A Multimodal Stimulation Cell Culture Bioreactor for Tissue Engineering: A Numerical Modelling Approach
Abstract
:1. Introduction
2. Materials and Methods
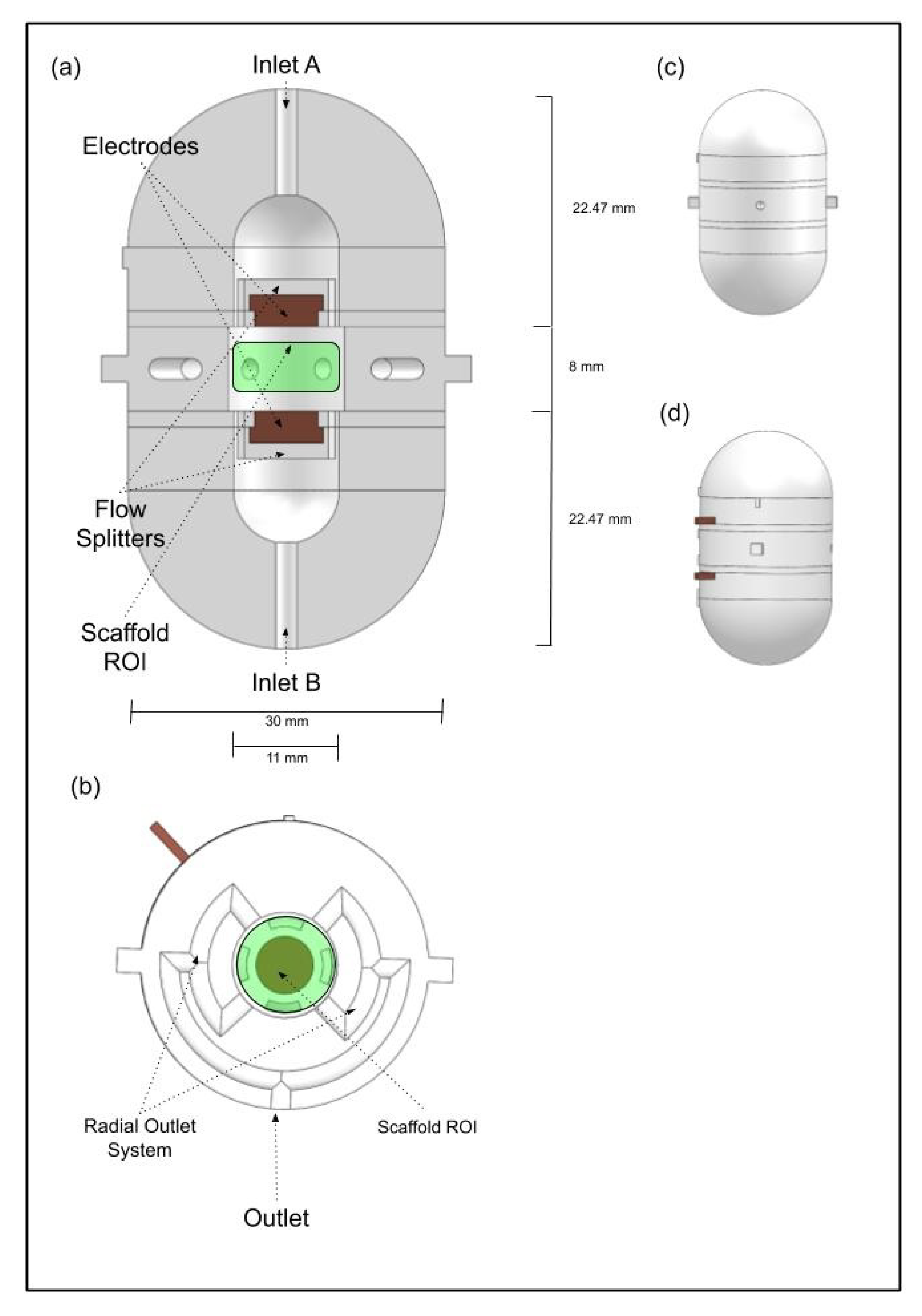
2.1. Multimodal Stimulation Bioreactor Design
2.2. Material Samples Production
2.3. In Vitro Cytotoxicity Tests
2.4. Multimodal Stimulation
3. Results and Discussion
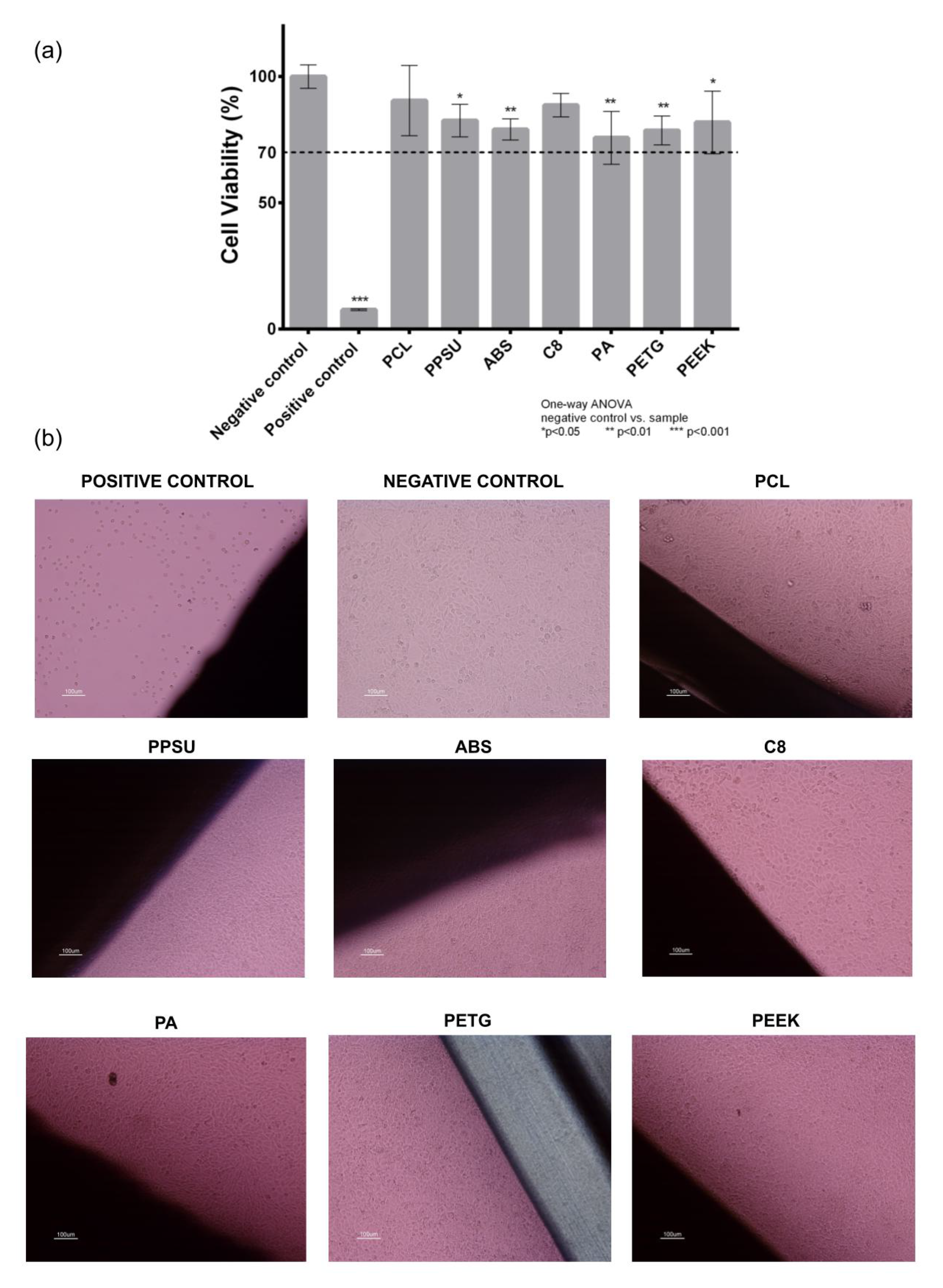
3.1. Cytotoxicity Study
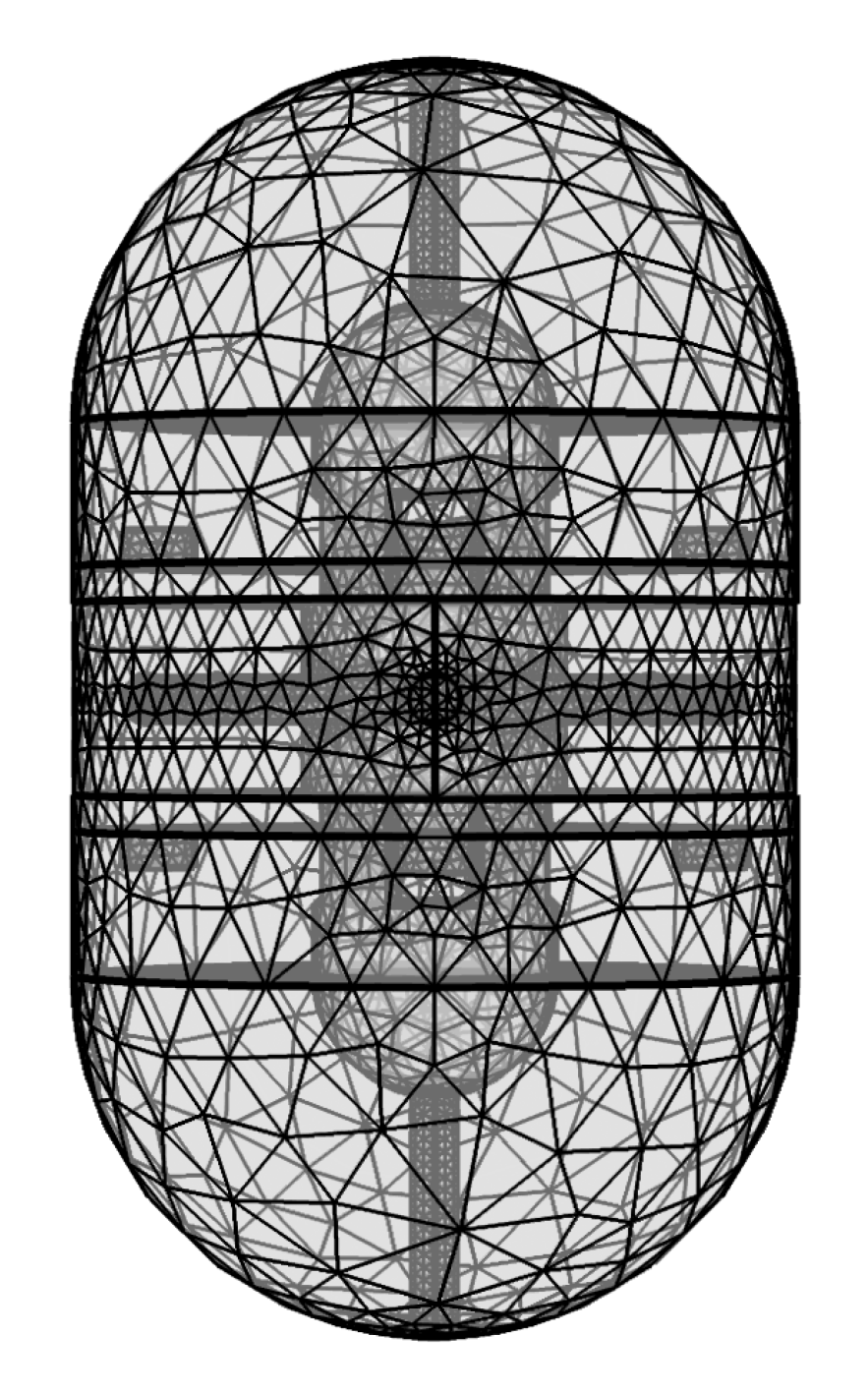
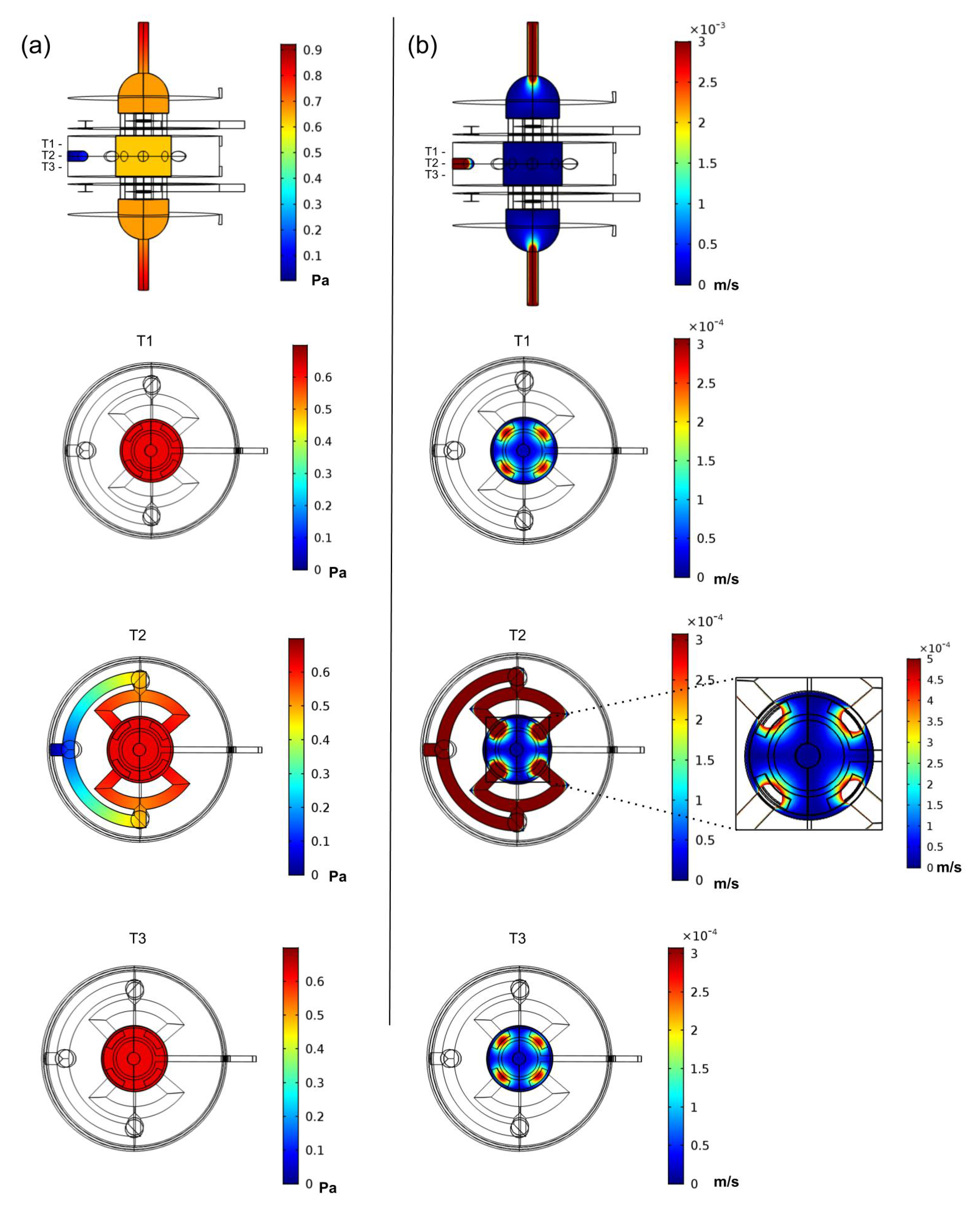
3.2. Finite Element Analysis Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mekala, N.K.; Baadhe, R.R.; Potumarthi, R. Mass transfer aspects of 3D cell cultures in tissue engineering. Asia-Pac. J. Chem. Eng. 2014, 9, 318–329. [Google Scholar] [CrossRef]
- Bhaskar, B.; Owen, R.; Bahmaee, H.; Rao, P.S.; Reilly, G.C. Design and Assessment of a Dynamic Perfusion Bioreactor for Large Bone Tissue Engineering Scaffolds. Appl. Biochem. Biotechnol. 2018, 2, 185–555. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef] [PubMed]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D printing applications in bone tissue engineering. J. Clin. Orthop. Trauma 2020, 11, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Dhinakaran, V.; Kumar, K.P.M.; Ram, P.M.B.; Ravichandran, M.; Vinayagamoorthy, M. A review on recent advancements in fused deposition modeling. Mater. Today Proc. 2020, in press. [Google Scholar] [CrossRef]
- Golebiowska, A.A.; Kim, H.S.; Camci-Unal, G.; Nukavarapu, S.P. Integration of Technologies for Bone Tissue Engineering. Encycl. Tissue Eng. Regen. Med. 2019, 1, 243–259. [Google Scholar]
- Selden, C.; Fuller, B. Role of Bioreactor Technology in Tissue Engineering for Clinical Use and Therapeutic Target Design. Bioengineering 2018, 5, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.F.; Freitas, D.; Tojeira, A.; Almeida, H.A.; Alves, N.; Bártolo, P.J. Computer modelling and simulation of a bioreactor for tissue engineering. Int. J. Comput. Integr. Manuf. 2014, 27, 946–959. [Google Scholar] [CrossRef]
- Zhao, F.; van Rietbergen, B.; Ito, K.; Hofmann, S. Flow rates in perfusion bioreactors to maximise mineralisation in bone tissue engineering in vitro. J. Biomech. 2018, 79, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Mobini, S.; Leppik, L.; Barker, J.H. Direct current electrical stimulation chamber for treating cells in vitro. BioTechniques 2016, 60, 95–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses, J.; Alves, N.; Moura, C.; Datta, A.; Ferreira, F.; Amado, S.; Pascoal-Faria, P. Electrical Stimulation of Bioscaolds for Tissue Engineering: A Numerical Analysis. In Proceedings of the ICNAAM 2019—17th International Conference of Numerical Analysis and Applied Mathematics, Rhodes, Greece, 23–28 September 2019. [Google Scholar]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part:5 Tests for Citotoxicity (ISO EN 10993-5) and Part 12: SamplePreparation and Reference Materials (ISO EN 10993-12); International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Visone, R.; Talò, G.; Lopa, S.; Rasponi, M.; Moretti, M. Enhancing all-in-one bioreactors by combining interstitial perfusion, electrical stimulation, on-line monitoring and testing within a single chamber for cardiac constructs. Sci. Rep. 2018, 8, 16944. [Google Scholar] [CrossRef] [PubMed]
- Online Database of Engineering Materials Information Resource. 2020. Available online: http:www.matweb.com (accessed on 4 April 2020).
- Chen, X.D. Laminar-to-Turbulence Transition Revealed Through a Reynolds Number Equivalence. Engineering 2019, 5, 576–579. [Google Scholar] [CrossRef]
- Plonsey, R.; Heppner, D.B. Considerations of Quasi-Stationarity in Electrophysiological Systems. Bull. Math. Biophys. 1967, 29, 657. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, P.; Corson, D.R.; Lorrain, F. Fundamentals of Electromagnetic Phenomena; W.H. Freeman and Company: New York, NY, USA, 2000; pp. 170–171. [Google Scholar]
- Belleville, P.; Guillet, F.; Pons, A.; Deseure, J.; Merlin, G.; Druart, F.; Ramousse, J.; Grindler, E. Low voltage water electrolysis: Decoupling hydrogen production using bioelectrochemical system. Int. J. Hydrogen Energy 2018, 43, 14867–14875. [Google Scholar] [CrossRef]
- Brighton, C.T.; Okereke, E.; Pollack, S.R.; Clark, C.C. In Vitro Bone-Cell Response to a Capacitively Coupled Electrical Field. Basic Sci. Pathol. 1991, III, 255–262. [Google Scholar] [CrossRef]
- Titushkin, I.A.; Cho, M.R. Controlling Cellular Biomechanics of Human Mesenchymal Stem Cells. In Proceedings of the 2009 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 2090–2093. [Google Scholar]
- Kim, I.S.; Song, J.K.; Song, Y.M.; Cho, H.T.; Lee, T.H.; Lim, S.S.; Kim, S.J.; Hwang, S.J. Novel Effect of Biphasic Electric Current on In Vitro Osteogenesis and Cytokine Production in Human Mesenchymal Stromal Cells. Tissue Eng. Part A 2009, 15, 2411–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppik, L.; Bhavsar, M.B.; Oliveira, K.M.C.; Eischen-Loges, M.; Mobini, S.; Barker, J.H. Construction and Use of an Electrical Stimulation Chamber for Enhancing Osteogenic Differentiation in Mesenchymal Stem/Stromal Cells In Vitro. JoVE 2019, 143, e59127. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample ID | Material | Supplier | AM Methodology |
|---|---|---|---|
| PCL | Polycaprolactone | FACILAN™ PCL 100, 750 GRAM (MW: 50,000 g/mol), 1.75 MM, 3D4MAKERS, Haarlem, The Netherlands | 3DP Technology: FDM; 3D Printer: Creatbot F430; Nozzle Diameter: 0.4 mm; Nozzle Temperature: 165 °C; Heated Bed Temperature: 35 °C; Chamber Temperature: 20 °C; Layer Thickness: 0.02 mm; Printing Speed: 60 mm/s; Infil: 100%. |
| PA | Polyamide/Nylon | PA Powder, 3D SYSTEMS | 3DP Technology: SLS; 3D Printer: 3D System sPro 60 HD-HS; CO2 Laser 70W; Layer Thickness: 0.1 mm; Scanning Speed: 6 m/s; Construction Chamber Temperature: 173 °C; Feeding Chamber Temperature: 135°C. |
| PETG | Polyethylene Terephthalate Glycol-modified | PETG FILAMENT, 750 GRAM, 1.75MM, 3D4MAKERS, Haarlem, The Netherlands | 3DP Technology: FDM; 3D Printer: Creatbot F430; Nozzle Diameter: 0.4 mm; Nozzle Temperature: 260 °C; Heated Bed Temperature: 110 °C; Chamber Temperature: 60 °C; Layer Thickness: 0.02 mm; Printing Speed: 60 mm/s; Infil: 100%. |
| ABS | Acrylonitrile- Butadiene- Styrene | ABS FILAMENT, 750 GRAM, 1.75MM, 3D4MAKERS, Haarlem, The Netherlands | 3DP Technology: FDM; 3D Printer: Creatbot F430; Nozzle Diameter: 0.4 mm; Nozzle Temperature: 230 °C; Heated Bed Temperature: 50 °C; Chamber Temperature: 35 °C; Layer Thickness: 0.02 mm; Printing Speed: 60 mm/s; Infil: 100%. |
| C8 | Proprietary Polymer Composite produced by ELOGIOAM 3D MATERIALS | FACILAN™ C8 FILAMENT, 750 GRAM, 1.75MM, 3D4MAKERS, Haarlem, The Netherlands | 3DP Technology: FDM; 3D Printer: Creatbot F430; Nozzle Diameter: 0.4 mm; Nozzle Temperature: 195 °C; Heated Bed Temperature: 35 °C; Chamber Temperature: 20 °C; Layer Thickness: 0.02 mm; Printing Speed: 60 mm/s; Infil: 100%. |
| PPSU | Polyphenylsulfone | PPSU FILAMENT, 500 GRAM, 1.75 MM, 3D4MAKERS, Haarlem, The Netherlands | 3DP Technology: FDM; 3D Printer: Creatbot F430; Nozzle Diameter: 0.4 mm; Nozzle Temperature: 380 °C; Heated Bed Temperature: 120 °C; Chamber Temperature: 60 °C; Layer Thickness: 0.02 mm; Printing Speed: 60 mm/s; Infil: 100%. |
| PEEK | Polyether Ether Ketone | PEEK FILAMENT, 500 GRAM, 1.75 MM, 3D4MAKERS, Haarlem, The Netherlands | 3DP Technology: FDM; 3D Printer: Creatbot F430; Nozzle Diameter: 0.4 mm; Nozzle Temperature: 390 °C; Heated Bed Temperature: 120 °C; Chamber Temperature: 60 °C; Layer Thickness: 0.02 mm; Printing Speed: 60 mm/s; Infill: 100%. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meneses, J.; C. Silva, J.; R. Fernandes, S.; Datta, A.; Castelo Ferreira, F.; Moura, C.; Amado, S.; Alves, N.; Pascoal-Faria, P. A Multimodal Stimulation Cell Culture Bioreactor for Tissue Engineering: A Numerical Modelling Approach. Polymers 2020, 12, 940. https://doi.org/10.3390/polym12040940
Meneses J, C. Silva J, R. Fernandes S, Datta A, Castelo Ferreira F, Moura C, Amado S, Alves N, Pascoal-Faria P. A Multimodal Stimulation Cell Culture Bioreactor for Tissue Engineering: A Numerical Modelling Approach. Polymers. 2020; 12(4):940. https://doi.org/10.3390/polym12040940
Chicago/Turabian StyleMeneses, João, João C. Silva, Sofia R. Fernandes, Abhishek Datta, Frederico Castelo Ferreira, Carla Moura, Sandra Amado, Nuno Alves, and Paula Pascoal-Faria. 2020. "A Multimodal Stimulation Cell Culture Bioreactor for Tissue Engineering: A Numerical Modelling Approach" Polymers 12, no. 4: 940. https://doi.org/10.3390/polym12040940






