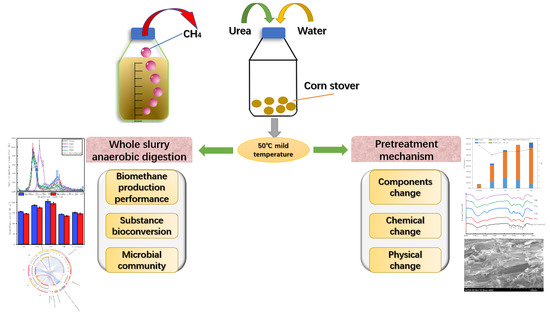
Biomethane Yield, Physicochemical Structures, and Microbial Community Characteristics of Corn Stover Pretreated by Urea Combined with Mild Temperature Hydrotherm
Abstract
:1. Introduction
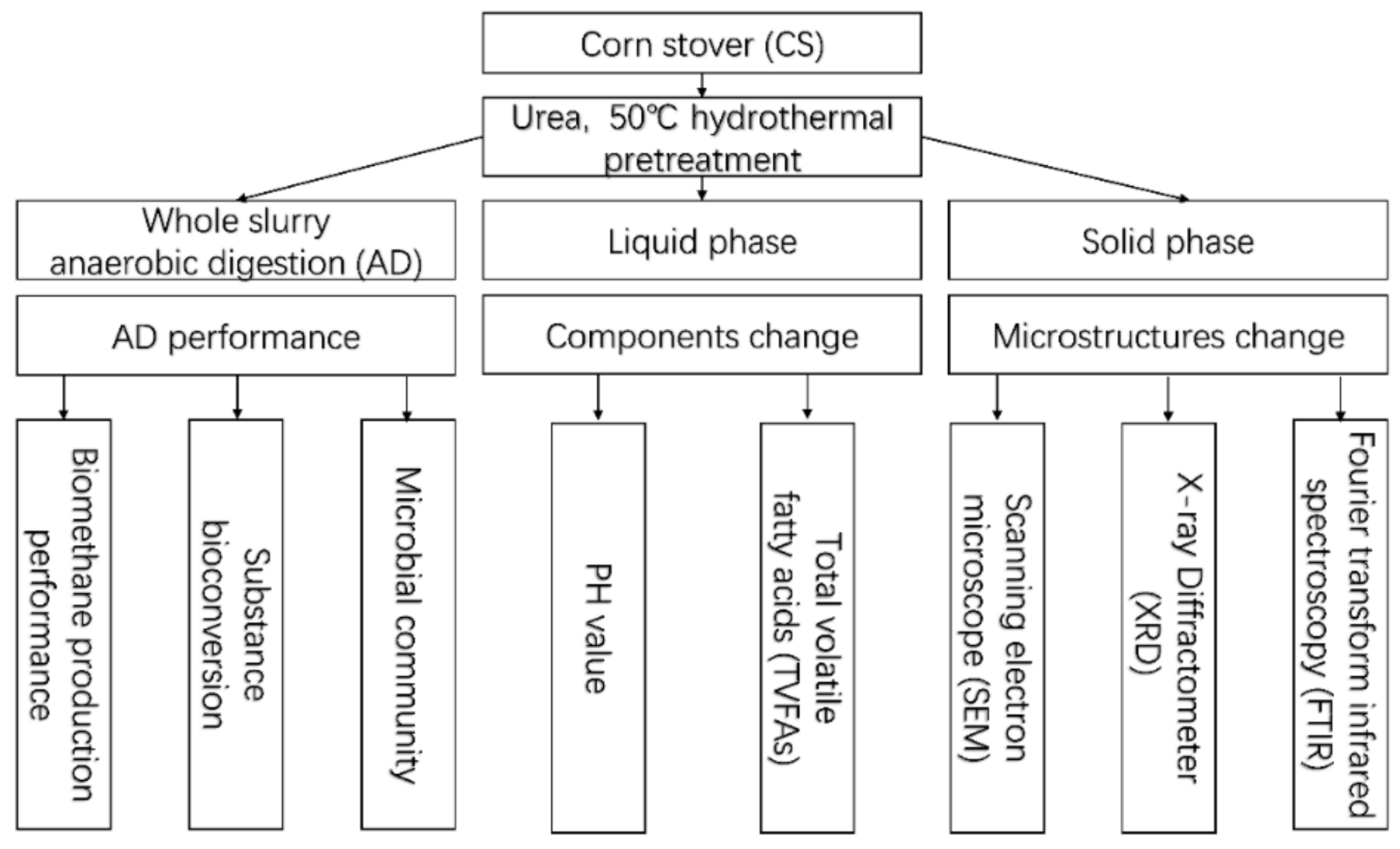
2. Material and Methods
2.1. Feedstock and Inoculum
2.2. Experimental Design
2.2.1. Pretreatment Methods
2.2.2. Anaerobic Digestion
2.2.3. Analytical Methods
2.2.4. Data Analysis
3. Results and Discussion
3.1. AD Performance
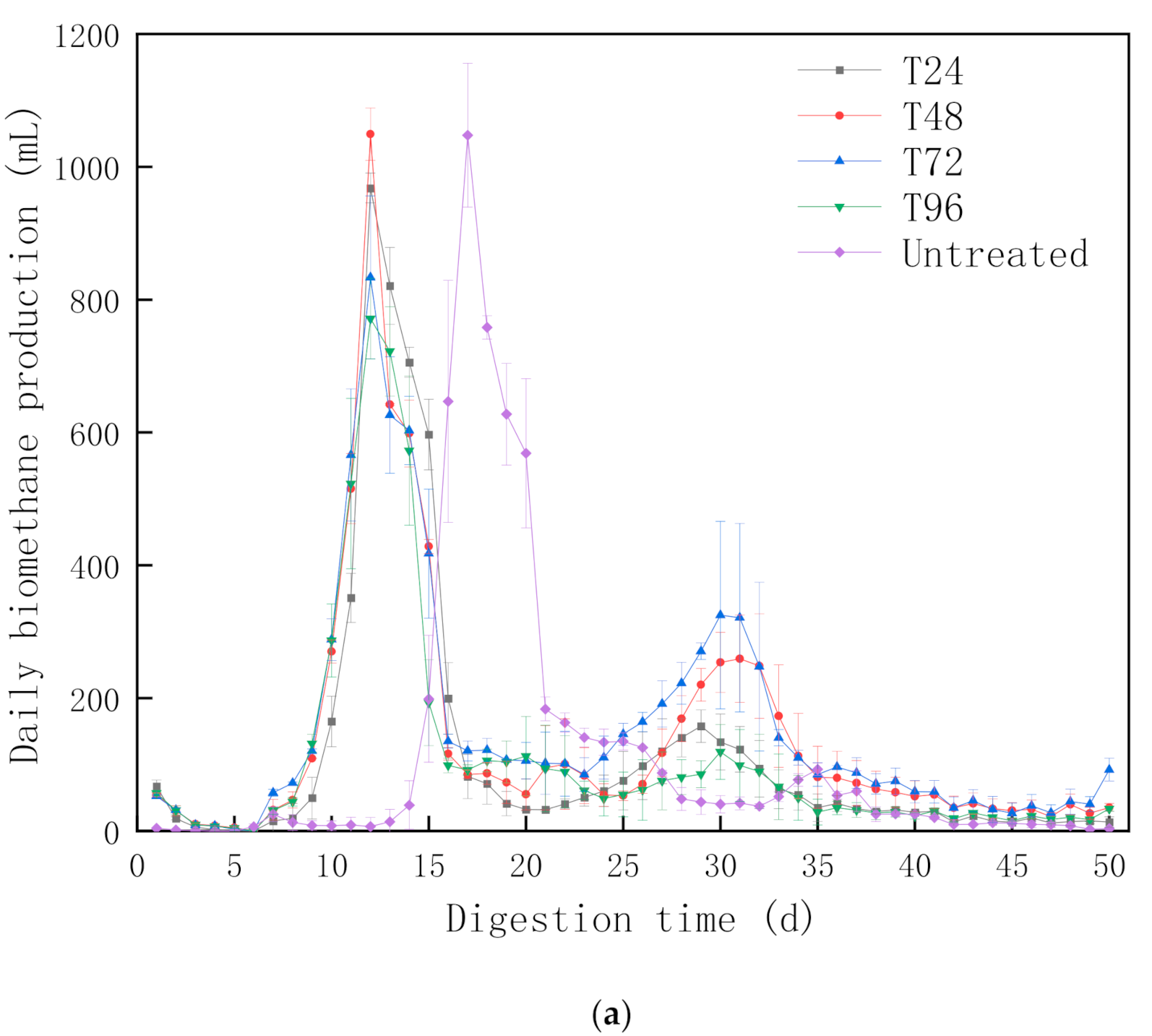
3.1.1. Biomethane Production
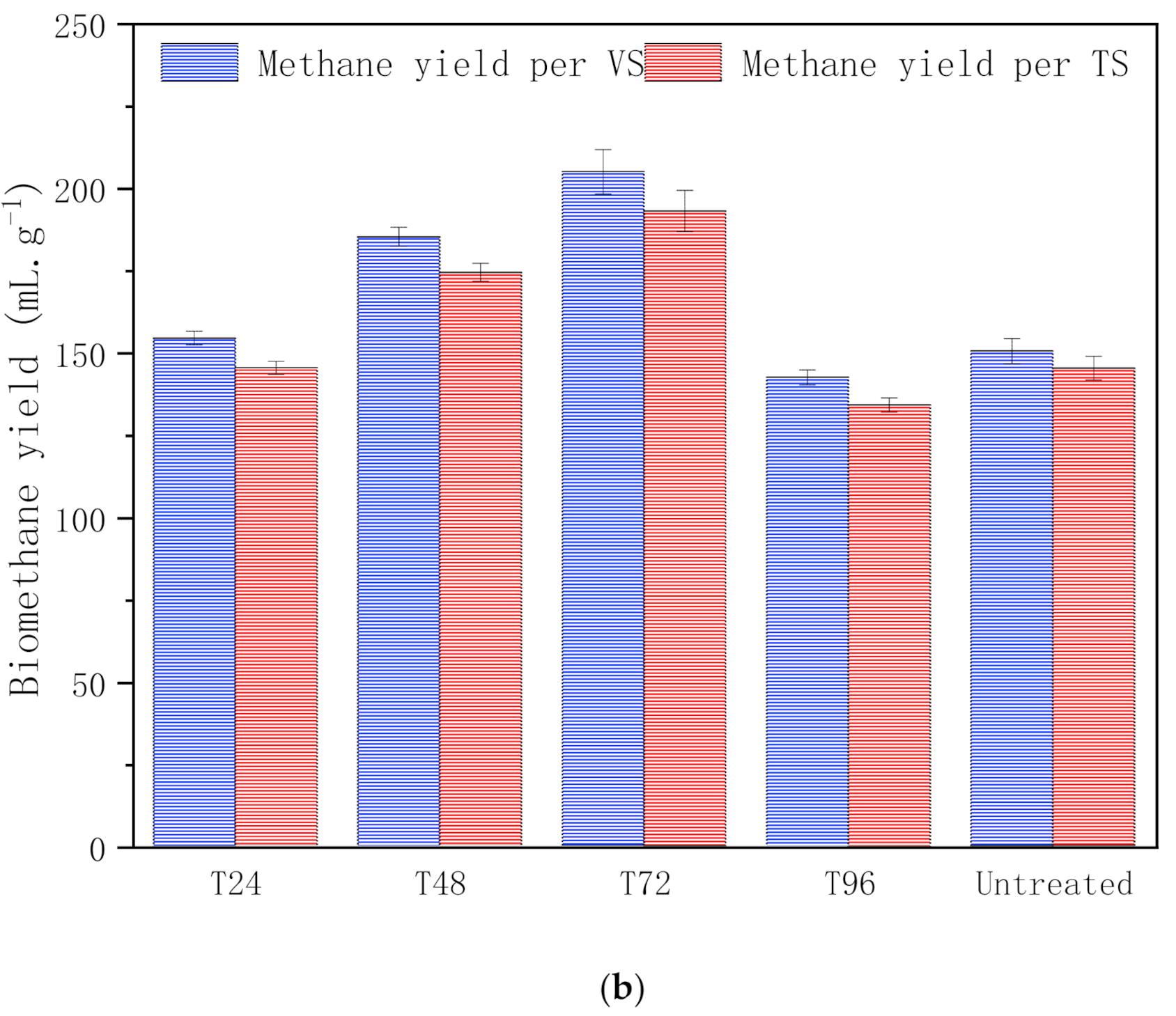
3.1.2. Biomethane Yield
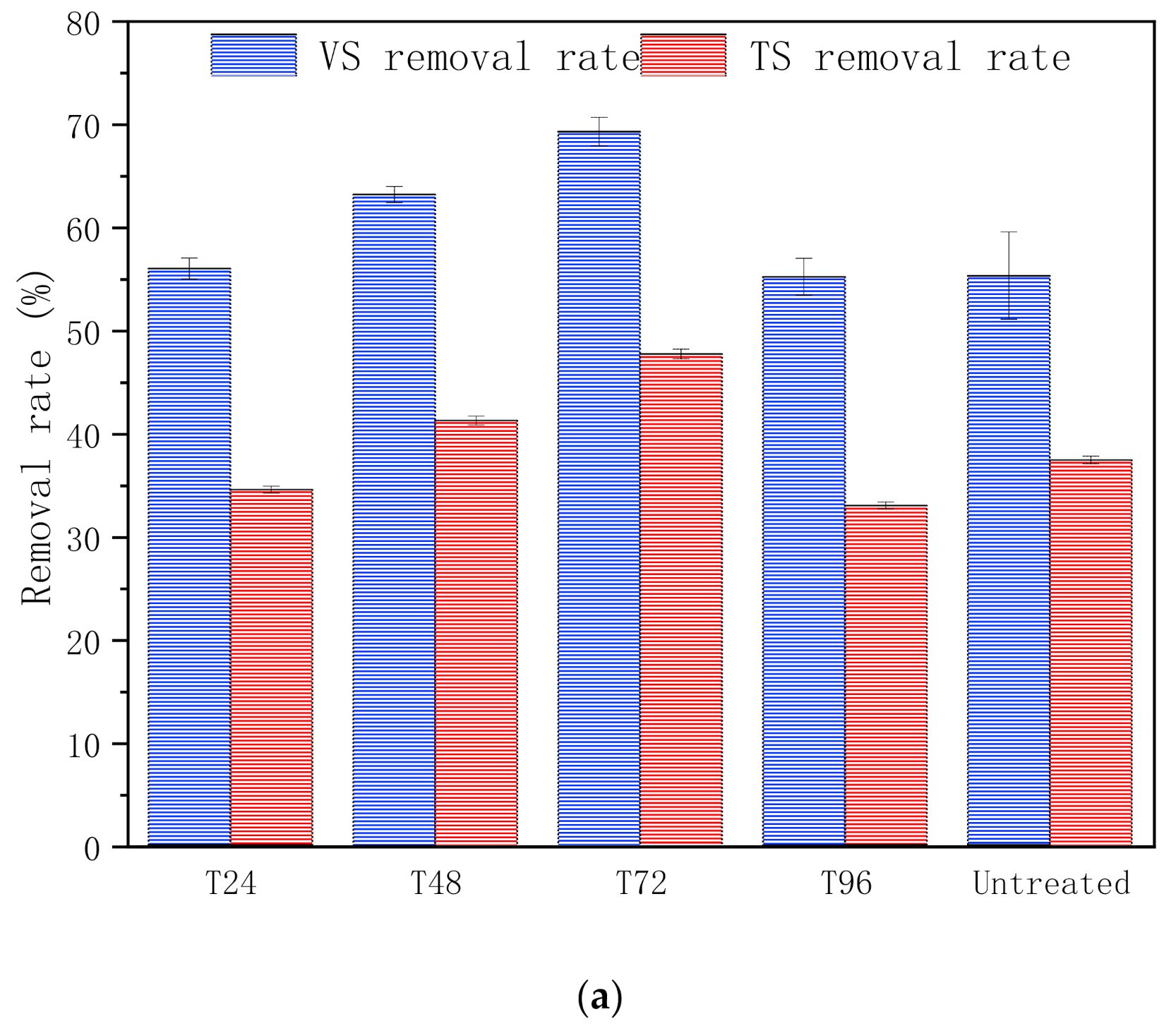
3.1.3. Substance Bioconversion
3.2. Pretreatment Mechanism
3.2.1. Total Volatile Fatty Acids (TVFAs)
3.2.2. Scanning Electron Microscope (SEM)
3.2.3. X-ray Diffractometer (XRD)
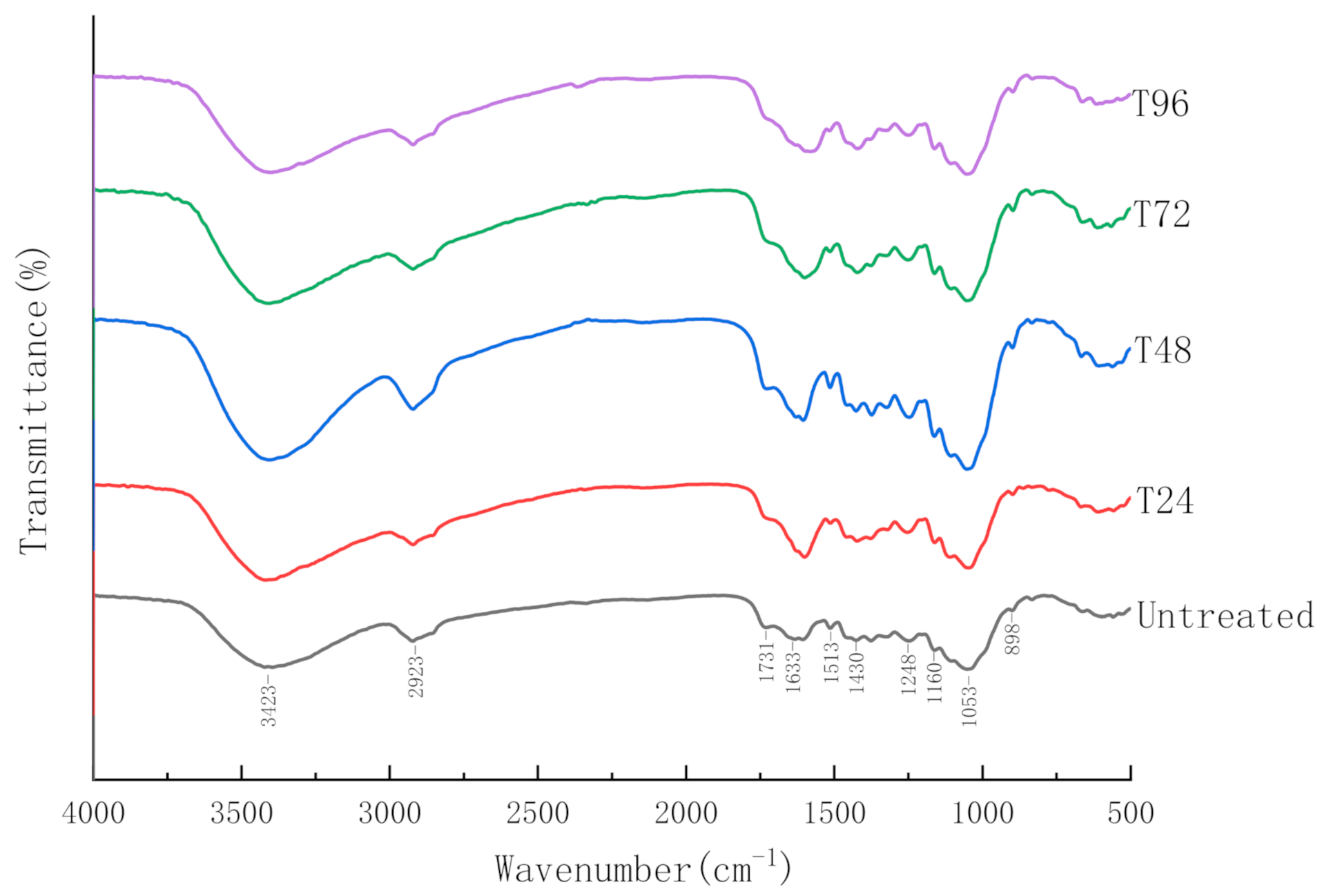
3.2.4. Fourier-Transform Infrared Spectroscopy (FTIR)
3.3. Microbial Community Analyses
3.3.1. Diversity and Richness
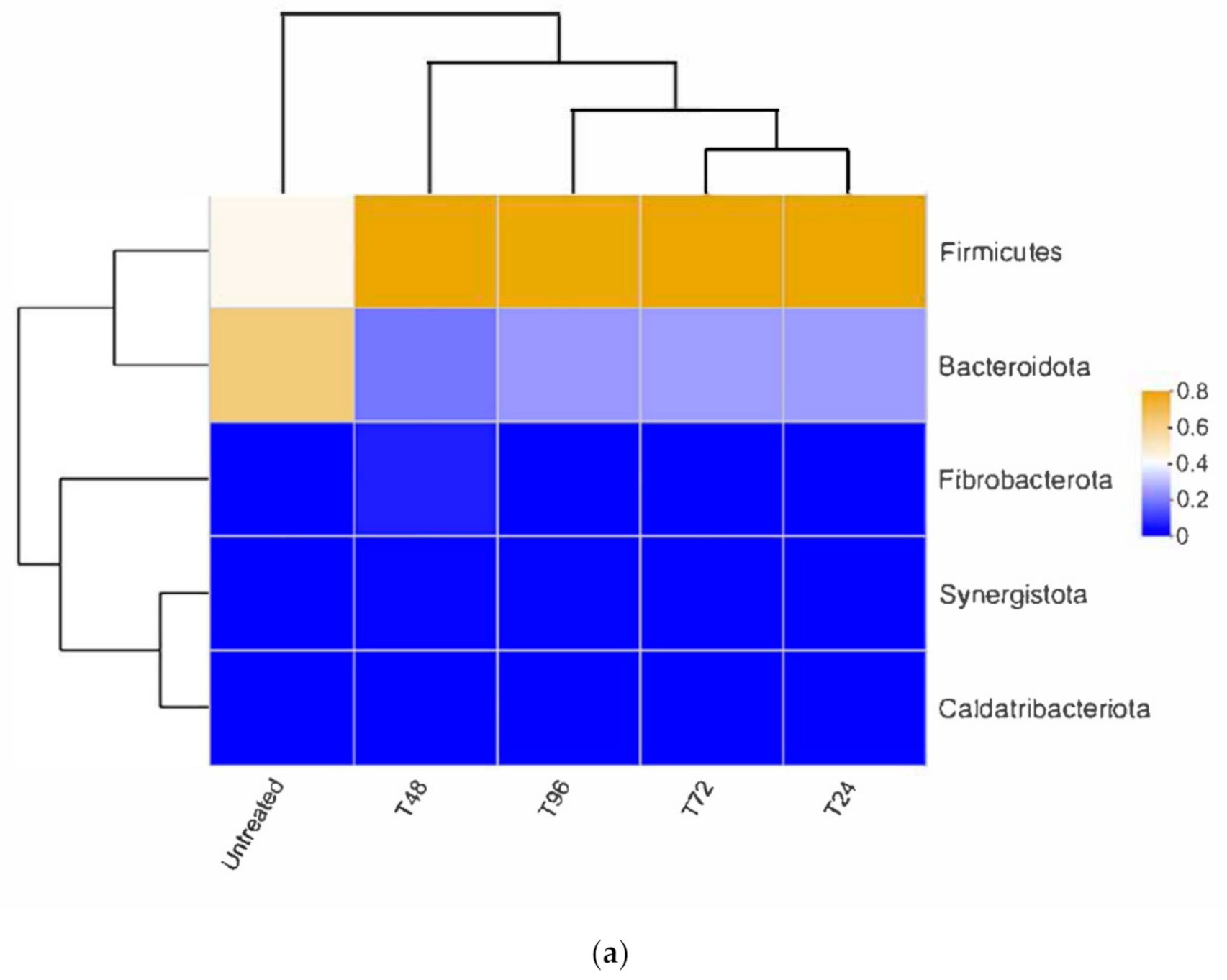
3.3.2. Bacterial Composition
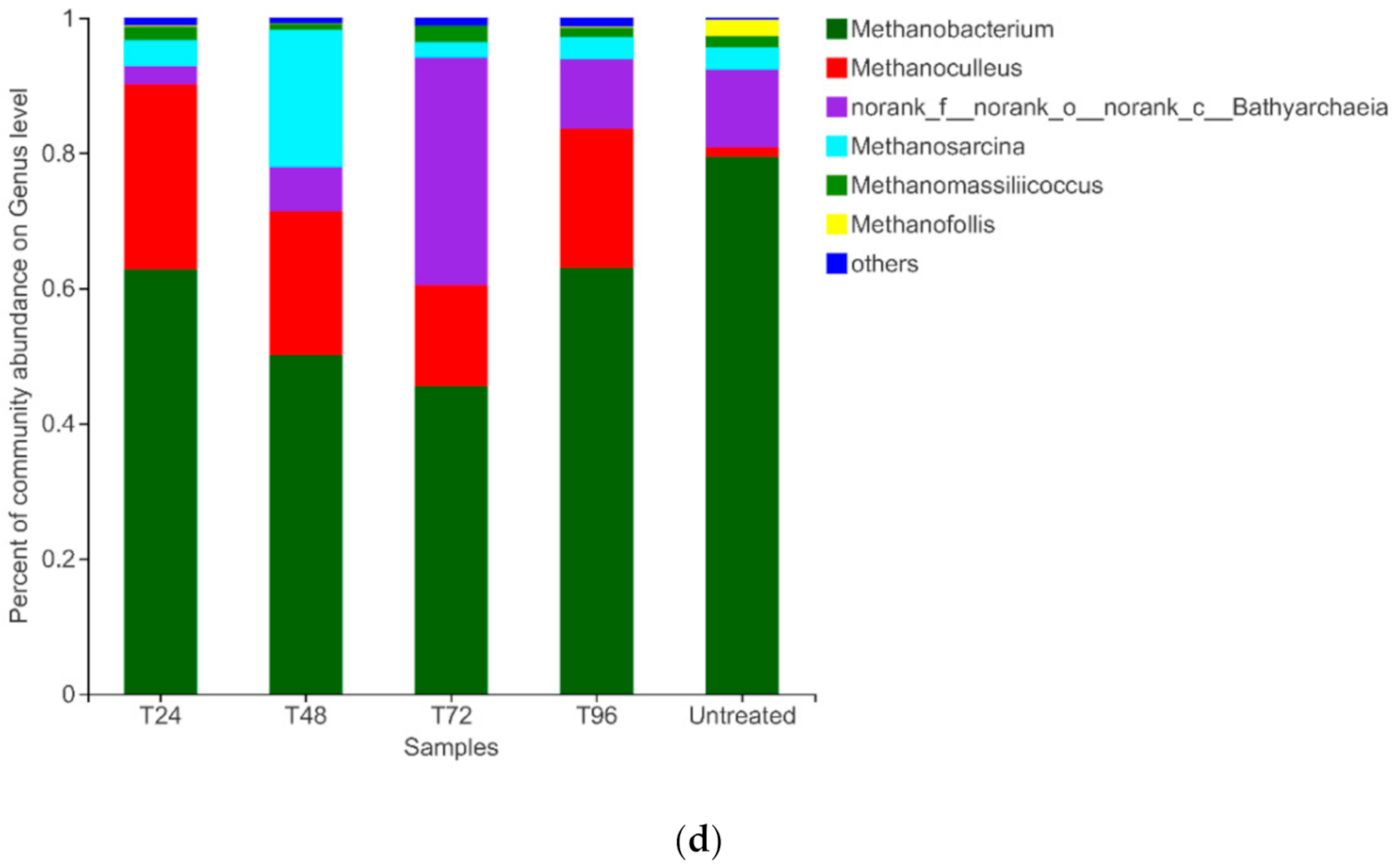
3.3.3. Archaeal composition
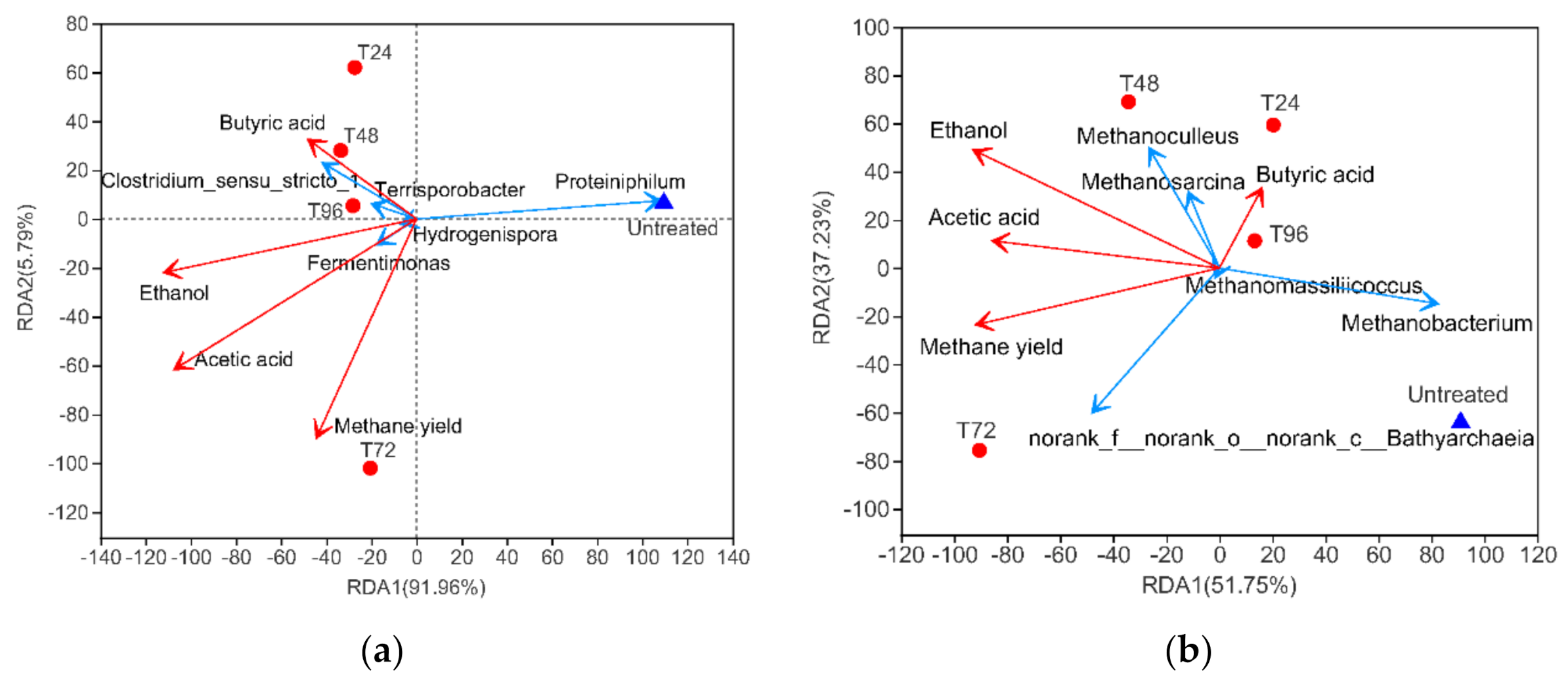
3.3.4. Correlation between Microbial Community and Environmental Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| CS | corn stover |
| AD | anaerobic digestion |
| TS | total solid |
| TC | total carbon |
| TN | total nitrogen |
| TH | total hydrogen |
| TO | total oxygen |
| VS | total volatile solid |
| MLSS | mixed liquid suspended solids |
| DMP | daily methane production |
| TVFAs | total volatile fatty acids |
| BD | biodegradability |
| SEM | scanning electron microscope |
| XRD | X-ray Diffractometer |
| FTIR | fourier transform infrared spectroscopy |
| CrI | crystallinity index |
| RDA | redundancy analysis |
References
- The National Bureau of Statistics. China Statistical Yearbook. Available online: http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm (accessed on 28 October 2020).
- Zhao, S.G.; Li, G.D.; Zheng, N.; Wang, J.Q.; Yu, Z.T. Steam explosion enhances digestibility and fermentation of corn stover by facilitating ruminal microbial colonization. Bioresour. Technol. 2018, 253, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Aho, M.; Paakkinen, K.; Taipale, R. Quality of deposits during grate combustion of corn stover and wood chip blends. Fuel 2013, 104, 476–487. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Z.; Pan, S.C.; Shoup, L.M.; Felix, T.L.; Perkins, J.B.; May, O.; Singh, V. Fungal pretreatment to improve digestibility of corn stover for animal feed. Trans. Asabe 2017, 60, 973–979. [Google Scholar] [CrossRef]
- Powers, S. Quantifying Cradle-to-Farm Gate Life-Cycle Impacts Associated with Fertilizer Used for Corn, Soybean, and Stover Production. Battelle Natl. Renew. Energy Lab. 2005. [Google Scholar] [CrossRef] [Green Version]
- Sharma, H.K.; Xu, C.B.; Qin, W.S. Biological Pretreatment of Lignocellulosic Biomass for Biofuels and Bioproducts: An Overview. Waste Biomass Valorization 2019, 10, 235–251. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Stamatelatou, K.; Antonopoulou, G.; Ntaikou, I.; Lyberatos, G. The Effect of Physical, Chemical, and Biological Pretreatments of Biomass on its Anaerobic Digestibility and Biogas Production. In Biogas Production; Scrivener Publishing LLC: Salem, MA, USA, 2012; pp. 55–90. [Google Scholar] [CrossRef]
- Zheng, M.; Li, L.; Li, X.; Xiong, J.; Mei, T.; Chen, G. The Effects of Alkaline Pretreatment Parameters on Anaerobic Biogasification of Corn Stover. Energy Sources Part A Recovery Util. Environ. Eff. 2010, 32, 1918–1925. [Google Scholar] [CrossRef]
- He, Y.F.; Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S. Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuels 2008, 22, 2775–2781. [Google Scholar] [CrossRef]
- Jaffar, M.; Pang, Y.Z.; Yuan, H.R.; Zou, D.X.; Liu, Y.P.; Zhu, B.N.; Korai, R.M.; Li, X.J. Wheat straw pretreatment with KOH for enhancing biomethane production and fertilizer value in anaerobic digestion. Chin. J. Chem. Eng. 2016, 24, 404–409. [Google Scholar] [CrossRef]
- Yuan, H.R.; Li, R.P.; Zhang, Y.T.; Li, X.J.; Liu, C.M.; Meng, Y.; Lin, M.N.; Yang, Z.Y. Anaerobic digestion of ammonia-pretreated corn stover. Biosyst. Eng. 2015, 129, 142–148. [Google Scholar] [CrossRef]
- Li, Q.; Yang, F.L.; Zheng, G.X.; Guan, Z.J. Effects of urea ammonia pretreatment on the batch anaerobic fermentation efficiency of corn stovers. Int. J. Agric. Biol. Eng. 2019, 12, 169–173. [Google Scholar] [CrossRef]
- Mohsenzadeh, A.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Alkali pretreatment of softwood spruce and hardwood birch by NaOH/thiourea, NaOH/urea, NaOH/urea/thiourea, and NaOH/PEG to improve ethanol and biogas production. J. Chem. Technol. Biotechnol. 2012, 87, 1209–1214. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Bergeron, A.D.; Davaritouchaee, M. Methane recovery from anaerobic digestion of urea-pretreated wheat straw. Renew. Energy 2017, 115, 139–148. [Google Scholar] [CrossRef]
- Yu, Q.; Cui, S.F.; Sun, C.; Liu, R.H.; Sarker, M.; Guo, Z.J.; Lai, R.Y. Synergistic Effects of Anaerobic Co-Digestion of Pretreated Corn Stover with Chicken Manure and Its Kinetics. Appl. Biochem. Biotechnol. 2021, 193, 515–532. [Google Scholar] [CrossRef]
- Li, J.; Wachemo, A.C.; Yuan, H.R.; Zuo, X.Y.; Li, X.J. Evaluation of system stability and anaerobic conversion performance for corn stover using combined pretreatment. Waste Manag. 2019, 97, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, X.S.; Guo, Y.; Zhang, B.W.; Chen, X.Y.; Yu, Q.; Zhuang, X.S.; Yuan, Z.H. Mild Urea/KOH pretreatment to enhance enzymatic hydrolysis of corn stover with liquid waste recovery for plant growth. J. Clean. Prod. 2021, 284, 9. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, C.; Liu, R.H.; Yellezuome, D.; Zhu, X.P.; Bai, R.F.; Liu, M.Q.; Sun, M.Z. Anaerobic co-digestion of corn stover and chicken manure using continuous stirred tank reactor: The effect of biochar addition and urea pretreatment. Bioresour. Technol. 2021, 319, 8. [Google Scholar] [CrossRef]
- Sato, A.; Soeprijanto, S.; Widjaja, A. Influence of Alkaline Hydrothermal Pretreatment of Rice Straw on Biomass Composition. Int. Energy J. 2019, 19, 115–124. [Google Scholar]
- Song, Z.; Sun, X.; Yang, G.; Yan, Z.; Yuan, Y.; Li, D.; Li, X.; Liu, X. Effect of NaOH pretreatment on methane yield of corn straw at different temperatures by anaerobic digestion. Huagong Xuebao/CIESC J. 2014, 65, 1876–1882. [Google Scholar] [CrossRef]
- Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S.; Yuan, H.R. Improving biodegradability and biogas production of corn stover through sodium hydroxide solid state pretreatment. Energy Fuels 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Li, X.; Li, L.Q.; Zheng, M.X.; Fu, G.Z.; Lar, J.S. Anaerobic Co-Digestion of Cattle Manure with Corn Stover Pretreated by Sodium Hydroxide for Efficient Biogas Production. Energy Fuels 2009, 23, 4635–4639. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Wahid, Z.A. Achievements and perspectives of anaerobic co-digestion: A review. J. Clean. Prod. 2018, 194, 359–371. [Google Scholar] [CrossRef]
- Li, J.; Wachemo, A.C.; Yu, G.Q.; Li, X.J. Enhanced anaerobic digestion performance of corn stalk pretreated with freezing-thawing and ammonia: An experimental and theoretical study. J. Clean. Prod. 2020, 247, 12. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Mustafa, A.M.; Li, H.; Radwan, A.A.; Sheng, K.C.; Chen, X. Effect of hydrothermal and Ca(OH)(2) pretreatments on anaerobic digestion of sugarcane bagasse for biogas production. Bioresour. Technol. 2018, 259, 54–60. [Google Scholar] [CrossRef]
- Yao, Y.Q.; He, M.L.; Ren, Y.B.; Ma, L.Y.; Luo, Y.; Sheng, H.M.; Xiang, Y.; Zhang, H.; Li, Q.; An, L.Z. Anaerobic digestion of poplar processing residues for methane production after alkaline treatment. Bioresour. Technol. 2013, 134, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Zhang, R.H.; He, Y.F.; Liu, X.Y.; Chen, C.; Liu, G.Q. Thermophilic Solid-State Anaerobic Digestion of Alkaline-Pretreated Corn Stover. Energy Fuels 2014, 28, 3759–3765. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wang, L.G.; Dai, Z.Q.; Zhang, R.H.; Chen, C.; Liu, G.Q. Effect of organic loading, feed-to-inoculum ratio, and pretreatment on the anaerobic digestion of tobacco stalks. Bioresour. Technol. 2020, 298, 8. [Google Scholar] [CrossRef]
- Yuan, H.R.; Song, X.C.; Guan, R.L.; Zhang, L.; Li, X.J.; Zuo, X.Y. Effect of low severity hydrothermal pretreatment on anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 294, 7. [Google Scholar] [CrossRef]
- Bolado-Rodriguez, S.; Toquero, C.; Martin-Juarez, J.; Travaini, R.; Garcia-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Hattori, S. Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes Environ. 2008, 23, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Song, X.C.; Wachemo, A.C.; Zhang, L.; Bai, T.Q.; Li, X.J.; Zuo, X.Y.; Yuan, H.R. Effect of hydrothermal pretreatment severity on the pretreatment characteristics and anaerobic digestion performance of corn stover. Bioresour. Technol. 2019, 289, 8. [Google Scholar] [CrossRef] [PubMed]
- Laser, M.; Schulman, D.; Allen, S.G.; Lichwa, J.; Antal, M.J.; Lynd, L.R. A comparison of liquid hot water and steam pretreatments of sugar cane bagasse for bioconversion to ethanol. Bioresour. Technol. 2002, 81, 33–44. [Google Scholar] [CrossRef]
- Xiang, C.X.; Tian, D.; Hu, J.G.; Huang, M.; Shen, F.; Zhang, Y.Z.; Yang, G.; Zeng, Y.M.; Deng, S.H. Why can hydrothermally pretreating lignocellulose in low severities improve anaerobic digestion performances? Sci. Total Environ. 2021, 752, 10. [Google Scholar] [CrossRef]
- Zhang, H.; Ning, Z.F.; Khalid, H.; Zhang, R.H.; Liu, G.Q.; Chen, C. Enhancement of methane production from Cotton Stalk using different pretreatment techniques. Sci. Rep. 2018, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.W.; Zhu, M.Q.; Li, M.F.; Wei, Q.; Sun, R.C. Effects of hydrothermal treatment on enhancing enzymatic hydrolysis of rapeseed straw. Renew. Energy 2019, 134, 446–452. [Google Scholar] [CrossRef]
- Liu, L.; Sun, J.S.; Li, M.; Wang, S.H.; Pei, H.S.; Zhang, J.S. Enhanced enzymatic hydrolysis and structural features of corn stover by FeCl3 pretreatment. Bioresour. Technol. 2009, 100, 5853–5858. [Google Scholar] [CrossRef]
- Wahab, M.A.; Jellali, S.; Jedidi, N. Ammonium biosorption onto sawdust: FTIR analysis, kinetics and adsorption isotherms modeling. Bioresour. Technol. 2010, 101, 5070–5075. [Google Scholar] [CrossRef]
- Ge, D.D.; You, Z.P.; Chen, S.Y.; Liu, C.C.; Gao, J.F.; Lv, S.T. The performance of asphalt binder with trichloroethylene: Improving the efficiency of using reclaimed asphalt pavement. J. Clean. Prod. 2019, 232, 205–212. [Google Scholar] [CrossRef]
- Guan, R.L.; Li, X.J.; Wachemo, A.C.; Yuan, H.R.; Liu, Y.P.; Zou, D.X.; Zuo, X.Y.; Gu, J.Y. Enhancing anaerobic digestion performance and degradation of lignocellulosic components of rice straw by combined biological and chemical pretreatment. Sci. Total Environ. 2018, 637, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Nieves, D.C.; Karimi, K.; Horvath, I.S. Improvement of biogas production from oil palm empty fruit bunches (OPEFB). Ind. Crop. Prod. 2011, 34, 1097–1101. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.Q.; Yuan, H.R.; Li, X.J. Anaerobic bioconversion efficiency of rice straw in continuously stirred tank reactor systems applying longer hydraulic retention time and higher load: One-stage vs. Two-stage. Bioresour. Technol. 2021, 321. [Google Scholar] [CrossRef]
- Xu, X.J.; Wang, W.Q.; Chen, C.; Xie, P.; Liu, W.Z.; Zhou, X.; Wang, X.T.; Yuan, Y.; Wang, A.J.; Lee, D.J.; et al. Bioelectrochemical system for the enhancement of methane production by anaerobic digestion of alkaline pretreated sludge. Bioresour. Technol. 2020, 304, 9. [Google Scholar] [CrossRef]
- Sun, L.; Liu, T.; Muller, B.; Schnurer, A. The microbial community structure in industrial biogas plants influences the degradation rate of straw and cellulose in batch tests. Biotechnol. Biofuels 2016, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Lanjekar, V.B.; Marathe, N.P.; Shouche, Y.S.; Ranade, D.R. Clostridium punense sp nov., an obligate anaerobe isolated from healthy human faeces. Int. J. Syst. Evol. Microbiol. 2015, 65, 4749–4756. [Google Scholar] [CrossRef]
- Cho, H.U.; Kim, Y.M.; Park, J.M. Changes in microbial communities during volatile fatty acid production from cyanobacterial biomass harvested from a cyanobacterial bloom in a river. Chemosphere 2018, 202, 306–311. [Google Scholar] [CrossRef]
- Bi, S.J.; Qiao, W.; Xiong, L.P.; Mandy, A.; Wandera, S.M.; Yin, D.M.; Dong, R.J. Improved high solid anaerobic digestion of chicken manure by moderate in situ ammonia stripping and its relation to metabolic pathway. Renew. Energy 2020, 146, 2380–2389. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Wang, J.F.; Li, Y.; Zhu, T.T.; Yu, Q.L.; Wang, T.T.; Liang, S.; Zhang, Y.B. Why do DIETers like drinking: Metagenomic analysis for methane and energy metabolism during anaerobic digestion with ethanol. Water Res. 2020, 171, 14. [Google Scholar] [CrossRef] [PubMed]
- Guan, R.; Yuan, H.; Zhang, L.; Zuo, X.; Li, X. Combined pretreatment using CaO and liquid fraction of digestate of rice straw: Anaerobic digestion performance and electron transfer. Chin. J. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Ziganshina, E.E.; Belostotskiy, D.E.; Bulynina, S.S.; Ziganshin, A.M. Effect of magnetite on anaerobic digestion of distillers grains and beet pulp: Operation of reactors and microbial community dynamics. J. Biosci. Bioeng. 2021, 131, 290–298. [Google Scholar] [CrossRef]
- Li, L.H.; He, S.B.; Sun, Y.M.; Kang, X.H.; Jiang, J.F.; Yuan, Z.H.; Liu, D.F. Anaerobic co-digestion of Pennisetum hybrid and pig manure: A comparative study of performance and microbial community at different mixture ratio and organic loading rate. Chemosphere 2020, 247, 10. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Wang, Z.Y.; Lu, T.D.; Liu, J.B.; Wang, Y.W.; Shen, P.H.; Wei, Y.S. Response and mechanisms of the performance and fate of antibiotic resistance genes to nano-magnetite during anaerobic digestion of swine manure. J. Hazard. Mater. 2019, 366, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Tang, Y.; Pavlostathis, S.G. Hydrothermal pretreatment of sewage sludge for enhanced anaerobic digestion: Resource transformation and energy balance. Chem. Eng. J. 2021, 410, 127430. [Google Scholar] [CrossRef]
- Guan, R.L.; Yuan, H.R.; Wachemo, A.C.; Li, X.J.; Zuo, X.Y.; Zou, D.X.; Liu, Y.P.; Gu, J.Y. Effect of Narrow Feeding Regimes on Anaerobic Digestion Performance and Microbial Community Structure of Rice Straw in Continuously Stirred Tank Reactors. Energy Fuels 2018, 32, 11587–11594. [Google Scholar] [CrossRef]
- Sorokin, D.Y.; Toshchakov, S.V.; Kolganova, T.V.; Kublanov, I.V. Halo(natrono)archaea isolated from hypersaline lakes utilize cellulose and chitin as growth substrates. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomberg, M.; Montonen, L.; Timonen, S. Anaerobic Eury- and Crenarchaeota inhabit ectomycorrhizas of boreal forest Scots pine. Eur. J. Soil Biol. 2010, 46, 356–364. [Google Scholar] [CrossRef]
- Tejerizo, G.T.; Kim, Y.S.; Maus, I.; Wibberg, D.; Winkler, A.; Off, S.; Puhler, A.; Scherer, P.; Schluter, A. Genome sequence of Methanobacterium congolense strain Buetzberg, a hydrogenotrophic, methanogenic archaeon, isolated from a mesophilic industrial-scale biogas plant utilizing bio-waste. J. Biotechnol. 2017, 247, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Achinas, S.; Zhao, J.; Geurkink, B.; Krooneman, J.; Willem Euverink, G.J. Co-digestion of cow and sheep manure: Performance evaluation and relative microbial activity. Renew. Energy 2020, 153, 553–563. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Achinas, S.; Zhang, Z.H.; Krooneman, J.; Euverink, G.J.W. The biomethanation of cow manure in a continuous anaerobic digester can be boosted via a bioaugmentation culture containing Bathyarchaeota. Sci. Total Environ. 2020, 745. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.F.; Wachemo, A.C.; Yuan, H.R.; Li, X.J. Enhanced hydrolysis and acidification strategy for efficient co-digestion of pretreated corn stover with chicken manure: Digestion performance and microbial community structure. Sci. Total Environ. 2020, 720, 12. [Google Scholar] [CrossRef] [PubMed]
- Evans, P.N.; Parks, D.H.; Chadwick, G.L.; Robbins, S.J.; Orphan, V.J.; Golding, S.D.; Tyson, G.W. Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics. Science 2015, 350, 434–438. [Google Scholar] [CrossRef] [Green Version]




| Group | Pretreatment Time | I002 a | Iam b | CrI (%) |
|---|---|---|---|---|
| Untreated | 0 h | 32,933.3 | 17,608.3 | 46.5 |
| T24 | 24 h | 34,575 | 17,950 | 48.1 |
| T48 | 48 h | 32,783.3 | 16,958.3 | 48.3 |
| T72 | 72 h | 35,650 | 18,275 | 48.7 |
| T96 | 96 h | 35,075 | 19,408.3 | 44.7 |
| Peak Position (cm−1) | Functional Groups |
|---|---|
| 898 | β-(1,4)-glycosidic bond (C-O-C) |
| 1052 | ether bond in hemicellulose (C-O-C) |
| 1160, 1248 | C-O stretching of COOH and C-O vibration of guaiacyl ring |
| 1430, 1515 | aromatic skeleton vibration |
| 1732 | carbonyl group on ester bond between hemicellulose and lignin (C=O) |
| 2919 | -CH3, -CH2, -CH in aliphatic compounds |
| 3420 | Intermolecular and intramolecular hydrogen bond (-OH) |
| Group | Richness | Diversity | Coverage | ||||
|---|---|---|---|---|---|---|---|
| Sobs | Ace | Chao | Shannon | Simpson | |||
| Bacteria | T24 | 384 | 476.47 | 511.66 | 3.54 | 0.07 | 0.99 |
| T48 | 436 | 535.10 | 545.02 | 3.75 | 0.06 | 0.99 | |
| T72 | 467 | 566.79 | 544.68 | 4.05 | 0.043 | 0.99 | |
| T96 | 455 | 538.08 | 532.34 | 3.87 | 0.06 | 0.99 | |
| Untreated | 329 | 417.63 | 404.62 | 2.65 | 0.27 | 0.99 | |
| Archaea | T24 | 24 | 27.47 | 26 | 1.45 | 0.35 | 0.99 |
| T48 | 20 | 21.74 | 20 | 1.66 | 0.25 | 0.99 | |
| T72 | 25 | 27.65 | 26.5 | 1.62 | 0.26 | 0.99 | |
| T96 | 21 | 21.75 | 21 | 1.56 | 0.33 | 0.99 | |
| Untreated | 25 | 25.96 | 25.33 | 1.86 | 0.22 | 0.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Yuan, H.; Zuo, X.; Chang, Y.; Li, X. Biomethane Yield, Physicochemical Structures, and Microbial Community Characteristics of Corn Stover Pretreated by Urea Combined with Mild Temperature Hydrotherm. Polymers 2021, 13, 2207. https://doi.org/10.3390/polym13132207
Lu Y, Yuan H, Zuo X, Chang Y, Li X. Biomethane Yield, Physicochemical Structures, and Microbial Community Characteristics of Corn Stover Pretreated by Urea Combined with Mild Temperature Hydrotherm. Polymers. 2021; 13(13):2207. https://doi.org/10.3390/polym13132207
Chicago/Turabian StyleLu, Yao, Hairong Yuan, Xiaoyu Zuo, Yanqing Chang, and Xiujin Li. 2021. "Biomethane Yield, Physicochemical Structures, and Microbial Community Characteristics of Corn Stover Pretreated by Urea Combined with Mild Temperature Hydrotherm" Polymers 13, no. 13: 2207. https://doi.org/10.3390/polym13132207