Recent Advances in Pharmaceutical and Medical Applications in the Area of Selected Porphyrinoids Connected with PLGA or PLGA-Based Modalities
Abstract
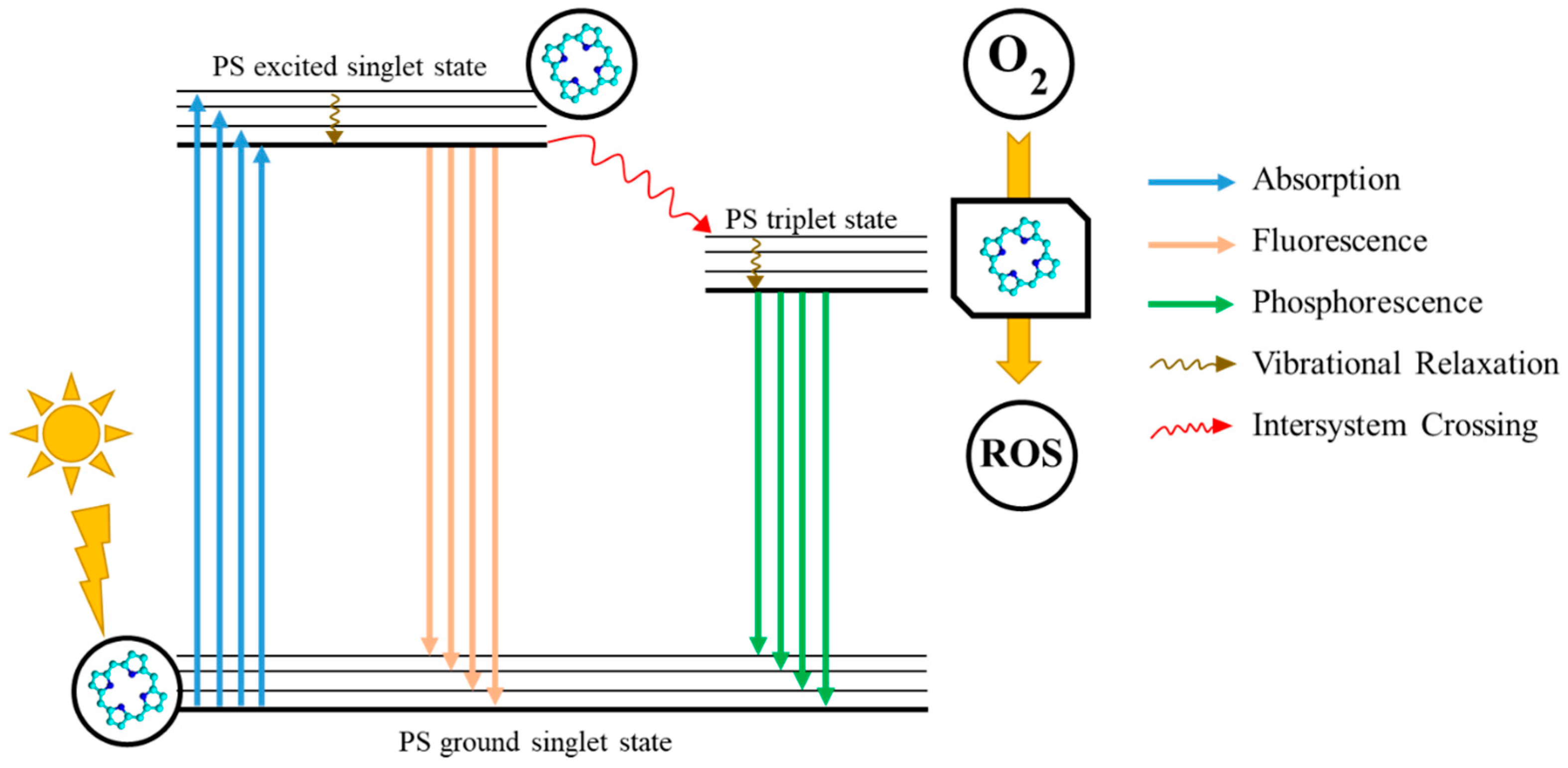
1. Introduction
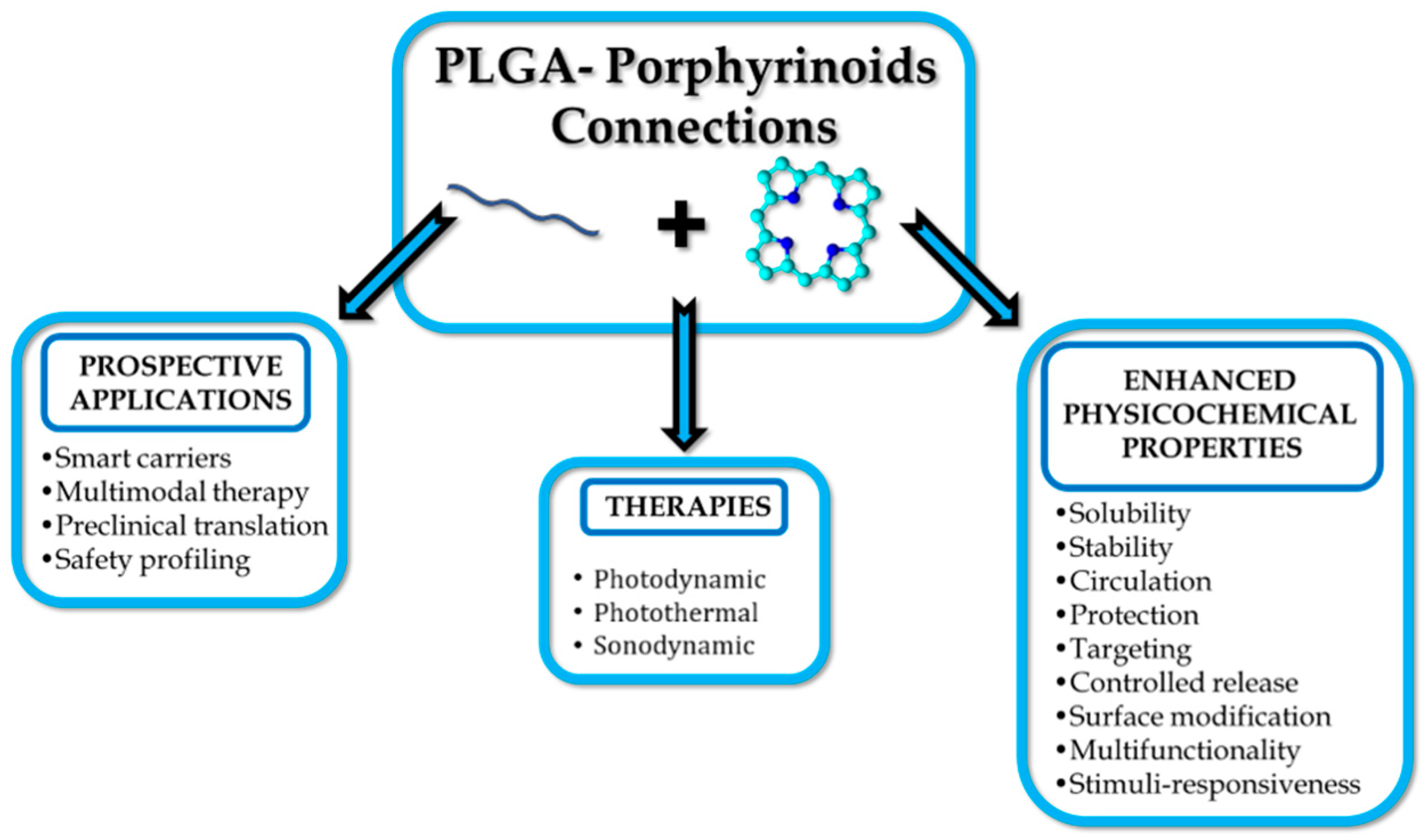
2. Selected Porphyrinoids in Connection with PLGA
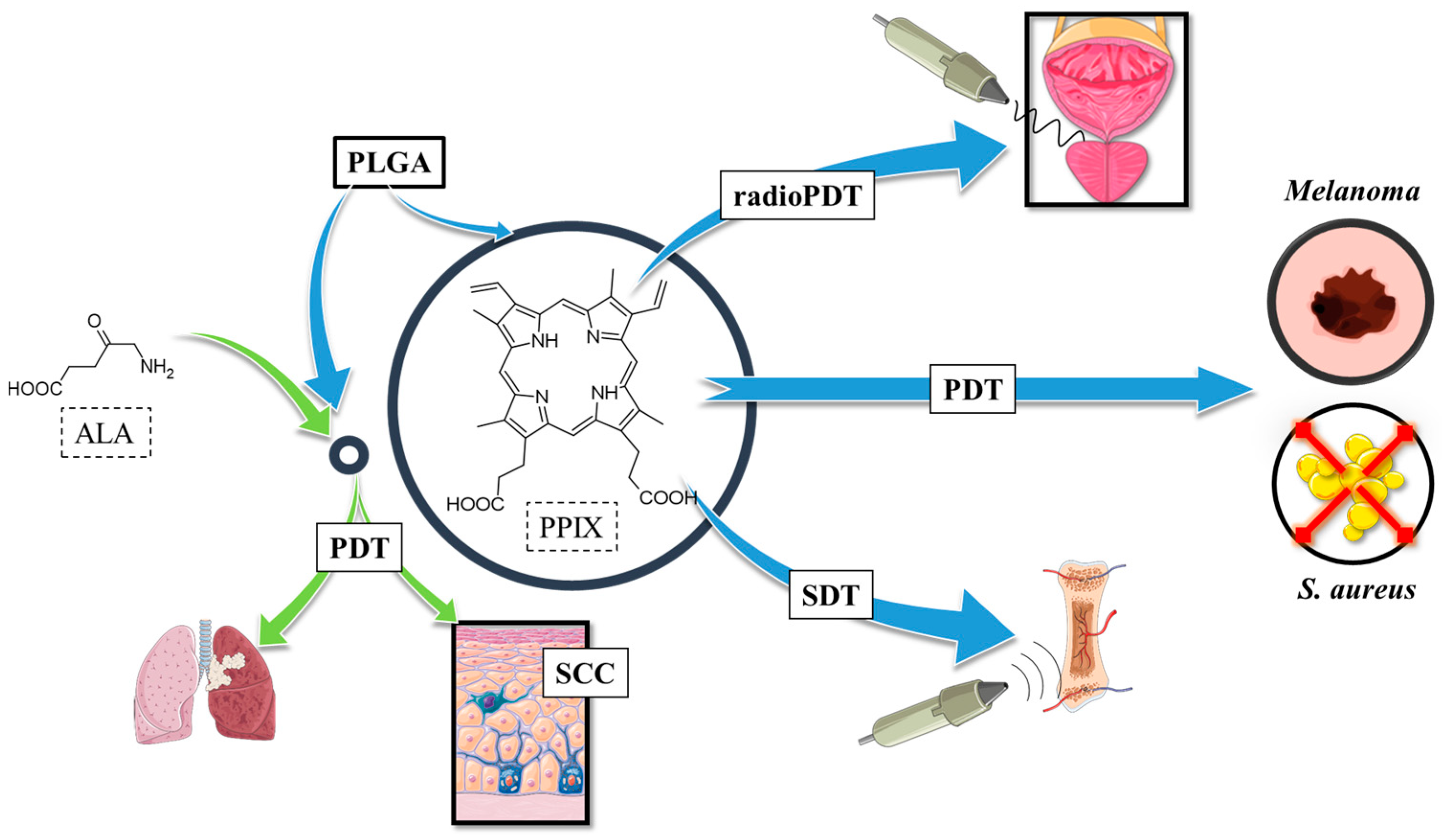
2.1. Protoporphyrin IX in Polymeric Nanoparticles
2.1.1. Protoporphyrin IX in PLGA Nanoparticles
2.1.2. Protoporphyrin IX in Connection with Bioactive Substances
2.1.3. Various Activation Approaches of Protoporphyrin IX in Connection with PLGA and Other Biopolymers
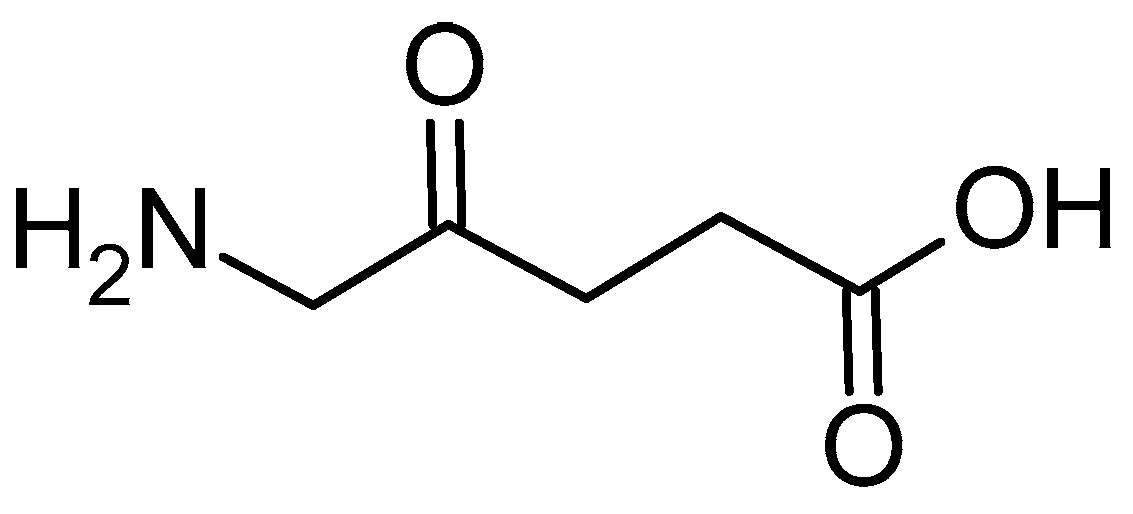
2.1.4. 5-Aminolevulinic Acid in Connection with PLGA and Other Polymers
2.1.5. Concluding Remarks for PPIX and 5-ALA Encapsulation in PLGA
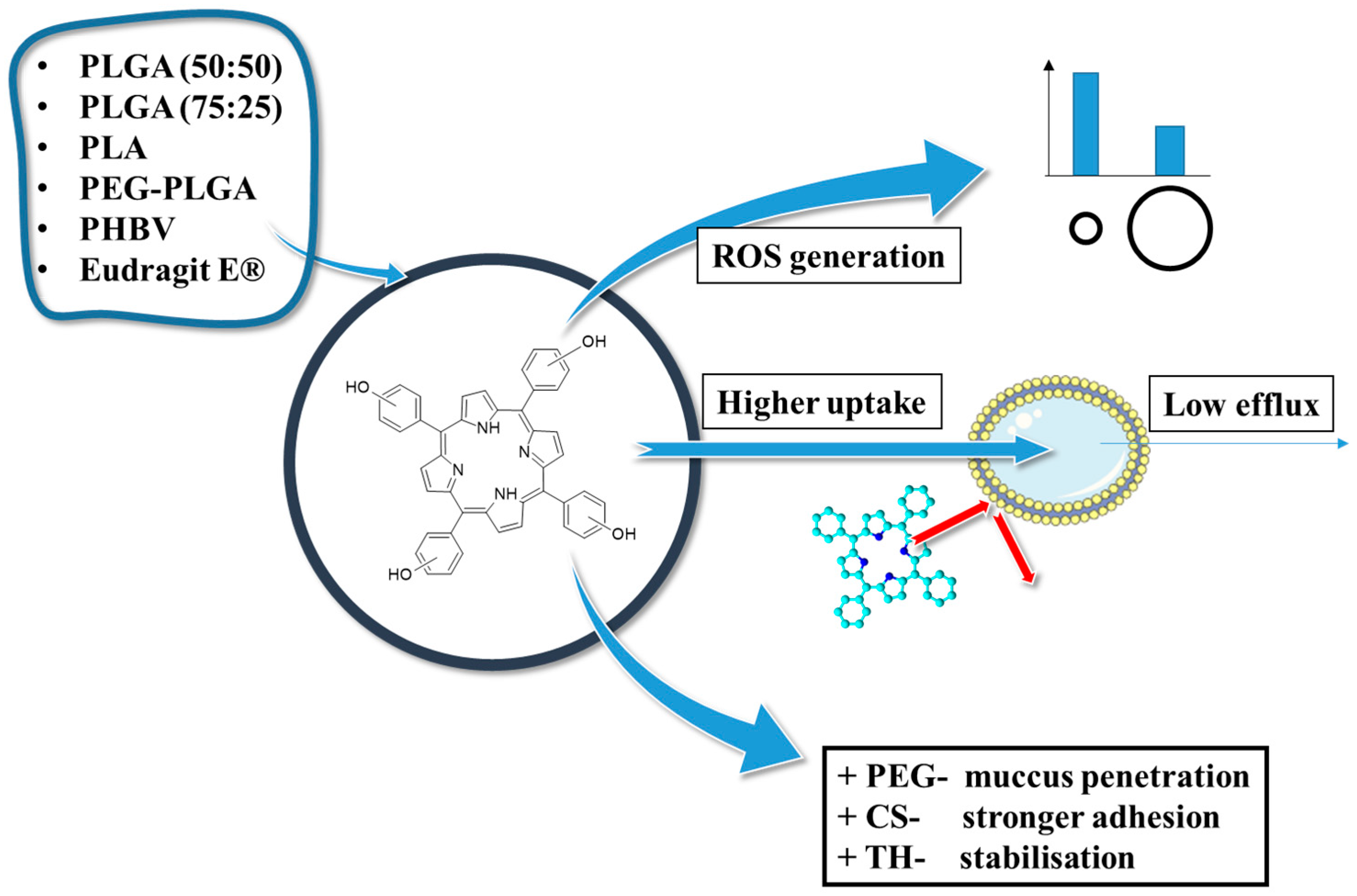
2.2. THPP in PLGA Nanoparticles
2.2.1. THPP in Connection with PLGA
2.2.2. THPP in Connection with Various Polymers
2.2.3. Concluding Remarks on the THPP Connection with PLGA
2.3. Chlorin e6 in PLGA Nanoparticles
2.3.1. Chlorin e6 in Connection with PLGA
2.3.2. Chlorin e6 in Connection with PLGA in PLGA-Based Modalities
2.3.3. Concluding Remarks on the Chlorin e6 Connection with Polymers
2.4. Other Porphyrinoids in Connection with PLGA-Based Nanoparticles
2.4.1. Verteporfin in Connection with PLGA-Based Polymers
2.4.2. Metal Tetraphenylporphyrins in Connection with PLGA-Based Polymers
2.4.3. Hematin in Connection with PLGA-Based Polymers
2.4.4. TMPyP in Connection with PLGA-Based Polymers
2.4.5. TCPP in Connection with PLGA-Based Polymers
2.4.6. Several Further Selected Porphyrins in Connection with PLGA-Based Polymers
2.4.7. Concluding Remarks
3. The Analysis of Particle Size, Zeta Potential and Encapsulation Efficiencies of Selected Porphyrinoids in PLGA
4. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| [EP+D] | epidermis plus dermis |
| 5-ALA | 5-aminolevulinic acid |
| AFM | Atomic Force Microscope |
| ALA | alanine |
| ART | artemisinin |
| ATP | adenosine triphosphate |
| AuNPs | gold nanoparticles |
| BBD | Box–Behnken design |
| bFGF | fibroblast growth factor |
| BNCT | boron neutron capture therapy |
| BRET | bioluminescence resonance energy transfer |
| BSA | bovine serum albumin |
| CAM | chick embryo chorioallantoic membrane |
| CDS | chondroitin sulphate |
| Ce6 | Chlorin e6 |
| Chl | chlorophyllin copper complex |
| CisPt | Cisplatin |
| CLP | cecal ligation and puncture |
| CMC | critical micelle concentration |
| CS | chitosan |
| DCC | N,N’-dicyclohexylcarbodiimide |
| DCM | dichloromethane |
| DDS | drug-delivery system |
| DHR123 | dihydrorhodamine 123 |
| DLC | Drug-Loading Content |
| DLS | dynamic light scattering |
| DMAP | 4-dimethylaminopyridine |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| DOX | doxycycline |
| DPA | 9,10-diphenylantracene |
| DPPC | 1,2-dipalmitoyl-sn-glycero-3-phosphocholine |
| DPPG | 1,2-dipalmitoyl-sn-glycero-3-phosphoglycerol |
| DSC | differential scanning calorimetry |
| DSPE-PEG-COOH | (1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[carboxyl(poly-etylene glycol)-200] |
| DVDMS | sinoporphyrin sodium |
| EC50 | half maximal effective concentration |
| EDC | 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| EDX | Energy-dispersive X-ray |
| EE | Encapsulation Efficiency |
| EPR | Enhanced permeability and retention |
| EtOAc | ethyl acetate |
| Eudragit E® | poly(butyl methacrylate-co-(2-dimethylaminoethyl) methacrylate-co-methylmethacrylate |
| FA | folic acid |
| FBS | fetal bovine serum |
| FDA | Food and Drug Administration |
| FRET | Förster resonance energy transfer |
| FTIR | Fourier-transform infrared spectroscopy |
| GCTB | giant cell tumours of bone |
| GOx | glucose oxidase |
| GSH | glutathione |
| GT | gelatin |
| HAp | hydroxyapatite |
| HA | hyaluronic acid |
| HA-b-PLGA | hyaluronic acid-block-poly(D,L-lactide-co-glycolide) |
| HAT | hyaluronic acid tyramine |
| HBA | carboxyl phenylhydrazine |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HepG2 | human hepatoma |
| HPLC-DAD | High-Performance Liquid Chromatography with Diode-Array Detection |
| IC50 | half maximal inhibitory concentration |
| ICG | indocyanine green |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectrometry |
| Iso | isolienisinine |
| KB | human nasopharyngeal epidermal carcinoma |
| LA-ICP-MS | Laser Ablation Inductively Coupled Plasma Mass Spectrometry |
| LDH | lactate dehydrogenase |
| LPS | lipopolysaccharide |
| MDR | multidrug-resistant |
| mMSCs | mesenchymal stem cells |
| MN | microneedle |
| MOF | metal–organic framework |
| MPO | myeloperoxidase |
| MPs | meso-tetraphenylporphyrins |
| MR/PA | magnetic resonance/photoacoustic |
| MRI | magnetic resonance imaging |
| mTHPC | 5,10,15,20-tetrakis(pentafluorophenyl)porphyrin |
| mTHPP | 5,10,15,20-tetrakis(3-hydroxyphenyl)porphin |
| mTHPP-Pd | [5,10,15,20-tetrakis(3-hydroxyphenyl)porphyrinato]palladium(II) |
| MTT | 3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide |
| NHS | N-hydroxysuccinimide |
| NIR | near-infrared |
| NMR | Nuclear Magnetic Resonance spectroscopy |
| NPs | nanoparticles |
| NR | Nile Red |
| O/W | oil in water |
| OA | oleic acid |
| OCDs | osteochondral defects |
| OVA | ovalbumin |
| PACT | Photodynamic Antimicrobial Chemotherapy |
| Pba, Pheo-a | pheophorbide a |
| PBG | porphobilinogen |
| PBS | phosphate-buffered saline |
| PC3 | prostate cancer cells |
| PCL | polycaprolactone |
| PDD | Photodynamic Diagnostics |
| PDI | polydispersity index |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PET | positron emission tomography |
| PHBV | poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PLA | polylactic acid |
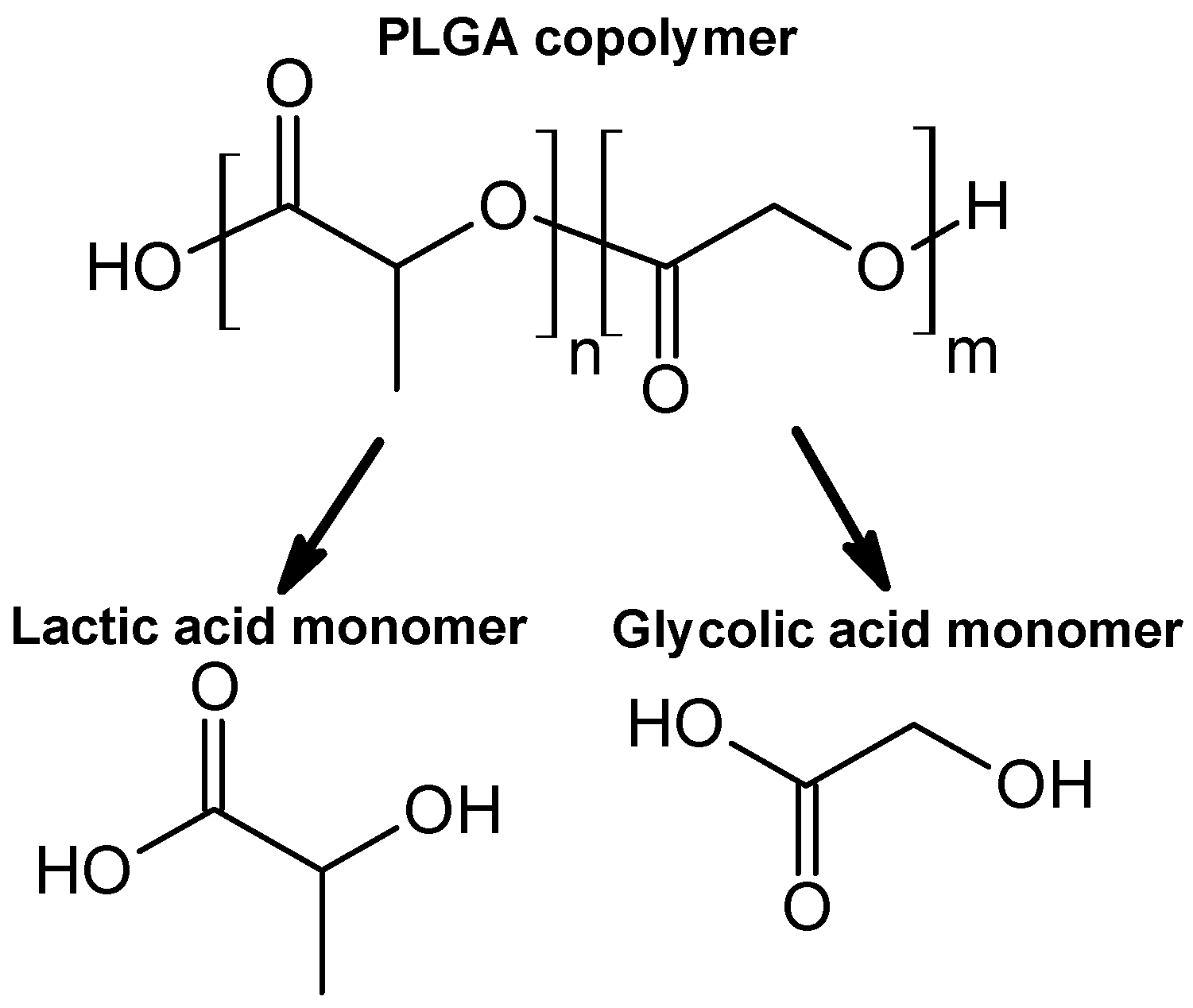
| PLGA | poly(lactic-co-glycolic acid) |
| PPIX | protoporphyrin IX |
| PS | photosensitizer |
| pTHPP | 5,10,15,20-tetrakis(4-hydroxyphenyl)porphin |
| PtOEP | platinum octaethylporphyrin |
| PTT | photothermal therapy |
| PVA | poly(vinyl alcohol) |
| QDs | quantum dots |
| ROS | reactive oxygen species |
| RT-PCR | Real-Time Reverse-Transcription Polymerase Chain Reaction |
| SC | stratum corneum |
| SCC | squamous cell carcinoma |
| SDT | Sonodynamic Therapy |
| SEM | Scanning Electron Microscope |
| SOSG | Singlet Oxygen Sensor Green |
| TA | tannic acid |
| TCPP | 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin |
| TEA | triethylamine |
| TEM | Transmission Electron Microscopy |
| TH | trehalose |
| THF | tetrahydrofuran |
| TMPyP | meso-tetrakis(1-methylpyridinium-4-yl) |
| TPP | 5,10,15,20-tetraphenylporphyrin |
| TRAP | Telomerase Repeated Amplification Protocol |
| TTA-UC | Triplet-Triplet Annihilation Upconversion |
| Tyr | tyramine |
| UCNPs | upconversion nanoparticles |
| US | ultrasound |
| UV-Vis | Ultraviolet-Visible spectroscopy |
| VP | Verteporfin, Visudyne® |
| VSV | vesicular stomatitis virus |
| w1/o/w2 | water-in-oil-in-water |
| XRD | X-Ray Diffraction |
| XRPD | X-Ray Powder Diffraction |
References
- Niculescu, A.G.; Grumezescu, A.M. Photodynamic Therapy—An up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Correia, J.H.; Rodrigues, J.A.; Pimenta, S.; Dong, T.; Yang, Z. Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions. Pharmaceutics 2021, 13, 1332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic Therapy—Mechanisms, Photosensitizers and Combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Bte Rahmat, J.N.; Zhang, Y. Advanced Techniques for Performing Photodynamic Therapy in Deep-Seated Tissues. Biomaterials 2022, 291, 121875. [Google Scholar] [CrossRef]
- Hu, Z. Photodynamic Therapy as an Emerging Treatment Modality for Cancer and Non-Cancer Diseases. J. Anal. Bioanal. Tech. 2014, S1, e001. [Google Scholar] [CrossRef]
- Lan, M.; Zhao, S.; Liu, W.; Lee, C.S.; Zhang, W.; Wang, P. Photosensitizers for Photodynamic Therapy. Adv. Healthc. Mater. 2019, 8, 1900132. [Google Scholar] [CrossRef]
- Kou, J.; Dou, D.; Yang, L. Porphyrin Photosensitizers in Photodynamic Therapy and Its Applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Fan, J.; Chao, H.; Peng, X. Recent Progress in Photosensitizers for Overcoming the Challenges of Photodynamic Therapy: From Molecular Design to Application. Chem. Soc. Rev. 2021, 50, 4185–4219. [Google Scholar] [CrossRef]
- Glowacka-Sobotta, A.; Czarczynska-Goslinska, B.; Ziental, D.; Wysocki, M.; Michalak, M.; Güzel, E.; Sobotta, L. Versatile Porphyrin Arrangements for Photodynamic Therapy—A Review. Nanomaterials 2024, 14, 1879. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Joralemon, M.J.; McRae, S.; Emrick, T. PEGylated Polymers for Medicine: From Conjugation to Self-Assembled Systems. Chem. Commun. 2010, 46, 1377–1393. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-Based Nanoparticles: An Overview of Biomedical Applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- De las Heras Alarcón, C.; Pennadam, S.; Alexander, C. Stimuli Responsive Polymers for Biomedical Applications. Chem. Soc. Rev. 2005, 34, 276–285. [Google Scholar] [CrossRef]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Faraji, F.; Torghabeh, F.M.; Faraji, F.; Mansouri, K.; Abam, F.; Shohaimi, S.; Akbari, H.; Mohammadi, M. Polymer-Based Drug Delivery Systems for Anticancer Drugs: A Systematic Review. Cancer Treat. Res. Commun. 2022, 32, 100605. [Google Scholar] [CrossRef] [PubMed]
- Vilar, G.; Tulla-Puche, J.; Albericio, F. Polymers and Drug Delivery Systems. Curr. Drug Deliv. 2012, 9, 367–394. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines Based Drug Delivery Systems for Anti-Cancer Targeting and Treatment. Curr. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef]
- Martins, C.; Sousa, F.; Araújo, F.; Sarmento, B. Functionalizing PLGA and PLGA Derivatives for Drug Delivery and Tissue Regeneration Applications. Adv. Healthc. Mater. 2018, 7, 1701035. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kim, C.S.; Saylor, D.M.; Koo, D. Polymer Degradation and Drug Delivery in PLGA-Based Drug–Polymer Applications: A Review of Experiments and Theories. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1692–1716. [Google Scholar] [CrossRef]
- Mehraban, N.; Musich, P.R.; Freeman, H.S. Synthesis and Encapsulation of a New Zinc Phthalocyanine Photosensitizer into Polymeric Nanoparticles to Enhance Cell Uptake and Phototoxicity. Appl. Sci. 2019, 9, 401. [Google Scholar] [CrossRef]
- de Toledo, M.C.M.C.; da Silva Abreu, A.; Carvalho, J.A.; Ambrósio, J.A.R.; da Silva Godoy, D.; Pinto, B.C.D.S.; Junior, M.B.; Simioni, A.R. Zinc Phthalocyanine Tetrasulfonate-Loaded Polyelectrolytic PLGA Nanoparticles for Photodynamic Therapy Applications. Photodiagn. Photodyn. Ther. 2020, 32, 101966. [Google Scholar] [CrossRef]
- Fadel, M.; Kassab, K.; Abdel Fadeel, D. Zinc Phthalocyanine-Loaded PLGA Biodegradable Nanoparticles for Photodynamic Therapy in Tumor-Bearing Mice. Lasers Med. Sci. 2010, 25, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Borzęcka, W.; Domiński, A.; Kowalczuk, M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials 2021, 11, 2426. [Google Scholar] [CrossRef]
- Rak, J.; Pouckova, P.; Benes, J.; Vetvicka, D. Drug Delivery Systems for Phthalocyanines for Photodynamic Therapy. Anticancer Res. 2019, 39, 3323–3339. [Google Scholar] [CrossRef]
- Hussain, Z.; Qi, Q.; Zhu, J.; Anderson, K.E.; Ma, X. Protoporphyrin IX-Induced Phototoxicity: Mechanisms and Therapeutics. Pharmacol. Ther. 2023, 248, 108487. [Google Scholar] [CrossRef]
- Sitte, E.; Senge, M.O. The Red Color of Life Transformed—Synthetic Advances and Emerging Applications of Protoporphyrin IX in Chemical Biology. Eur. J. Org. Chem. 2020, 2020, 3171–3191. [Google Scholar] [CrossRef] [PubMed]
- Nyman, E.S.; Hynninen, P.H. Research Advances in the Use of Tetrapyrrolic Photosensitizers for Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2004, 73, 1–28. [Google Scholar] [CrossRef]
- Peng, Q.; Berg, K.; Moan, J.; Kongshaug, M.; Nesland, J.M. 5-Aminolevulinic Acid-Based Photodynamic Therapy: Principles and Experimental Research. Photochem. Photobiol. 1997, 65, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in Nanomaterials for Photodynamic Therapy Applications: Status and Challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.L.; Del Ciampo, J.O.; Rossetti, F.C.; Bentley, M.V.L.B.; Pierre, M.B.R. Improved in Vitro and in Vivo Cutaneous Delivery of Protoporphyrin IX from PLGA-Based Nanoparticles. Photochem. Photobiol. 2013, 89, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.L.; Del Ciampo, J.O.; Cristina Rossetti, F.; Badra Bentley, M.V.L.; Riemma Pierre, M.B. PLGA Nanoparticles as Delivery Systems for Protoporphyrin Ix in Topical Pdt: Cutaneous Penetration of Photosensitizer Observed by Fluorescence Microscopy. J. Nanosci. Nanotechnol. 2013, 13, 6533–6540. [Google Scholar] [CrossRef]
- da Silva, D.B.; da Silva, C.L.; Davanzo, N.N.; da Silva Souza, R.; Correa, R.J.; Tedesco, A.C.; Riemma Pierre, M.B. Protoporphyrin IX (PpIX) Loaded PLGA Nanoparticles for Topical Photodynamic Therapy of Melanoma Cells. Photodiagn. Photodyn. Ther. 2021, 35, 102317. [Google Scholar] [CrossRef]
- Izquierdo, N.; Gamez, E.; Alejo, T.; Mendoza, G.; Arruebo, M. Antimicrobial Photodynamic Therapy Using Encapsulated Protoporphyrin IX for the Treatment of Bacterial Pathogens. Materials 2024, 17, 1717. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Li, J.; Huang, H.; Sun, X.; Lv, Y. Development and Evaluation of Hyaluronic Acid-Based Polymeric Micelles for Targeted Delivery of Photosensitizer for Photodynamic Therapy in Vitro. J. Drug Deliv. Sci. Technol. 2018, 48, 414–421. [Google Scholar] [CrossRef]
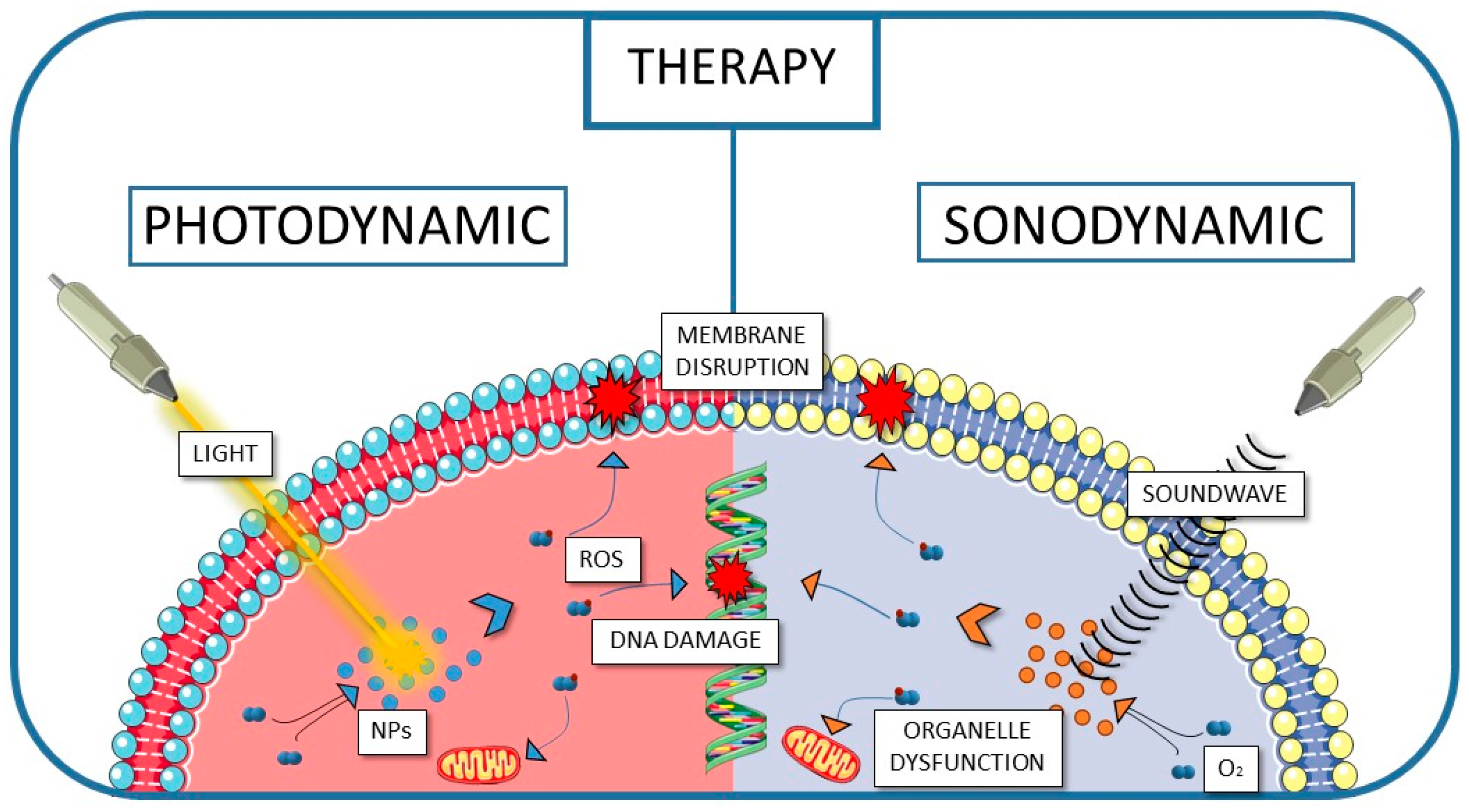
- Wan, G.Y.; Liu, Y.; Chen, B.W.; Liu, Y.Y.; Wang, Y.S.; Zhang, N. Recent Advances of Sonodynamic Therapy in Cancer Treatment. Cancer Biol. Med. 2016, 13, 325–338. [Google Scholar] [CrossRef]
- Yan, P.; Liu, L.H.; Wang, P. Sonodynamic Therapy (SDT) for Cancer Treatment: Advanced Sensitizers by Ultrasound Activation to Injury Tumor. ACS Appl. Bio Mater. 2020, 3, 3456–3475. [Google Scholar] [CrossRef]
- Dong, H.-Q.; Fu, X.-F.; Wang, M.-Y.; Zhu, J. Research Progress on Reactive Oxygen Species Production Mechanisms in Tumor Sonodynamic Therapy. World J. Clin. Cases 2023, 11, 5193–5203. [Google Scholar] [CrossRef]
- He, W.; Li, C.; Zhao, S.; Li, Z.; Wu, J.; Li, J.; Zhou, H.; Yang, Y.; Xu, Y.; Xia, H. Integrating Coaxial Electrospinning and 3D Printing Technologies for the Development of Biphasic Porous Scaffolds Enabling Spatiotemporal Control in Tumor Ablation and Osteochondral Regeneration. Bioact. Mater. 2024, 34, 338–353. [Google Scholar] [CrossRef]
- Prieto, M.; Rwei, A.Y.; Alejo, T.; Wei, T.; Lopez-Franco, M.T.; Mendoza, G.; Sebastian, V.; Kohane, D.S.; Arruebo, M. Light-Emitting Photon-Upconversion Nanoparticles in the Generation of Transdermal Reactive-Oxygen Species. ACS Appl. Mater. Interfaces 2017, 9, 41737–41747. [Google Scholar] [CrossRef]
- Zou, X.; Yao, M.; Ma, L.; Hossu, M.; Han, X.; Juzenas, P.; Chen, W. X-Ray-Induced Nanoparticle-Based Photodynamic Therapy of Cancer. Nanomedicine 2014, 9, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, D.; Sengupta, J.; Pink, D.; Raturi, A.; Chen, H.; Usmani, N.; Kumar, P.; Lewis, J.D.; Narain, R.; Moore, R.B. PEG-PLGA Nanospheres Loaded with Nanoscintillators and Photosensitizers for Radiation-Activated Photodynamic Therapy. Acta Biomater. 2020, 117, 335–348. [Google Scholar] [CrossRef]
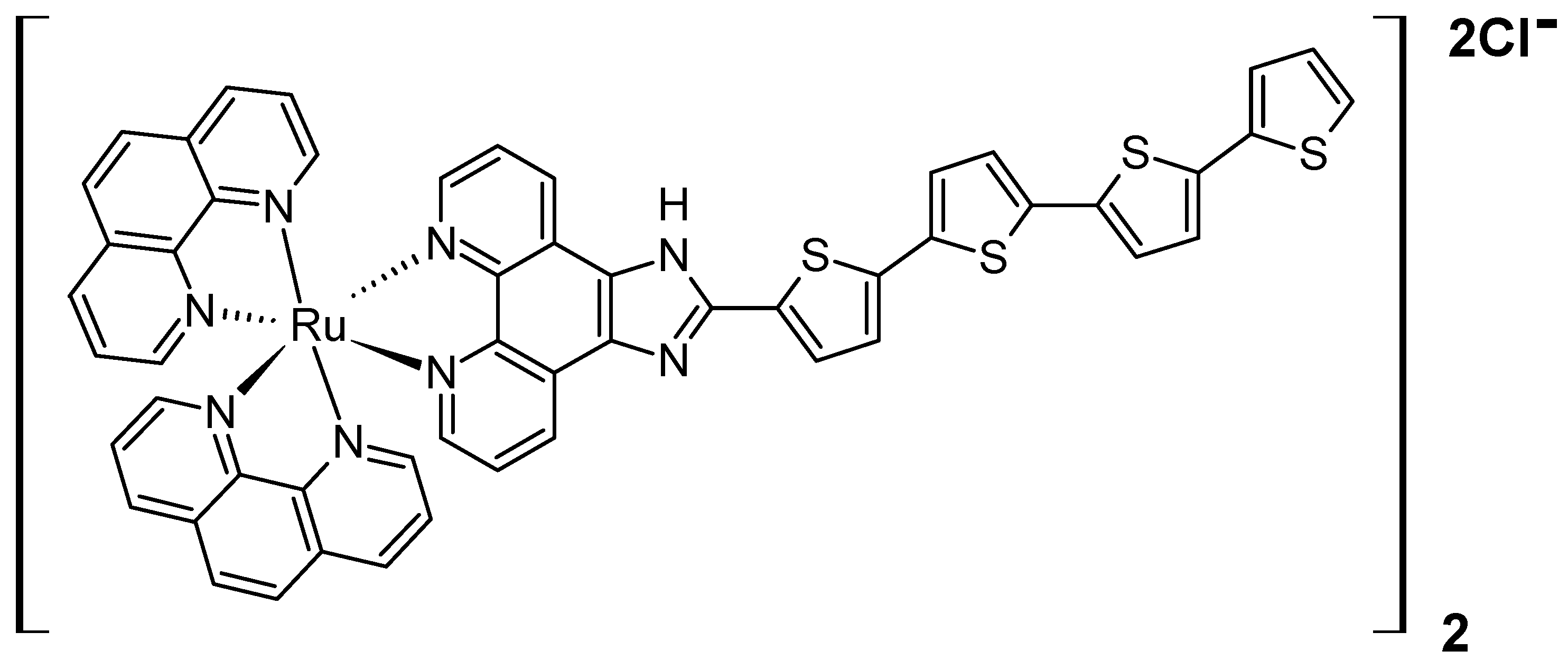
- Azad, A.K.; Lilge, L.; Usmani, N.H.; Lewis, J.D.; Cole, H.D.; Cameron, C.G.; McFarland, S.A.; Dinakaran, D.; Moore, R.B. High Quantum Efficiency Ruthenium Coordination Complex Photosensitizer for Improved Radiation-Activated Photodynamic Therapy. Front. Oncol. 2023, 13, 1244709. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Wang, X.; Zhao, F.; Luan, H.; Tu, Q.; Huang, Z.; Wang, H.; Wang, H. In Vitro Evaluation of 5-Aminolevulinic Acid (ALA) Loaded PLGA Nanoparticles. Int. J. Nanomed. 2013, 8, 2669–2676. [Google Scholar] [CrossRef]
- Wang, X.; Shi, L.; Tu, Q.; Wang, H.; Zhang, H.; Wang, P.; Zhang, L.; Huang, Z.; Zhao, F.; Luan, H.; et al. Treating Cutaneous Squamous Cell Carcinoma Using 5-Aminolevulinic Acid Polylactic-Co-Glycolic Acid Nanoparticle-Mediated Photodynamic Therapy in a Mouse Model. Int. J. Nanomed. 2015, 10, 347–355. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Hao, Y.; Li, L.; Zheng, C.; Zhao, H.; Niu, M.; Yin, Y.; Zhang, Z.; Zhang, Y. Tumor-Targeting Core-Shell Structured Nanoparticles for Drug Procedural Controlled Release and Cancer Sonodynamic Combined Therapy. J. Control. Release 2018, 286, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, Y.; Luo, Q.; Liu, Z.; Man, Y.; Liu, J.; Lu, Y.; Zheng, L. Carrier Cascade Target Delivery of 5-Aminolevulinic Acid Nanoplatform to Enhance Antitumor Efficiency of Photodynamic Therapy against Lung Cancer. J. Photochem. Photobiol. B Biol. 2024, 258, 112999. [Google Scholar] [CrossRef]
- Konan, Y.N.; Berton, M.; Gurny, R.; Allémann, E. Enhanced Photodynamic Activity of Meso-Tetra(4-Hydroxyphenyl)Porphyrin by Incorporation into Sub-200 Nm Nanoparticles. Eur. J. Pharm. Sci. 2003, 18, 241–249. [Google Scholar] [CrossRef]
- Konan, Y.N.; Cerny, R.; Favet, J.; Berton, M.; Gurny, R.; Allémann, E. Preparation and Characterization of Sterile Sub-200 Nm Meso-Tetra(4-Hydroxylphenyl)Porphyrin-Loaded Nanoparticles for Photodynamic Therapy. Eur. J. Pharm. Biopharm. 2003, 55, 115–124. [Google Scholar] [CrossRef]
- Konan, Y.N.; Chevallier, J.; Gurny, R.; Allémann, E. Encapsulation of P-THPP into Nanoparticles: Cellular Uptake, Subcellular Localization and Effect of Serum on Photodynamic Activity. Photochem. Photobiol. 2003, 77, 638–644. [Google Scholar] [CrossRef]
- Vargas, A.; Pegaz, B.; Debefve, E.; Konan-Kouakou, Y.; Lange, N.; Ballini, J.P.; Van Den Bergh, H.; Gurny, R.; Delie, F. Improved Photodynamic Activity of Porphyrin Loaded into Nanoparticles: An in Vivo Evaluation Using Chick Embryos. Int. J. Pharm. 2004, 286, 131–145. [Google Scholar] [CrossRef]
- Vargas, A.; Lange, N.; Arvinte, T.; Cerny, R.; Gurny, R.; Delie, F. Toward the Understanding of the Photodynamic Activity of M-THPP Encapsulated in PLGA Nanoparticles: Correlation between Nanoparticle Properties and in Vivo Activity. J. Drug Target. 2009, 17, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Grünebaum, J.; Söbbing, J.; Mulac, D.; Langer, K. Nanoparticulate Carriers for Photodynamic Therapy of Cholangiocarcinoma: In Vitro Comparison of Various Polymer-Based Nanoparticles. Int. J. Pharm. 2015, 496, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Lirdprapamongkol, K.; Svasti, J.; Bergkvist, M.; Jouan-Hureaux, V.; Arnoux, P.; Frochot, C.; Barberi-Heyob, M.; Niamsiri, N. Polymer-Lipid-PEG Hybrid Nanoparticles as Photosensitizer Carrier for Photodynamic Therapy. J. Photochem. Photobiol. B Biol. 2017, 173, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Pramual, S.; Lirdprapamongkol, K.; Jouan-Hureaux, V.; Barberi-Heyob, M.; Frochot, C.; Svasti, J.; Niamsiri, N. Overcoming the Diverse Mechanisms of Multidrug Resistance in Lung Cancer Cells by Photodynamic Therapy Using PTHPP-Loaded PLGA-Lipid Hybrid Nanoparticles. Eur. J. Pharm. Biopharm. 2020, 149, 218–228. [Google Scholar] [CrossRef]
- Forouharshad, M.; Ajalloueian, F. Tunable Self-Assembled Stereocomplexed-Polylactic Acid Nanoparticles as a Drug Carrier. Polym. Adv. Technol. 2022, 33, 246–253. [Google Scholar] [CrossRef]
- Mahlert, L.; Anderski, J.; Mulac, D.; Langer, K. The Impact of Gastrointestinal Mucus on Nanoparticle Penetration—In Vitro Evaluation of Mucus-Penetrating Nanoparticles for Photodynamic Therapy. Eur. J. Pharm. Sci. 2019, 133, 28–39. [Google Scholar] [CrossRef]
- Anderski, J.; Mahlert, L.; Mulac, D.; Langer, K. Mucus-Penetrating Nanoparticles: Promising Drug Delivery Systems for the Photodynamic Therapy of Intestinal Cancer. Eur. J. Pharm. Biopharm. 2018, 129, 1–9. [Google Scholar] [CrossRef]
- Niehoff, A.C.; Moosmann, A.; Söbbing, J.; Wiehe, A.; Mulac, D.; Wehe, C.A.; Reifschneider, O.; Blaske, F.; Wagner, S.; Sperling, M.; et al. A Palladium Label to Monitor Nanoparticle-Assisted Drug Delivery of a Photosensitizer into Tumor Spheroids by Elemental Bioimaging. Metallomics 2014, 6, 77–81. [Google Scholar] [CrossRef]
- Hak, A.; Ali, M.S.; Sankaranarayanan, S.A.; Shinde, V.R.; Rengan, A.K. Chlorin E6: A Promising Photosensitizer in Photo-Based Cancer Nanomedicine. ACS Appl. Bio Mater. 2023, 6, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Cai, M.; Zhu, R.; Fu, T.; Du, Y.; Kong, J.; Zhang, Y.; Qu, C.; Dong, X.; Ni, J.; et al. Antitumor Effect of Photodynamic Therapy/Sonodynamic Therapy/Sono-Photodynamic Therapy of Chlorin E6 and Other Applications. Mol. Pharm. 2023, 20, 875–885. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Szymczyk, J.; Pawska, A.; Wysocki, M.; Janiak, D.; Ziental, D.; Ptaszek, M.; Güzel, E.; Sobotta, L. Chlorin Activity Enhancers for Photodynamic Therapy. Molecules 2025, 30, 2810. [Google Scholar] [CrossRef]
- Park, J.H.; Moon, Y.H.; Bang, I.S.; Kim, Y.C.; Kim, S.A.; Ahn, S.G.; Yoon, J.H. Antimicrobial Effect of Photodynamic Therapy Using a Highly Pure Chlorin E6. Lasers Med. Sci. 2010, 25, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Park, G.Y.; Oh, K.T.; Oh, N.M.; Kwag, D.S.; Youn, Y.S.; Oh, Y.T.; Park, J.W.; Lee, E.S. Multifunctional Poly (Lactide-Co-Glycolide) Nanoparticles for Luminescence/Magnetic Resonance Imaging and Photodynamic Therapy. Int. J. Pharm. 2012, 434, 257–263. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-Based Nanoparticles for MR Imaging-Guided Ferroptosis in Combination with Photodynamic Therapy to Enhance Cancer Treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Huang, T.; Xu, X.; Cheng, C.; Wang, J.; Yang, L. Cooperative Phototherapy Based on Bimodal Imaging Guidance for the Treatment of Uveal Melanoma. J. Nanobiotechnol. 2023, 21, 146. [Google Scholar] [CrossRef]
- Liu, P.; Zhou, Y.; Shi, X.; Yuan, Y.; Peng, Y.; Hua, S.; Luo, Q.; Ding, J.; Li, Y.; Zhou, W. A Cyclic Nano-Reactor Achieving Enhanced Photodynamic Tumor Therapy by Reversing Multiple Resistances. J. Nanobiotechnol. 2021, 19, 149. [Google Scholar] [CrossRef]
- Liang, J.; Jin, X.; Chen, B.; Hu, J.; Huang, Q.; Wan, J.; Hu, Z.; Wang, B. Doxorubicin-Loaded PH-Responsive Nanoparticles Coated with Chlorin E6 for Drug Delivery and Synergetic Chemo-Photodynamic Therapy. Nanotechnology 2020, 31, 195103. [Google Scholar] [CrossRef]
- Lv, X.; Min, J.; Huang, J.; Wang, H.; Wei, S.; Huang, C.; Dai, J.; Chen, Z.; Zhou, H.; Xu, Y.; et al. Simultaneously Controlling Inflammation and Infection by Smart Nanomedicine Responding to the Inflammatory Microenvironment. Adv. Sci. 2024, 11, 2403934. [Google Scholar] [CrossRef]
- Son, J.; Lee, D.; Yoo, J.; Park, C.; Koo, H. A Comparative Study of the Effect of Drug Hydrophobicity on Nanoparticle Drug Delivery in Vivo Using Two Photosensitizers. Nanomed. Nanotechnol. Biol. Med. 2020, 24, 102151. [Google Scholar] [CrossRef]
- Pegaz, B.; Debefve, E.; Borle, F.; Ballini, J.P.; van den Bergh, H.; Kouakou-Konan, Y.N. Encapsulation of Porphyrins and Chlorins in Biodegradable Nanoparticles: The Effect of Dye Lipophilicity on the Extravasation and the Photothrombic Activity. A Comparative Study. J. Photochem. Photobiol. B Biol. 2005, 80, 19–27. [Google Scholar] [CrossRef]
- Oba, T. Photosensitizer Nanoparticles for Photodynamic Therapy. Curr. Bioact. Compd. 2007, 3, 239–251. [Google Scholar] [CrossRef]
- Clement, S.; Chen, W.; Deng, W.; Goldys, E.M. X-Ray Radiation-Induced and Targeted Photodynamic Therapy with Folic Acid-Conjugated Biodegradable Nanoconstructs. Int. J. Nanomed. 2018, 13, 3553–3570. [Google Scholar] [CrossRef]
- Bazylińska, U.; Kulbacka, J.; Chodaczek, G. Nanoemulsion Structural Design in Co-Encapsulation of Hybrid Multifunctional Agents: Influence of the Smart Plga Polymers on the Nanosystem-Enhanced Delivery and Electro-Photodynamic Treatment. Pharmaceutics 2019, 11, 405. [Google Scholar] [CrossRef] [PubMed]
- Mollaeva, M.R.; Yabbarov, N.; Sokol, M.; Chirkina, M.; Mollaev, M.D.; Zabolotskii, A.; Seregina, I.; Bolshov, M.; Kaplun, A.; Nikolskaya, E. Optimization, Characterization and Pharmacokinetic Study of Meso-tetraphenylporphyrin Metal Complex-loaded Plga Nanoparticles. Int. J. Mol. Sci. 2021, 22, 12261. [Google Scholar] [CrossRef]
- Amin, M.L.; Kim, D.; Kim, S.J. Development of Hematin Conjugated PLGA Nanoparticle for Selective Cancer Targeting. Eur. J. Pharm. Sci. 2016, 91, 138–143. [Google Scholar] [CrossRef]
- González-Delgado, J.A.; Castro, P.M.; Machado, A.; Araújo, F.; Rodrigues, F.; Korsak, B.; Ferreira, M.; Tomé, J.P.C.; Sarmento, B. Hydrogels Containing Porphyrin-Loaded Nanoparticles for Topical Photodynamic Applications. Int. J. Pharm. 2016, 510, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.C.; Woranovicz-Barreira, S.M.; Faustino, M.A.F.; Fernandes, R.; Neves, M.G.P.M.S.; Tomé, A.C.; Gomes, N.C.M.; Almeida, A.; Cavaleiro, J.A.S.; Cunha, Â.; et al. Photodynamic Inactivation of Penicillium Chrysogenum Conidia by Cationic Porphyrins. Photochem. Photobiol. Sci. 2011, 10, 1735–1743. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Zheng, Y.; Pan, X.; Huang, Y.; Kang, Y.; Zhong, W.; Xu, K. Enzyme-Mediated Fabrication of Nanocomposite Hydrogel Microneedles for Tunable Mechanical Strength and Controllable Transdermal Efficiency. Acta Biomater. 2024, 174, 127–140. [Google Scholar] [CrossRef] [PubMed]
- Ito, F.; Yamada, H.; Kanamura, K.; Kawakami, H. Preparation of Biodegradable Polymer Nanospheres Containing Manganese Porphyrin (Mn-Porphyrin). J. Inorg. Organomet. Polym. Mater. 2019, 29, 1010–1018. [Google Scholar] [CrossRef]
- Ohse, T.; Kawakami, H.; Morita, A.; Nagaoka, S. Mn-Porphyrin Derivatives as an Antioxidant for Medical Devices. J. Biomater. Sci. Polym. Ed. 1999, 10, 917–929. [Google Scholar] [CrossRef]
- Laster, B.H.; Isaacson, C.; Perets, E.; Msamra, M.; Priel, E.; Kalef-Ezra, J.; Kost, J. Keeping Those Telomeres Short! An Innovative Intratumoral Long-Term Drug Delivery System. J. Cancer Res. Clin. Oncol. 2015, 141, 23–34. [Google Scholar] [CrossRef]
- Hu, Z.; Pan, Y.; Wang, J.; Chen, J.; Li, J.; Ren, L. Meso-Tetra (Carboxyphenyl) Porphyrin (TCPP) Nanoparticles Were Internalized by SW480 Cells by a Clathrin-Mediated Endocytosis Pathway to Induce High Photocytotoxicity. Biomed. Pharmacother. 2009, 63, 155–164. [Google Scholar] [CrossRef]
- Gong, X.; Milic, T.; Xu, C.; Batteas, J.D.; Drain, C.M. Preparation and Characterization of Porphyrin Nanoparticles. J. Am. Chem. Soc. 2002, 124, 14290–14291. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Wang, J.; Qiu, Y.; Qi, Z.; Song, D.; Wang, M. Tcpp-Isoliensinine Nanoparticles for Mild-Temperature Photothermal Therapy. Int. J. Nanomed. 2021, 16, 6797–6806. [Google Scholar] [CrossRef] [PubMed]
- Bruno, C.; Waeckerle-Men, Y.; Håkerud, M.; Kündig, T.M.; Gander, B.; Johansen, P. Photosensitizer and Light Pave the Way for Cytosolic Targeting and Generation of Cytosolic CD8 T Cells Using PLGA Vaccine Particles. J. Immunol. 2015, 195, 166–173. [Google Scholar] [CrossRef]
- Elberskirch, L.; Le Harzic, R.; Scheglmann, D.; Wieland, G.; Wiehe, A.; Mathieu-Gaedke, M.; Golf, H.R.A.; von Briesen, H.; Wagner, S. A HET-CAM Based Vascularized Intestine Tumor Model as a Screening Platform for Nano-Formulated Photosensitizers. Eur. J. Pharm. Sci. 2022, 168, 106046. [Google Scholar] [CrossRef]
- Geier, G.R.; Lindsey, J.S. Effects of Aldehyde or Dipyrromethane Substituents on the Reaction Course Leading to Meso-Substituted Porphyrins. Tetrahedron 2004, 60, 11435–11444. [Google Scholar] [CrossRef]
- Xu, Q.; Boylan, N.J.; Cai, S.; Miao, B.; Patel, H.; Hanes, J. Scalable Method to Produce Biodegradable Nanoparticles That Rapidly Penetrate Human Mucus. J. Control. Release 2013, 170, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Galliani, M.; Signore, G. Poly(Lactide-Co-Glycolide) Nanoparticles Co-Loaded with Chlorophyllin and Quantum Dots as Photodynamic Therapy Agents. Chempluschem 2019, 84, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Mai, B.; Jia, M.; Liu, S.; Sheng, Z.; Li, M.; Gao, Y.; Wang, X.; Liu, Q.; Wang, P. Smart Hydrogel-Based DVDMS/BFGF Nanohybrids for Antibacterial Phototherapy with Multiple Damaging Sites and Accelerated Wound Healing. ACS Appl. Mater. Interfaces 2020, 12, 10156–10169. [Google Scholar] [CrossRef]
- Park, J.S.; Yang, H.N.; Woo, D.G.; Jeon, S.Y.; Park, K.H. Multilineage Differentiation of Human-Derived Dermal Fibroblasts Transfected with Genes Coated on PLGA Nanoparticles plus Growth Factors. Biomaterials 2013, 34, 582–597. [Google Scholar] [CrossRef]
- Presley, K.F.; Reinsch, B.M.; Cybyk, D.B.; Ly, J.T.; Schweller, R.M.; Dalton, M.J.; Lannutti, J.J.; Grusenmeyer, T.A. Oxygen Sensing Performance of Biodegradable Electrospun Nanofibers: Influence of Fiber Composition and Core-Shell Geometry. Sens. Actuators B Chem. 2021, 329, 129191. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Zhang, Z.; Duan, D.; Zhang, Z.; Liu, H.; Liu, T.; Liu, Z. Tracing Boron with Fluorescence and Positron Emission Tomography Imaging of Boronated Porphyrin Nanocomplex for Imaging-Guided Boron Neutron Capture Therapy. ACS Appl. Mater. Interfaces 2018, 10, 43387–43395. [Google Scholar] [CrossRef]
- Chen, M.; Gao, S.; Dong, M.; Song, J.; Yang, C.; Howard, K.A.; Kjems, J.; Besenbacher, F. Chitosan/SiRNA Nanoparticles Encapsulated in PLGA Nanofibers for SiRNA Delivery. ACS Nano 2012, 6, 4835–4844. [Google Scholar] [CrossRef]
- Vepris, O.; Eich, C.; Feng, Y.; Fuentes, G.; Zhang, H.; Kaijzel, E.L.; Cruz, L.J. Optically Coupled PtOEP and DPA Molecules Encapsulated into PLGA-Nanoparticles for Cancer Bioimaging. Biomedicines 2022, 10, 1070. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Zhou, W.; Zhao, J.; Peng, Q.; Zhang, L.; Wang, Z.; Li, P.; Li, R. Hypoxia Modulation by Dual-Drug Nanoparticles for Enhanced Synergistic Sonodynamic and Starvation Therapy. J. Nanobiotechnology 2021, 19, 87. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic Efficacy of Nanoparticles and Routes of Administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Ricci-Júnior, E.; Marchetti, J.M. Preparation, Characterization, Photocytotoxicity Assay of PLGA Nanoparticles Containing Zinc (II) Phthalocyanine for Photodynamic Therapy Use. J. Microencapsul. 2006, 23, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Hung, H.I.; Klein, O.J.; Peterson, S.W.; Rokosh, S.R.; Osseiran, S.; Nowell, N.H.; Evans, C.L. PLGA Nanoparticle Encapsulation Reduces Toxicity While Retaining the Therapeutic Efficacy of EtNBS-PDT in Vitro. Sci. Rep. 2016, 6, 33234. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, G.; Jin, S.; Xu, L.; Zhao, C.X. Development of High-Drug-Loading Nanoparticles. Chempluschem 2020, 85, 2143–2157. [Google Scholar] [CrossRef] [PubMed]
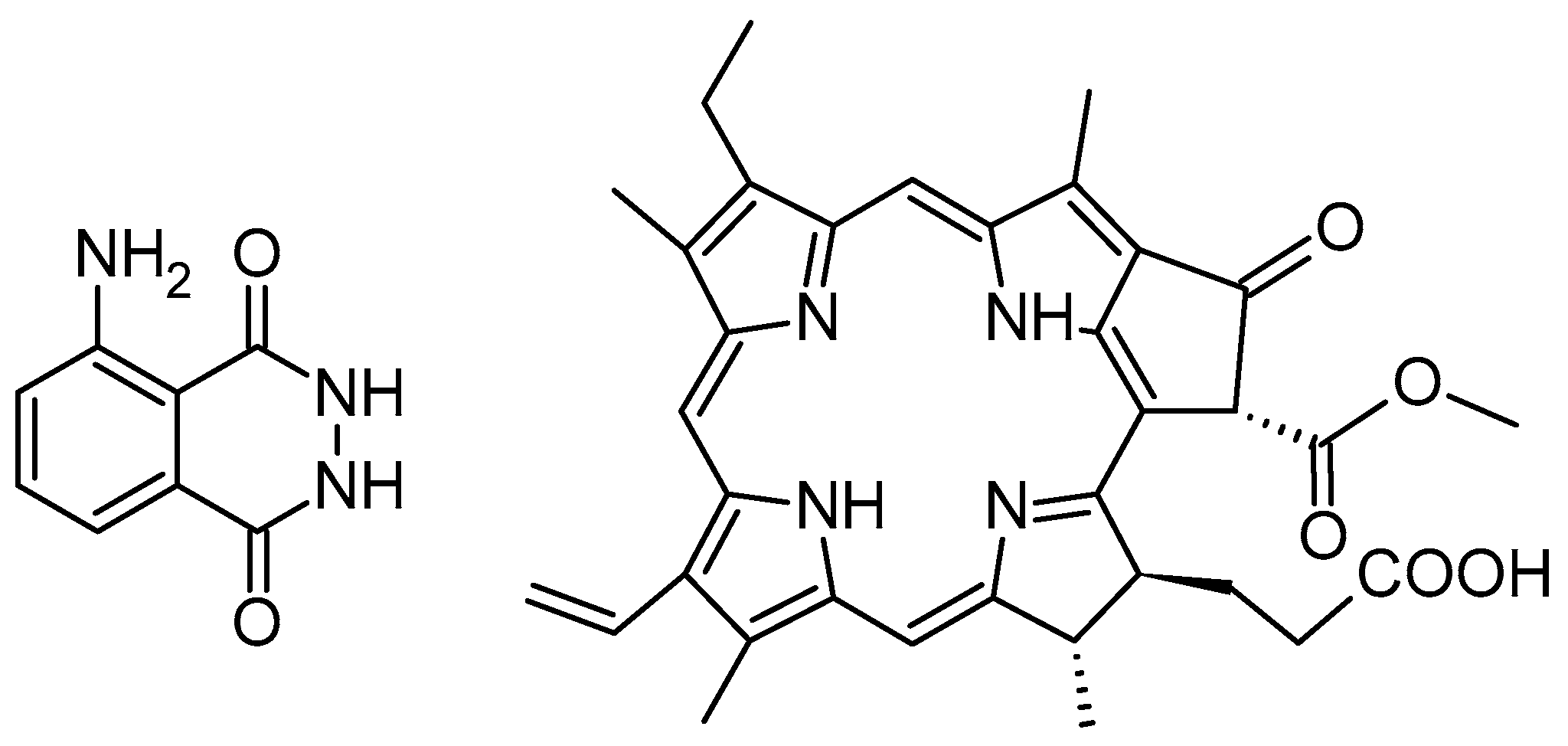
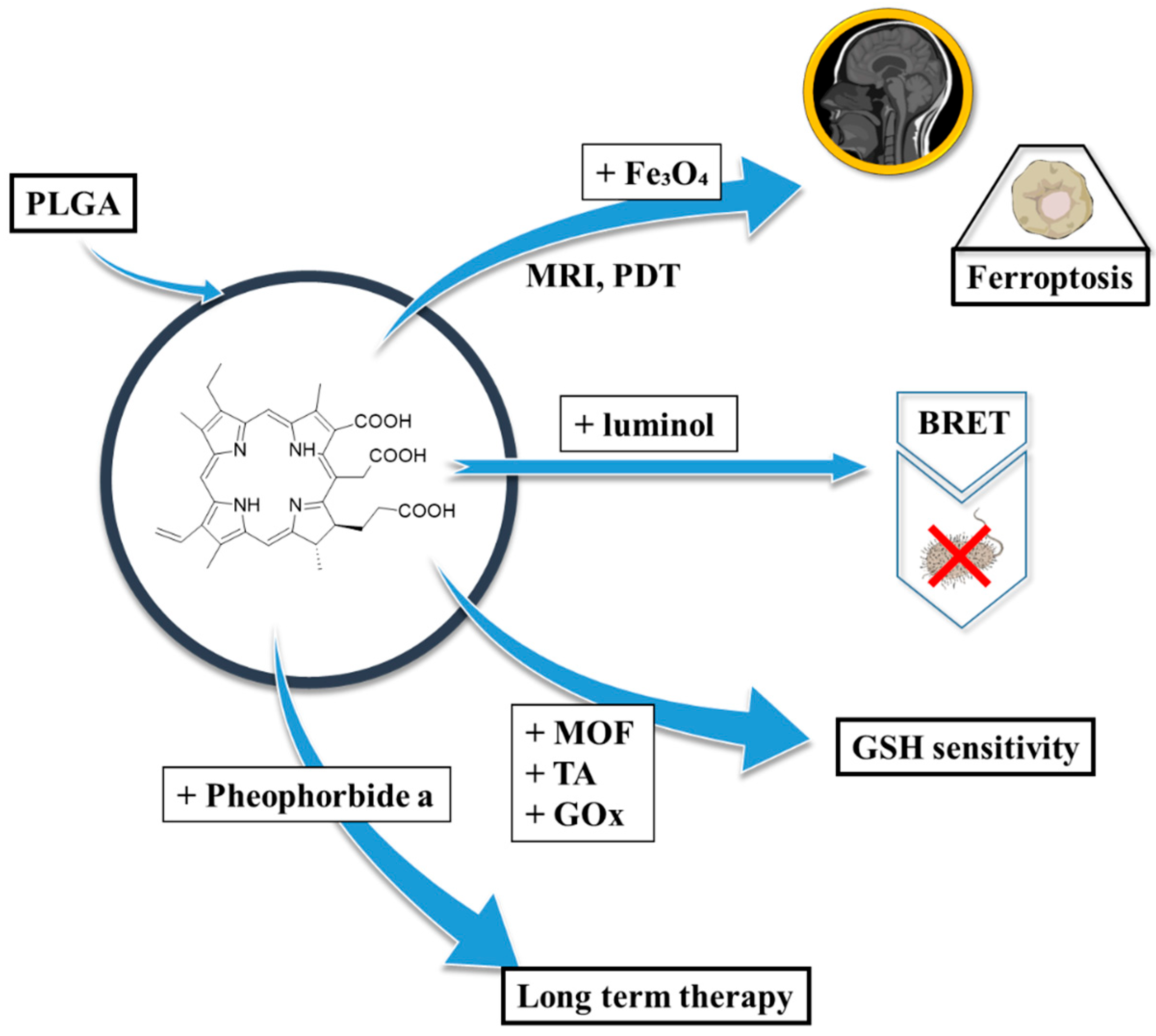
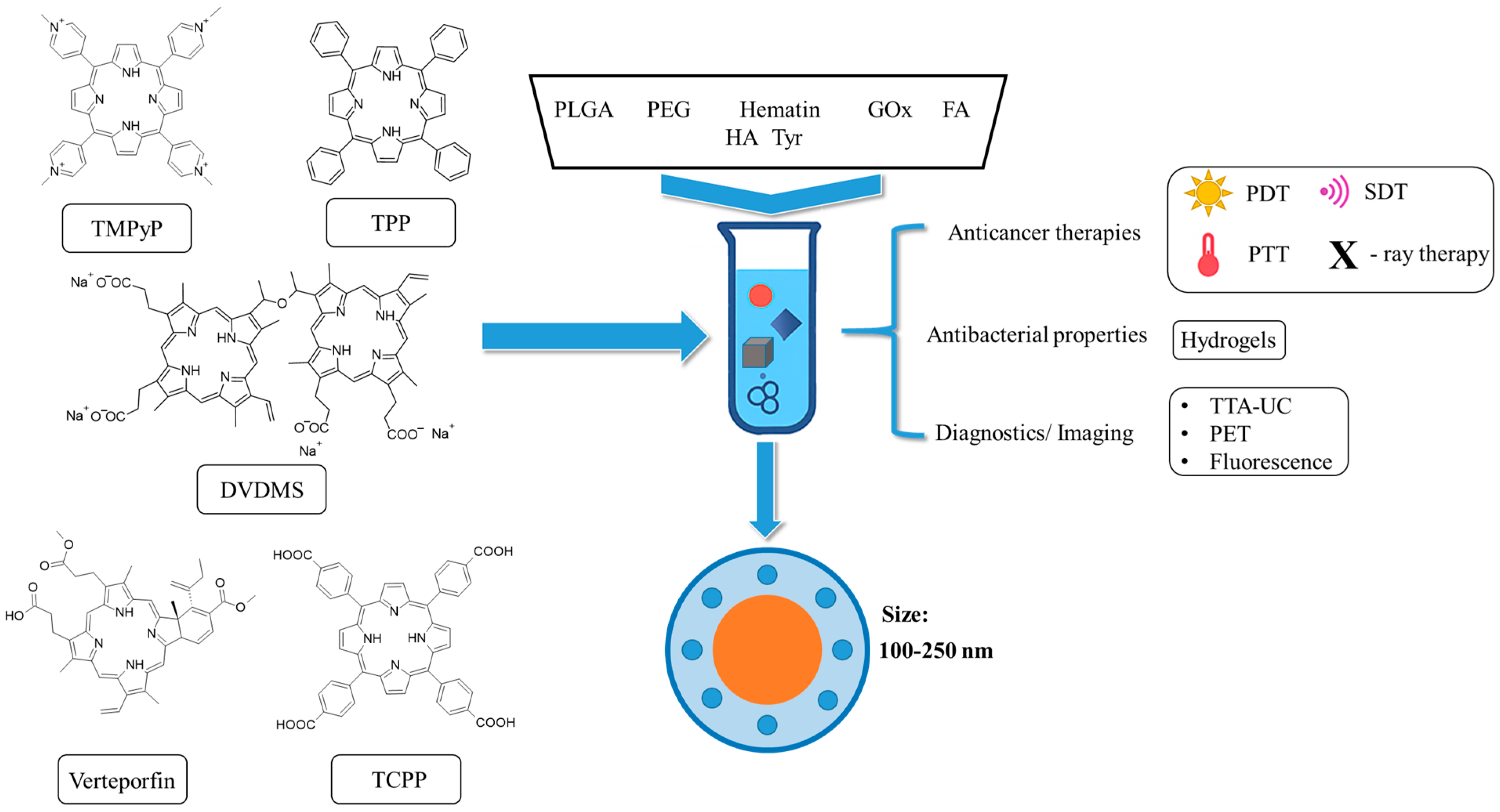
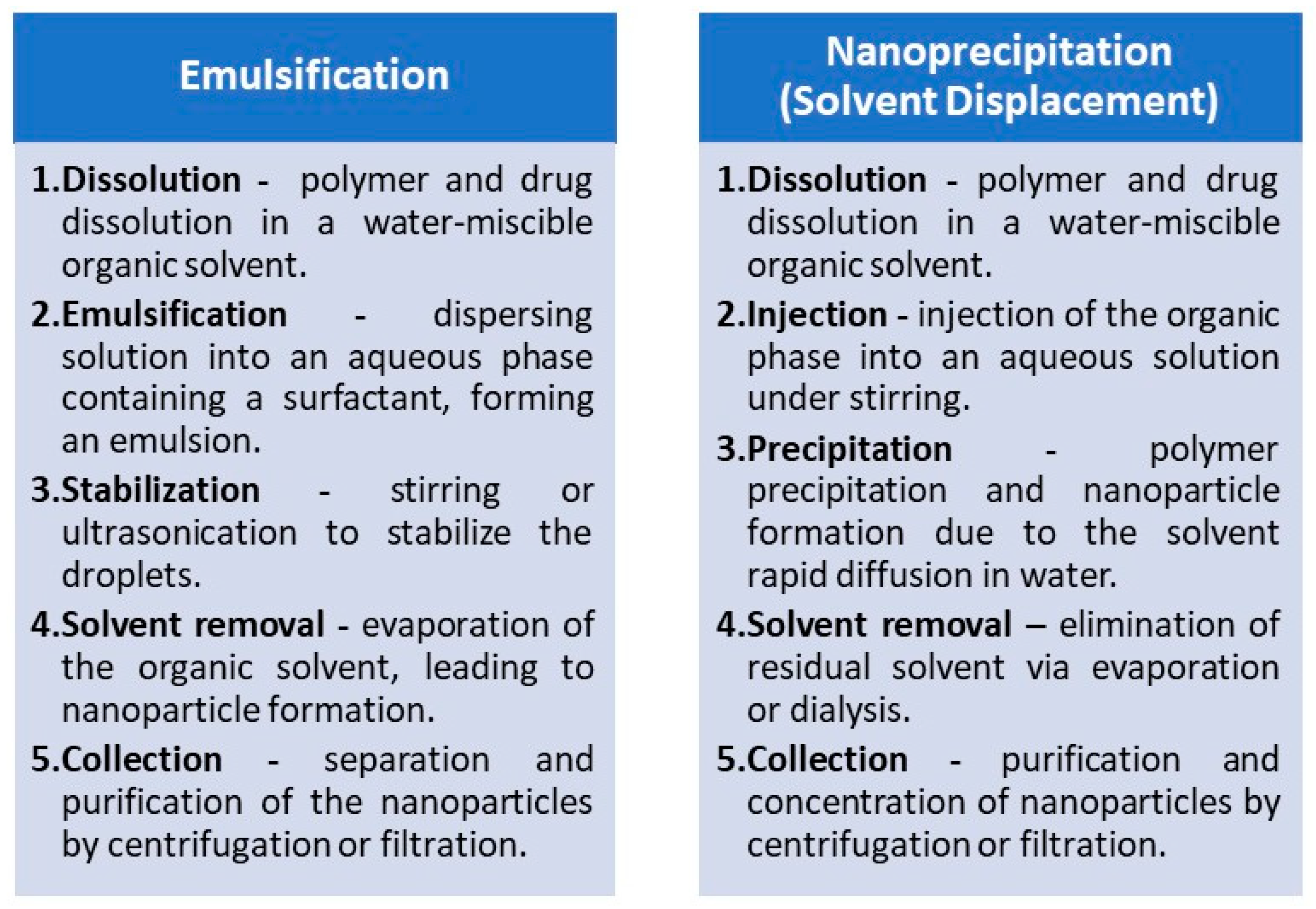
| Preparation Method | LA to GA Ratio | Size [nm] | Zeta Potential [mV] | PDI | Ref. |
|---|---|---|---|---|---|
| nanoprecipitation | 50:50 | 176.6 ± 17.55 | −38.0 ± 7.41 | 0.056 | [33] |
| nanoprecipitation | 50:50 | 200.0 ± 20 | Not specified | 0.05 ± 0.004 | [34] |
| nanoprecipitation | 50:50 | 200.0 ± 20 | Not specified | 0.05 | [35] |
| single-emulsion-solvent evaporation | 50:50 | 116.3 ± 36.7 | −11.9 ± 0.6 | - | [36] |
| single-emulsion-solvent evaporation | 50:50 | 241 ± 4 | −20 ± 1 | 0.07 ± 0.03 | [75] |
| emulsion-solvent evaporation | 50:50 | 99.6 ± 8.21 | −25.31 ± 1.04 | - | [78] |
| evaporation method | 50:50 and 75:25 | 117 ± 5 to 126 ± 7 | From −26.7 ± 1.5 to −25.9 ± 1.2 | 0.08 ± 0.02 to 0.14 ± 0.02 | [79] |
| Not specified | 50:50 | 180 ± 80 | −24 ± 6 | 0.2 | [98] |
| Name | Type of NP | LA to GA Ratio | Size [nm] | Zeta Potential [mV] | PDI | EE | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Preparation Method | DLS | TEM/SEM | ||||||
| PPIX/PLGA | nanoparticles | 50:50 | 290.0 ± 67.21 | - | −32.3 ± 8.16 | 0.233 | 67.7% | [33] |
| nanoprecipitation | ||||||||
| PPIX/PLGA | nanoparticles | 50:50 | 280 ± 60.2 | - | - | 0.22 ± 0.1 | 68.0 ± 12.0% | [34] |
| nanoprecipitation | ||||||||
| PPIX/PLGA | nanoparticles | 50:50 | 278 ± 60 | - | - | 0.22 | - | [35] |
| nanoprecipitation | ||||||||
| PPIX/HA-b-PLGA | core-shell micelles | 50:50 | 213.4 | 150 | −24.3 | 0.152 | 42.9 ± 2.0% | [37] |
| solvent dialysis | ||||||||
| PPIX/PLGA | nanoparticles | 50:50 | 320 | - | - | - | - | |
| nanoprecipitation | ||||||||
| GT-PLGA | shell-core nanofibers | NS | - | 110–150 µm | - | - | - | [41] |
| coaxial electrospinning technol. | ||||||||
| PPIX/GT-CS/PLGA | shell-core nanofibers | NS | - | 110–150 µm | - | - | - | |
| coaxial electrospinning technol. | ||||||||
| PPIX/GT-HA/PLGA | shell-core nanofibers | NS | - | 110–150 µm | - | - | - | |
| coaxial electrospinning technol. | ||||||||
| PPIX/PLGA | nanoparticles | 50:50 | 121.5 ± 44.5 | 33.6 ± 9 | −12.2 ± 1 | - | 13.7 ± 1.7 wt.% | [36] |
| single-emulsion-solvent evapor. | ||||||||
| UCNPs/PPIX/PLGA-PEG | nanoparticles | NS | - | 288.1 ± 49.2 | - | - | 6.24 ± 0.8 wt.% | [42] |
| nanoprecipitation | ||||||||
| LaF3:Ce3+/PPIX/PLGA | microspheres | NS | - | approx. 2 µm | - | - | - | [43] |
| emulsion/evaporation technique | ||||||||
| LaF3:Ce3+(NSCs)/PPIX/PEG-PLGA | core-shell structures | NS | 90 to 120 | - | −15 to −30 | <0.3 | 90% | [44] |
| nanoprecipitation | ||||||||
| PPIX/NSCs/PEG-PLGA | nanoparticles | NS | 96 ± 6 | - | −27.4 ± 0.5 | <0.25 | - | [45] |
| rac-[Ru(phen)2(IP-4T)](Cl)2/NSCs/PEG-PLGA | nanoparticles | NS | 118 ± 3 | - | −17.4 ± 0.7 | <0.25 | - | |
| Name | Type of NP | LA to GA Ratio | Size [nm] | Zeta Potential [mV] | PDI | EE | Ref. |
|---|---|---|---|---|---|---|---|
| Preparation Method | |||||||
| pTHPP/PLGA (50:50) | nanoparticles | 50:50 | 117 ± 7 | - | 0.20 | 76.9 ± 3.4% | [50] |
| emulsification-diffusion | |||||||
| pTHPP/PLGA (75:25) | nanoparticles | 75:25 | 118 ± 2 | - | 0.20 | 77.0 ± 7.0% | |
| emulsification-diffusion | |||||||
| pTHPP/PLA | nanoparticles | - | 125 ± 1 | - | 0.16 | 72.8 ± 8.7% | |
| emulsification-diffusion | |||||||
| pTHPP/PLGA (50:50) | nanoparticles | 50:50 | 93 ± 0–145 ± 1 | −5.8 ± 0.3 to −4.7 ± 1.4 | - | 56.2 ± 10.5–76.9 ± 3.4% | [51] |
| emulsification-diffusion | |||||||
| pTHPP/PLGA (75:25) | nanoparticles | 75:25 | 95 ± 6–157 ± 7 | −6.6 ± 1.9 to −4.2 ± 0.7 | - | 47.0 ± 3.2–77.4 ± 7.0% | |
| emulsification-diffusion | |||||||
| pTHPP/PLA | nanoparticles | - | 104 ± 1–134 ± 6 | −7.8 ± 1.1 to −4.3 ± 0.8 | - | 61.2 ± 0.5–91.1 ± 6.4% | |
| emulsification-diffusion | |||||||
| pTHPP/PLGA | nanoparticles | 50:50 | 117 ± 7 | - | 0.2 | [53] | |
| emulsification-diffusion | |||||||
| mTHPP/PLGA (6% PVA) | nanoparticles | 50:50 | 593 ± 15 | - | 0.08 | [54] | |
| emulsification-diffusion | |||||||
| mTHPP/PLGA (9% PVA) | nanoparticles | 50:50 | 285 ± 7 | - | 0.05 | ||
| emulsification-diffusion | |||||||
| mTHPP/PLGA (17% PVA) | nanoparticles | 50:50 | 117 ± 8 | - | 0.04 | ||
| emulsification-diffusion | |||||||
| mTHPP/PLGA | nanoparticles | 50:50 | 245.6 ± 9.9 | −41.8 ± 3.2 | 0.07 ± 0.01 | [55] | |
| emulsification-diffusion | |||||||
| mTHPP/PLA | nanoparticles | - | 285.6 ± 15.3 | −37.1 ± 5.6 | 0.16 ± 0.05 | ||
| emulsification-diffusion | |||||||
| mTHPP/ Eudragit-E® | nanoparticles | - | 213.6 ± 5.6 | +55.0 ± 3.4 | 0.06 ± 0.02 | ||
| emulsification-diffusion | |||||||
| pTHPP/PLGA | core-shell nanoparticles | 50:50 | 88.5 ± 3.4–94.6 ± 2.7 | −43.2 ± 1.6 to −48.6 ± 1.9 | 0.06 to 0.11 | 73.12 ± 3.17–88.91 ± 2.07% | [56] |
| nanoprecipitation | |||||||
| pTHPP/PHBV | core-shell nanoparticles | - | 213.7 ± 4.0–230 ± 5.9 | −35.4 ± 2.5 to −39.7 ± 1.6 | 0.10 to 0.14 | 67.47 ± 3.61–77.91 ± 3.83% | |
| nanoprecipitation | |||||||
| pTHPP/PLHNPs | PLGA-lipid hybrid NPs | 50:50 | 70.4 ± 1.4 | −39.2 ± 0.8 | - | 88.91 ± 2.07% | [57] |
| nanoprecipitation | |||||||
| Homo and sc-PLA/pTHPP@PLGA | nanoparticles in PLGA nanofibers | 75:25 | 200–500/546 | - | 0.1 to 0.4 | - | [58] |
| nanoprecipitation and electrospining | |||||||
| mTHPP-PLGA | nanoparticles | 50:50 | 107.6 ± 6.8 | −29.7 ± 2.6 | 0.07 ± 0.01 | - | [59] |
| solvent displacement | |||||||
| mTHPP-CS-PLGA | nanoparticles | 50:50 | 120.1 ± 4.2 | +10.3 ± 0.6 | 0.20 ± 0.01 | - | |
| solvent displacement | |||||||
| mTHPP-PLGA-PEG | nanoparticles | NS | 93.4 ± 2.3 | −19.8 ± 1.7 | 0.04 ± 0.01 | - | |
| solvent displacement | |||||||
| mTHPP-PLGA | nanoparticles | 50:50 | 107.6 ± 6.8 | −29.7 ± 2.6 | 0.07 ± 0.01 | - | [60] |
| solvent displacement | |||||||
| mTHPP-CS-PLGA | nanoparticles | 50:50 | 120.1 ± 4.2 | +10.3 ± 0.6 | 0.20 ± 0.01 | - | |
| solvent displacement | |||||||
| mTHPP-PLGA-PEG | nanoparticles | NS | 93.4 ± 2.3 | −19.8 ± 1.7 | 0.04 ± 0.01 | - | |
| solvent displacement | |||||||
| mTHPP-Pd/PLGA | nanoparticles | NS | 250 | - | <0.1 | - | [61] |
| emulsion diffusion |
| Name | Type of NP | LA to GA Ratio | Size [nm] | Zeta Potential [mV] | PDI | EE | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Preparation Method | DLS | TEM | ||||||
| Fe3O4-PLGA-Ce6 | nanoparticles | NS | ~100 | 85 | −30.1 | [67] | ||
| emulsion diffusion | ||||||||
| CPNPs (Ce6/PLGA) | nanoparticles | 50:50 | 232.58 ± 5.73 | −18.57 ± 1.875 | 0.036 | [68] | ||
| double-emulsion solvent evaporation | ||||||||
| FTCPNPs (Ce6/PLGA/Fe3+-TA) | core-shell NPs | 50:50 | 246.32 ± 5.34 | −29.70 ± 1.819 | 0.086 | 82.09 ± 2.332% (Ce6) | ||
| double-emulsion solvent evaporation | ||||||||
| PTFCG | core-shell nanocomposites | NS | ~175 | −33.4 | [69] | |||
| solvent exchange and evaporation | ||||||||
| PTFCG-M | core-shell nanocomposites | NS | +17.3 | |||||
| in situ growth | ||||||||
| PTFCG-MH | core-shell nanocomposites | NS | ~205 | ~160 | −21.7 | |||
| electrostatic coating | ||||||||
| DOX/PB-Ce6 | core-shell structures | NS | 164.18–531.17 | −38.4 | 56.18 ± 3.06% (DOX) | [70] | ||
| double emulsion-solvent evaporation | 46.16 ± 2.81% (Ce6) | |||||||
| Lum/Ce6-PLGA | nanoparticles | NS | 120 | [71] | ||||
| O/W single-emulsion | ||||||||
| Ce6-PLGA | nanoparticles | NS | ~160 | [72] | ||||
| self-assembly in aqueous conditions | ||||||||
| PLA/Ce6 | nanoparticles | NS | 183 ± 30 | 0.046 | 10.4 ± 1.4% | [73] | ||
| salting-out or emulsification-diffusion | ||||||||
| Name | Type of NP | LA to GA Ratio | Size [nm] | Zeta Potential [mV] | PDI | EE | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Preparation Method | DLS | TEM | ||||||
| PLA/TPP | nanoparticles | NS | 210 ± 11 | - | - | 0.323 | 87.2 ± 1.0% | [73] |
| salting-out | ||||||||
| PLA/TCPP | nanoparticles | NS | 194 ± 12 | - | - | 0.219 | 20.9 ± 0.5% | |
| salting-out | ||||||||
| PLA/Pheo | nanoparticles | NS | 233 ± 10 | - | - | 0.256 | 14.4 ± 0.7% | |
| salting-out | ||||||||
| PLGA-VP | nanoparticles | 50:50 | 241 ± 4–252 ± 4 | −20 ±1 to −23.3 ± 0.1 | 0.03 ± 0.01–0.09 ± 0.02 | - | 4.5–39.6 µM | [75] |
| single emulsion solvent evaporation | ||||||||
| NiTPP/PLGA | nanoparticles | 50:50 | 322.9 ± 9.7 | - | −14.7 ± 1.7 | 0.172 | 24.1 ± 0.9% | [77] |
| single emulsion solvent evaporation | ||||||||
| CoTPP/PLGA | nanoparticles | 50:50 | 344.5 ± 15.6 | - | −10.7 ± 2.3 | 0.191 | 79.7 ± 2.2% | |
| single emulsion solvent evaporation | ||||||||
| MnClTPP/PLGA | nanoparticles | 50:50 | 205.2 ± 10.2 | - | +18.1 ± 1.6 mV | 0.140 | 79.9 ± 1.8% | |
| single emulsion solvent evaporation | ||||||||
| PLGA-Arg | nanoparticles | 50:50 | 121.1 ± 11.19 | - | +6.02 ± 2.11 | - | - | [78] |
| emulsion solvent evaporation | ||||||||
| PLGA-Arg-Hematin | nanoparticles | 50:50 | 127.5 ± 9.93 | - | −15.19 ± 2.43 | - | - | |
| emulsion solvent evaporation | ||||||||
| PLGA + VP + CisPt | nanoparticles | 50:50 | 193 ± 6 | - | −9 ± 1 | 0.16 ± 0.01 | 92 ± 1% (CisPt) 97 ± 3% (VP) | [76] |
| double-emulsion solvent evaporation | ||||||||
| PLGA-PEG + VP + CisPt | nanoparticles | 50:50 | 187 ± 5 | - | −4 ± 1 | 0.12 ± 0.01 | 88 ± 1% (CisPt) 92 ± 1% (VP) | |
| double-emulsion solvent evaporation | ||||||||
| PLGA-FA + VP + CisPt | nanoparticles | 50:50 | 200 ± 7 | - | −15 ± 2 | 0.20 ± 0.02 | 90 ± 2% (CisPt) 95 ± 3% (VP) | |
| double-emulsion solvent evaporation | ||||||||
| PLGA-FA + VP | nanoparticles | 50:50 | 197 ± 7 | - | −16 ± 2 | 0.22 ± 0.02 | 96 ± 3 (VP) | |
| double-emulsion solvent evaporation | ||||||||
| PLGA-FA + CisPt | nanoparticles | 50:50 | 194 ± 6 | - | −16 ± 2 | 0.25 ± 0.02 | 92 ± 2 (CisPt) | |
| double-emulsion solvent evaporation | ||||||||
| PLGA-FA | nanoparticles | 50:50 | 189 ± 5 | - | −17 ± 3 | 0.10 ± 0.01 | - | |
| double-emulsion solvent evaporation | ||||||||
| TMPyP-PLGA | nanoparticles | 50:50 | 118 ± 5–133 ± 3 | - | −26.7 ± 3.0 to −21.6 ± 1.0 | 0.17 ± 0.04 to 0.18 ± 0.03 | 55.8 ± 1.1 to 92.5 ± 35% | [79] |
| evaporation method | ||||||||
| MP/TMPyP | nanoparticles | 75:25 | 117 | - | - | 0.066 | 59.7% | [81] |
| double-emulsion solvent evaporation | ||||||||
| TCPP/Iso/PEG-b-PLGA | nanoparticles | 50:50 | 87–108 | - | - | 0.2–0.4 | - | [87] |
| anti-solvent precipitation process | ||||||||
| PLGA/OVA | microparticles | 50:50 | 0.4–1.3 µm | - | −20 to–12 | - | 69% | [88] |
| double-emulsion solvent evaporation | ||||||||
| PLGA/OVA/TPCS2a | microparticles | 50:50 | 0.4–1.3 µm | - | −20 to–12 | - | 58% | |
| double-emulsion solvent evaporation | ||||||||
| PLGA-mTHPC-CP | nanoparticles | NS | 124.1 ± 2.8 | - | −52.8 ± 1.5 | 0.03 ± 0.01 | - | [89] |
| double-emulsion solvent evaporation | ||||||||
| PLGA-mTHPC-F127 | nanoparticles | NS | 115.4 ± 0.8 | - | −46,5 ± 4.5 | 0.09 ± 0.01 | - | |
| double-emulsion solvent evaporation | ||||||||
| PLGA-(Chl)-QD | nanoparticles | 75:25 | 169–220 | - | −30 | - | 20% (Chl) 90% (QDs) | [92] |
| nanoprecipitation method | ||||||||
| PLGA-bFGF | nanoparticles | 50:50 | 415 ± 25.6 | - | −9.86 ± 1.5 | - | - | [93] |
| double-emulsion solvent evaporation | ||||||||
| TBPP/PLGA | nanoparticles | 75:25 | ~100 | - | −39 | 0.1 | - | [96] |
| dialysis method | ||||||||
| TTA-UC PLGA | nanoparticles | 50:50 | 200 ± 50 | - | −31 ± 5 | 0.4 | 24.4% (PtOEP) 39.6% (DPA) | [98] |
| PLGA/PMnC/GOx | nanoparticles | 50:50 | 278.3 ± 40.96 | - | −26.7 ± 3.29 | - | 95.83 ± 1.35% (PMnC) 28.80 ± 1.96% (GOx) | [99] |
| double-emulsion solvent evaporation | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koza, P.; Kubiak, J.; Goslinski, T.; Koczorowski, T. Recent Advances in Pharmaceutical and Medical Applications in the Area of Selected Porphyrinoids Connected with PLGA or PLGA-Based Modalities. Polymers 2025, 17, 3190. https://doi.org/10.3390/polym17233190
Koza P, Kubiak J, Goslinski T, Koczorowski T. Recent Advances in Pharmaceutical and Medical Applications in the Area of Selected Porphyrinoids Connected with PLGA or PLGA-Based Modalities. Polymers. 2025; 17(23):3190. https://doi.org/10.3390/polym17233190
Chicago/Turabian StyleKoza, Patrycja, Jakub Kubiak, Tomasz Goslinski, and Tomasz Koczorowski. 2025. "Recent Advances in Pharmaceutical and Medical Applications in the Area of Selected Porphyrinoids Connected with PLGA or PLGA-Based Modalities" Polymers 17, no. 23: 3190. https://doi.org/10.3390/polym17233190
APA StyleKoza, P., Kubiak, J., Goslinski, T., & Koczorowski, T. (2025). Recent Advances in Pharmaceutical and Medical Applications in the Area of Selected Porphyrinoids Connected with PLGA or PLGA-Based Modalities. Polymers, 17(23), 3190. https://doi.org/10.3390/polym17233190







