Micropatterned Poly(3,4-ethylenedioxythiophene) Thin Films with Improved Color-Switching Rates and Coloration Efficiency
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rai, V.; Singh, R.S.; Blackwood, D.J.; Zhili, D. A review on recent advances in electrochromic devices: A material approach. Adv. Energy Mater. 2020, 22, 2000082. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, J.; Lin, G.; Wang, S.; Zhou, Y.; Yin, J.; Long, Y. Smart Windows: Electro-, Thermo-, Mechano-, Photochromics, and Beyond. Adv. Energy Mater. 2019, 9, 1902066. [Google Scholar] [CrossRef]
- Granqvist, C.G.; Arvizu, M.A.; Pehlivan, İ.B.; Qu, H.Y.; Wen, R.T.; Niklasson, G.A. Electrochromic materials and devices for energy efficiency and human comfort in buildings: A critical review. Electrochim. Acta 2018, 259, 1170–1182. [Google Scholar] [CrossRef] [Green Version]
- Gueye, M.N.; Carella, A.; Faure-Vincent, J.; Demadrille, R.; Simonato, J.P. Progress in understanding structure and transport properties of PEDOT-based materials: A critical review. Prog. Mater. Sci. 2020, 108, 100616. [Google Scholar] [CrossRef]
- Groenendaal, L.; Zotti, G.; Aubert, P.H.; Waybright, S.M.; Reynolds, J.R. Electrochemistry of poly (3, 4-alkylenedioxythiophene) derivatives. Adv. Mater. 2003, 15, 855–879. [Google Scholar] [CrossRef]
- Mishra, S.P.; Krishnamoorthy, K.; Sahoo, R.; Kumar, A. Synthesis and characterization of monosubstituted and disubstituted poly (3, 4-propylenedioxythiophene) derivatives with high electrochromic contrast in the visible region. J. Polym. Sci. A Polym. Chem. 2005, 43, 419–428. [Google Scholar] [CrossRef]
- Dominguez-Alfaro, A.; Gómez, I.J.; Alegret, N.; Mecerreyes, D.; Prato, M. 2D and 3D Immobilization of Carbon Nanomaterials into PEDOT via Electropolymerization of a Functional Bis-EDOT Monomer. Polymers 2021, 13, 436. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Ouyang, J. Review on application of PEDOTs and PEDOT:PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, J. Scientific importance of water-processable PEDOT–PSS and preparation, challenge and new application in sensors of its film electrode: A review. J. Polym. Sci. A Polym. Chem. 2017, 55, 1121–1150. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, R.J.; Dyer, A.L.; Reynolds, J.R. Electrochromic organic and polymeric materials for display applications. Displays 2006, 27, 2–18. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.Z.; Li, M.M.; Gu, C.; Wan, M.; Duvail, J.L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Cho, S.I.; Kwon, W.J.; Choi, S.-J.; Kim, P.; Park, S.-A.; Kim, J.; Son, S.J.; Xiao, R.; Kim, S.-H.; Lee, S.B. Nanotube-based ultrafast electrochromic display. Adv. Mater. 2005, 17, 171–175. [Google Scholar] [CrossRef]
- Karaca, G.Y.; Eren, E.; Cogal, G.C.; Uygun, E.; Oksuz, L.; Oksuz, A.U. Enhanced electrochromic characteristics induced by Au/PEDOT/Pt microtubes in WO3 based electrochromic devices. Opt. Mater. 2019, 88, 472–478. [Google Scholar] [CrossRef]
- Fonseca, S.M.; Moreira, T.; Parola, A.J.; Pinheiro, C.; Laia, C.A. PEDOT electrodeposition on oriented mesoporous silica templates for electrochromic devices. Sol. Energy Mater. Sol. Cells 2017, 159, 94–101. [Google Scholar] [CrossRef]
- DeLongchamp, D.; Hammond, P.T. Layer-by-layer assembly of PEDOT/polyaniline electrochromic devices. Adv. Mater. 2001, 13, 1455–1459. [Google Scholar] [CrossRef]
- Kang, J.H.; Oh, Y.J.; Paek, S.M.; Hwang, S.J.; Choy, J.H. Electrochromic device of PEDOT–PANI hybrid system for fast response and high optical contrast. Sol. Energy Mater. Sol. Cells 2009, 93, 2040–2044. [Google Scholar] [CrossRef]
- Kateb, M.; Safarian, S.; Kolahdouz, M.; Fathipour, M.; Ahamdi, V. An ultrafast, high contrast and transparent electrochromic display. Sol. Energy Mater. Sol. Cells 2016, 145, 200–205. [Google Scholar] [CrossRef]
- Pal, R.K.; Kundu, S.C.; Yadavalli, V.K. Biosensing using photolithographically micropatterned electrodes of PEDOT:PSS on ITO substrates. Sens. Actuators B Chem. 2017, 242, 140–147. [Google Scholar] [CrossRef]
- Chen, D.; Tan, H.; Xu, T.; Wang, W.; Chen, H.; Zhang, J. Micropatterned PEDOT with Enhanced Electrochromism and Electrochemical Tunable Diffraction. ACS Appl. Mater. Interfaces 2021, 13, 58011–58018. [Google Scholar] [CrossRef]
- Vidal, J.C.; Garcia-Ruiz, E.; Castillo, J.R. Recent advances in electropolymerized conducting polymers in amperometric biosensors. Microchim. Acta 2003, 143, 93–111. [Google Scholar] [CrossRef]
- Gharahcheshmeh, M.H.; Gleason, K.K. Device fabrication based on oxidative Chemical Vapor Deposition (oCVD) Synthesis of conducting polymers and related conjugated organic materials. Adv. Mater. Interfaces. 2019, 6, 1801564. [Google Scholar] [CrossRef] [Green Version]
- Brooke, R.; Edberg, J.; Crispin, X.; Berggren, M.; Engquist, I.; Jonsson, M.P. Greyscale and paper electrochromic polymer displays by UV patterning. Polymers 2019, 11, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.L.; Liao, L.J. Preparation of micropatterned polyaniline thin films with enhanced electrochromic properties by electrostatic field-assisted potentiostatic deposition. Sol. Energy Mater. Sol. Cells 2016, 145, 54–60. [Google Scholar] [CrossRef]
- Lin, C.L.; Ding, K.Y.; Chou, K.P. Direct electrodeposition of micropatterned Prussian blue films on indium tin oxide/polyethylene terephthalate substrate by electrostatic field-assisted potentiostatic and pulse potentiostatic deposition methods. Surf. Coat. Technol. 2018, 342, 226–232. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism: Fundamentals and Applications; VCH: Weinheim, Germany, 1995; pp. 54–55. [Google Scholar] [CrossRef]
- Tian, Y.; Dou, S.; Zhang, X.; Zhang, L.; Wang, L.; Zhao, J.; Zhao, X.; Li, Y. Synthesis of ordered bowl-like polyaniline film with enhanced electrochromic performances. Synth. Met. 2017, 232, 111–116. [Google Scholar] [CrossRef]
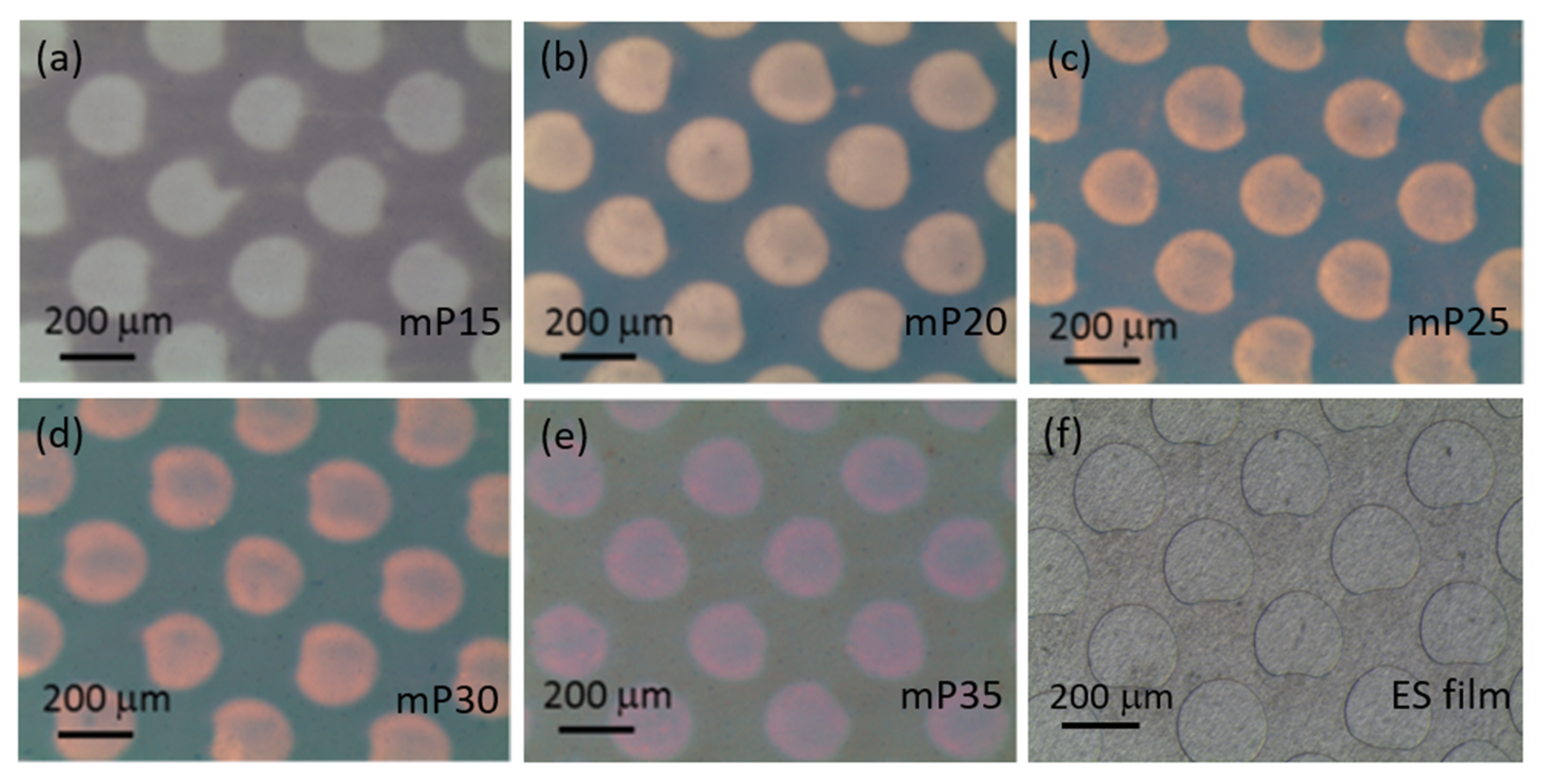
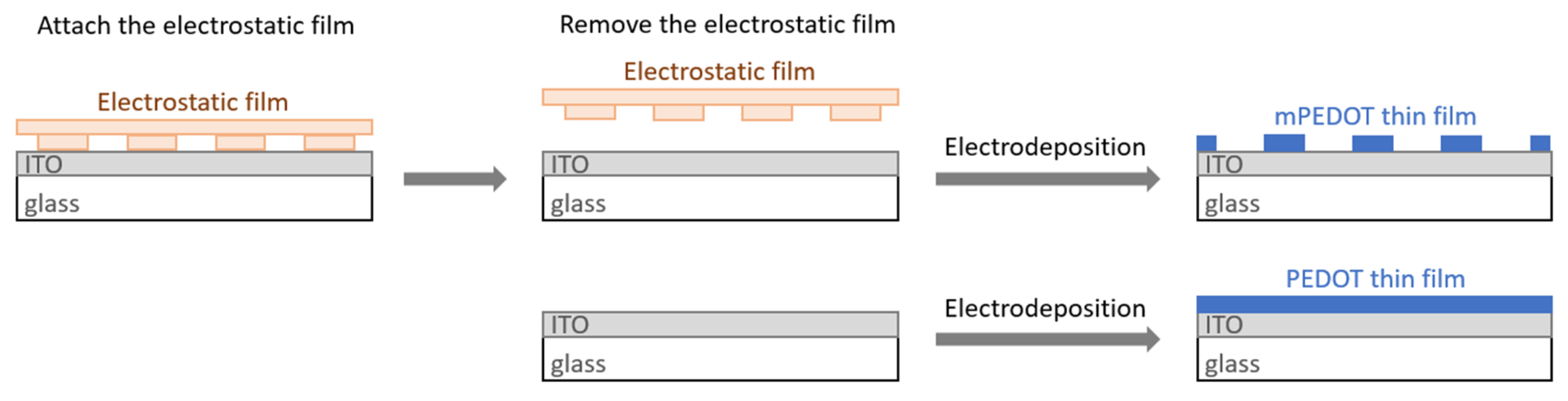
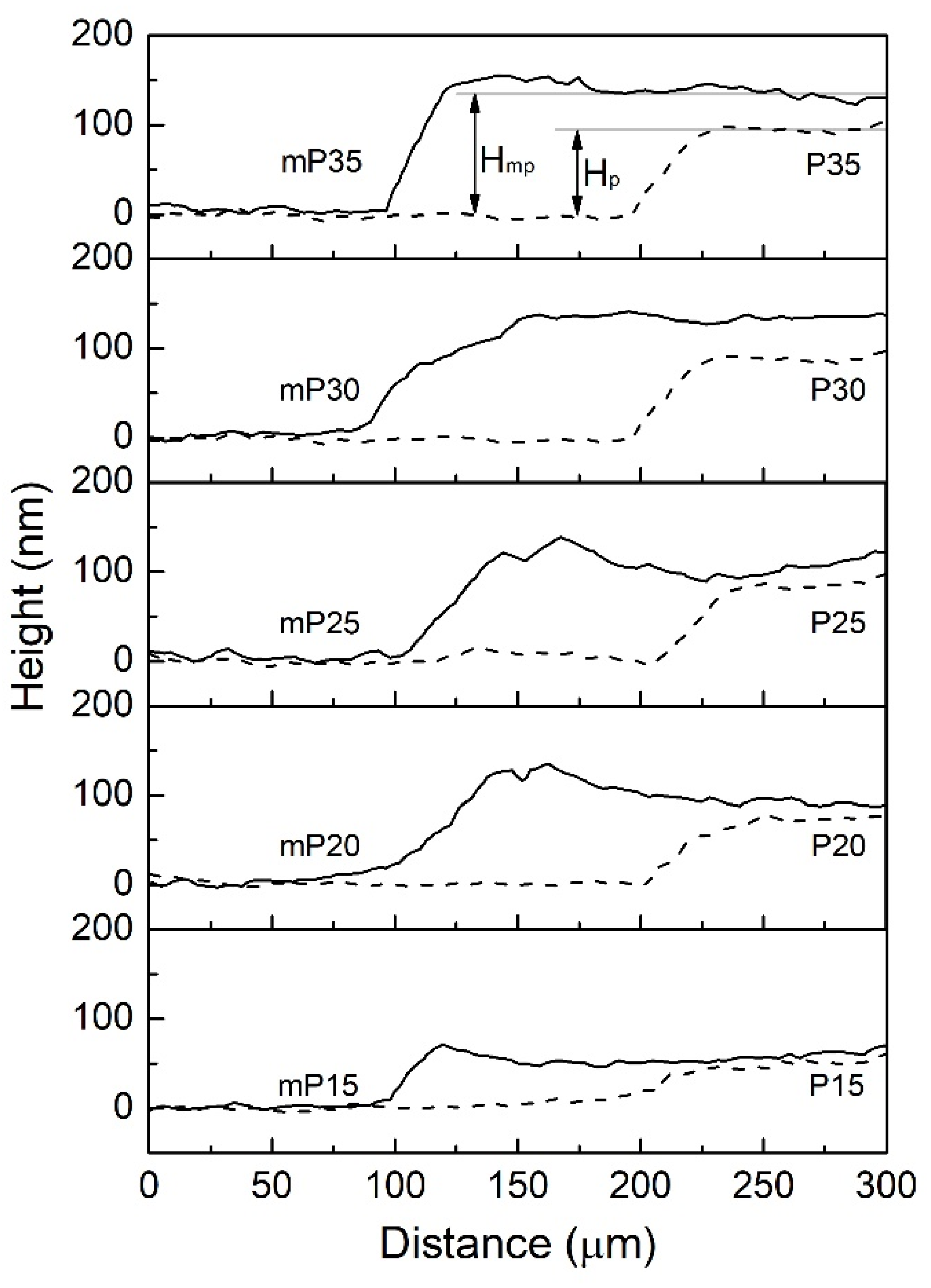

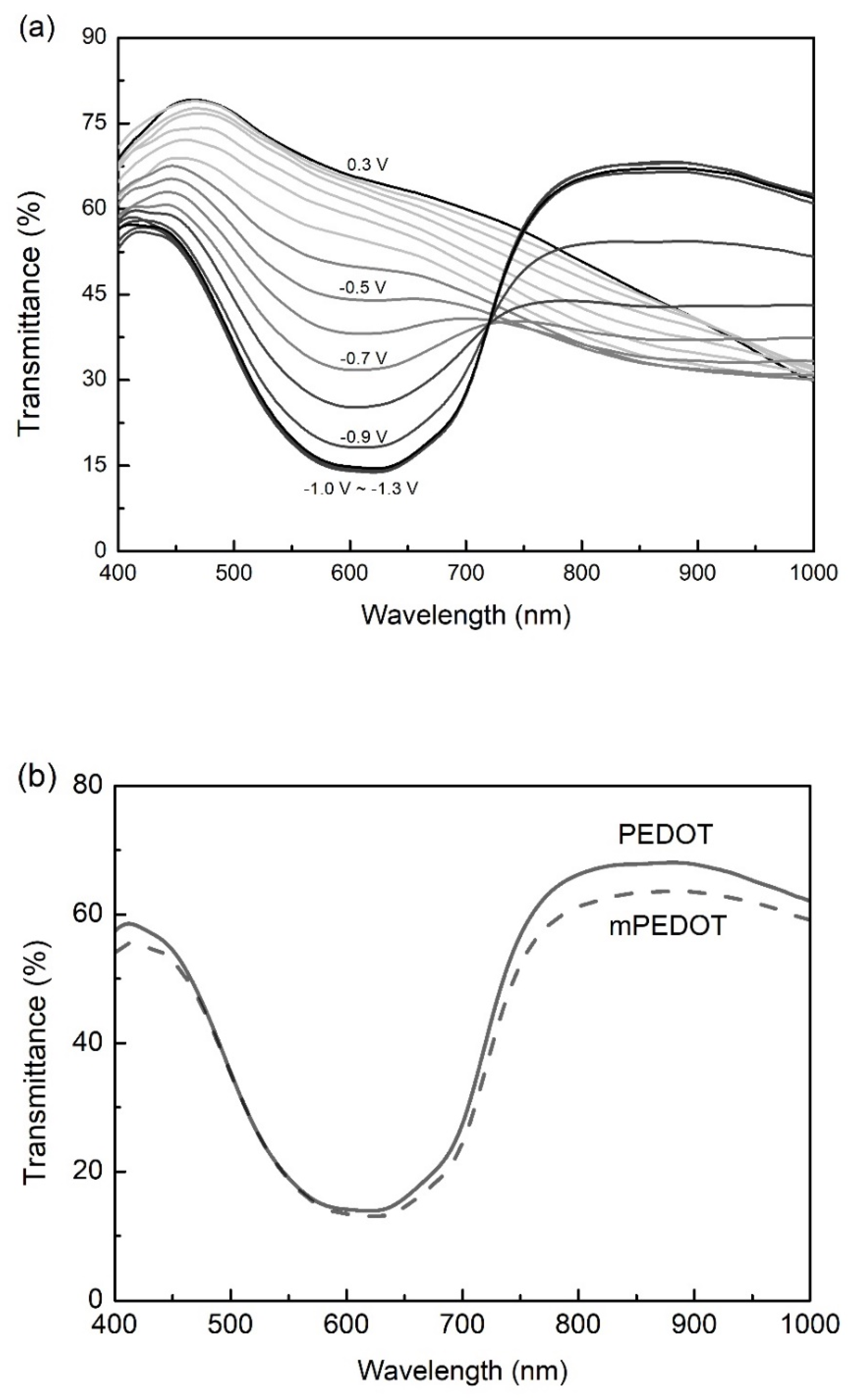
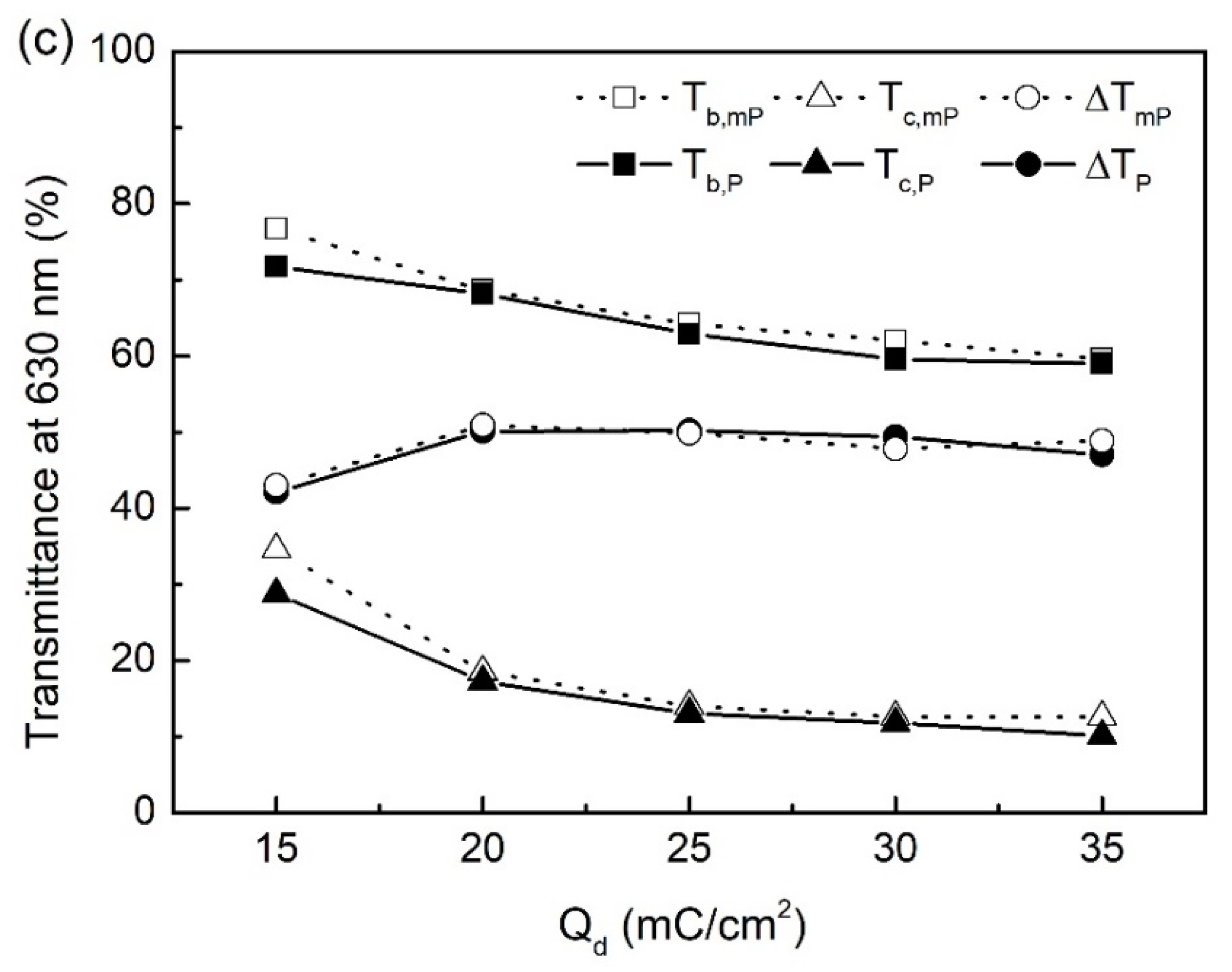
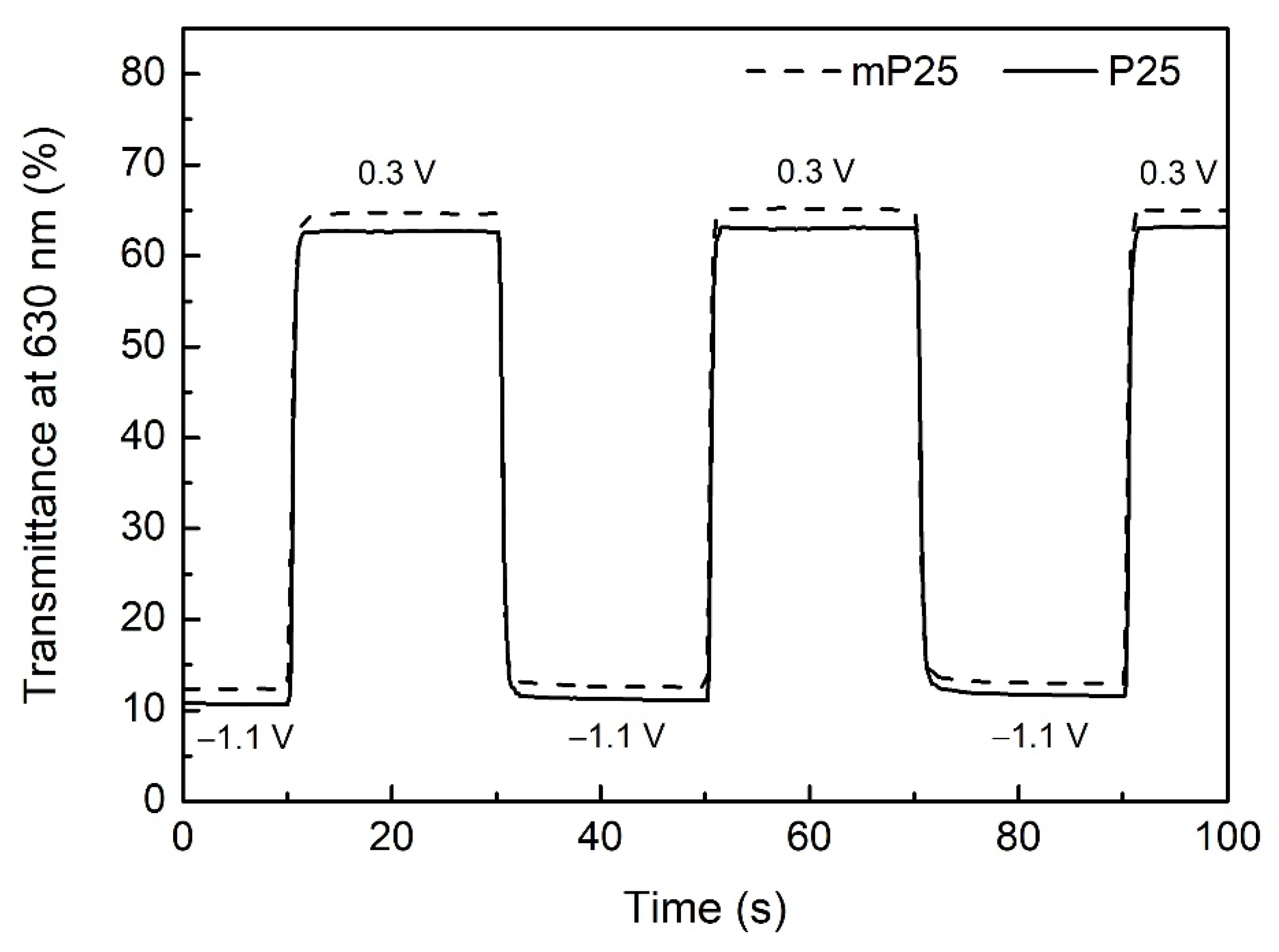
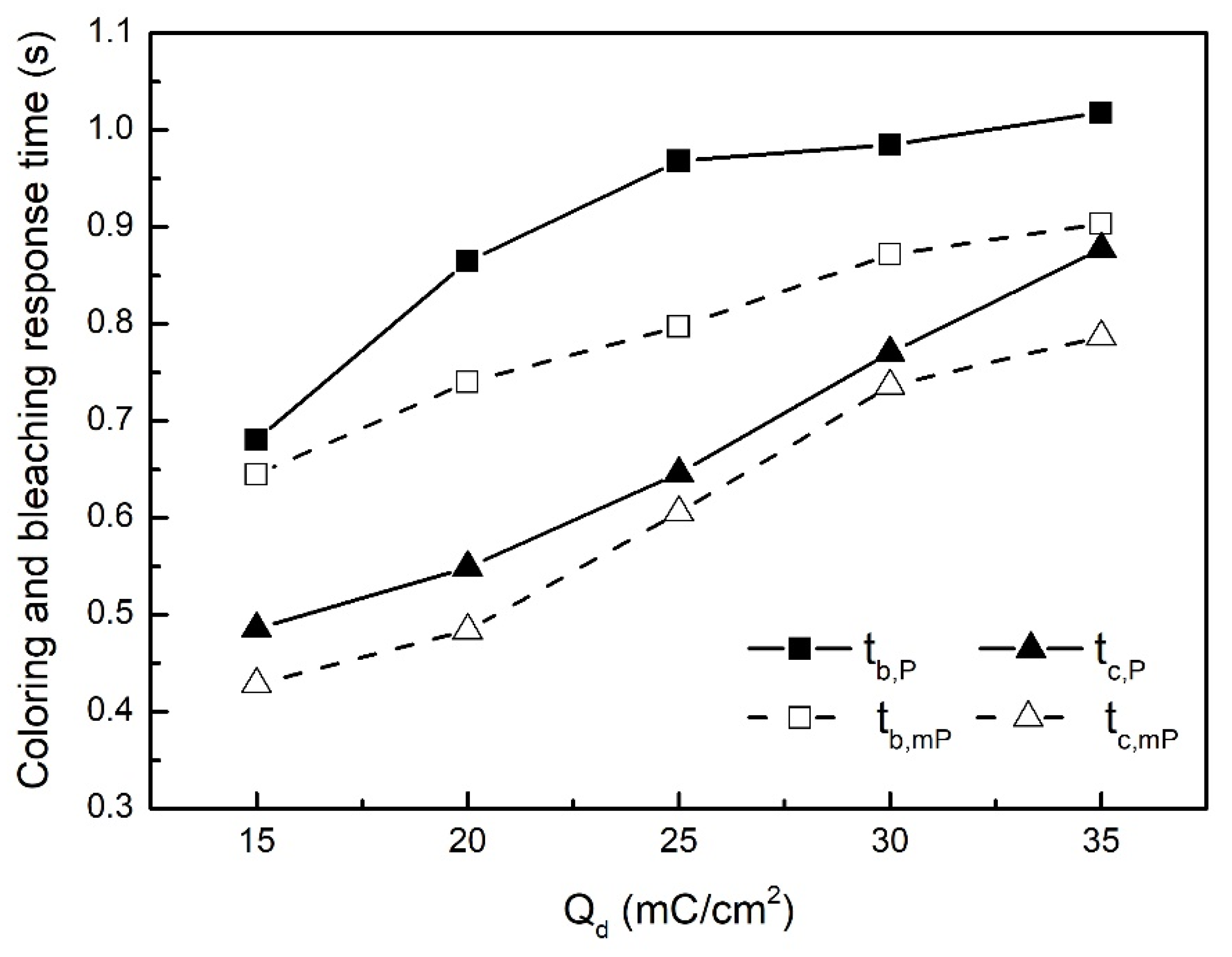
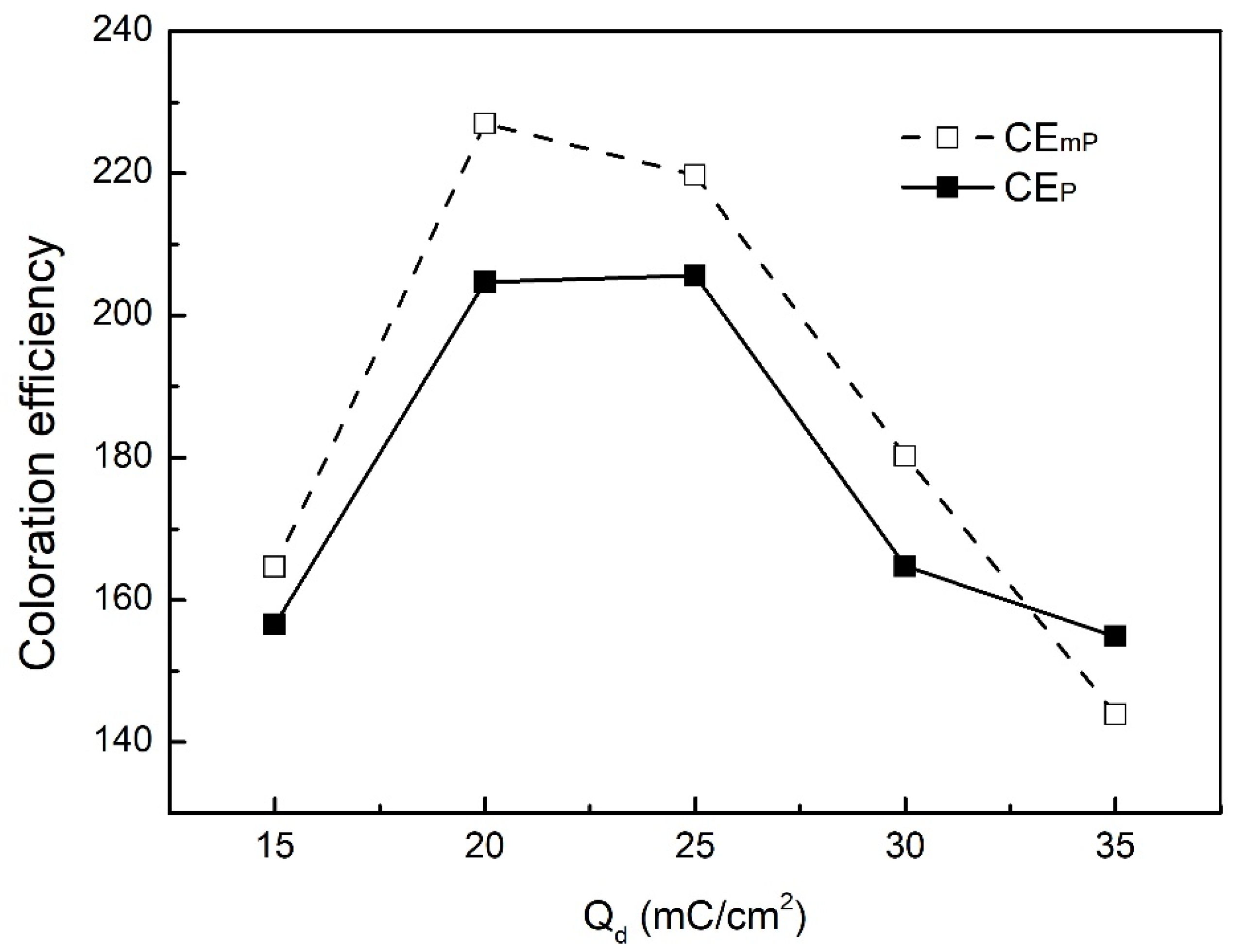
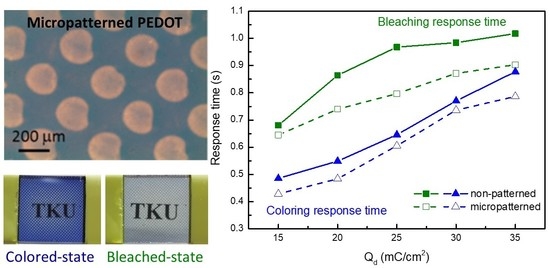
| Qd (mC/cm2) | mPEDOT HmP (nm) | PEDOT HP (nm) |
|---|---|---|
| 15 | 55 | 50 |
| 20 | 96 | 73 |
| 25 | 109 | 82 |
| 30 | 132 | 89 |
| 35 | 137 | 96 |
| Qd (mC/cm2) | Qc,mP (mC/cm2) | Qc,P (mC/cm2) | RPD (%) |
|---|---|---|---|
| 15 | 2.10 | 2.54 | −17.3% |
| 20 | 2.51 | 2.91 | −13.7% |
| 25 | 3.01 | 3.33 | −9.6% |
| 30 | 3.84 | 4.27 | −10.1% |
| 35 | 4.69 | 4.95 | −5.3% |
| Qd (mC/cm2) | mPEDOT | PEDOT | ||||
|---|---|---|---|---|---|---|
| Tb,mP (%) | Tc,mP (%) | ΔTmP (%) | Tb,P (%) | Tc,P (%) | ΔTP (%) | |
| 15 | 76.7 | 34.6 | 42.1 | 71.7 | 28.7 | 43.0 |
| 20 | 68.7 | 18.5 | 50.1 | 68.2 | 17.3 | 50.9 |
| 25 | 64.2 | 14.0 | 50.2 | 62.9 | 13.0 | 49.9 |
| 30 | 62.0 | 12.6 | 49.4 | 59.6 | 11.8 | 47.8 |
| 35 | 59.6 | 12.6 | 47.0 | 59.0 | 10.1 | 48.9 |
| Qd (mC/cm2) | mPEDOT | PEDOT | ||
|---|---|---|---|---|
| tc,mP (s) | tb,mP (s) | tc,P (s) | tb,P (s) | |
| 15 | 0.65 | 0.43 | 0.68 | 0.49 |
| 20 | 0.74 | 0.48 | 0.86 | 0.55 |
| 25 | 0.80 | 0.61 | 0.97 | 0.65 |
| 30 | 0.87 | 0.74 | 0.99 | 0.77 |
| 35 | 0.90 | 0.79 | 1.02 | 0.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-L.; Cheng, T.-L.; Wu, N.-J. Micropatterned Poly(3,4-ethylenedioxythiophene) Thin Films with Improved Color-Switching Rates and Coloration Efficiency. Polymers 2022, 14, 2951. https://doi.org/10.3390/polym14142951
Lin C-L, Cheng T-L, Wu N-J. Micropatterned Poly(3,4-ethylenedioxythiophene) Thin Films with Improved Color-Switching Rates and Coloration Efficiency. Polymers. 2022; 14(14):2951. https://doi.org/10.3390/polym14142951
Chicago/Turabian StyleLin, Cheng-Lan, Tzung-Lin Cheng, and Nian-Jheng Wu. 2022. "Micropatterned Poly(3,4-ethylenedioxythiophene) Thin Films with Improved Color-Switching Rates and Coloration Efficiency" Polymers 14, no. 14: 2951. https://doi.org/10.3390/polym14142951