrochemical carcinoembryonic antigen immunosensor based on a novel Au nanoparticles–graphene–chitosan nanocomposite cryogel electrode. Anal. Chim. Acta 2015, 853, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Chong, M.-H.; Park, M.; Kim, H.-Y.; Park, S.-J. Effect of chemically reduced graphene oxide on epoxy nanocomposites for flexural behaviors. Carbon Lett. 2014, 15, 67–70. [Google Scholar] [CrossRef]
- Teng, C.-C.; Ma, C.-C.M.; Lu, C.-H.; Yang, S.-Y.; Lee, S.-H.; Hsiao, M.-C.; Yen, M.-Y.; Chiou, K.-C.; Lee, T.-M. Thermal conductivity and structure of non-covalent functionalized graphene/epoxy composites. Carbon 2011, 49, 5107–5116. [Google Scholar] [CrossRef]
- Chu, K.; Li, W.; Dong, H.; Tang, F. Modeling the thermal conductivity of graphene nanoplatelets reinforced composites. EPL Europhys. Lett. 2012, 100, 36001–36005. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Lin, W.-N.; Huang, Y.-L.; Tien, H.-W.; Wang, J.-Y.; Ma, C.-C.M.; Li, S.-M.; Wang, Y.-S. Synergetic effects of graphene platelets and carbon nanotubes on the mechanical and thermal properties of epoxy composites. Carbon 2011, 49, 793–803. [Google Scholar] [CrossRef]
- Pu, N.-W.; Peng, Y.-Y.; Wang, P.-C.; Chen, C.-Y.; Shi, J.-N.; Liu, Y.-M.; Ger, M.-D.; Chang, C.-L. Application of nitrogen-doped graphene nanosheets in electrically conductive adhesives. Carbon 2014, 67, 449–456. [Google Scholar] [CrossRef]
- Zhao, Q.; Hao, S. Toughening mechanism of epoxy resins with micro/nano particles. J. Compos. Mater. 2007, 41, 201–219. [Google Scholar] [CrossRef]
- Zhao, Q.; Hoa, S.; Ouellette, P. Progressive failure of triaxial woven fabric (TWF) composites with open holes. Compos. Struct. 2004, 65, 419–431. [Google Scholar] [CrossRef]
- Bastwros, M.; Kim, G.-Y.; Zhu, C.; Zhang, K.; Wang, S.; Tang, X.; Wang, X. Effect of ball milling on graphene reinforced Al6061 composite fabricated by semi-solid sintering. Compos. Part B 2014, 60, 111–118. [Google Scholar] [CrossRef]
- Wu, H.; Rook, B.; Drzal, L.T. Dispersion optimization of exfoliated graphene nanoplatelet in polyetherimide nanocomposites: Extrusion, precoating, and solid state ball milling. Polym. Compos. 2013, 34, 426–432. [Google Scholar] [CrossRef]
- Yu, M.; Shao, D.; Lu, F.; Sun, X.; Sun, H.; Hu, T.; Wang, G.; Sawyer, S.; Qiu, H.; Lian, J. ZnO/graphene nanocomposite fabricated by high energy ball milling with greatly enhanced lithium storage capability. Electrochem. Commun. 2013, 34, 312–315. [Google Scholar] [CrossRef]
- Jiang, X.; Drzal, L.T. Reduction in percolation threshold of injection molded high-density polyethylene/exfoliated graphene nanoplatelets composites by solid state ball milling and solid state shear pulverization. J. Appl. Polym. Sci. 2011, 124, 525–535. [Google Scholar] [CrossRef]
- Wu, H.; Zhao, W.; Chen, G. One-pot in situ ball milling preparation of polymer/graphene nanocomposites. J. Appl. Polym. Sci. 2012, 125, 3899–3903. [Google Scholar] [CrossRef]
- Xu, J.; Jeon, I.-Y.; Seo, J.-M.; Dou, S.; Dai, L.; Baek, J.-B. Edge-selectively halogenated graphene nanoplatelets (XGnPs, X = Cl, Br, or I) prepared by ball-milling and used as anode materials for lithium-ion batteries. Adv. Mater. 2014, 26, 7317–7323. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, G. Fabrication of graphene/epoxy resin composites with much enhanced thermal conductivity via ball milling technique. J. Appl. Polym. Sci. 2014, 131, 40565–40569. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Prieto, P.; Prato, M.; Va, E. Exfoliation of graphite with triazine derivatives under ball-milling conditions: Preparation of few-layer graphene via selective noncovalent interactions. ACS Nano 2014, 8, 563–571. [Google Scholar]
- Xu, J.; Shui, J.; Wang, J.; Wang, M.; Liu, H.; Dou, S.X.; Jeon, I. Sulfur–graphene nanostructured cathodes via ball-milling for high-performance lithium–sulfur batteries. ACS Nano 2014, 8, 10920–10930. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Cintas, P. Sonication-assisted fabrication and post-synthetic modifications of graphene-like materials. Chemistry 2010, 16, 5246–5259. [Google Scholar] [CrossRef] [PubMed]
- Yi, M.; Shen, Z.; Zhang, X.; Ma, S. Vessel diameter and liquid height dependent sonication-assisted production of few-layer graphene. J. Mater. Sci. 2012, 47, 8234–8244. [Google Scholar] [CrossRef]
- Ciesielski, A.; Samorì, P. Graphene via sonication assisted liquid-phase exfoliation. Chem. Soc. Rev. 2014, 43, 381–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, L.A.L.; Bao, Q.; Lin, M.; Deng, S.; Goh, B.M.; Loh, K. P. Room-temperature synthesis of soluble carbon nanotubes by the sonication of graphene oxide nanosheets. J. Am. Chem. Soc. 2009, 131, 16832–16837. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Esfandiar, A. Wrapping bacteria by graphene nanosheets for isolation from environment, reactivation by sonication, and inactivation by near-infrared irradiation. J. Phys. Chem. B 2011, 115, 6279–6288. [Google Scholar] [CrossRef] [PubMed]
- Polyakova Stolyarova, E.Y.; Rim, K.T.; Eom, D.; Douglass, K.; Opila, R.L.; Heinz, T.F.; Teplyakov, A.V.; Flynn, G.W. Scanning tunneling microscopy and X-ray photoelectron spectroscopy studies of graphene films prepared by sonication-assisted dispersion. ACS Nano 2011, 5, 6102–6108. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Loomis, J.; King, B.; Panchapakesan, B. Synergy among binary (MWNT, SLG) nano-carbons in polymer nano-composites: A Raman study. Nanotechnology 2012. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Kaloni, T.P.; Zhu, Z.Y.; Schwingenschlögl, U. Oxidation of graphene in ozone under ultraviolet light. Appl. Phys. Lett. 2012. [Google Scholar] [CrossRef]
- Gracia-espino, E.; Hu, G.; Shchukarev, A.; Wa, T. Understanding the interface of six-shell cuboctahedral and icosahedral palladium clusters on reduced graphene oxide: Experimental and theoretical study. J. Am. Chem. Soc. 2014, 136, 6626–6633. [Google Scholar] [CrossRef] [PubMed]
- Velizhanin, K.A.; Dandu, N.; Solenov, D. Electromigration of bivalent functional groups on graphene. Phys. Rev. B 2014. [Google Scholar] [CrossRef]
- Radovic, L.R.; Suarez, A.; Vallejos-Burgos, F.; Sofo, J.O. Oxygen migration on the graphene surface. 2. Thermochemistry of basal-plane diffusion (hopping). Carbon 2011, 49, 4226–4238. [Google Scholar] [CrossRef]
- Radovic, L.R.; Silva-Tapia, A.B.; Vallejos-Burgos, F. Oxygen migration on the graphene surface. 1. Origin of epoxide groups. Carbon 2011, 49, 4218–4225. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Santamaría, R.; Granda, M.; Ares, P.; Rodríguez-Reinoso, F.; Menéndez, R. The effect of the parent graphite on the structure of graphene oxide. Carbon 2012, 50, 275–282. [Google Scholar] [CrossRef]
- Šljivančanin, Ž.; Milošević, A.S.; Popović, Z.S.; Vukajlović, F.R. Binding of atomic oxygen on graphene from small epoxy clusters to a fully oxidized surface. Carbon 2013, 54, 482–488. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Han, H.S.; Jeon, S. One-step chemical reduction of graphene oxide with oligothiophene for improved electrocatalytic oxygen reduction reactions. Carbon 2013, 61, 164–172. [Google Scholar] [CrossRef]
- Yuan, F.-Y.; Zhang, H.-B.; Li, X.; Ma, H.-L.; Li, X.-Z.; Yu, Z.-Z. In situ chemical reduction and functionalization of graphene oxide for electrically conductive phenol formaldehyde composites. Carbon 2014, 68, 653–661. [Google Scholar] [CrossRef]
- Jiang, X.; Nisar, J.; Pathak, B.; Zhao, J.; Ahuja, R. Graphene oxide as a chemically tunable 2-D material for visible-light photocatalyst applications. J. Catal. 2013, 299, 204–209. [Google Scholar] [CrossRef]
- Park, J.S.; Yu, L.; Lee, C.S.; Shin, K.; Han, J.H. Liquid-phase exfoliation of expanded graphites into graphene nanoplatelets using amphiphilic organic molecules. J. Colloid Interface Sci. 2014, 417, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Karger-Kocsis, J.; Friedrich, K. Microstructure-related fracture toughness and fatigue crack growth behaviour in toughened, anhydride-cured epoxy resins. Compos. Sci. Technol. 1993, 48, 263–272. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Gremmels, J. Use of hygrothermal decomposed polyester–urethane waste for the impact modification of epoxy resins. J. Appl. Polym. Sci. 2000, 5, 1139–1151. [Google Scholar] [CrossRef]
- Smith, G.; Bedrov, D.; Li, L.; Byutner, O. A molecular dynamics simulation study of the viscoelastic properties of polymer nanocomposites. J. Chem. Phys. 2002, 117, 9478–9489. [Google Scholar] [CrossRef]
- Corcione, C.E.; Freuli, F.; Maffezzoli, A. The aspect ratio of epoxy matrix nanocomposites reinforced with graphene stacks. Polym. Eng. Sci. 2013, 53, 531–539. [Google Scholar] [CrossRef]
- Ramos-Galicia, L.; Mendez, L.N.; Martínez-Hernández, A.L.; Espindola-Gonzalez, A.; Galindo-Esquivel, I.R.; Fuentes-Ramirez, R.; Velasco-Santos, C. Improved performance of an epoxy matrix as a result of combining graphene oxide and reduced graphene. Int. J. Polym. Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Li, Z.; Young, R.J.; Wang, R.; Yang, F.; Hao, L.; Jiao, W.; Liu, W. The role of functional groups on graphene oxide in epoxy nanocomposites. Polymer (Guildf) 2013, 54, 5821–5829. [Google Scholar] [CrossRef]
- Liu, W.; Koh, K.L.; Lu, J.; Yang, L.; Phua, S.; Kong, J.; Chen, Z.; Lu, X. Simultaneous catalyzing and reinforcing effects of imidazole-functionalized graphene in anhydride-cured epoxies. J. Mater. Chem. 2012. [Google Scholar] [CrossRef]
- Yang, H.; Shan, C.; Li, F.; Zhang, Q.; Han, D.; Niu, L. Convenient preparation of tunably loaded chemically converted graphene oxide/epoxy resin nanocomposites from graphene oxide sheets through two-phase extraction. J. Mater. Chem. 2009, 19, 8856. [Google Scholar] [CrossRef]
- Galpaya, D.; Wang, M.; George, G.; Motta, N.; Waclawik, E.; Yan, C. Preparation of graphene oxide/epoxy nanocomposites with significantly improved mechanical properties. J. Appl. Phys. 2014. [Google Scholar] [CrossRef]
- Li, W.; Dichiara, A.; Bai, J. Carbon nanotube–graphene nanoplatelet hybrids as high-performance multifunctional reinforcements in epoxy composites. Compos. Sci. Technol. 2013, 74, 221–227. [Google Scholar] [CrossRef]
- Cao, L.; Liu, X.; Na, H.; Wu, Y.; Zheng, W.; Zhu, J. How a bio-based epoxy monomer enhanced the properties of diglycidyl ether of bisphenol A (DGEBA)/graphene composites. J. Mater. Chem. A 2013, 1, 5081–5088. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Tang, L.-C.; Yan, D.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Improved dispersion and interface in the graphene/epoxy composites via a facile surfactant-assisted process. Compos. Sci. Technol. 2013, 82, 60–68. [Google Scholar] [CrossRef]
- Ghaleb, Z.A.; Mariatti, M.; Ariff, Z.M. Properties of graphene nanopowder and multi-walled carbon nanotube-filled epoxy thin-film nanocomposites for electronic applications: The effect of sonication time and filler loading. Compos. Part A 2014, 58, 77–83. [Google Scholar] [CrossRef]
- King, J.A.; Klimek, D.R.; Miskioglu, I.; Odegard, G.M. Mechanical properties of graphene nanoplatelet/epoxy composites. J. Appl. Polym. Sci. 2013, 128, 4217–4223. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Pornwannchai, W.; Hu, Y.; Kandola, B. The effect of graphene presence in flame retarded epoxy resin matrix on the mechanical and flammability properties of glass fiber-reinforced composites. Compos. Part A 2013, 53, 88–96. [Google Scholar] [CrossRef]
- Serena Saw, W.P.; Mariatti, M. Properties of synthetic diamond and graphene nanoplatelet-filled epoxy thin film composites for electronic applications. J. Mater. Sci. Mater. Electron. 2011, 23, 817–824. [Google Scholar] [CrossRef]
- Zaman, I.; Kuan, H.-C.; Meng, Q.; Michelmore, A.; Kawashima, N.; Pitt, T.; Zhang, L.; Gouda, S.; Luong, L.; Ma, J. A Facile Approach to Chemically Modified Graphene and its Polymer Nanocomposites. Adv. Funct. Mater. 2012, 22, 2735–2743. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-H.; Hsu, M.-H.; Chang, K.-C.; Lai, M.-C.; Liu, P.-J.; Chuang, T.-L.; Yeh, J.-M.; Liu, W.-R. Physical study of room-temperature-cured epoxy/thermally reduced graphene oxides with various contents of oxygen-containing groups. Polym. Int. 2014, 63, 1765–1770. [Google Scholar] [CrossRef]
- Yang, Y.; Rigdon, W.; Huang, X.; Li, X. Enhancing graphene reinforcing potential in composites by hydrogen passivation induced dispersion. Sci. Rep. 2013, 3, 2086–2093. [Google Scholar] [CrossRef] [PubMed]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 2014, 4, 4375–4382. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Yuan, Z.; Lu, S.; Liu, K.; Li, S.; Yang, L.; Yu, J. Mechanical and thermal properties of epoxy composites containing graphene oxide and liquid crystalline epoxy. Fibers Polym. 2014, 15, 326–333. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, G.; Ren, P.; Wang, Y.; Cui, X. In situ polymerization of graphene oxide and cyanate ester–epoxy with enhanced mechanical and thermal properties. Appl. Surf. Sci. 2014, 316, 549–557. [Google Scholar] [CrossRef]
- Qi, B. Enhanced thermal and mechanical properties of epoxy composites by mixing thermotropic liquid crystalline epoxy grafted graphene oxide. Express Polym. Lett. 2014, 8, 467–479. [Google Scholar] [CrossRef]
- Lu, S.; Li, S.; Yu, J.; Yuan, Z.; Qi, B. Epoxy nanocomposites filled with thermotropic liquid crystalline epoxy grafted graphene oxide. RSC Adv. 2013, 3, 8915. [Google Scholar] [CrossRef]
- Shen, X.-J.; Liu, Y.; Xiao, H.-M.; Feng, Q.-P.; Yu, Z.-Z.; Fu, S.-Y. The reinforcing effect of graphene nanosheets on the cryogenic mechanical properties of epoxy resins. Compos. Sci. Technol. 2012, 72, 1581–1587. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Kan, Y.; Qian, X.; Hu, Y. In situ preparation of functionalized graphene oxide/epoxy nanocomposites with effective reinforcements. J. Mater. Chem. 2011, 21, 13290–13298. [Google Scholar] [CrossRef]
- Meng, Q.; Jin, J.; Wang, R.; Kuan, H.-C.; Ma, J.; Kawashima, N.; Michelmore, A.; Zhu, S.; Wang, C.H. Processable 3-nm thick graphene platelets of high electrical conductivity and their epoxy composites. Nanotechnology 2014, 25, 125707–125719. [Google Scholar] [CrossRef] [PubMed]
- Atif, R.; Shyha, I.; Inam, F. Modeling and experimentation of multi-layered nanostructured graphene-epoxy nanocomposites for enhanced thermal and mechanical properties. J. Compos. Mater. 2016. [Google Scholar] [CrossRef]
- Yu, A.; Ramesh, P.; Itkis, M.E.; Bekyarova, E.; Haddon, R.C. Graphite nanoplatelet—epoxy composite thermal interface materials. J. Phys. Chem. C 2007, 111, 7565–7569. [Google Scholar] [CrossRef]
- Yavari, F.; Fard, H.R.; Pashayi, K.; Rafiee, M.a.; Zamiri, A.; Yu, Z.; Ozisik, R.; Borca-Tasciuc, T.; Koratkar, N. Enhanced thermal conductivity in a nanostructured phase change composite due to low concentration graphene additives. J. Phys. Chem. C 2011, 115, 8753–8758. [Google Scholar] [CrossRef]
- Ganguli, S.; Roy, A.K.; Anderson, D.P. Improved thermal conductivity for chemically functionalized exfoliated graphite/epoxy composites. Carbon 2008, 46, 806–817. [Google Scholar] [CrossRef]
- Fukushima, H.; Drzal, L.T.; Rook, B.P.; Rich, M.J. Thermal conductivity of exfoliated graphite nanocomposites. J. Therm. Anal. Calorim. 2006, 85, 235–238. [Google Scholar] [CrossRef]
- Xie, S.H.; Liu, Y.Y.; Li, J.Y. Comparison of the effective conductivity between composites reinforced by graphene nanosheets and carbon nanotubes. Appl. Phys. Lett. 2008, 92, 1–3. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, R.; Wong, C.P. Modeling of thermal conductivity of graphite nanosheet composites. J. Electron. Mater. 2010, 39, 268–272. [Google Scholar] [CrossRef]
- Nan, C.-W.; Birringer, R.; Clarke, D.R.; Gleiter, H. Effective thermal conductivity of particulate composites with interfacial thermal resistance. J. Appl. Phys. 1997, 81, 6692–6699. [Google Scholar] [CrossRef]
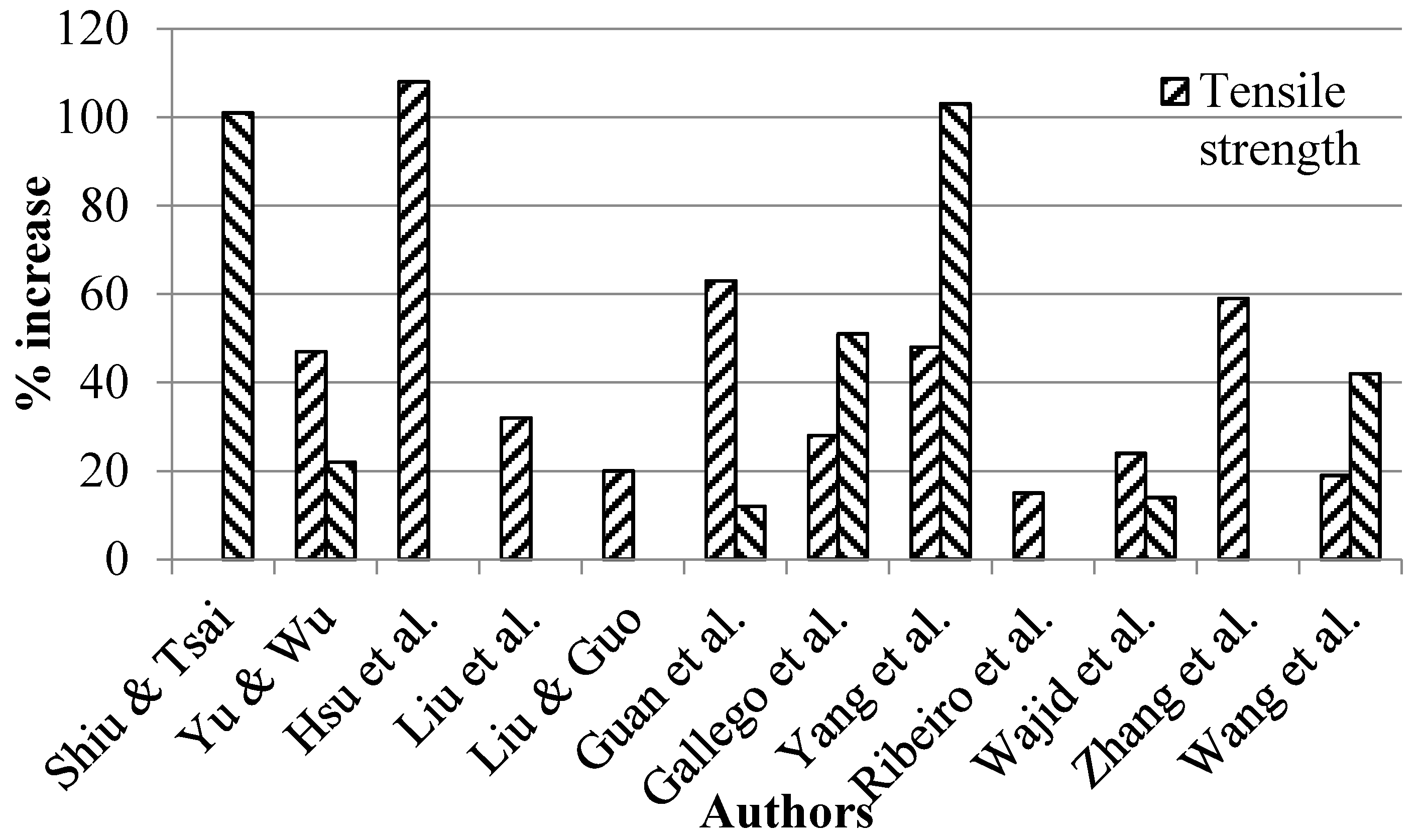
- Shiu, S.-C.; Tsai, J.-L. Characterizing thermal and mechanical properties of graphene/epoxy nanocomposites. Compos. Part B 2014, 56, 691–697. [Google Scholar] [CrossRef]
- Yu, G.; Wu, P. Effect of chemically modified graphene oxide on the phase separation behaviour and properties of an epoxy/polyetherimide binary system. Polym. Chem. 2014, 5, 96–104. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, Z.; Tjiu, W.W.; Lv, J.; Wei, C. Preparation and characterization of epoxy nanocomposites containing surface-modified graphene oxide. J. Appl. Polym. Sci. 2014, 131, 40236–40242. [Google Scholar] [CrossRef]
- Liu, F.; Guo, K. Reinforcing epoxy resin through covalent integration of functionalized graphene nanosheets. Polym. Adv. Technol. 2014, 25, 418–423. [Google Scholar] [CrossRef]
- Guan, L.-Z.; Wan, Y.-J.; Gong, L.-X.; Yan, D.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. Toward effective and tunable interphases in graphene oxide/epoxy composites by grafting different chain lengths of polyetheramine onto graphene oxide. J. Mater. Chem. A 2014, 2, 15058–15069. [Google Scholar] [CrossRef]
- Martin-Gallego, M.; Bernal, M.M.; Hernandez, M.; Verdejo, R.; Lopez-Manchado, M.A. Comparison of filler percolation and mechanical properties in graphene and carbon nanotubes filled epoxy nanocomposites. Eur. Polym. J. 2013, 49, 1347–1353. [Google Scholar] [CrossRef]
- Ribeiro, H.; Silva, W.M.; Rodrigues, M.-T.F.; Neves, J.C.; Paniago, R.; Fantini, C.; Calado, H.D.R.; Seara, L.M.; Silva, G.G. Glass transition improvement in epoxy/graphene composites. J. Mater. Sci. 2013, 48, 7883–7892. [Google Scholar] [CrossRef]
- Wajid, A.S.; Ahmed, H.S.T.; Das, S.; Irin, F.; Jankowski, A.F.; Green, M.J. High-Performance Pristine Graphene/Epoxy Composites With Enhanced Mechanical and Electrical Properties. Macromol. Mater. Eng. 2013, 298, 339–347. [Google Scholar] [CrossRef]
- Zhang, X.; Alloul, O.; He, Q.; Zhu, J.; Verde, M.J.; Li, Y.; Wei, S.; Guo, Z. Strengthened magnetic epoxy nanocomposites with protruding nanoparticles on the graphene nanosheets. Polymer (Guildf) 2013, 54, 3594–3604. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of graphene with grafted polyphosphamide for flame retardant epoxy composites: Synthesis, flammability and mechanism. Polym. Chem. 2014. [Google Scholar] [CrossRef]
- Hu, L.; Desai, T.; Keblinski, P. Thermal transport in graphene-based nanocomposite. J. Appl. Phys. 2011, 110, 1–6. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Li, W.; Qiu, S.; Zhu, C.; Wei, X.; Chen, M.; Liu, C.; Liao, S.; Gong, Y.; Mishra, A. K.; Liu, L. Ultrahigh Thermal Conductivity of Assembled Aligned Multilayer Graphene/Epoxy Composite. Chem. Mater. 2014, 26, 4459–4465. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; He, W.-Y.; Chu, Z.-D.; Liu, M.; Meng, L.; Dou, R.-F.; Zhang, Y.; Liu, Z.; Nie, J.-C.; He, L. Strain and curvature induced evolution of electronic band structures in twisted graphene bilayer. Nat. Commun. 2013, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K.; Guinea, F. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.-W.; Stormer, H. L.; Kim, P. Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 2005, 438, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Katsnelson, M.I.; Grigorieva, I.V.; Dubonos, S.V.; Firsov, A.A. Two-dimensional gas of massless Dirac fermions in graphene. Nature 2005, 438, 197–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Levendorf, M.; Goncher, S.; Schiros, T.; Pálová, L.; Zabet-Khosousi, A.; Rim, K. T.; Gutiérrez, C.; Nordlund, D.; Jaye, C.; Hybertsen, M.; Reichman, D.; Flynn, G. W.; Park, J.; Pasupathy, A. N. Local atomic and electronic structure of boron chemical doping in monolayer graphene. Nano Lett. 2013, 13, 4659–4665. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Kawakami, R.K.; Gmitra, M.; Fabian, J. Graphene spintronics. Nat. Nanotechnol. 2014, 9, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Kandare, E.; Khatibi, A.A.; Yoo, S.; Wang, R.; Ma, J.; Olivier, P.; Gleizes, N.; Wang, C.H. Improving the through-thickness thermal and electrical conductivity of carbon fibre/epoxy laminates by exploiting synergy between graphene and silver nano-inclusions. Compos. Part A 2015, 69, 72–82. [Google Scholar] [CrossRef]
- Tang, B.; Hu, G.; Gao, H.; Hai, L. Application of graphene as filler to improve thermal transport property of epoxy resin for thermal interface materials. Int. J. Heat Mass Transf. 2015, 85, 420–429. [Google Scholar] [CrossRef]
- Burger, N.; Laachachi, A.; Mortazavi, B.; Ferriol, M.; Lutz, M.; Toniazzo, V.; Ruch, D. Alignments and network of graphite fillers to improve thermal conductivity of epoxy-based composites. Int. J. Heat Mass Transf. 2015, 89, 505–513. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, S.; Xiao, X.; Gao, J.; Pan, L.; He, Z.; Yu, J. Enhanced thermal and mechanical properties of epoxy composites by mixing noncovalently functionalized graphene sheets. Polym. Bull. 2014, 72, 453–472. [Google Scholar] [CrossRef]
- Wang, F.; Drzal, L.T.; Qin, Y.; Huang, Z. Mechanical properties and thermal conductivity of graphene nanoplatelet/epoxy composites. J. Mater. Sci. 2014, 50, 1082–1093. [Google Scholar] [CrossRef]
- Zhou, T.; Nagao, S.; Sugahara, T.; Koga, H.; Nogi, M.; Suganuma, K.; Nge, T.T.; Nishina, Y. Facile identification of the critical content of multi-layer graphene oxide for epoxy composite with optimal thermal properties. RSC Adv. 2015, 5, 20376–20385. [Google Scholar] [CrossRef]
- Zeng, C.; Lu, S.; Song, L.; Xiao, X.; Gao, J.; Pan, L.; He, Z.; Yu, J. Enhanced thermal properties in a hybrid graphene–alumina filler for epoxy composites. RSC Adv. 2015, 5, 35773–35782. [Google Scholar] [CrossRef]
- Tang, D.; Su, J.; Yang, Q.; Kong, M.; Zhao, Z.; Huang, Y.; Liao, X.; Liu, Y. Preparation of alumina-coated graphite for thermally conductive and electrically insulating epoxy composites. RSC Adv. 2015, 5, 55170–55178. [Google Scholar] [CrossRef]
- Pan, L.; Ban, J.; Lu, S.; Chen, G.; Yang, J.; Luo, Q.; Wu, L.; Yu, J. Improving thermal and mechanical properties of epoxy composites by using functionalized graphene. RSC Adv. 2015, 5, 60596–60607. [Google Scholar] [CrossRef]
- Wang, R.; Zhuo, D.; Weng, Z.; Wu, L.; Cheng, X.; Zhou, Y.; Wang, J.; Xuan, B. A novel nanosilica/graphene oxide hybrid and its flame retarding epoxy resin with simultaneously improved mechanical, thermal conductivity, and dielectric properties. J. Mater. Chem. A 2015, 3, 9826–9836. [Google Scholar] [CrossRef]
- Zha, J.-W.; Zhu, T.-X.; Wu, Y.-H.; Wang, S.-J.; Li, R.K.Y.; Dang, Z.-M. Tuning of thermal and dielectric properties for epoxy composites filled with electrospun alumina fibers and graphene nanoplatelets through hybridization. J. Mater. Chem. C 2015, 3, 7195–7202. [Google Scholar] [CrossRef]
- Zhou, T. Targeted kinetic strategy for improving the thermal conductivity of epoxy composite containing percolating multi-layer graphene oxide chains. Express Polym. Lett. 2015, 9, 608–623. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, J.; Dai, W.; Song, Y.; Wang, D.; Zeng, L.; Jiang, N. Enhanced Thermal and Electrical Properties of Epoxy Composites Reinforced With Graphene Nanoplatelets. Polym. Compos. 2015. [Google Scholar] [CrossRef]
- Pu, X.; Zhang, H.-B.; Li, X.; Gui, C.; Yu, Z.-Z. Thermally conductive and electrically insulating epoxy nanocomposites with silica-coated graphene. RSC Adv. 2014, 4, 15297–15303. [Google Scholar] [CrossRef]
- Fu, Y.-X.; He, Z.-X.; Mo, D.-C.; Lu, S.-S. Thermal conductivity enhancement of epoxy adhesive using graphene sheets as additives. Int. J. Therm. Sci. 2014, 86, 276–283. [Google Scholar] [CrossRef]
- Esposito Corcione, C.; Maffezzoli, A. Transport properties of graphite/epoxy composites: Thermal, permeability and dielectric characterization. Polym. Test. 2013, 32, 880–888. [Google Scholar] [CrossRef]
- Min, C.; Yu, D.; Cao, J.; Wang, G.; Feng, L. A graphite nanoplatelet/epoxy composite with high dielectric constant and high thermal conductivity. Carbon 2013, 55, 116–125. [Google Scholar] [CrossRef]
- Hsiao, M.-C.; Ma, C.-C.M.; Chiang, J.-C.; Ho, K.-K.; Chou, T.-Y.; Xie, X.; et al. Thermally conductive and electrically insulating epoxy nanocomposites with thermally reduced graphene oxide-silica hybrid nanosheets. Nanoscale 2013, 5, 5863–5871. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Wang, X.; Cheng, P.; Wang, T.; Xiong, D.; Wang, X. Improving the thermal conductivity of epoxy resin by the addition of a mixture of graphite nanoplatelets and silicon carbide microparticles. Express Polym. Lett. 2013, 7, 585–594. [Google Scholar] [CrossRef]
- Raza, M.A.; Westwood, A.V.K.; Stirling, C. Effect of processing technique on the transport and mechanical properties of graphite nanoplatelet/rubbery epoxy composites for thermal interface applications. Mater. Chem. Phys. 2012, 132, 63–73. [Google Scholar] [CrossRef]
- Kim, J.; Yim, B.; Kim, J.; Kim, J. The effects of functionalized graphene nanosheets on the thermal and mechanical properties of epoxy composites for anisotropic conductive adhesives (ACAs). Microelectron. Reliab. 2012, 52, 595–602. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. Thermal conductivity of a graphene oxide–carbon nanotube hybrid/epoxy composite. Carbon 2012, 50, 5429–5440. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J.; Kim, J. The influence of Al(OH)3-coated graphene oxide on improved thermal conductivity and maintained electrical resistivity of Al2O3/epoxy composites. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Huang, X.; Zhi, C.; Jiang, P. Toward Effective Synergetic Effects from Graphene Nanoplatelets and Carbon Nanotubes on Thermal Conductivity of Ultrahigh Volume Fraction Nanocarbon Epoxy Composites. J. Phys. Chem. C 2012, 116, 23812–23820. [Google Scholar] [CrossRef]
- Martin-gallego, M.; Verdejo, R.; Khayet, M.; Maria, J.; De Zarate, O.; Essalhi, M.; Lopez-manchado, M.A. Thermal conductivity of carbon nanotubes and graphene in epoxy nanofluids and nanocomposites. Nanoscale Res. Lett. 2011, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tien, D.H.; Joonkyu, P.; Sang, A.H.; Muneer, A.; Yongho, S.; Koo, S. Electrical and Thermal Conductivities of Stycast 1266 Epoxy/Graphite Composites. J. Korean Phys. Soc. 2011, 59, 2760–2764. [Google Scholar]
- Yu, A.; Ramesh, P.; Sun, X.; Bekyarova, E.; Itkis, M.E.; Haddon, R.C. Enhanced thermal conductivity in a hybrid graphite nanoplatelet—Carbon nanotube filler for epoxy composites. Adv. Mater. 2008, 20, 4740–4744. [Google Scholar] [CrossRef]
- Wu, S.; Ladani, R.B.; Zhang, J.; Bafekrpour, E.; Ghorbani, K.; Mouritz, A.P.; Kinloch, A.J.; Wang, C.H. Aligning multilayer graphene flakes with an external electric field to improve multifunctional properties of epoxy nanocomposites. Carbon 2015, 94, 607–618. [Google Scholar] [CrossRef]
- Liu, X.; Sun, X.; Wang, Z.; Shen, X.; Wu, Y.; Kim, J.-K. Planar Porous Graphene Woven Fabric/Epoxy Composites with Exceptional Electrical, Mechanical Properties, and Fracture Toughness. ACS Appl. Mater. Interfaces 2015, 7, 21455–21464. [Google Scholar] [CrossRef] [PubMed]
- Ming, P.; Zhang, Y.; Bao, J.; Liu, G.; Li, Z.; Jiang, L.; Cheng, Q. Bioinspired highly electrically conductive graphene–epoxy layered composites. RSC Adv. 2015, 5, 22283–22288. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, Z.-G.; Li, X.; Zhang, H.-B.; Hong, S.; Yu, Z.-Z. Electrically conductive rubbery epoxy/diamine-functionalized graphene nanocomposites with improved mechanical properties. Compos. Part B Eng. 2014, 67, 564–570. [Google Scholar] [CrossRef]
- Dou, S.; Qi, J.; Guo, X.; Yu, C. Preparation and adhesive performance of electrical conductive epoxy-acrylate resin containing silver-plated graphene. J. Adhes Sci. Technol. 2014, 28, 1556–1567. [Google Scholar] [CrossRef]
- Tang, G.; Jiang, Z.-G.; Li, X.; Zhang, H.-B.; Yu, Z.-Z. Simultaneous functionalization and reduction of graphene oxide with polyetheramine and its electrically conductive epoxy nanocomposites. Chin. J. Polym. Sci. 2014, 32, 975–985. [Google Scholar] [CrossRef]
- Monti, M.; Rallini, M.; Puglia, D.; Peponi, L.; Torre, L.; Kenny, J.M. Morphology and electrical properties of graphene–epoxy nanocomposites obtained by different solvent assisted processing methods. Compos. Part A Appl. Sci. Manuf. 2013, 46, 166–172. [Google Scholar] [CrossRef]
- Suherman, H.; Sulong, A.B.; Sahari, J. Effect of the compression molding parameters on the in-plane and through-plane conductivity of carbon nanotubes/graphite/epoxy nanocomposites as bipolar plate material for a polymer electrolyte membrane fuel cell. Ceram. Int. 2013, 39, 1277–1284. [Google Scholar] [CrossRef]
- Mancinelli, P.; Heid, T.F.; Fabiani, D.; Saccani, A.; Toselli, M.; Frechette, M.F.; Savoie, S.; David, E. Electrical conductivity of graphene-based epoxy nanodielectrics. In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Shenzhen, China, 20–23 October 2013; pp. 772–775.
- Al-Ghamdi, A.A.; Al-Hartomy, O.A.; Al-Solamy, F.; Al-Ghamdi, A.A.; El-Tantawy, F. Electromagnetic wave shielding and microwave absorbing properties of hybrid epoxy resin/foliated graphite nanocomposites. J. Appl. Polym. Sci. 2013, 127, 2227–2234. [Google Scholar] [CrossRef]
- Kim, J.; Im, H.; Kim, J.; Kim, J. Thermal and electrical conductivity of Al(OH)3 covered graphene oxide nanosheet/epoxy composites. J. Mater. Sci. 2011, 47, 1418–1426. [Google Scholar] [CrossRef]
- Fan, Z.; Zheng, C.; Wei, T.; Zhang, Y.; Lu, G. Effect of Carbon Black on Electrical Property of Graphite Nanoplatelets/Epoxy Resin Composites. Polym. Eng. Sci. 2009, 49, 2041–2045. [Google Scholar] [CrossRef]
- Jović, N.; Dudić, D.; Montone, A.; Antisari, M.V.; Mitrić, M.; Djoković, V. Temperature dependence of the electrical conductivity of epoxy/expanded graphite nanosheet composites. Scr. Mater. 2008, 58, 846–849. [Google Scholar] [CrossRef]
- Li, J.; Ma, P.C.; Chow, W.S.; To, C.K.; Tang, B.Z.; Kim, J.-K. Correlations between Percolation Threshold, Dispersion State, and Aspect Ratio of Carbon Nanotubes. Adv. Funct. Mater. 2007, 17, 3207–3215. [Google Scholar] [CrossRef]
- Sandler, J.; Shaffer, M.S.; Prasse, T.; Bauhofer, W.; Schulte, K.; Windle, A. Development of a dispersion process for carbon nanotubes in an epoxy matrix and the resulting electrical properties. Polymer (Guildf) 1999, 40, 5967–5971. [Google Scholar] [CrossRef]
- Raza, M.A.; Westwood, A.; Stirling, C. Effect of processing technique on the transport and mechanical properties of vapour grown carbon nanofibre/rubbery epoxy composites for electronic packaging applications. Carbon 2012, 50, 84–97. [Google Scholar] [CrossRef]
- Mas, B.; Fernández-Blázquez, J.P.; Duval, J.; Bunyan, H.; Vilatela, J.J. Thermoset curing through Joule heating of nanocarbons for composite manufacture, repair and soldering. Carbon 2013, 63, 523–529. [Google Scholar] [CrossRef]
- Chang, K.-C.; Hsu, M.-H.; Lu, H.-I.; Lai, M.-C.; Liu, P.-J.; Hsu, C.-H.; Ji, W.-F.; Chuang, T.-L.; Wei, Y.; Yeh, J.-M.; Liu, W.-R. Room-temperature cured hydrophobic epoxy/graphene composites as corrosion inhibitor for cold-rolled steel. Carbon 2014, 66, 144–153. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Jiménez-Suárez, A.; Moriche, R.; Ureña, A. Graphene nanoplatelets thickness and lateral size influence on the morphology and behavior of epoxy composites. Eur. Polym. J. 2014, 53, 292–301. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Moriche, R.; Jiménez-Suárez, A.; Sanchez, M.; Ureña, A. Advantages and disadvantages of the addition of graphene nanoplatelets to epoxy resins. Eur. Polym. J. 2014, 61, 206–214. [Google Scholar] [CrossRef]

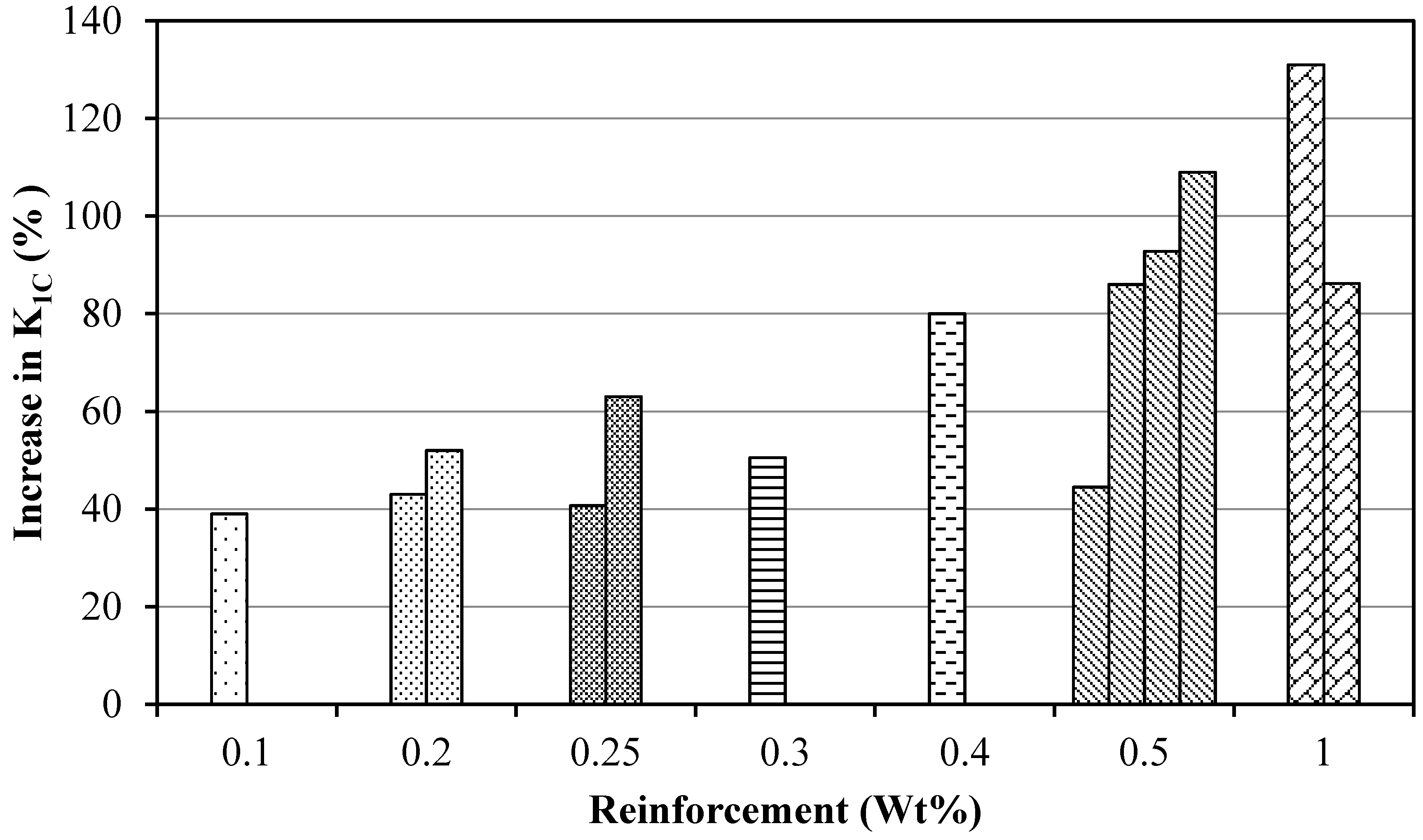
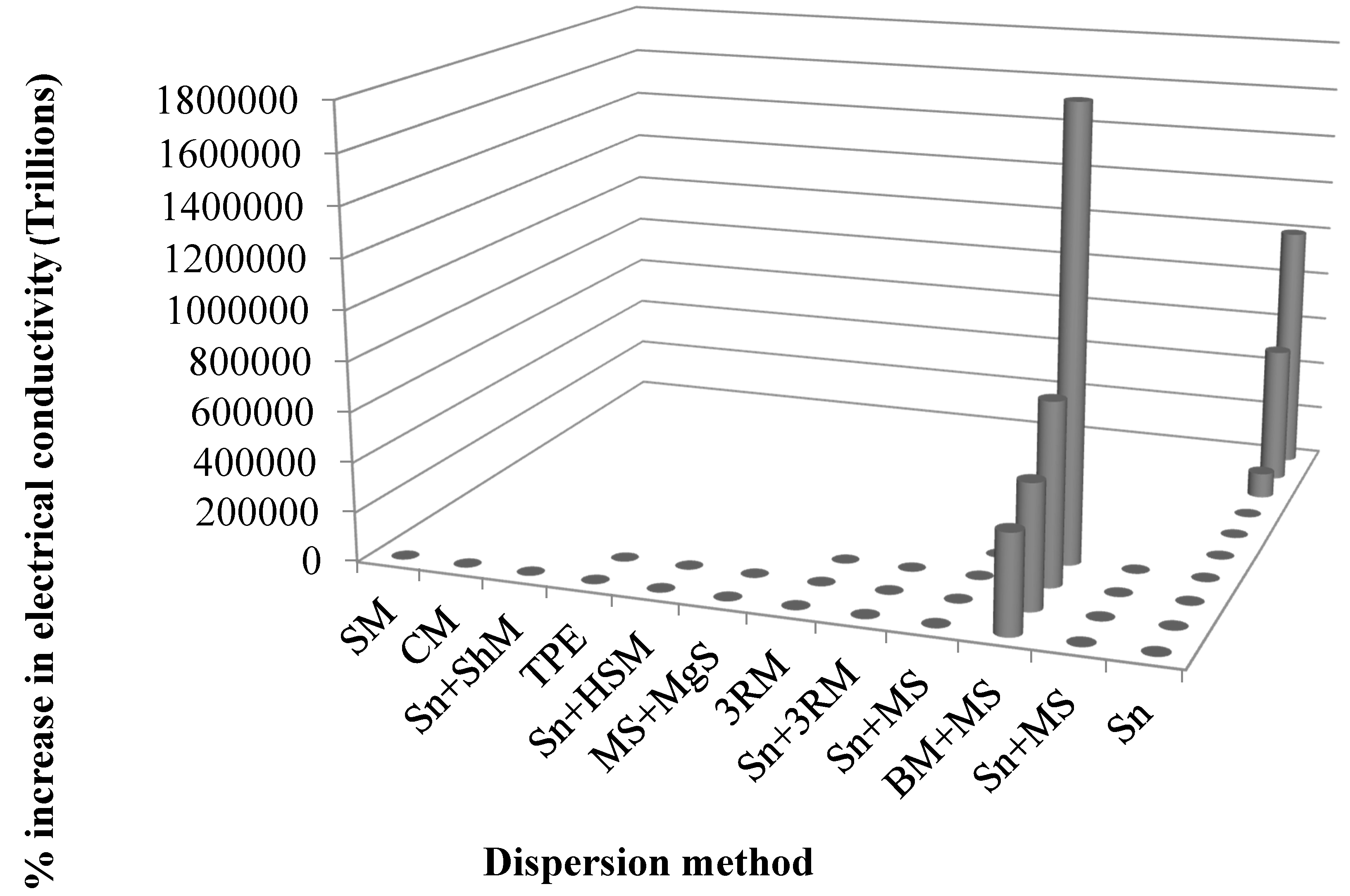
| Sr. | Authors | Year | Reinforcement/(wt %) | Dispersion method | % Increase in K1C (MPa·m1/2) | Remarks | Ref. | |
|---|---|---|---|---|---|---|---|---|
| 1 | Wan et al. | 2014 | GO (0.25 wt %) | Sn + BM | 25.6 | K1C drops after 0.25 wt % of reinforcement | [63] | |
| DGEBA-f-GO (0.25 wt %) | 40.7 | |||||||
| 2 | Sharmila et al. | 2014 | MERGO (0.25 wt %) | MS + USn | 63 | K1C drops after 0.25 wt % of reinforcement | [64] | |
| 3 | Zhang et al. | 2014 | GnPs (0.5 wt %) | Sn | 27.6 | Trend still increasing | [65] | |
| fGnPs (0.3 wt %) | 50.5 | K1C drops after 0.3 wt % of reinforcement | ||||||
| 4 | Moghadam et al. | 2014 | UG (0.5 wt %) | 3RM | 55 | K1C drops after 0.5 wt % of reinforcement | [66] | |
| GO (0.5 wt %) | 57 | |||||||
| G-NH2 (0.5 wt %) | 86 | |||||||
| G-Si (0.5 wt %) | 86 | |||||||
| 5 | Ma et al. | 2014 | m-GnP (1 wt %) | MS + Sn | 131 | K1C drops after 1 wt % of reinforcement of m-GnP | [59] | |
| 6 | Chandrasekaran et al. | 2014 | TRGO (0.5 wt %) | 3RM | 44.5 | Trend still increasing | [67] | |
| GNP (1 wt %) | 49 | K1C drops after 1 wt % | ||||||
| MWCNTs (0.5 wt %) | 12.7 | Trend still increasing | ||||||
| 7 | Wan et al. | 2014 | GO (0.1 wt %) | Sn + BM | 24 | K1C improves with silane functionalization | [68] | |
| Silane-f-GO (0.1 wt %) | 39 | |||||||
| 8 | Zaman et al. | 2014 | m-clay (2.5 wt %) | MS | 38 | K1C drops after 2.5 wt % m-clay | [69] | |
| m-GP (4 wt %) | 103 | Trend still increasing | ||||||
| 9 | Jiang et al. | 2014 | SATPGO (0.5 wt %) | USn | 92.8 | K1C drops after 0.5 wt % of reinforcement | [70] | |
| 10 | Shokrieh et al. | 2014 | GPLs (0.5 wt %) | Sn | 39 | K1C drops after 0.5 wt % of reinforcement | [71] | |
| GNSs (0.5 wt %) | 16 | |||||||
| 11 | Jia et al. | 2014 | GF (0.1 wt %) (resin infiltration) | None | 70 | K1C did not change much between 0.1 to 0.5 wt % | [58] | |
| 12 | Tang et al. | 2013 | Poorly dispersed RGO (0.2 wt %) | Sn | 24 | Trend still increasing | [72] | |
| Highly dispersed RGO (0.2 wt %) | Sn + BM | 52 | ||||||
| 13 | Wang et al. | 2013 | GO | 10.79 µm (0.5wt %) | USn | 12 | K1C drops after 0.5 wt % of reinforcement | [57] |
| 1.72 µm (0.5 wt %) | 61 | |||||||
| 0.70 µm (0.1 wt %) | 75 | |||||||
| 14 | Chandrasekaran et al. | 2013 | GNPs* (0.5 wt %) | 3RM | 43 | Dispersion and K1C improved with three roll milling | [73] | |
| 15 | Li et al. | 2013 | APTS-GO (0.5 wt %) | USn | 25 | Trend still increasing | [74] | |
| GPTS-GO (0.2 wt %) | 43 | K1C drops after 0.2 wt % of reinforcement | ||||||
| 16 | Shadlou et al. | 2013 | ND (0.5 wt %) | USn | No effect | Fracture toughness improvement is higher by CNF and GO (high aspect ratio) compared with that by spherical ND | [75] | |
| CNF (0.5 wt %) | 4.3 | |||||||
| GO (0.5 wt %) | 39.1 | |||||||
| 17 | Jiang et al. | 2013 | GO (0.1 wt %) | Sn | 31 | Trend remains same after 1 wt % of reinforcement | [76] | |
| ATS (1 wt %) | 58.6 | K1C drops after 0.1 wt % of reinforcement | ||||||
| ATGO (1 wt %) | 86.2 | The maximum improvement is achieved with functionalization | ||||||
| 18 | Liu et al. | 2013 | p-CNFs (0.4 wt %) | Sn | 41 | Trend still increasing | [77] | |
| m-CNFs (0.4 wt %) | 80 | |||||||
| 19 | Wang et al. | 2013 | ATP (1 wt %) | Sn | 14 | K1C drops after 0.1 wt % | [78] | |
| GO (0.2 wt %) | 19 | Trend still increasing after 0.2 wt % | ||||||
| ATP (1 wt %) + GO (0.2 wt %) | 27 | K1C drops with the further increase in ATP of reinforcement | ||||||
| 20 | Alishahi et al. | 2013 | ND (0.5 wt %) | Sn | −26.9 | Trend still increasing | [79] | |
| CNF (0.5 wt %) | 19 | |||||||
| GO (0.5 wt %) | 23 | |||||||
| CNT (0.5 wt %) | 23.8 | |||||||
| 21 | Ma et al. | 2013 | U-GnP (0.5 wt %) | MgSr + USn | 49 | Trend still increasing | [80] | |
| m-GnP (0.5 wt %) | 109 | |||||||
| 22 | Feng et al. | 2013 | Graphene (0.5 wt %) | Sn | 76 | K1C decreases after 0.5 wt % of reinforcement | [81] | |
| 23 | Chatterjee et al. | 2012 | GnPs (5 µm, 2 wt %) | 3RM | 60 | Trend still increasing | [82] | |
| GnPs (25 µm, 2 wt %) | 80 | |||||||
| CNTs (2 wt %) | 80 | |||||||
| CNT:GnP = (9:1) (2 wt %) | 76 | |||||||
| 24 | Chatterjee et al. | 2012 | EGNPs (0.1 wt %) | HPH + 3RM | 66 | K1C drops after 0.1 wt % of reinforcement | [83] | |
| 25 | Zaman et al. | 2011 | GP (2.5 wt %) | Sn + MS | 57 | The surface modification significantly improved the K1C | [84] | |
| m-GP (4 wt %) | 90 | |||||||
| 26 | Rana et al. | 2011 | CNFs | Sn + MS | 40 | K1C is dependent upon mixing time | [85] | |
| 27 | Bortz et al. | 2011 | GO (0.5 wt %) | 3RM | 60 | K1C drops after 0.5 wt % of reinforcement | [86] | |
| 28 | Zhang et al. | 2010 | CNFs (0.5 wt %) | 3RM | 19.4 | Trend still increasing | [87] | |
| SCFs (15 wt %) | 125.8 | |||||||
| SCF (10 wt %)/CNF (0.75 wt %) | 210 | |||||||
| 29 | Fang et al. | 2010 | GNs | MS + Sn | 93.8 | Better results with combination of MS and Sn | [88] | |
| 30 | Jana et al. | 2009 | GP with “puffed” structure (5 wt %) | Sn | 28 | Trend still increasing | [89] | |
| 31 | Rafiee et al. | 2009 | SWNT (0.1 wt %) | Sn + MS | 17 | Graphene platelets have more influence on K1C than CNTs | [90] | |
| MWNT (0.1 wt %) | 20 | |||||||
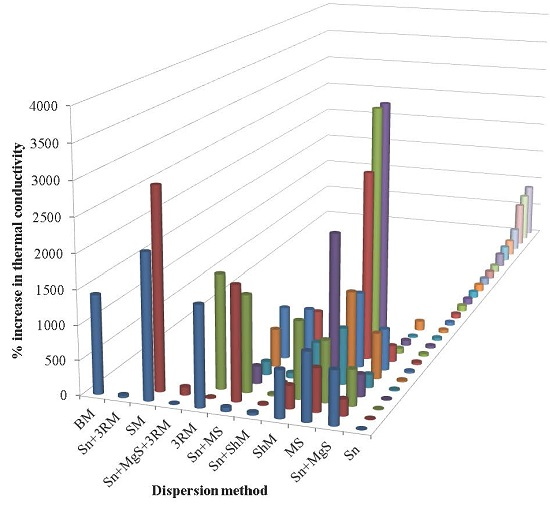
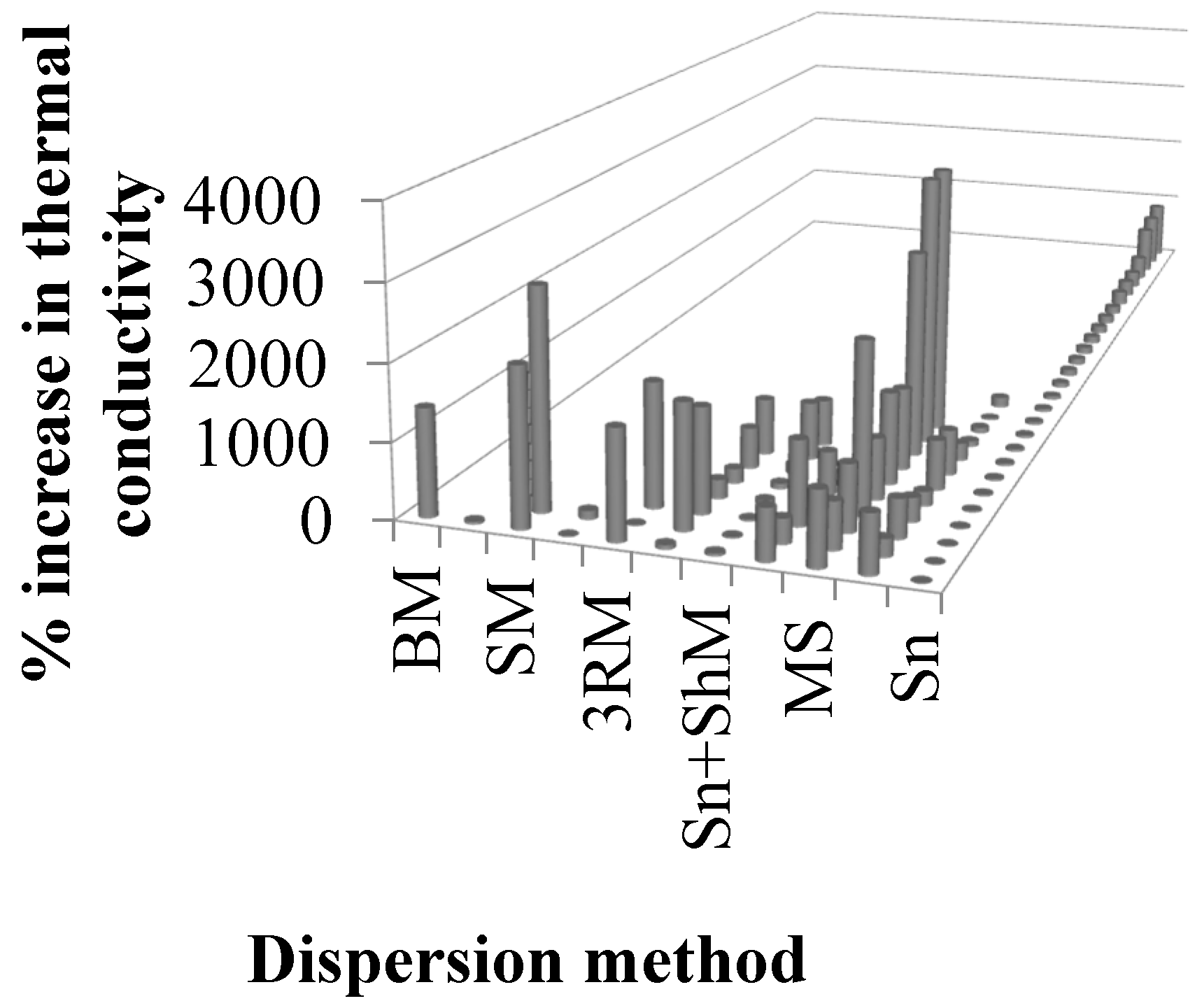
| Sr. | Authors | Year | Reinforcement (wt %) | Dispersion method | % Increase in thermal conductivity | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Kandre et al. | 2015 | GnP (1.9 wt %) | Sn | 9 | The simultaneous inclusion of GnPs and SnP/SnW at a combined loading of 1 vol % resulted in about 40% enhancement in the through-thickness thermal conductivity, while the inclusion of GnP at the same loading resulted in only 9% improvement. A higher increment with simultaneous addition of GnP and SnP/SnW can be attributed to synergistic effects. | [202] |
| SnP/(0.09 wt %) | 18 | ||||||
| SnW/(0.09 wt %) | 8 | ||||||
| GnP (1.9 wt %), SnP (0.09 wt %) | 38 | ||||||
| GnP (1.9 wt %), SnW (0.09 wt %) | 40 | ||||||
| 2 | Tang et al. | 2015 | Three-dimensional graphene network (3DGNs) (30 wt %) | None | 1,900 | (Composites produced using layer-by-layer dropping method.) The filler with large size is more effective in increasing the thermal conductivity of epoxy because of continuous transmission of acoustic phonons and minimum scattering at the interface due to reduced interfacial area. High intrinsic thermal conductivity of graphene is the major reason for the obtained high thermal conductivity of nanocomposites. | [203] |
| Chemically reduced graphene oxide (RGO) (30 wt %) | Sn + MS | 1,650 | |||||
| Natural graphite powder (NG) (30 wt %) | 1,400 | ||||||
| 3 | Burger et al. | 2015 | Graphite flakes (12 wt %) (GRA-12) | Sn + MgSr | 237.5 | As the filler/matrix interfaces increase, the thermal resistance increases due to phonon scattering. In order to improve the thermal conductivity of a composite, it is better to structure a sample with an adapted morphology than trying to have the best dispersion. A 3D-network was first prepared with graphite foils oriented through the thickness of the sample and then stabilized with DGEBA/DDS resin. The produced composite sample was called as “Network”. In “fibers”, all the graphite flakes were aligned through the thickness of sample. When a DGEBA interface layer was applied in “fiber”, the sample was called “Fiber + 1 interface”. When two DGEBA interface layers was applied in “fiber” the sample was called as “Fiber + 2 interfaces”. | [204] |
| Graphite flakes (15 wt %) (GRA-15) | 325 | ||||||
| Graphite flakes (14–15 wt %) (Network) | 775 | ||||||
| Graphite flakes (11–12 wt %) (Fibers) | 666.7 | ||||||
| Graphite flakes (11–12 wt %) (Fiber + 1 interface) | 608.3 | ||||||
| Graphite flakes (11–12 wt %) (Fiber + 2 interface) | 237.5 | ||||||
| 4 | Zeng et al. | 2015 | Liquid crystal perylene bisimides polyurethane (LCPU) modified reduced graphene oxide (RGO) (1 wt %) | Sn | 44.4 | Along with the increase in thermal conductivity, the impact and flexural strengths increased up to 68.8% and 48.5%, respectively, at 0.7 wt % LCPU/RGO. | [205] |
| 5 | Wang et al. | 2015 | GnPs, 1 µm, (GnP-C750) | Sn + MgSr + 3RM | 9.1 | The increase in thermal conductivity is higher in the case of larger particle size than smaller particle size. | [206] |
| GnPs, 5 µm | 115 | ||||||
| 6 | Zhou et al. | 2015 | Multi-layer graphene oxide (MGO) (2 wt %) | Sn | 95.5 | The thermal conductivity decreases after 2 wt % MGO. | [207] |
| 7 | Zeng et al. | 2015 | Al2O3 nanoparticles (30 wt %) | Sn | 50 | The thermal conductivity can be improved by using hybrid fillers. | [208] |
| Aminopropyltriethoxy-silane modified Al2O3 nanoparticles (Al2O3-APS) (30 wt %) | 68.8 | ||||||
| Liquid-crystal perylene-bisimide polyurethane (LCPBI) functionalized reduced graphene oxide (RGO) and Al2O3-APS (LCPBI/RGO/Al2O3-APS) | 106.2 | ||||||
| 8 | Tang et al. | 2015 | Al2O3 (18.4 wt %) | Sn + MS | 59.1 | The increase in thermal conductivity decreases with Al2O3 coating of graphite. | [209] |
| Graphite (18.4 wt %) | 254.6 | ||||||
| Al2O3-coated graphite (Al2O3-graphite) (18.4 wt %) | 195.5 | ||||||
| 9 | Pan et al. | 2015 | Perylene bisimide (PBI)-hyper-branched polyglycerol (HPG) modified reduced graphene oxide (RGO), (PBI-HPG/RGO) (1 wt %) | Sn | 37.5 | The filler was observed to be uniformly dispersed, resulting in strong interfacial thermal resistance. | [210] |
| 10 | Wang et al. | 2015 | SiO2, 15 nm, (1 wt %) | Sn | 14.3 | SiO2 nanoparticles are more effective in increasing thermal conductivity than GO. The maximum improvement in thermal conductivity was observed in the case of hybrid filler. | [211] |
| GO (1 wt %) | 4.8 | ||||||
| As-prepared nanosilica/graphene oxide hybrid (m-SGO) (1 wt %) | 28.6 | ||||||
| 11 | Zha et al. | 2015 | GNPs (3.7 wt %), Al2O3 nanoparticles (ANPs), (65 wt %) | Sn + MS | 550.4 | Al2O3 nanofibers are more effective in improving thermal conductivity than Al2O3 nanoparticles. | [212] |
| GNPs (3.7 wt %), Al2O3 fibers (Afs) (65 wt %) | 756.7 | ||||||
| 12 | Zhou et al. | 2015 | Multi-layer graphene oxide (MGO) (2 wt %) | Sn | 104.8 | The thermal conductivity decreases after 2 wt % MGO. | [213] |
| 13 | Wang et al. | 2015 | GNPs (8 wt %) | MS | 627 | The thermal conductivity increases with GNPs at the loss of Vickers microhardness after 1 wt % of GNP. | [214] |
| 14 | Pu et al. | 2014 | RGO (1 wt %) | Sn + MgSr | 21.8 | The thermal conductivity decreases after 1 wt % RGO. The silica layer on S-graphene makes electrically conducting graphene insulating, reduces the modulus mismatch between the filler and matrix, and improves the interfacial interactions of the nanocomposites, which results in enhanced thermal conductivity. | [215] |
| 3-aminopropyl triethoxysilane (APTES) functionalized graphene oxide (A-graphene) (8 wt %) | 47.1 | ||||||
| Silica-coated A-graphene (S-graphene) (8 wt %) | 76.5 | ||||||
| 15 | Fu et al. | 2014 | Graphite (44.30 wt %) | MS | 888.2 | The maximum improvement in thermal conductivity was observed in the case of graphene sheets with thickness of 1.5 nm. | [216] |
| Graphite nanoflakes (16.81 wt %) | 982.3 | ||||||
| Graphene sheets (10.10 wt %) | 2258.8 | ||||||
| 16 | Li et al. | 2014 | Aligned MLG (AG) (11.8 wt %) | Sn | 16670 | The alignment of MLG causes an exceptional improvement in thermal conductivity and exceeds other filler-based epoxy nanocomposites. | [193] |
| 17 | Guo and Chen | 2014 | GNPs (25 wt %) | Sn | 780 | Ball milling is more effective in improving the thermal conductivity of GNP/epoxy than sonication. The thermal conductivity decreases when ball milling is carried out for more than 30 h. | [126] |
| GNPs (25 wt %) | BM | 1420 | |||||
| 18 | Corcione and Maffezzoli | 2013 | Natural graphite (NG) (1 wt %) | Sn | 24.1 | The thermal conductivity decreases with increasing wt % of NG after 1 wt %. The thermal conductivity decreases after 2 wt % of GNPs. The maximum improvement in thermal conductivity was observed with expanded graphite. | [217] |
| GNPs (2 wt %) | 89.8 | ||||||
| Expanded graphite (EGS) (3 wt %) | 232.1 | ||||||
| 19 | Chandrasekaran et al. | 2013 | GNP (2 wt %) | 3RM | 14 | The thermal conductivity increases with increasing temperature. | [73] |
| 20 | Min et al. | 2013 | GNPs (5 wt %) | Sn | 240 | High aspect ratio of GNPs and oxygen functional groups play a significant role in improving thermal conductivity of nanocomposites. | [218] |
| 21 | Hsiao et al. | 2013 | Silica (1 wt %) | Sn + ShM | 19 | The existence of the intermediate silica layer enhances the interfacial attractions between TRGO and epoxy and improved dispersion state, which caused a significant increase in thermal conductivity. | [219] |
| Thermally reduced graphene oxide (TRGO) (1 wt %) | 26.5 | ||||||
| Silica nanosheets (Silica-NS) (1 wt %) | 37.5 | ||||||
| TRGO-silica-NS (1 wt %) | 61.5 | ||||||
| 22 | Zhou et al. | 2013 | Untreated GNPs (12 wt %) | Sn + MgSr | 139.3 | Silane functionalization can significantly improve thermal conductivity of GNP/epoxy. | [220] |
| Silane-treated COOH-MWCNTs (6 wt %) | 192.9 | ||||||
| Silane-treated GNPs (6 wt %) | 525 | ||||||
| 23 | Raza et al. | 2012 | GNPs, 5 µm, 30 wt %, in rubbery epoxy | MS | 818.6 | The thermal conductivity increases with increasing particle size. The particle size distribution significantly influences the thermal conductivity. GNPs with a broad particle size distribution gave higher thermal conductivity than the particles with a narrow particle size distribution, due to the availability of smaller particles that can bridge gaps between larger particles. | [221] |
| GNPs, 5 µm, 20 wt %, in rubbery epoxy | ShM | 332.6 | |||||
| GNPs, 15 µm, 25 wt %, in rubbery epoxy | MS | 1228.4 | |||||
| GNPs, 15 µm, 25 wt %, in rubbery epoxy | ShM | 1118.2 | |||||
| GNPs, 20 µm, 20 wt %, in rubbery epoxy | ShM | 684.6 | |||||
| GNPs, 20 µm, 12 wt %, in glassy epoxy | ShM | 567.6 | |||||
| GNPs, 15 µm, 20 wt %, in glassy epoxy | MS | 683 | |||||
| 24 | Kim et al. | 2012 | GO (3 wt %) | Sn | 90.4 | The increase in thermal conductivity decreases with Al(OH)3 coating on GO. | [222] |
| Al(OH)3-coated graphene oxide (Al-GO) (3 wt %) | 35.1 | ||||||
| 25 | Chatterjee et al. | 2012 | Amine functionalized expanded graphene nanoplatelets (EGNPs) (2 wt %) | Sn + 3RM | 36 | The EGNPs form a conductive network in the epoxy matrix allowing for increased thermal conductivity. | [83] |
| 26 | Im and Kim | 2012 | Thermally conductive graphene oxide (GO) (50 wt %) | Sn | 111 | The thermal conductivity decreases after 50 wt %, which can be attributed to residual epoxy that forms an insulting layer on reinforcement. MWCNT helps the formation of 3D network structure. | [223] |
| Thermally conductive graphene oxide (GO) (50 wt %), MWCNTs (0.36 wt %) | 203.4 | ||||||
| 27 | Heo et al. | 2012 | Al2O3 (80 wt %), GO (5 wt %) | 3RM | 1,650 | The increase in thermal conductivity decreases with Al(OH)3 coating of GO. | [224] |
| Al(OH)3-coated GO (5 wt %) | 1,450 | ||||||
| 28 | Huang et al. | 2012 | MWNTs (65 wt %) | MS | 1,100 | GNPs are more effective in improving thermal conductivity than MWNTs. The maximum improvement in thermal conductivity was observed in the case of hybrid fillers. | [225] |
| GNPs (65 wt %) | 2,750 | ||||||
| MWNTs (38 wt %), GNPs (38 wt %) | 3,600 | ||||||
| 29 | Teng et al. | 2011 | MWNT (4 wt %) | Sn | 160 | GNPs showed a significantly greater increase in thermal conductivity than MWNTs. The maximum improvement in thermal conductivity is shown by non-covalent functionalized GNS, which can be attributed to high surface area and uniform dispersion of GNS. | [114] |
| GNPs(4 wt %) | 700 | ||||||
| Poly(glycidyl methacrylate containing localized pyrene groups (Py-PGMA) functionalized GNPs (Py-PGMA-GNS) | 860 | ||||||
| 30 | Gallego et al. | 2011 | MWNTs (1 wt %) in nanofluids | ShM | 66.7 | The layered structure of MWNTs enables an efficient phonon transport through the inner layers, while SWNTs present a higher resistance to heat flow at the interface, due to its higher surface area. The f-MWNTs have functional groups on their surface, acting as scattering points for the phonon transport. | [226] |
| f-MWNTs (0.6 wt %) in nanofluids | 20 | ||||||
| SWNTs (0.6 wt %) in nanofluids | 20 | ||||||
| Functionalized graphene sheet (FGS) (1 wt %) in nanofluids | 0 | ||||||
| GO (1 wt %) in nanofluids | 0 | ||||||
| MWNTs(1 wt %) in nanocomposites | 72.7 | ||||||
| Functionalized graphene sheet (FGS) (1 wt %) in nanocomposites | 63.6 | ||||||
| 31 | Tien et al. | 2011 | Graphene flakes (12 wt %) | Sn | 350 | The thermal conductivity increases exponentially with increasing wt % of graphene flakes. | [227] |
| 32 | Ganguli et al. | 2008 | Exfoliated graphite flakes (20 wt %) | SM | 2,087.2 | The thermal conductivity increases with chemical functionalization. | [177] |
| Chemically functionalized graphite flakes (20 wt %) | 2,907.2 | ||||||
| 33 | Yu et al. | 2008 | Carbon black (CB) (10 wt %) | Sn + ShM | 75 | The hybrid filler demonstrates a strong synergistic effect and surpasses the performance of the individual SWNT and GNP filler. | [228] |
| SWNTs (10 wt %) | 125 | ||||||
| GNPs (10 wt %) | 625 | ||||||
| GNPs (7.5 wt %), SWNTs (2.5 wt %) | 775 |
| Sr. | Authors | Year | Reinforcement/wt % | Dispersion method | % Increase in electrical conductivity | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | Wu et al. | 2015 | GNPs (1.5 wt %), transverse to alignment | Sn + 3RM | 1 × 107 | The maximum thermal conductivity was observed in the case of aligned GNPs. | [229] |
| GNPs (3 wt %), randomly oriented | 1 × 108 | ||||||
| GNPs (3 wt %), parallel to alignment | 1 × 1010 | ||||||
| 2 | Liu et al. | 2015 | Graphene woven fabric (GWF) (0.62 wt %) | None. | 1 × 1013 | (Samples were produced using resin infiltration.) The average number of graphene layers in GWFs varied between 4 and 12. | [230] |
| 3 | Ming et al. | 2015 | Graphene foam (GF) (80 wt %) | None. | 8 × 102 | (Samples were produced using hot pressing.) The electrical conductivity of pure graphene foam (GF) is 2.9 S-cm-1, which is much lower than graphene, which can be because of the presence of structural defects. | [231] |
| 5 | Ghaleb et al. | 2014 | GNPs (1.1 wt %) | Sn | 1.39 × 106 | GNPs are more effective in improving the thermal conductivity of epoxy than MWCNTs. | [159] |
| MWCNTs (1.9 wt %) | 1.62 × 105 | ||||||
| 6 | Tang et al. | 2014 | GO (5 wt %) | Sn + HSM | 1.92 × 109 | The surface functionalization of GO can significantly improve the electrical conductivity of GO–epoxy. | [232] |
| Diamine polyetheramine functionalized GO (GO-D230) (5 wt %) | 1.92 × 1012 | ||||||
| 7 | Dou et al. | 2014 | Silver plated graphene (Ag-G) (25 wt %) | Sn + MS | 4.13 × 102 | Ag–graphene can be used in electronic applications due to its high electrical conductivity. | [233] |
| 8 | Tang et al. | 2014 | GO (3.6 wt %) | Sn | 1 × 1018 | The surface functionalization significantly improves electrical conductivity. | [234] |
| Polyetheramine refluxed GO (GO-D2000) (3.6 wt %) | 1 × 1017 | ||||||
| 9 | Monti et al. | 2013 | GNPs (3 wt %) | Sn + MS | 2.08 × 105 | The samples were produced using chloroform. | [235] |
| GNPs (3 wt %) | 1.16 × 105 | The samples were produced using tetrahydrofuran. | |||||
| 10 | Wajid et al. | 2013 | GNPs (0.24 wt %) | Sn + MS | 2.22 × 103 | The samples were produced using dimethylformamide. | [189] |
| 11 | Chandrakekaran et al. | 2013 | GNPs (1 wt %) | Sn + ShM | 1 × 104 | 3RM is more effective in improving the electrical conductivity of epoxy than sonication and high speed shear mixing. | [73] |
| GNPs (2 wt %) | 3RM | 1 × 108 | |||||
| 12 | Suherman et al. | 2013 | GNPs (80 wt %), CNTs (5 wt %), through-plane | BM + MS | 7.30 × 1017 | The electrical conductivity significantly increases with hybrid filler. | [236] |
| GNPs (80 wt %), CNTs (5 wt %), in-plane | 1.80 × 1018 | ||||||
| GNPs (80 wt %), through-plane | 4 × 1017 | ||||||
| GNPs (80 wt %) in-plane | 5 × 1017 | ||||||
| 13 | Mancinelli et al. | 2013 | GO (0.5 wt %) | Sn | 240 | The conductivity was measured before post-curing. | [237] |
| GO (0.5 wt %) | 730 | The conductivity was measured after post-curing. | |||||
| Octadecylamine (ODA)-treated partially reduced and chemically modified GO (MGO) (0.5 wt %) | 550 | The conductivity was reduced after functionalization. | |||||
| GO (0.5 wt %) | Two phase extraction | 240 | The system was not fully cured during curing process. | ||||
| GO (0.5 wt %) | 7.80 × 103 | The conductivity significantly increased after post-curing. | |||||
| 14 | Al-Ghamdi et al. | 2013 | Foliated graphite nanosheets (FGNs) (40 wt %) | Centrifugal mixing | 9.90 × 103 | Dielectric properties of epoxy–FGN composites decreased with an increase in frequency. | [238] |
| 15 | Kim et al. | 2012 | Al(OH)3 functionalized GO (Al-GO) (3 wt %) | MS + MgSr | 75 | The increase in electrical conductivity decreases with Al(OH)3 functionalization of GO. | [239] |
| GO (3 wt %) | 115 | ||||||
| 16 | Heo et al. | 2012 | Al2O3 (80 wt %), Al(OH)3 functionalized GO (Al-GO) (5 wt %) | 3RM | 2.90 × 103 | The increase in electrical conductivity with Al(OH)3 functionalization decreased. The electrically insulating Al(OH)3 on the graphene oxide nanosheet can prevent electron tunneling and act as ion traps which block ion mobility, resulting in a decrease in the electrical properties of nanocomposites. | [224] |
| Al2O3 (80 wt %), GO (5 wt %) | 4.90 × 103 | ||||||
| 17 | Tien et al. | 2011 | Graphite flakes (14 wt %) | Sn | 4 × 107 | The percolation threshold was 8 wt %. | [227] |
| 18 | Fan et al. | 2009 | GNPs (5 wt %) | Sn + MS | 5.50 × 1010 | The maximum electrical conductivity was observed in the case of hybrid fillers. | [240] |
| GNPs (4.5 wt %), carbon black (CB) (0.5 wt %) | 5.50 × 1012 | ||||||
| 19 | Jovic et al. | 2008 | Expanded graphite (EG) (8 wt %) | Sn | 5.50 × 1017 | The electrical conductivity further increases with the application of electric field. | [241] |
| 20 | Li et al. | 2007 | MWCNTs (1 wt %) | Sn | 4.63 × 107 | The samples were produced using acetone. | [242] |
| 21 | Pecastaings et al. | 2004 | MWCNTs (20 wt %) | Sn + MS | 4.53 × 103 | The samples were produced using acetone. | [243] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atif, R.; Shyha, I.; Inam, F. Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review. Polymers 2016, 8, 281. https://doi.org/10.3390/polym8080281
Atif R, Shyha I, Inam F. Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review. Polymers. 2016; 8(8):281. https://doi.org/10.3390/polym8080281
Chicago/Turabian StyleAtif, Rasheed, Islam Shyha, and Fawad Inam. 2016. "Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review" Polymers 8, no. 8: 281. https://doi.org/10.3390/polym8080281
APA StyleAtif, R., Shyha, I., & Inam, F. (2016). Mechanical, Thermal, and Electrical Properties of Graphene-Epoxy Nanocomposites—A Review. Polymers, 8(8), 281. https://doi.org/10.3390/polym8080281