The Effect of the Temperature and Moisture to the Permeation Properties of PEO-Based Membranes for Carbon-Dioxide Separation
Abstract
:1. Introduction
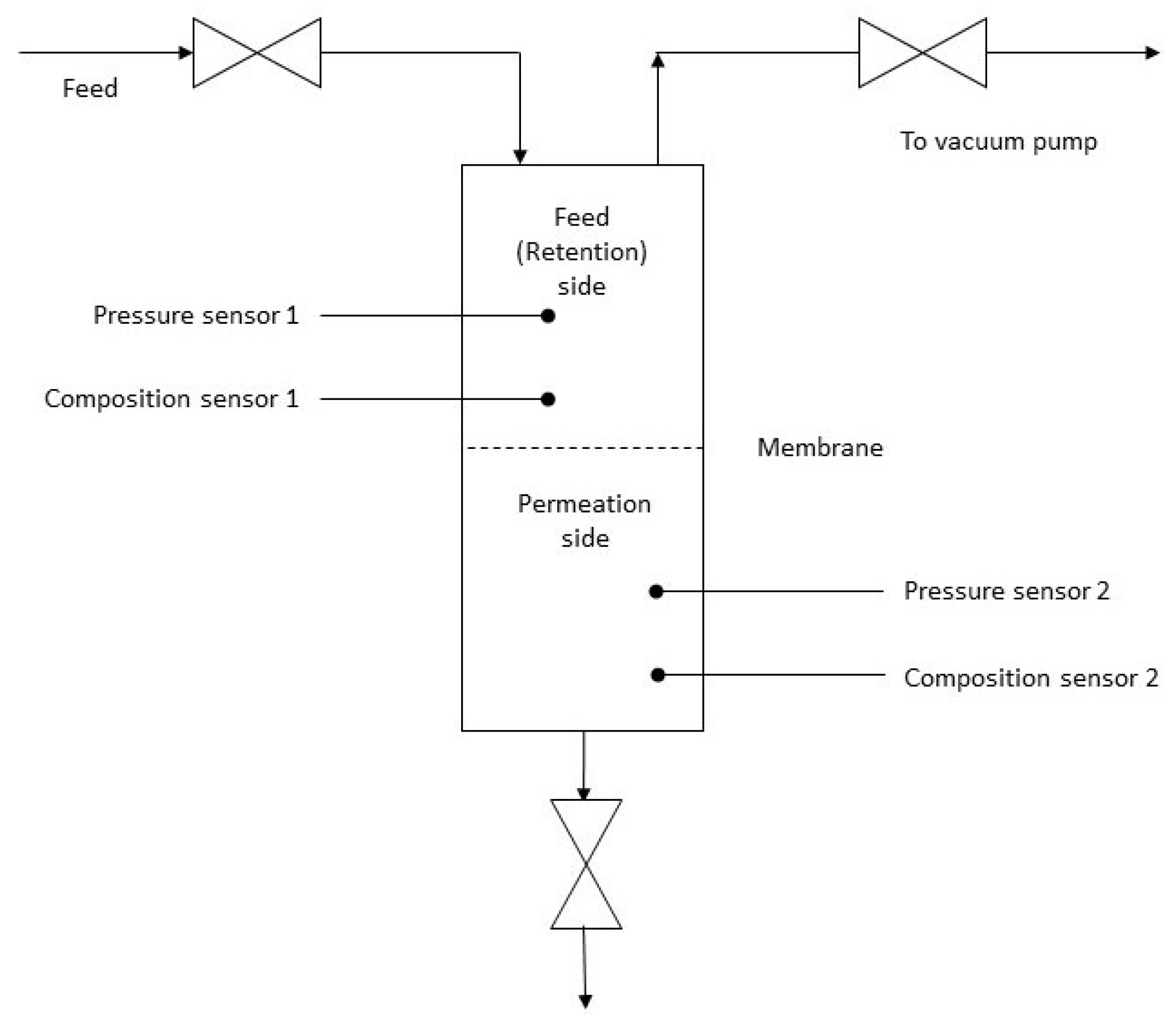
2. Materials and Methods
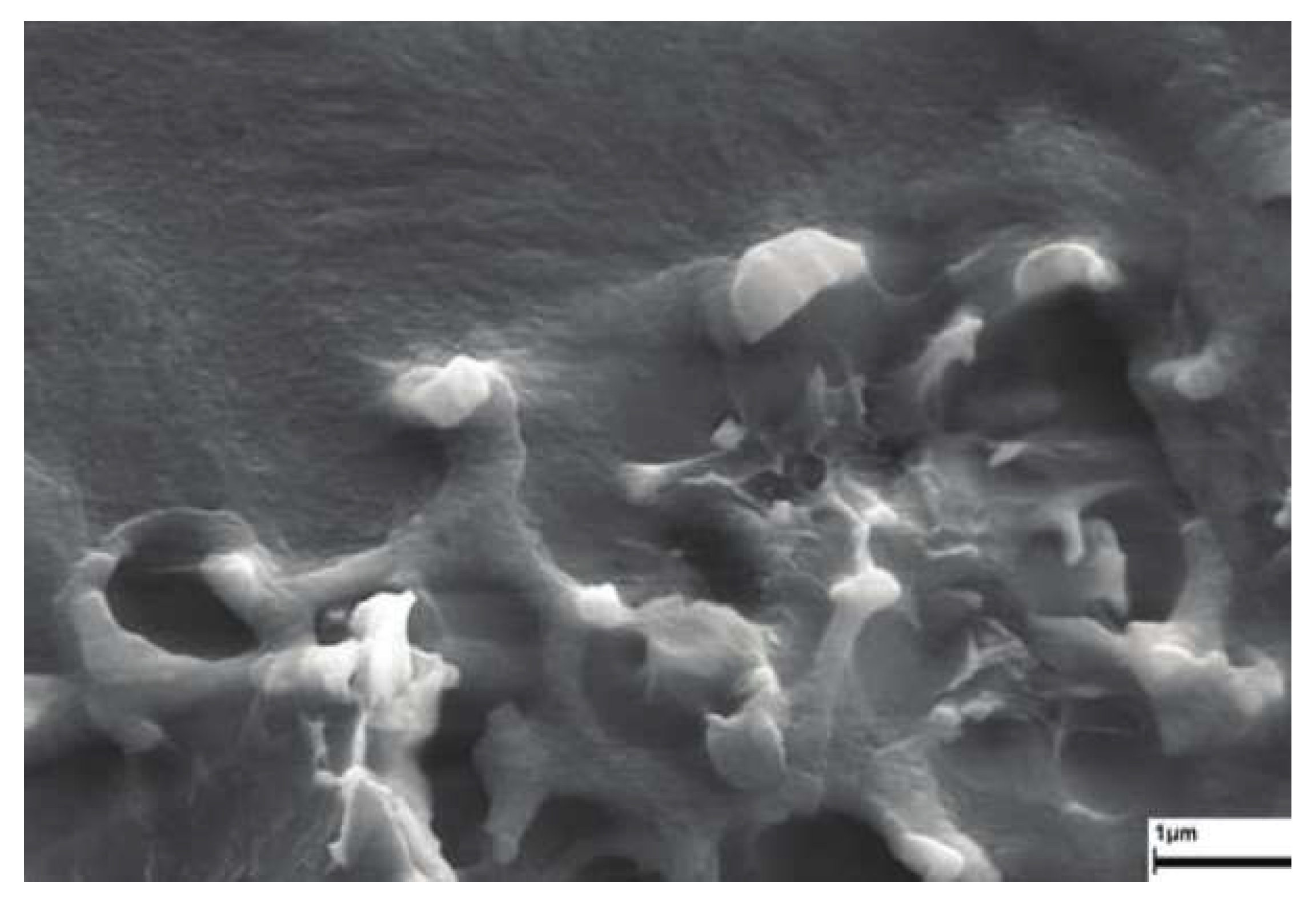
3. Results
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desideri, U.; Corbelli, R. CO2 capture in small size cogeneration plants: Technical and economical considerations. Energy Convers. Manag. 1998, 39. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [Green Version]
- Kuppan, C.S.; Chavali, M. CO2 sequestration: Processes and methodologies. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 619–668. [Google Scholar]
- Chang, P.-H.; Lee, T.-J.; Chang, Y.-P.; Chen, S.-Y. CO2 sorbents with scaffold-like Ca–Al layered double hydroxides as precursors for CO2 capture at high temperatures. ChemSusChem 2013, 6, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Vericella, J.J.; Baker, S.E.; Stolaroff, J.K.; Duoss, E.B.; Hardin, J.O.; Lewicki, J.; Glogowski, E.; Floyd, W.C.; Valdez, C.A.; Smith, W.L.; et al. Encapsulated liquid sorbents for carbon dioxide capture. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kang, S.W. CO2 separation with polymer/aniline composite membranes. Polymers 2020, 12, 1363. [Google Scholar] [CrossRef]
- Živković, L.A.; Pohar, A.; Likozar, B.; Nikačević, N.M. Kinetics and reactor modeling for CaO sorption-enhanced high-temperature water–gas shift (SE–WGS) reaction for hydrogen production. Appl. Energy 2016, 178, 844–855. [Google Scholar] [CrossRef]
- Živković, L.; Pohar, A.; Likozar, B.; Nikačević, N. Reactor conceptual design by optimization for hydrogen production through intensified sorption- and membrane-enhanced water-gas shift reaction. Chem. Eng. Sci. 2019, 211, 115174. [Google Scholar] [CrossRef]
- Ješić, D.; Lašič Jurković, D.; Pohar, A.; Suhadolnik, L.; Likozar, B. Engineering photocatalytic and photoelectrocatalytic CO2 reduction reactions: Mechanisms, intrinsic kinetics, mass transfer resistances, reactors and multi-scale modelling simulations. Chem. Eng. J. 2021, 407, 126799. [Google Scholar] [CrossRef]
- Nunes, S.P.; Peinemann, K.V. Membrane Technology in the Chemical Industry; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Koros, W.J.; Fleming, G.K.; Jordan, S.M.; Kim, T.H.; Hoehn, H.H. Polymeric membrane materials for solution-diffusion based permeation separations. Prog. Polym. Sci. 1988, 13, 339–401. [Google Scholar] [CrossRef]
- Yampolskii, Y.; FInkelshtein, E. Membrane Materials for Gas and Separation: Synthesis and Application of Silicon-Containing Polymers; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Zare, A.; Perna, L.; Nogalska, A.; Ambrogi, V.; Cerruti, P.; Tylkowski, B.; García-Valls, R.; Giamberini, M. Polymer blends for improved CO2 capture membranes. Polymers 2019, 11, 1662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Materials Science of Membranes for Gas and Vapor Separation, Wiley. Available online: https://www.wiley.com/en-us/Materials+Science+of+Membranes+for+Gas+and+Vapor+Separation-p-9780470853450 (accessed on 29 May 2021).
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons: Chichester, UK, 2012. [Google Scholar]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57–74. [Google Scholar] [CrossRef]
- Jankowski, A.; Grabiec, E.; Nocoń-Szmajda, K.; Marcinkowski, A.; Janeczek, H.; Wolińska-Grabczyk, A. Polyimide-based membrane materials for CO2 separation: A Comparison of segmented and aromatic (Co) polyimides. Membranes 2021, 11, 274. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Bruno, R.; Monteleone, M.; Fuoco, A.; Ferrando Soria, J.; Pardo, E.; Armentano, D.; Jansen, J.C. Glassy PEEK-WC vs. rubbery Pebax®1657 polymers: Effect on the gas transport in CuNi-MOF based mixed matrix membranes. Appl. Sci. 2020, 10, 1310. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.; Freeman, B.D. Gas solubility, diffusivity and permeability in poly(ethylene oxide). J. Membr. Sci. 2004, 239, 105–117. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, X.; Zhang, L.; Howarter, J.A.; Baba, A.A.; Neelameggham, N.R.; Wang, C.; Sun, Z.; Peng, H.; Guillen, D.P. Energy Technology 2021: Carbon Dioxide Management and Other Technologies; Baba, A.A., Zhang, L., Guillen, D.P., Neelameggham, N.R., Peng, H., Zhong, Y., Eds.; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2021. [Google Scholar]
- Chen, J.C.; Feng, X.; Penlidis, A. Gas permeation through poly(ether-b-amide) (PEBAX 2533) block copolymer membranes. Sep. Sci. Technol. 2005, 39, 149–164. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas transport properties of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 2051–2062. [Google Scholar] [CrossRef]
- Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-blended PCL/PEO electrospun nanofibers with functional properties enhanced by plasma processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Bondar, V.I.; Freeman, B.D.; Pinnau, I. Gas sorption and characterization of poly(ether-b-amide) segmented block copolymers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 2463–2475. [Google Scholar] [CrossRef]
- Yoshino, M.; Ito, K.; Kita, H.; Okamoto, K.-I. Effects of hard-segment polymers on CO2/N2 gas-separation properties of poly(ethylene oxide)-segmented copolymers. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Buttersack, C.; Rudolph, H.; Mahrholz, J.; Buchholz, K. High specific interaction of polymers with the pores of hydrophobic zeolites. Langmuir 1996, 12, 3101–3106. [Google Scholar] [CrossRef]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. Tailor-made polymeric membranes based on segmented block copolymers for CO2 separation. Adv. Funct. Mater. 2008, 18, 2815–2823. [Google Scholar] [CrossRef] [Green Version]
- Car, A.; Stropnik, C.; Yave, W.; Peinemann, K.-V. PEG modified poly(amide-b-ethylene oxide) membranes for CO2 separation. J. Membr. Sci. 2008, 307, 88–95. [Google Scholar] [CrossRef]
- Chong, K.C.; Lai, S.O.; Lau, W.J.; Thiam, H.S.; Ismail, A.F.; Roslan, R.A. Preparation, characterization, and performance evaluation of polysulfone hollow fiber membrane with PEBAX or PDMS coating for oxygen enhancement process. Polymers 2018, 10, 126. [Google Scholar] [CrossRef] [Green Version]
- Scholes, C. Water resistant composite membranes for carbon dioxide separation from methane. Appl. Sci. 2018, 8, 829. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Dai, Z.; Deng, L. Effects of the morphology of the ZIF on the CO2 separation performance of MMMs. Ind. Eng. Chem. Res. 2020, 59, 14458–14466. [Google Scholar] [CrossRef]
- Hsu, P.-Y.; Hu, T.-Y.; Kumar, S.R.; Chang, C.-H.; Wu, K.C.-W.; Tung, K.-L.; Lue, S.J. Highly zeolite-loaded polyvinyl alcohol composite membranes for alkaline fuel-cell electrolytes. Polymers 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Goh, P.S.; Wong, K.C.; Yogarathinam, L.T.; Ismail, A.F.; Abdullah, M.S.; Ng, B.C. Surface modifications of nanofillers for carbon dioxide separation nanocomposite membrane. Symmetry 2020, 12, 1102. [Google Scholar] [CrossRef]
- Megías-Sayago, C.; Bingre, R.; Huang, L.; Lutzweiler, G.; Wang, Q.; Louis, B. CO2 adsorption capacities in zeolites and layered double hydroxide materials. Front. Chem. 2019, 7. [Google Scholar] [CrossRef] [Green Version]
- Frisch, H.L. The time lag in diffusion. J. Phys. Chem. 1957, 61, 93–95. [Google Scholar] [CrossRef]
- Qiu, J.; Zheng, J.-M.; Peinemann, K.-V. Gas transport properties of poly(trimethylsilylpropyne) and ethylcellulose filled with different molecular weight trimethylsilylsaccharides: Impact on fractional free volume and chain mobility. Macromolecules 2007, 40, 3213–3222. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Shishatskii, A.; Yampol’Skii, Y.; Peinemann, K.-V. Effects of film thickness on density and gas permeation parameters of glassy polymers. J. Membr. Sci. 1996, 112, 275–285. [Google Scholar] [CrossRef]

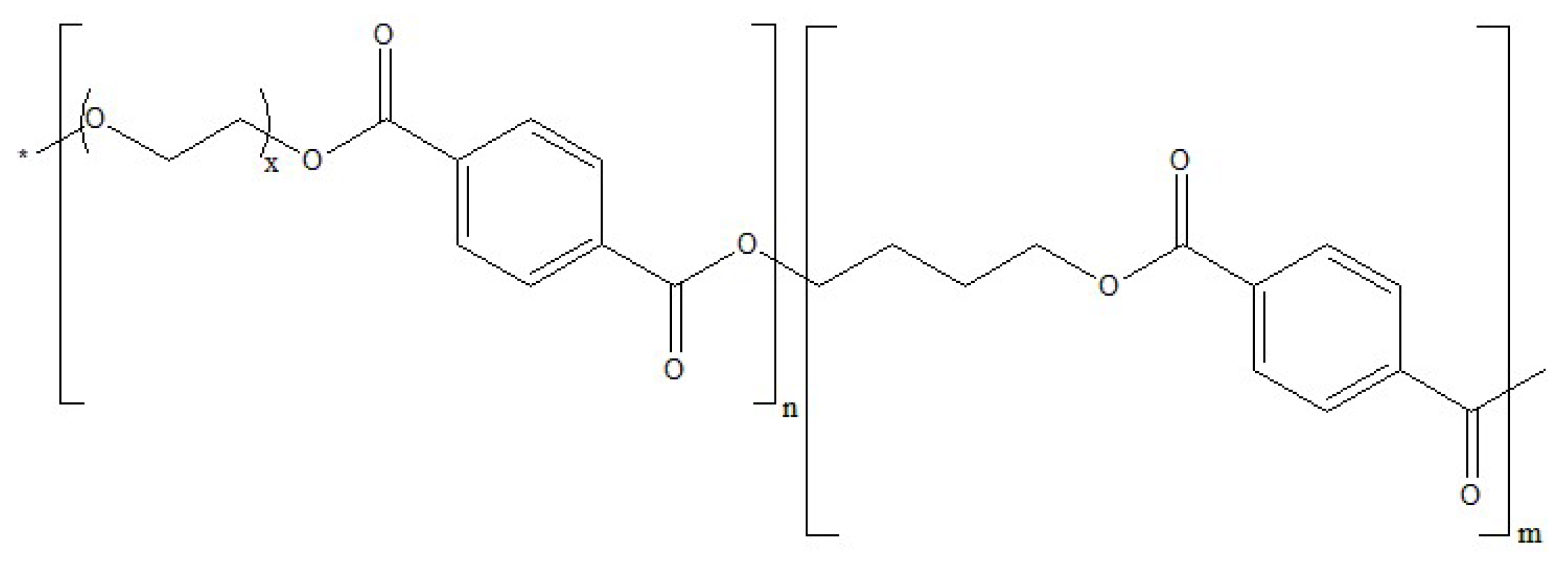

| Sample | Polymer | Zeolite | Additive | Thickness, µm |
|---|---|---|---|---|
| PB | PEBAX | - | - | 207 |
| PBT | PEBAX | ITR | TMAB | 188 |
| PBW | PEBAX | IWS | TMAB | 197 |
| PA | Polyactive | - | - | 220 |
| PAT | Polyactive | ITR | TMAB | 235 |
| PAW | Polyactive | IWS | TMAB | 230 |
| Sample | P(CO2), Barrer | P(H2), Barrer | P(O2), Barrer | P(N2), Barrer | α (CO2/H2) | α (CO2/O2) | α (CO2/N2) |
|---|---|---|---|---|---|---|---|
| PB | 118 | 13.7 | 5.2 | 2.1 | 8.6 | 22.5 | 55 |
| PBT | 123 | 13.6 | 5.2 | 2 | 9 | 23.7 | 62 |
| PBW | 119 | 12.9 | 4.9 | 2 | 9.2 | 24 | 60 |
| PA | 110 | 12.5 | 5.4 | 1.8 | 8.8 | 20.5 | 60 |
| PAT | 120 | 14 | 5.9 | 2 | 8.6 | 20.3 | 58 |
| PAW | 118 | 13.1 | 5.8 | 2 | 9 | 20.3 | 60 |
| Sample | P(CO2), Barrer | P(H2), Barrer | P(O2), Barrer | P(N2), Barrer | α (CO2/H2) | α (CO2/O2) | α (CO2/N2) |
|---|---|---|---|---|---|---|---|
| PB | 120 | 13.7 | 5.2 | 2.1 | 8.8 | 22.7 | 56 |
| PBT | 127 | 13.9 | 5.3 | 2 | 9.1 | 23.7 | 63 |
| PBW | 118 | 12.9 | 4.9 | 2 | 9.4 | 24.1 | 60 |
| PA | 115 | 12.8 | 5.5 | 1.9 | 9 | 20.8 | 61 |
| PAT | 123 | 14 | 6 | 2.1 | 8.8 | 20.3 | 60 |
| PAW | 120 | 13.2 | 5.9 | 2 | 9.1 | 20.4 | 60 |
| Sample | P(CO2), Barrer | P(H2), Barrer | P(O2), Barrer | P(N2), Barrer | α (CO2/H2) | α (CO2/O2) | α (CO2/N2) |
|---|---|---|---|---|---|---|---|
| PB | 121 | 13.8 | 5.3 | 2.1 | 8.9 | 23 | 56.5 |
| PBT | 130 | 14.3 | 5.4 | 2.1 | 9.1 | 23.9 | 63 |
| PBW | 122 | 13 | 5 | 2 | 9.4 | 24.3 | 61 |
| PA | 117 | 12.9 | 5.6 | 1.9 | 9.1 | 21 | 61 |
| PAT | 125 | 14.2 | 6.1 | 2.1 | 8.8 | 20.5 | 61 |
| PAW | 121 | 13.2 | 5.9 | 2 | 9.2 | 20.6 | 62 |
| Sample | P(CO2), Barrer | P(H2), Barrer | P(O2), Barrer | P(N2), Barrer | α (CO2/H2) | α (CO2/O2) | α (CO2/N2) |
|---|---|---|---|---|---|---|---|
| PB | 125 | 14 | 5.4 | 2.2 | 8.9 | 23 | 57 |
| PBT | 133 | 14.3 | 5.5 | 2.1 | 9.3 | 24 | 64 |
| PBW | 123 | 13 | 5.1 | 2 | 9.5 | 24.3 | 61 |
| PA | 121 | 13.3 | 5.7 | 2 | 9.1 | 21.3 | 62 |
| PAT | 126 | 14.2 | 6.1 | 2.1 | 8.9 | 20.6 | 61 |
| PAW | 124 | 13.3 | 6 | 2.1 | 9.3 | 21 | 61 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nedeljkovic, D. The Effect of the Temperature and Moisture to the Permeation Properties of PEO-Based Membranes for Carbon-Dioxide Separation. Polymers 2021, 13, 2053. https://doi.org/10.3390/polym13132053
Nedeljkovic D. The Effect of the Temperature and Moisture to the Permeation Properties of PEO-Based Membranes for Carbon-Dioxide Separation. Polymers. 2021; 13(13):2053. https://doi.org/10.3390/polym13132053
Chicago/Turabian StyleNedeljkovic, Dragutin. 2021. "The Effect of the Temperature and Moisture to the Permeation Properties of PEO-Based Membranes for Carbon-Dioxide Separation" Polymers 13, no. 13: 2053. https://doi.org/10.3390/polym13132053






