Polyvinylpyrrolidone-Functionalized NiCo2O4 Electrodes for Advanced Asymmetric Supercapacitor Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
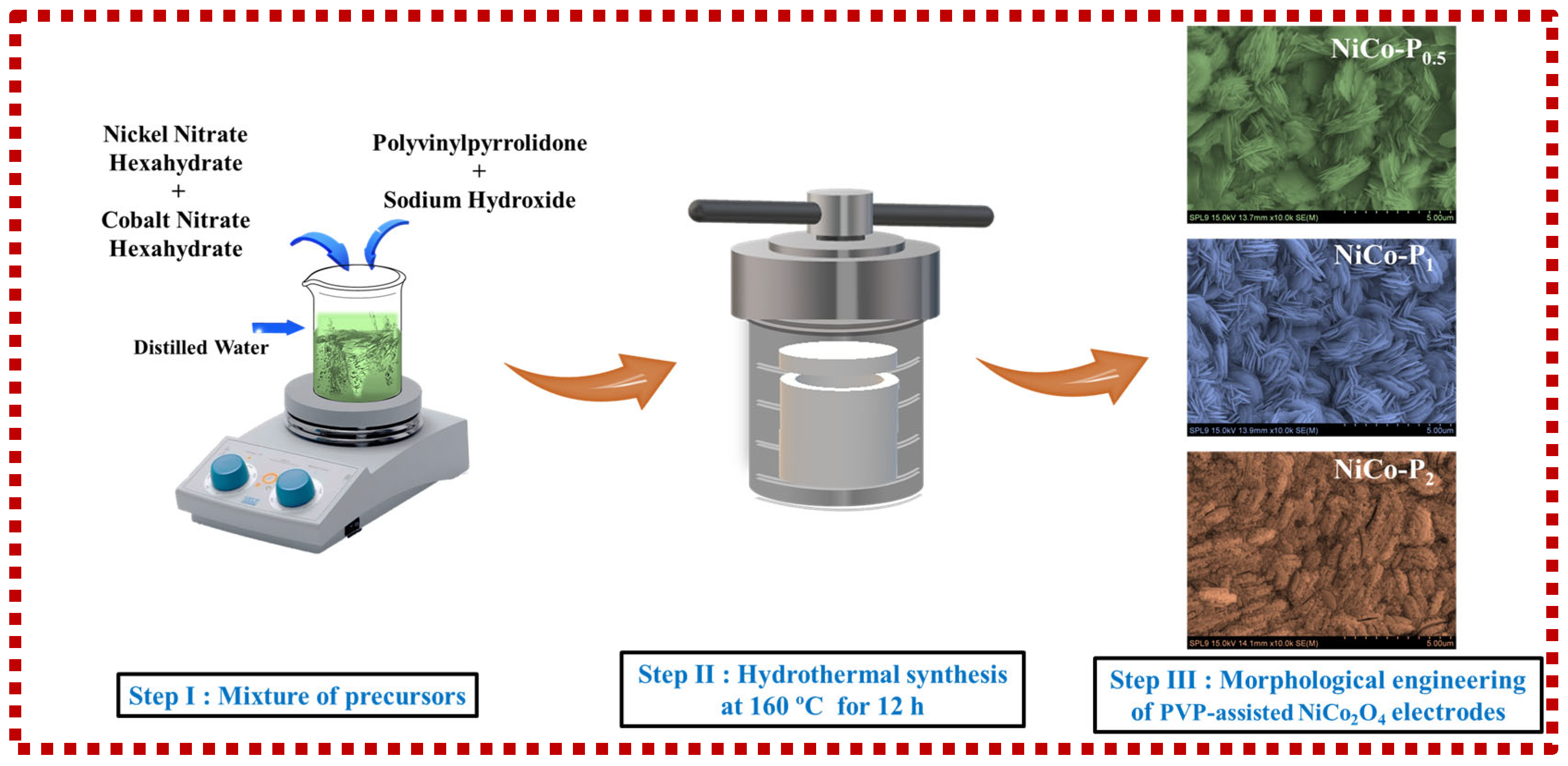
2.2. Synthesis of NiCo2O4 Electrodes
2.3. Sample Characterization and Electrochemical Measurements
3. Results and Discussion
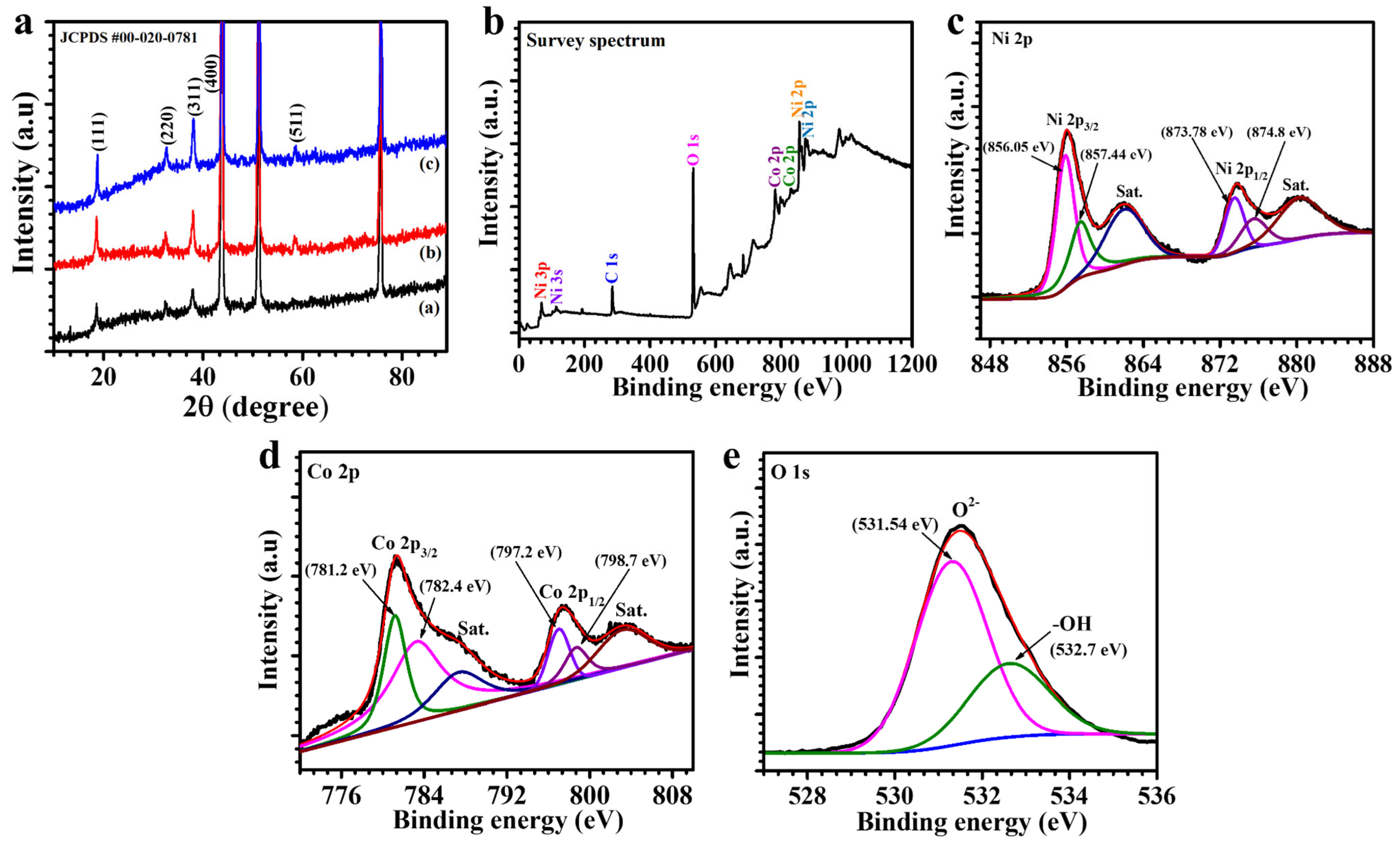
3.1. X-Ray Diffraction Elucidation
3.2. X-Ray Photoelectron Spectroscopy
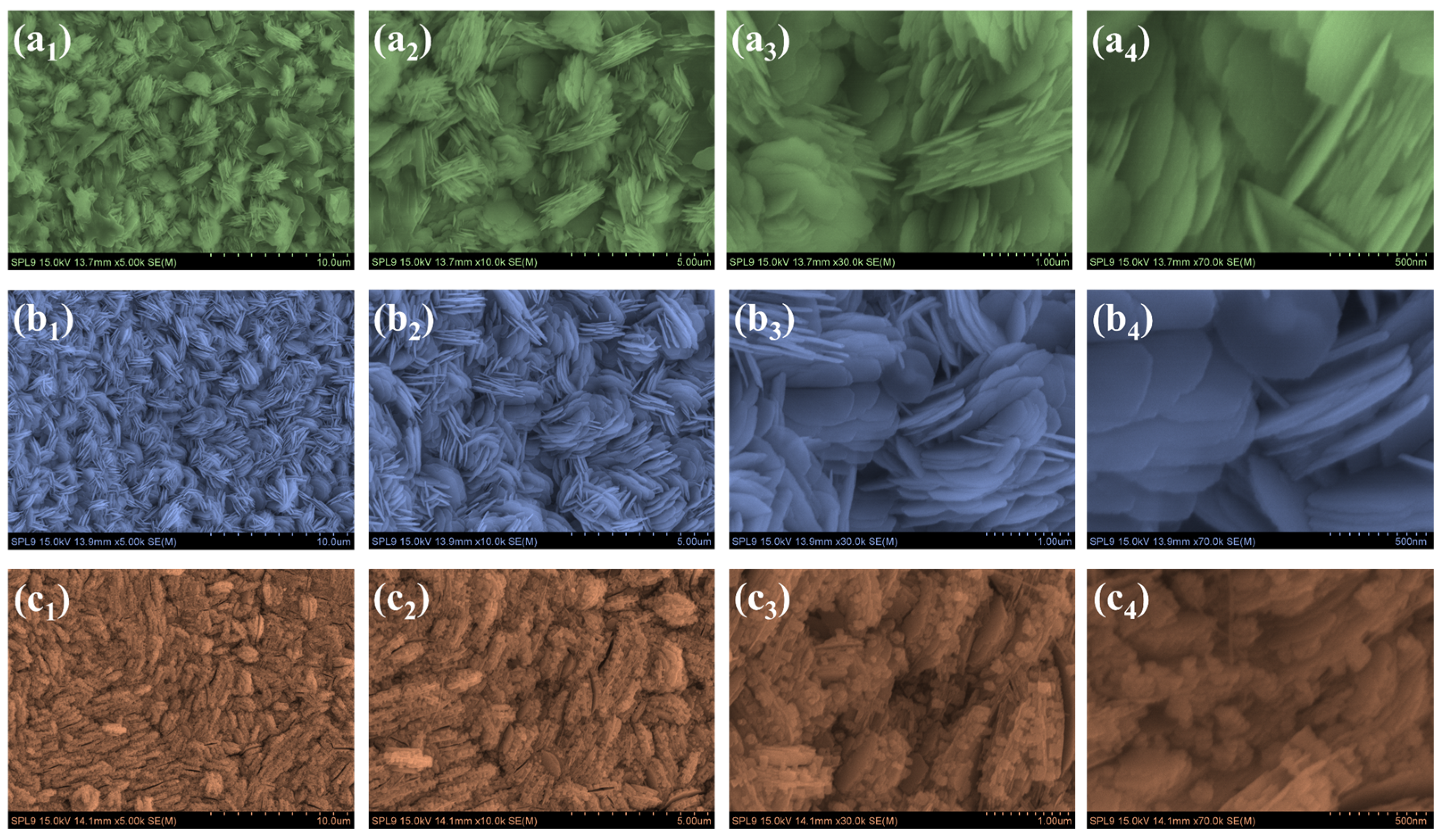
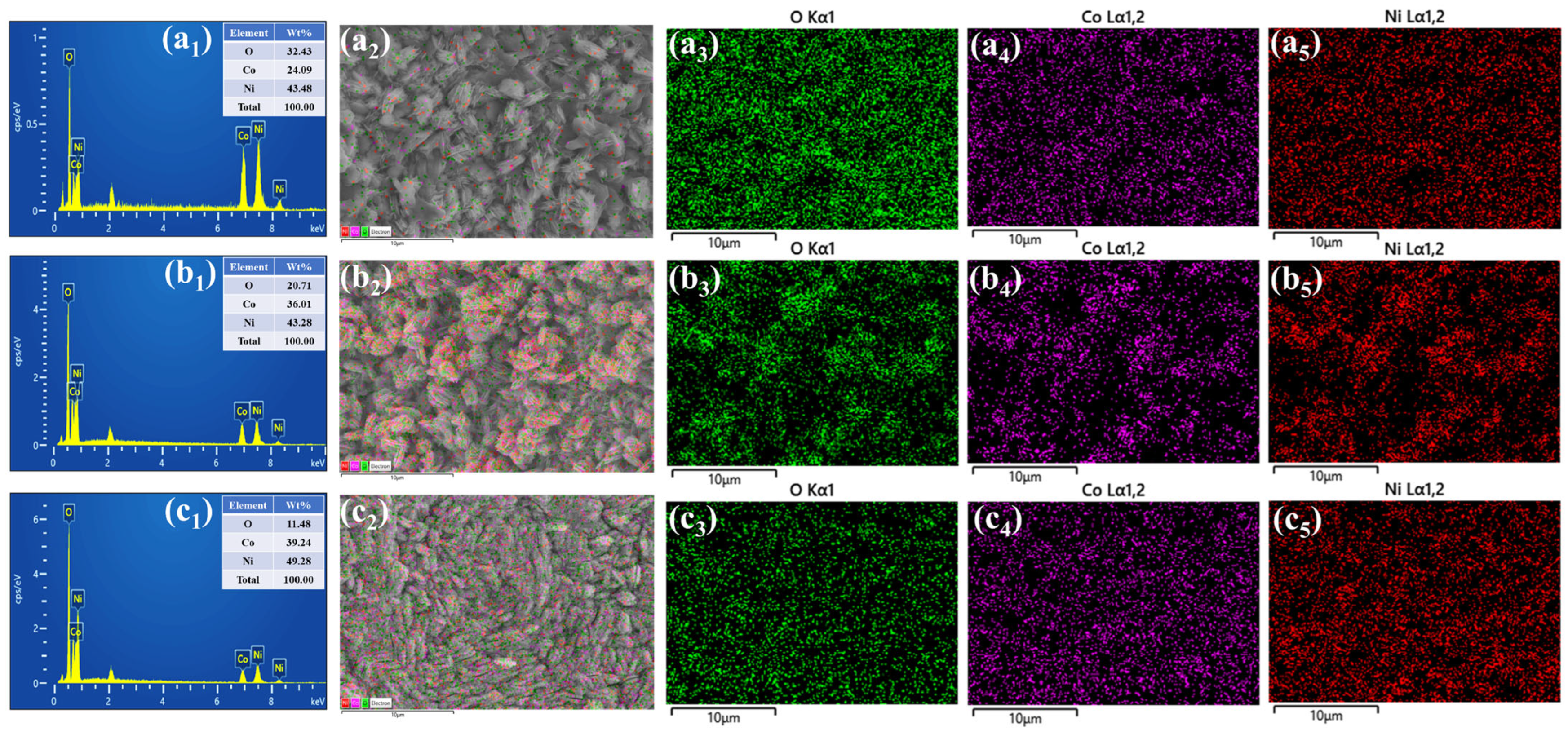
3.3. Morphological and Elemental Composition
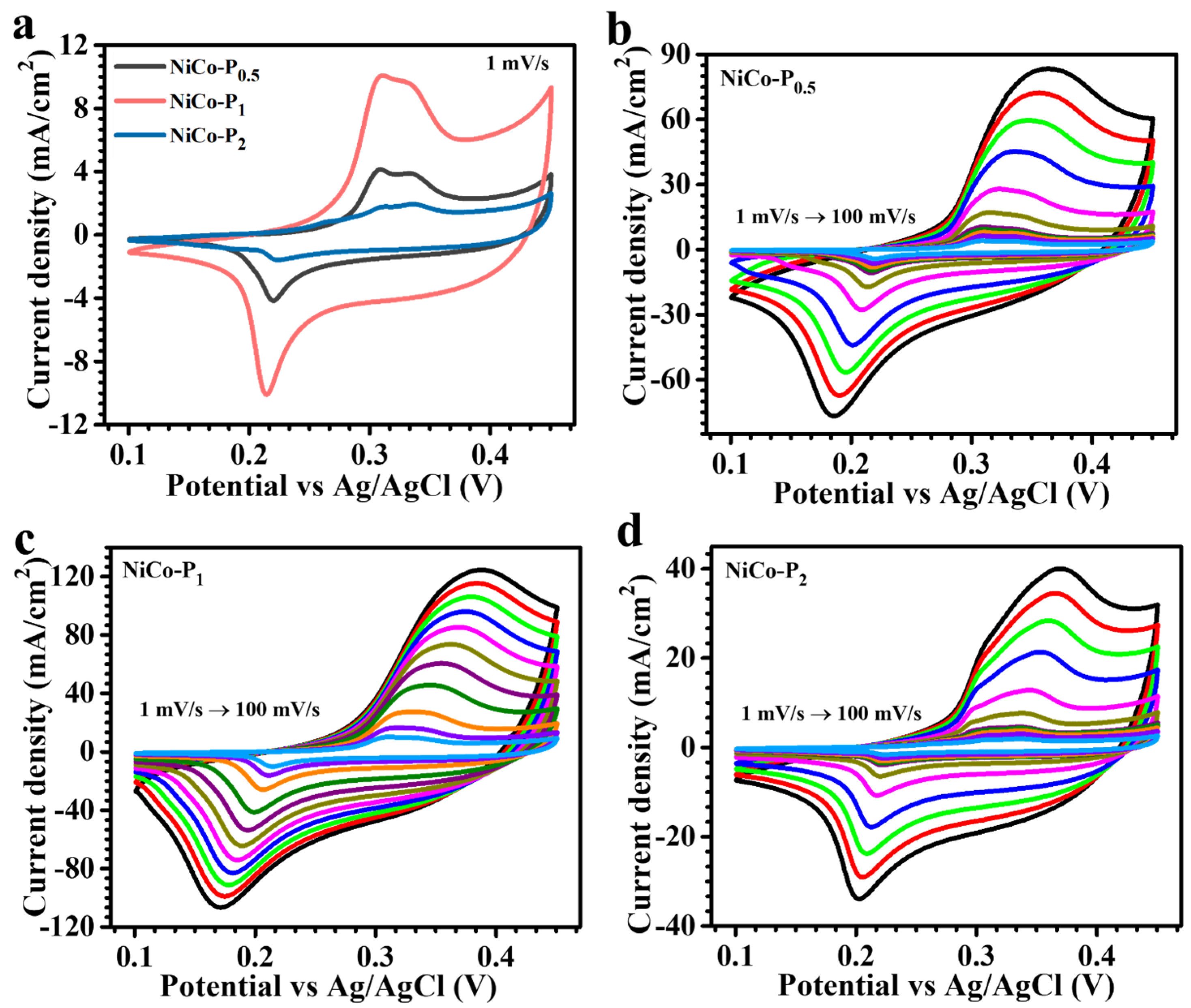
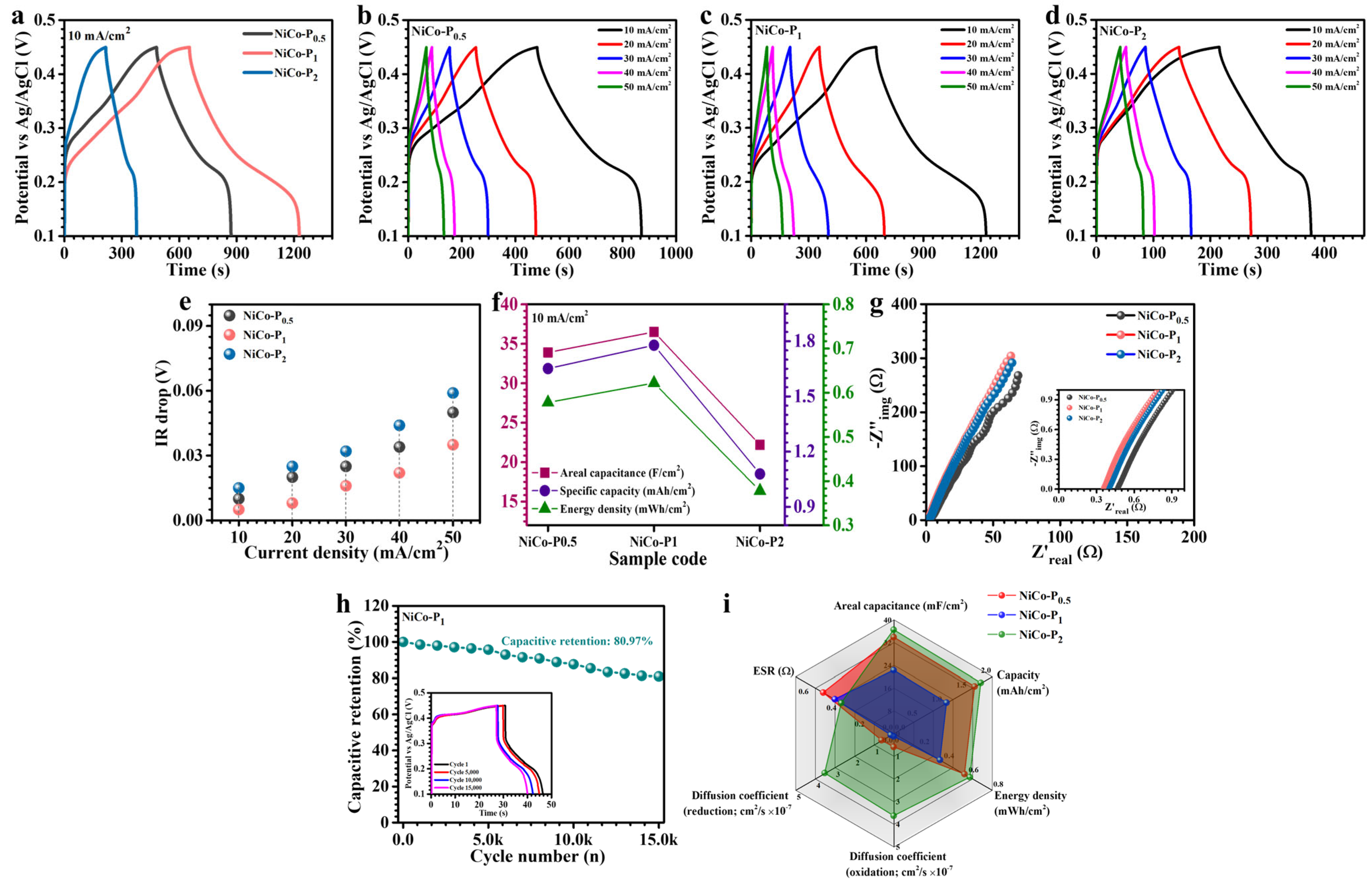
3.4. Electrochemical Analysis
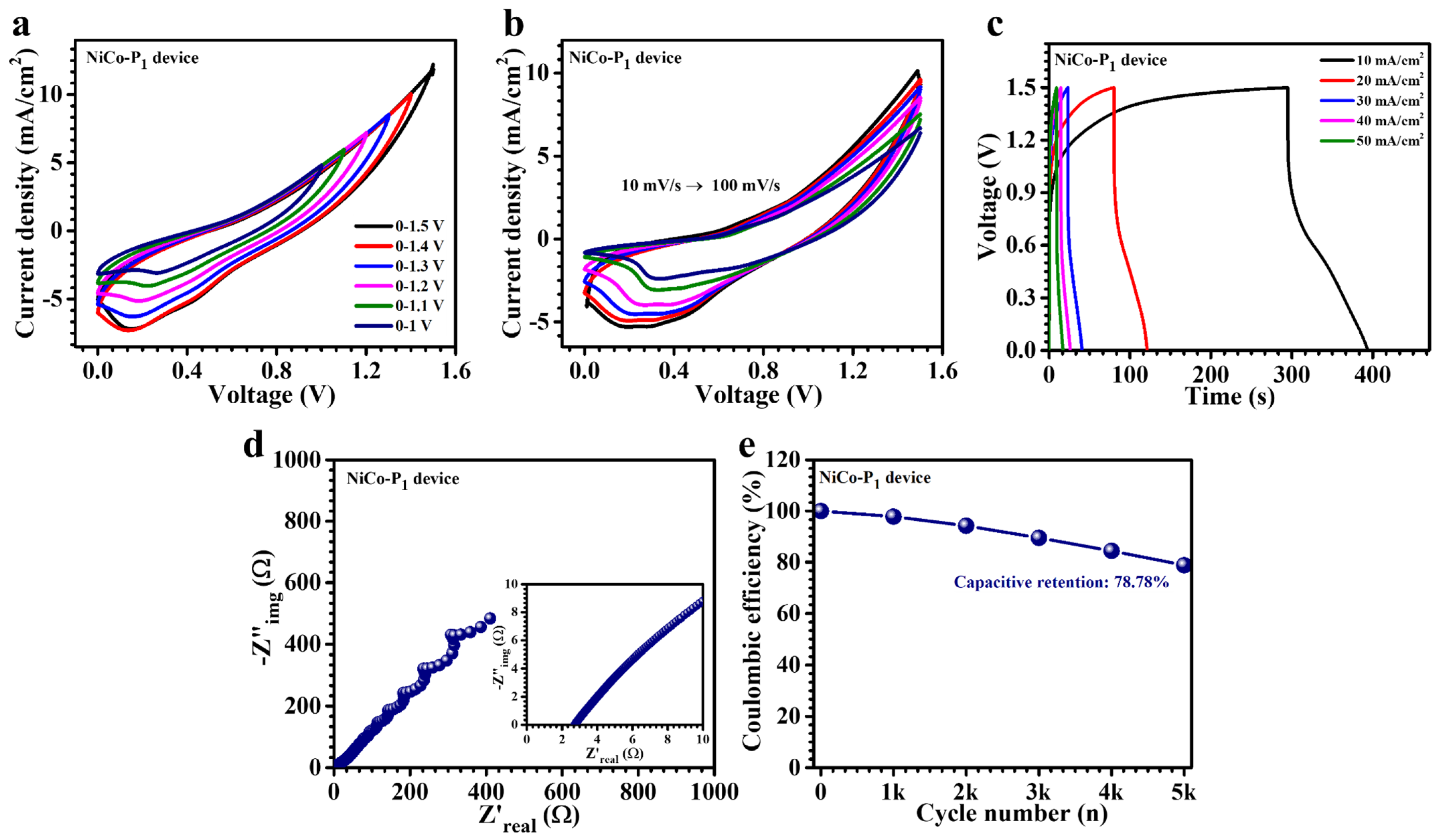
3.5. Electrochemical Performance of Asymmetric Supercapacitor Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chatterjee, D.P.; Nandi, A.K. A Review on the Recent Advances in Hybrid Supercapacitors. J. Mater. Chem. A 2021, 9, 15880–15918. [Google Scholar] [CrossRef]
- Choi, H.J.; Jung, S.M.; Seo, J.M.; Chang, D.W.; Dai, L.; Baek, J.B. Graphene for Energy Conversion and Storage in Fuel Cells and Supercapacitors. Nano Energy 2012, 1, 534–551. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon Nanomaterials for High-Performance Supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Tomy, M.; Rajappan, A.A.; Vm, V.; Suryabai, X.T. Emergence of Novel 2D Materials for High-Performance Supercapacitor Electrode Applications: A Brief Review. Energy Fuels 2021, 35, 19881–19900. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and Challenges for a Sustainable Energy Future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef]
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280. [Google Scholar] [CrossRef]
- Lim, J.M.; Jang, Y.S.; Van, T.; Nguyen, H.; Kim, J.S.; Yoon, Y.; Park, B.J.; Seo, D.H.; Lee, K.K.; Han, Z.; et al. Advances in High-Voltage Supercapacitors for Energy Storage Systems: Materials and Electrolyte Tailoring to Implementation. Nanoscale Adv. 2023, 5, 615–626. [Google Scholar] [CrossRef]
- Baena-Moncada, A.M.; Quispe-Garrido, V.; Antonio Cerron-Calle, G.; Bazan-Aguilar, A.; Ruiz-Montoya, J.G.; López, E.O. Advances in the Design and Application of Transition Metal Oxide-Based Supercapacitors. Open Chem. 2021, 19, 709–725. [Google Scholar] [CrossRef]
- Tundwal, A.; Kumar, H.; Binoj, B.J.; Sharma, R.; Kumar, G.; Kumari, R.; Dhayal, A.; Yadav, A.; Singh, D.; Kumar, P. Developments in Conducting Polymer-, Metal Oxide-, and Carbon Nanotube-Based Composite Electrode Materials for Supercapacitors: A Review. RSC Adv. 2024, 14, 9406–9439. [Google Scholar] [CrossRef]
- Dar, M.A.; Dinagaran, S.; Govindarajan, D.; Ahamed, S.R.; Habib, F.; Siva, C.; Moholkar, A.V.; Ahmad, Z.; Yatoo, M.A. Snx-0MnxS Nanomaterial Based Electrodes for Future-Generation Supercapacitor and Data Storage Devices. J. Alloys Compd. 2023, 958, 170523. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Lai-Peng, M.; Cheng, H.M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, 28–62. [Google Scholar] [CrossRef]
- Hao, L.; Li, X.; Zhi, L. Carbonaceous Electrode Materials for Supercapacitors. Adv. Mater. 2013, 25, 3899–3904. [Google Scholar] [CrossRef]
- Tatrari, G.; Ahmed, M.; Shah, F.U. Synthesis, Thermoelectric and Energy Storage Performance of Transition Metal Oxides Composites. Coord. Chem. Rev. 2024, 498, 215470. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, E.; He, W.; Deng, X.; Huang, J.; Ding, M.; Wei, X.; Liu, X.; Xu, X. NiCo2O4-Based Supercapacitor Nanomaterials. Nanomaterials 2017, 7, 41. [Google Scholar] [CrossRef]
- Li, Y.; Han, X.; Yi, T.; He, Y.; Li, X. Review and Prospect of NiCo2O4-Based Composite Materials for Supercapacitor Electrodes. J. Energy Chem. 2019, 31, 54–78. [Google Scholar] [CrossRef]
- Zhu, Y.; Ji, X.; Wu, Z.; Song, W.; Hou, H.; Wu, Z.; He, X.; Chen, Q.; Banks, C.E. Spinel NiCo2O4 for Use as a High-Performance Supercapacitor Electrode Material: Understanding of Its Electrochemical Properties. J. Power Sources 2014, 267, 888–900. [Google Scholar] [CrossRef]
- Yewale, M.A.; Kadam, R.A.; Kaushik, N.K.; Vattikuti, S.V.P.; Lingamdinne, L.P.; Koduru, J.R.; Shin, D.K. Hydrothermally Synthesized Microrods and Microballs of NiCo2O4 for Supercapacitor Application. Ceram. Int. 2022, 48, 22037–22046. [Google Scholar] [CrossRef]
- Liu, X.; Shi, S.; Xiong, Q.; Li, L.; Zhang, Y.; Tang, H.; Gu, C.; Wang, X.; Tu, J. Hierarchical NiCo2O4@NiCo2O4 Core/Shell Nanoflake Arrays as High-Performance Supercapacitor Materials. ACS Appl. Mater. Interfaces 2013, 5, 8790–8795. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Wang, W.; Luo, X.; Chen, C.; Zeng, Y.; Zhu, Z. NiCo2O4 with Oxygen Vacancies as Better Performance Electrode Material for Supercapacitor. Chem. Eng. J. 2018, 334, 864–872. [Google Scholar] [CrossRef]
- Mary, A.J.C.; Bose, A.C. Surfactant assisted ZnCo2O4 nanomaterial for supercapacitor application. Appl. Surf. Sci. 2018, 449, 105–112. [Google Scholar] [CrossRef]
- Li, Q.; Lu, C.; Chen, C.; Xie, L.; Liu, Y.; Li, Y.; Kong, Q.; Wang, H. Layered NiCo2O4/Reduced Graphene Oxide Composite as an Advanced Electrode for Supercapacitor. Energy Storage Mater. 2017, 8, 59–67. [Google Scholar] [CrossRef]
- Ulisso, D.M.; Mane, S.A.; Chavan, R.A.; Kamble, G.P.; Kolekar, S.S.; Ghule, A.V. Multilayered Core-Shell NiCo-Layered Double Hydroxide@NiCo2O4 Composite Electrode for High-Performance Supercapacitor. J. Alloys Compd. 2024, 980, 173563. [Google Scholar] [CrossRef]
- Park, J.; Jo, S.; Kitchamsetti, N.; Zaman, S.; Kim, D. The Development of NiCo2O4/PVP/PANI Heterogeneous Nanocomposites as an Advanced Battery-Type Electrode Material for High-Performing Supercapacitor Application. J. Alloys Compd. 2022, 926, 166815. [Google Scholar] [CrossRef]
- Huang, C.; Ding, Y.; Hao, C.; Zhou, S.; Wang, X.; Gao, H.; Zhu, L.; Wu, J. PVP-Assisted Growth of Ni-Co Oxide on N-Doped Reduced Graphene Oxide with Enhanced Pseudocapacitive Behavior. Chem. Eng. J. 2019, 378, 122202. [Google Scholar] [CrossRef]
- Yuan, C.; Li, J.; Hou, L.; Lin, J.; Zhang, X.; Xiong, S. Polymer-Assisted Synthesis of a 3D Hierarchical Porous Network-like Spinel NiCo2O4 Framework towards High-Performance Electrochemical Capacitors. J. Mater. Chem. A 2013, 1, 11145–11151. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Xu, J.; Jaber, F.; Musharavati, F.; Zalezhad, E.; Bae, S.; Hui, K.S.; Hui, K.N.; Liu, J. Synthesis and Characterization of a NiCo2C4@NiCo2C4 Hierarchical Mesoporous Nanoflake Electrode for Supercapacitor Applications. Nanomaterials 2020, 10, 1292. [Google Scholar] [CrossRef]
- Khalid, S.; Cao, C.; Wang, L.; Zhu, Y. Microwave Assisted Synthesis of Porous NiCo2O4 Microspheres: Application as High Performance Asymmetric and Symmetric Supercapacitors with Large Areal Capacitance. Sci. Rep. 2016, 6, 22699. [Google Scholar] [CrossRef]
- Zou, R.; Xu, K.; Wang, T.; He, G.; Liu, Q.; Liu, X.; Zhang, Z.; Hu, J. Chain-like NiCo2O4 Nanowires with Different Exposed Reactive Planes for High-Performance Supercapacitors. J. Mater. Chem. A 2013, 1, 8560–8566. [Google Scholar] [CrossRef]
- Tao, K.; Yang, Y.; Yang, C.; Ma, Q.; Han, L. Construction of NiCo2O4 Nanosheet-Decorated Leaf-like Co3O4 Nanoarrays from Metal-Organic Framework for High-Performance Hybrid Supercapacitors. Dalton Trans. 2019, 48, 14156–14163. [Google Scholar] [CrossRef]
- Lv, X.; Lei, T.; Wang, B.; Chen, W.; Jiao, Y.; Hu, Y.; Yan, Y.; Huang, J.; Chu, J.; Yan, C.; et al. An Efficient Separator with Low Li-Ion Diffusion Energy Barrier Resolving Feeble Conductivity for Practical Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1901800. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, X.; Zhang, Z.; You, J.; Liu, S.; Wang, Y. Macro-/Meso-Porous NiCo2O4 Synthesized by Template-Free Solution Combustion to Enhance the Performance of a Nonenzymatic Amperometric Glucose Sensor. Microchim. Acta 2020, 187, 64. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Han, Z.; Zheng, X.; Yao, S.; Yang, X.; Zhai, T. Core-Shell Structured Co3O4@NiCo2O4 Electrodes Grown on Flexible Carbon Fibers with Superior Electrochemical Properties. Nano Energy 2017, 31, 410–417. [Google Scholar] [CrossRef]
- Moradlou, O.; Sharifpour, H. Interconnected NiCo2S4—Coated NiO Nanosheet Arrays as Electrode Materials for High-Performance Supercapacitors. J. Energy Storage 2020, 32, 101886. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Son, L.T.; Van Thuy, V.; Thao, V.D.; Hatsukano, M.; Higashimine, K.; Maenosono, S.; Chun, S.-E.; Thu, T.V. Facile Synthesis of Mn-Doped NiCo2O4 Nanoparticles with Enhanced Electrochemical Performance for a Battery-Type Supercapacitor Electrode. Dalton Trans. 2020, 49, 6718–6729. [Google Scholar] [CrossRef] [PubMed]
- Abu El-Fadl, A.; Mohammed, M.M.M.; Mansour, H.R.; Nashaat, A.M.; Abbady, G. The Impact of Different Surfactants on the Structure, Surface Area, and Electrochemical Properties of Hydrothermally Synthesized Spinel NiCo2O4 Nanoparticles. J. Mater. Sci. Mater. Electron. 2023, 34, 584. [Google Scholar] [CrossRef]
- Amate, R.U.; Morankar, P.J.; Chavan, G.T.; Teli, A.M.; Desai, R.S.; Dalavi, D.S.; Jeon, C.W. Bi-Functional Electrochromic Supercapacitor Based on Hydrothermal-Grown 3D Nb2O5 Nanospheres. Electrochim. Acta 2023, 459, 142522. [Google Scholar] [CrossRef]
- Amedzo-Adore, M.; Han, J.I. Surfactant-Assisted NiCo2S4 for Redox Supercapacitors. Batteries 2024, 10, 360. [Google Scholar] [CrossRef]
- Wu, Z.; Zhu, Y.; Ji, X. NiCo2O4-Based Materials for Electrochemical Supercapacitors. J. Mater. Chem. A 2014, 2, 14759–14772. [Google Scholar] [CrossRef]
- Morankar, P.J.; Sreekanth, T.V.M.; Amate, R.U.; Yewale, M.A.; Teli, A.M.; Beknalkar, S.A.; Jeon, C.W. Asymmetric Supercapacitor Performance Enhancement Through Fe-Doped MoS2 Nanosheets Synthesized via Hydrothermal Method. Coatings 2024, 14, 1328. [Google Scholar] [CrossRef]
- Lan, Y.; Zhao, H.; Zong, Y.; Li, X.; Sun, Y.; Feng, J.; Wang, Y.; Zheng, X.; Du, Y. Phosphorization Boosts the Capacitance of Mixed Metal Nanosheet Arrays for High Performance Supercapacitor Electrodes. Nanoscale 2018, 10, 11775–11781. [Google Scholar] [CrossRef]
- Wei, F.; Xu, P.; Xu, C.; Han, M.; Ran, S.; Lv, Y. High-Rate Performance Zinc-Ion Hybrid Capacitors Constructed by Multi-Layered Carbon Nanosheet Cathode. Ionics 2022, 28, 1419–1426. [Google Scholar] [CrossRef]
- Padalkar, N.S.; Dubal, D.P.; Park, J.P. Self-Assembled Polyoxovanadate-Intercalated Layered Double Hydroxide Nanosheets Hybridized with Graphene Oxide for Extrinsic Supercapacitors. J. Mater. Chem. A 2024, 12, 13901–13914. [Google Scholar] [CrossRef]
- Sadavar, S.V.; Padalkar, N.S.; Shinde, R.B.; Patil, A.S.; Patil, U.M.; Magdum, V.V.; Chitare, Y.M.; Kulkarni, S.P.; Bulakhe, R.N.; Parale, V.G.; et al. Graphene Oxide as an Efficient Hybridization Matrix for Exploring Electrochemical Activity of Two-Dimensional Cobalt-Chromium-Layered Double Hydroxide-Based Nanohybrids. ACS Appl. Energy Mater. 2022, 5, 2083–2095. [Google Scholar] [CrossRef]
- Gunjakar, J.L.; Inamdar, A.I.; Hou, B.; Cha, S.; Pawar, S.M.; Abu Talha, A.A.; Chavan, H.S.; Kim, J.; Cho, S.; Lee, S.; et al. Direct Growth of 2D Nickel Hydroxide Nanosheets Intercalated with Polyoxovanadate Anions as a Binder-Free Supercapacitor Electrode. Nanoscale 2018, 10, 8953–8961. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ling, H.; Huang, Q.; Yang, Y.; Wang, X.; Chen, R.; Ling, H.; Huang, Q.; Yang, Y.; Wang, X. Interface Engineering on Cellulose-Based Flexible Electrode Enables High Mass Loading Wearable Supercapacitor with Ultrahigh Capacitance and Energy Density. Small 2022, 18, 2106356. [Google Scholar] [CrossRef] [PubMed]
- Šedajová, V.; Nandi, D.; Langer, P.; Lo, R.; Hobza, P.; Plachá, D.; Bakandritsos, A.; Zbořil, R. Direct Upcycling of Highly Efficient Sorbents for Emerging Organic Contaminants into High Energy Content Supercapacitors. J. Colloid Interface Sci. 2025, 692, 137481. [Google Scholar] [CrossRef]
- Wagh, K.S.; Mane, S.M.; Teli, A.M.; Shin, J.C.; Lee, J. Recent Advancements in Co3O4-Based Composites for Enhanced Electrocatalytic Water Splitting. Micromachines 2024, 15, 1450. [Google Scholar] [CrossRef]
- Reddy, B.J.; Vickraman, P.; Justin, A.S. Electrochemical Performance of Nitrogen-Doped Graphene Anchored Nickel Sulfide Nanoflakes for Supercapacitors. Appl. Surf. Sci. 2019, 483, 1142–1148. [Google Scholar] [CrossRef]
- Ye, Y.; Luo, Y.; Lou, J.; Chen, X.; Cheng, Y.J.; Xia, J.; Li, Y.; Guo, K. Hollow Spherical NiCo2S4@N-CNT Composites with High Energy Density for All-Solid-State Supercapacitors. ACS Appl. Energy Mater. 2023, 6, 6742–6751. [Google Scholar] [CrossRef]
- Huang, J.; Wei, J.; Xiao, Y.; Xu, Y.; Xiao, Y.; Wang, Y.; Tan, L.; Yuan, K.; Chen, Y. When Al-Doped Cobalt Sulfide Nanosheets Meet Nickel Nanotube Arrays: A Highly Efficient and Stable Cathode for Asymmetric Supercapacitors. ACS Nano 2018, 12, 3030–3041. [Google Scholar] [CrossRef]
- Zhou, W.; Kong, D.; Jia, X.; Ding, C.; Cheng, C.; Wen, G. NiCo2O4 Nanosheet Supported Hierarchical Core–Shell Arrays for High-Performance Supercapacitors. J. Mater. Chem. A 2014, 2, 6310–6315. [Google Scholar] [CrossRef]
- Yadav, K.; Ovhal, M.M.; Parmar, S.; Gaikwad, N.; Datar, S.; Kang, J.W.; Patro, T.U. NiCo2O4 Nanoneedle-Coated 3D Reticulated Vitreous Porous Carbon Foam for High-Performance All-Solid-State Supercapacitors. ACS Appl. Nano Mater. 2024, 7, 2312–2324. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Ye, J.; Wan, P.; Wei, H.; Zhao, S.; Li, T.; Hussain, S. NiCo2O4 Arrays Nanostructures on Nickel Foam: Morphology Control and Application for Pseudocapacitors. Ceram. Int. 2016, 42, 14976–14983. [Google Scholar] [CrossRef]
- Wei, H.; Guo, X.; Wang, Y.; Zhou, Z.; Lv, H.; Zhao, Y.; Gu, Z.; Chen, Z. Inherently porous Co3O4@ NiO core–shell hierarchical material for excellent electrochemical performance of supercapacitors. Appl. Surf. Sci. 2022, 574, 151487. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Chu, Y.; Li, L.; Yu, Q.; Zhu, Y.; Liu, G.; Hou, Q.; Zeng, R.; Zhao, L. Self-assembled Co3O4 nanostructure with controllable morphology towards high performance anode for lithium ion batteries. Electrochim. Acta 2016, 188, 909–916. [Google Scholar] [CrossRef]
- Bai, R.; Luo, X.; Zhen, D.; Ci, C.; Zhang, J.; Wu, D.; Cao, M.; Liu, Y. Facile Fabrication of Comb-like Porous NiCo2O4 Nanoneedles on Ni Foam as an Advanced Electrode for High-Performance Supercapacitor. Int. J. Hydrog. Energy 2020, 45, 32343–32354. [Google Scholar] [CrossRef]
- Zhang, G.; Lou, X.W. General Solution Growth of Mesoporous NiCo2O4 Nanosheets on Various Conductive Substrates as High-Performance Electrodes for Supercapacitors. Adv. Mater. 2013, 25, 976–979. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, T.; Yu, X.; Zhang, H.; Duan, H.; Lu, B. Nanoforest of Hierarchical Co3O4@NiCo2O4 Nanowire Arrays for High-Performance Supercapacitors. Nano Energy 2013, 2, 586–594. [Google Scholar] [CrossRef]
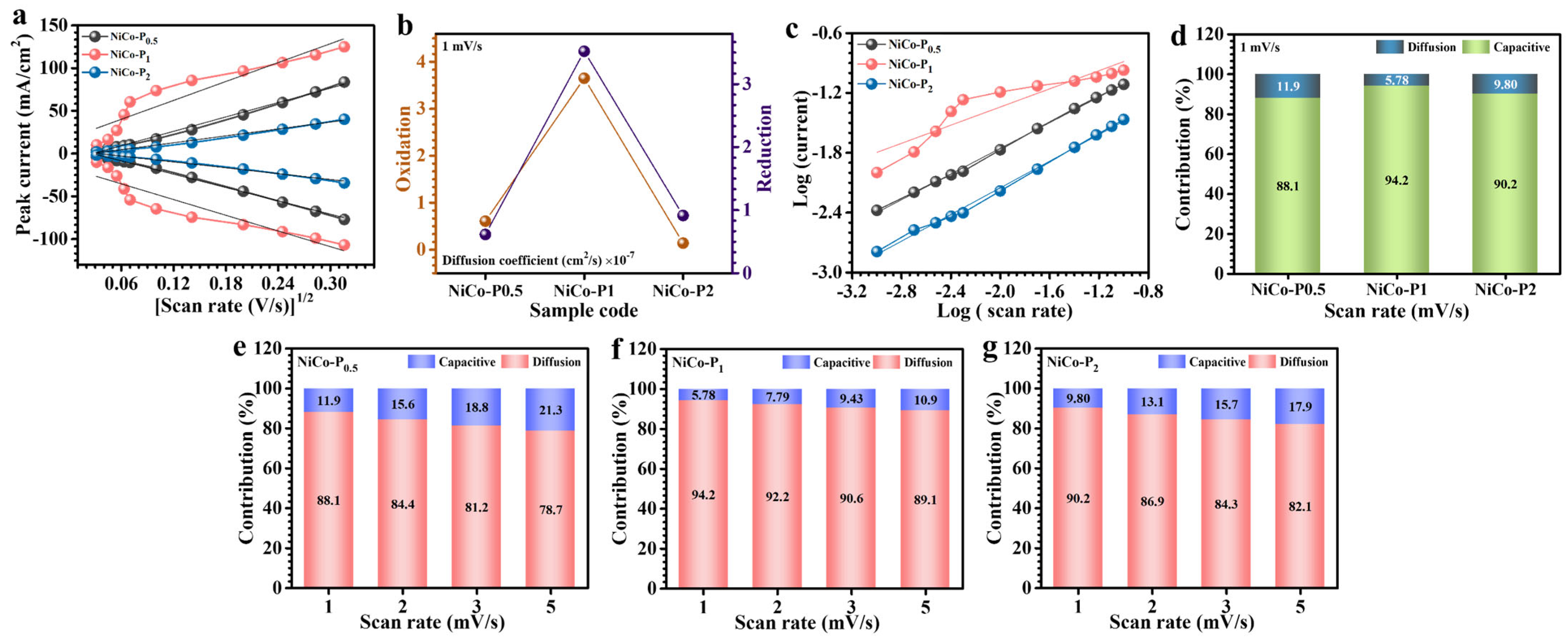
| Sample Code | Diffusion Coefficient (cm2/s) × 10−7 | b-Value | Rs (Ω) | |
|---|---|---|---|---|
| Oxidation | Reduction | |||
| NiCo-P0.5 | 0.608 | 0.615 | 0.64 | 0.503 |
| NiCo-P1 | 3.65 | 3.52 | 0.45 | 0.374 |
| NiCo-P2 | 0.14 | 0.15 | 0.66 | 0.42 |
| Sample Code | I (mA//cm2) | CA (F/cm2) | C (mAh/cm2) | ED (mWh/cm2) | PD (mW/cm2) |
|---|---|---|---|---|---|
| NiCo-P0.5 | 10 | 33.9 | 1.651 | 0.578 | 4.59 |
| 20 | 31.5 | 1.532 | 0.536 | 9.24 | |
| 30 | 29 | 1.413 | 0.494 | 13.45 | |
| 40 | 20.9 | 1.016 | 0.356 | 16.74 | |
| 50 | 17.1 | 0.833 | 0.292 | 20.00 | |
| NiCo-P1 | 10 | 36.5 | 1.778 | 0.622 | 3.36 |
| 20 | 31.8 | 1.548 | 0.542 | 6.59 | |
| 30 | 29.3 | 1.429 | 0.500 | 9.05 | |
| 40 | 23.5 | 1.143 | 0.400 | 11.64 | |
| 50 | 21.2 | 1.032 | 0.361 | 12.96 | |
| NiCo-P2 | 10 | 22.2 | 1.079 | 0.378 | 4.67 |
| 20 | 20.6 | 1.000 | 0.350 | 10.79 | |
| 30 | 17.1 | 0.833 | 0.292 | 15.75 | |
| 40 | 16.3 | 0.764 | 0.278 | 19.61 | |
| 50 | 13.9 | 0.675 | 0.236 | 26.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amate, R.U.; Bhosale, M.K.; Morankar, P.J.; Teli, A.M.; Jeon, C.-W. Polyvinylpyrrolidone-Functionalized NiCo2O4 Electrodes for Advanced Asymmetric Supercapacitor Application. Polymers 2025, 17, 1802. https://doi.org/10.3390/polym17131802
Amate RU, Bhosale MK, Morankar PJ, Teli AM, Jeon C-W. Polyvinylpyrrolidone-Functionalized NiCo2O4 Electrodes for Advanced Asymmetric Supercapacitor Application. Polymers. 2025; 17(13):1802. https://doi.org/10.3390/polym17131802
Chicago/Turabian StyleAmate, Rutuja U., Mrunal K. Bhosale, Pritam J. Morankar, Aviraj M. Teli, and Chan-Wook Jeon. 2025. "Polyvinylpyrrolidone-Functionalized NiCo2O4 Electrodes for Advanced Asymmetric Supercapacitor Application" Polymers 17, no. 13: 1802. https://doi.org/10.3390/polym17131802
APA StyleAmate, R. U., Bhosale, M. K., Morankar, P. J., Teli, A. M., & Jeon, C.-W. (2025). Polyvinylpyrrolidone-Functionalized NiCo2O4 Electrodes for Advanced Asymmetric Supercapacitor Application. Polymers, 17(13), 1802. https://doi.org/10.3390/polym17131802






