Effects of Bromination-Dehydrobromination on the Microstructure of Isotropic Pitch Precursors for Carbon Fibers
Abstract
:1. Introduction
2. Materials and Methods
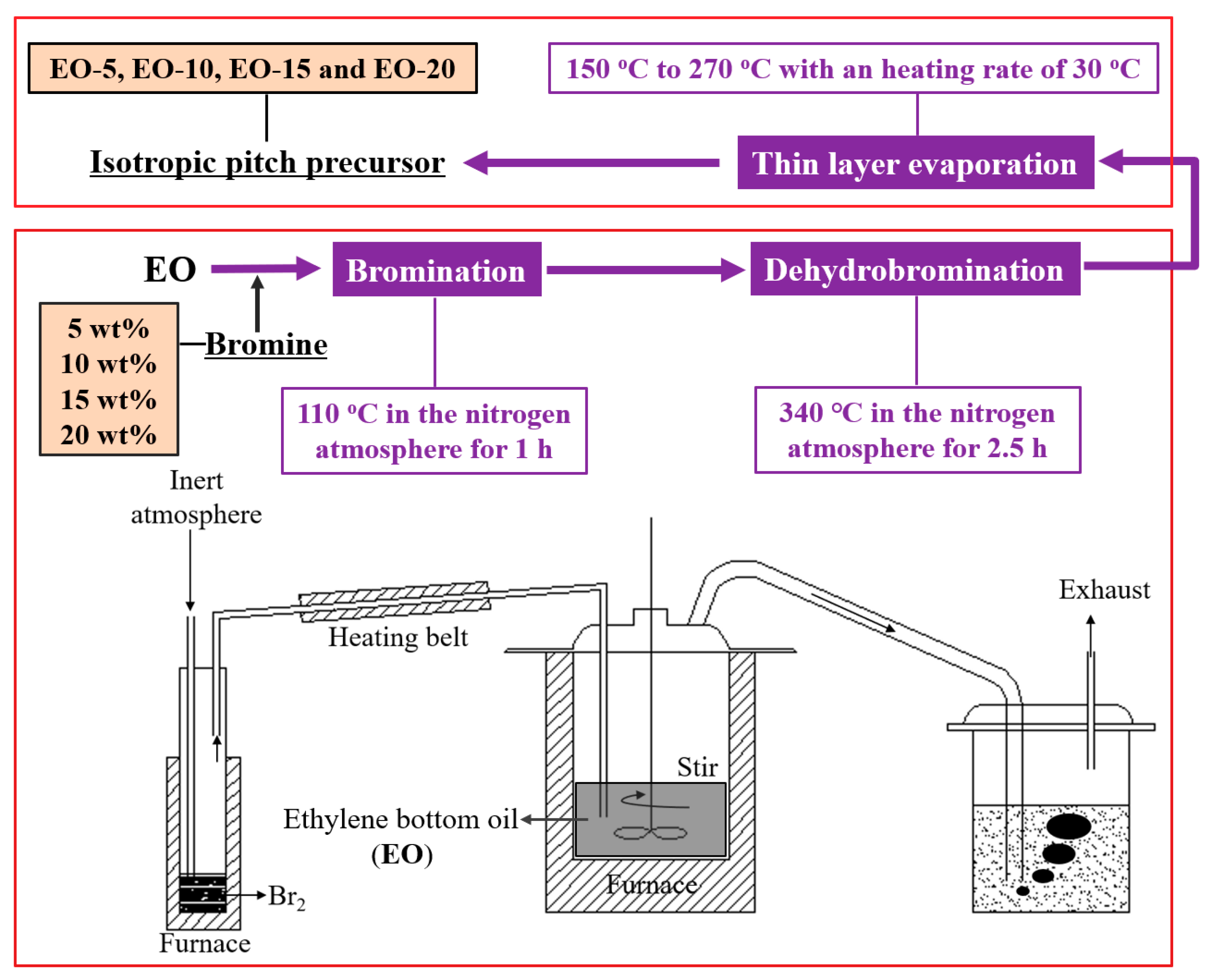
2.1. Materials and Preparation of Isotropic Pitch Precursors
2.2. Characterization of Isotropic Pitch Precursors
3. Results and Discussion
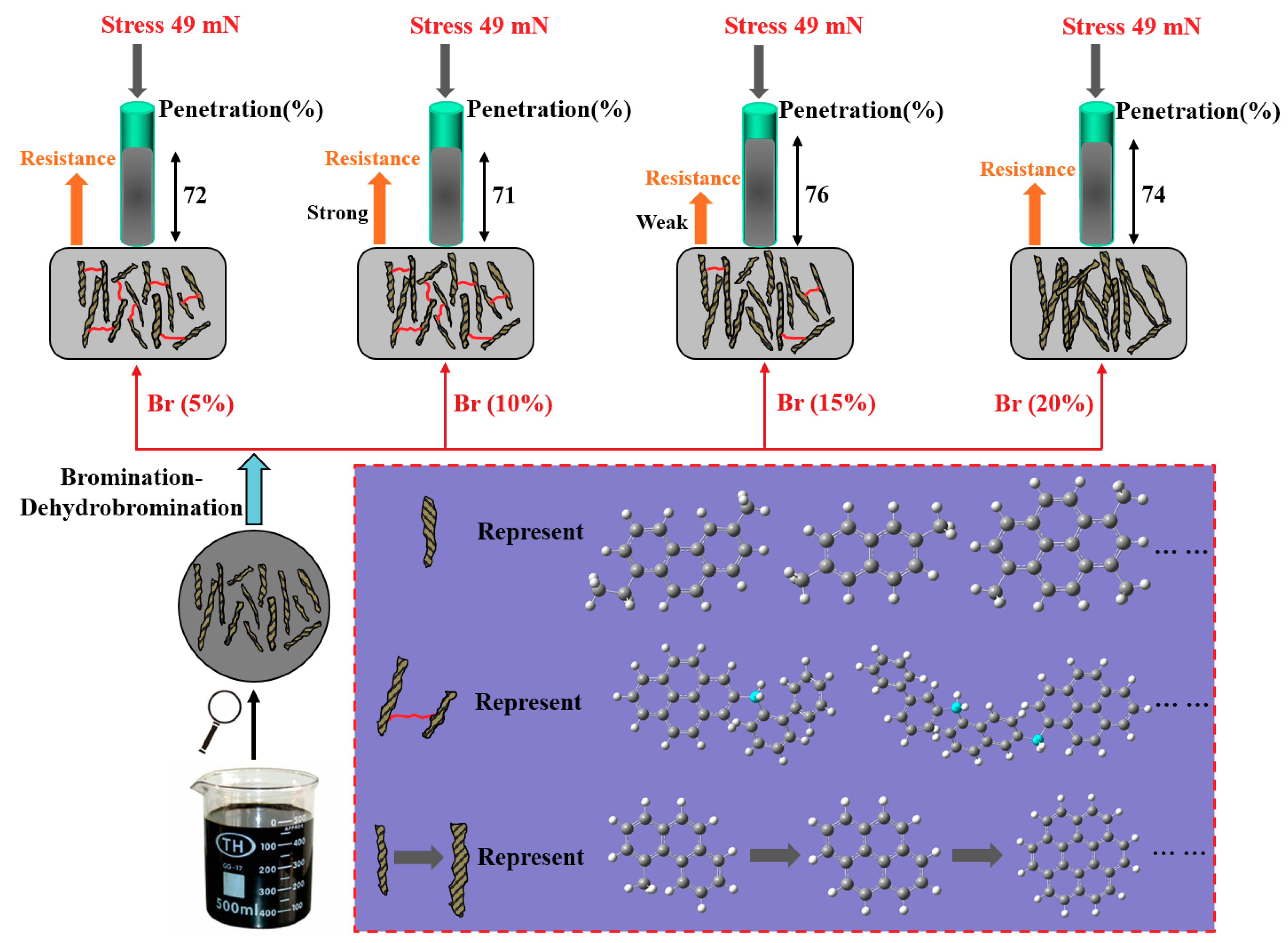
3.1. Basic Properties of Pitch Precursors
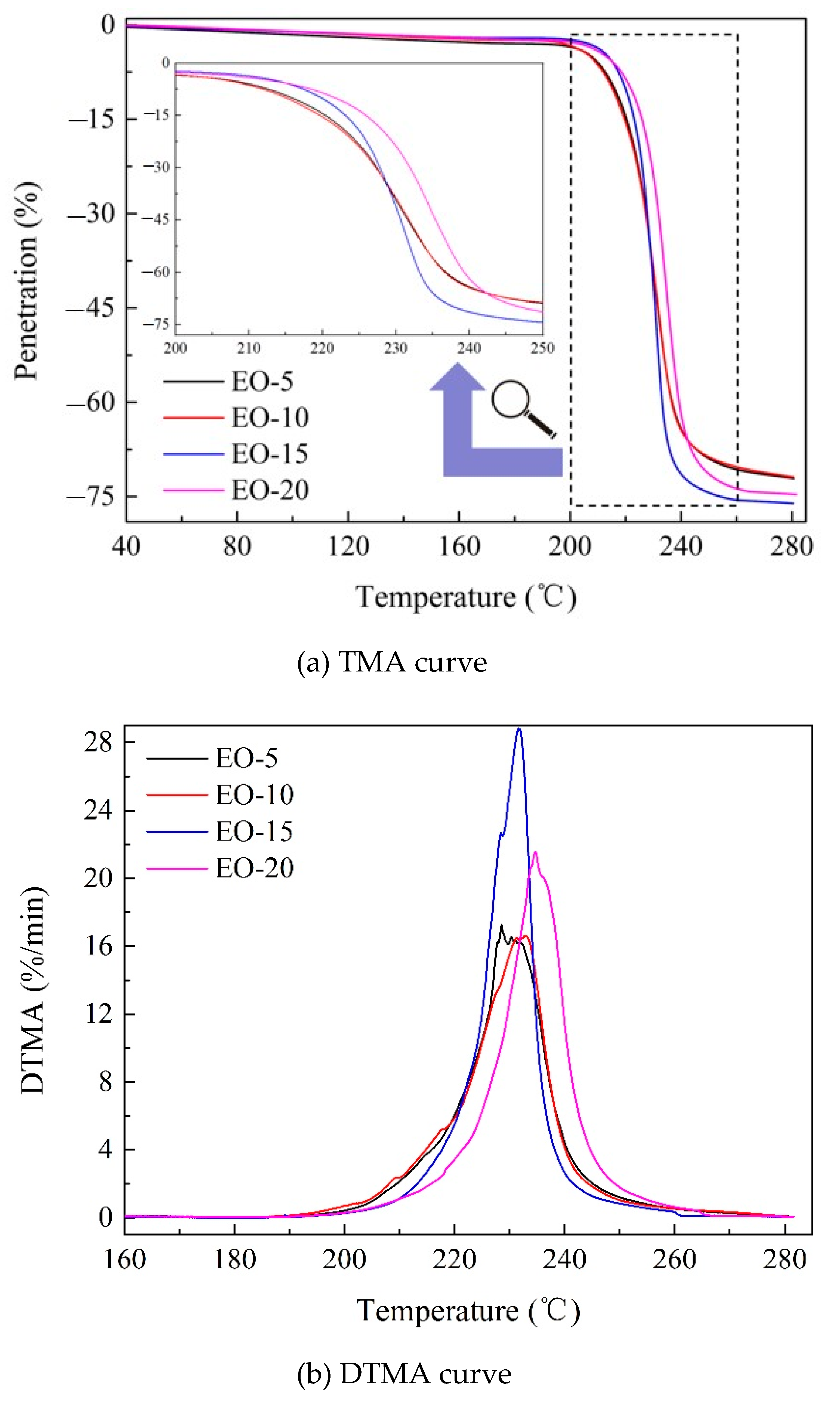
3.2. Thermal Mechanical Analysis
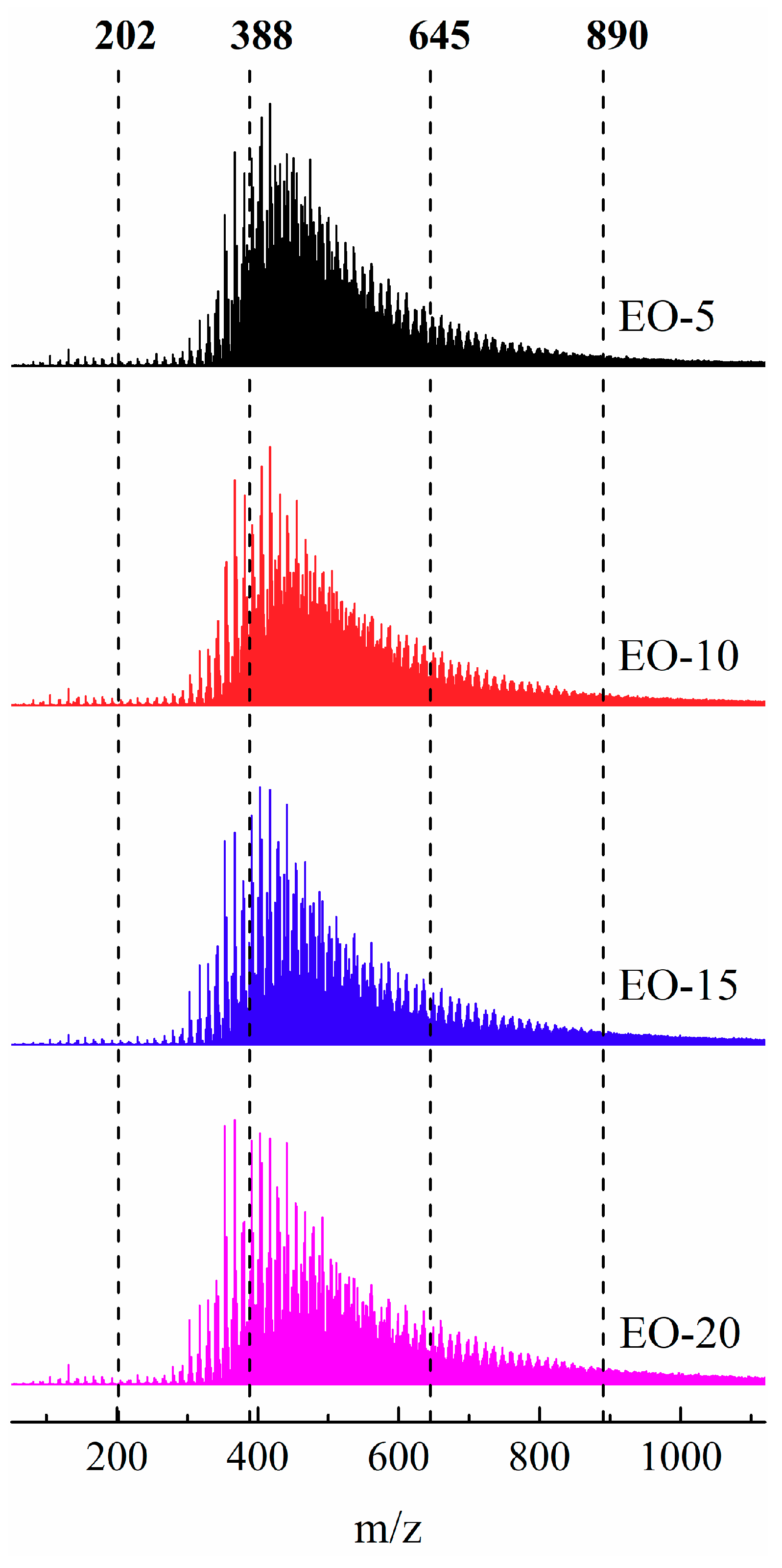
3.3. Molecular Weight Distributions
3.4. 13C NMR Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, J.; Chen, X.; Liang, D.; Xie, Q. Development of pitch-based carbon fibers: A review. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–21. [Google Scholar] [CrossRef]
- Miranda, A.T.; Bolzoni, L.; Barekar, N.; Huang, Y.; Shin, J.; Ko, S.H.; Mckay, B.J. Processing, structure and thermal conductivity correlation in carbon fiber reinforced aluminium metal matrix composites. Mater. Des. 2018, 156, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Xi, X.; Chung, D.D.L. Colossal electric permittivity discovered in polyacrylonitrile (PAN) based carbon fiber, with comparison of PAN-based and pitch-based carbon fibers. Carbon 2019, 145, 734–739. [Google Scholar] [CrossRef]
- Naito, K. Stress analysis and fracture toughness of notched polyacrylonitrile (PAN)-based and pitch-based single carbon fibers. Carbon 2018, 126, 346–359. [Google Scholar] [CrossRef]
- Asano, K. Thermal expansion behaviour of squeeze-cast aluminium matrix composites reinforced with PAN- and Pitch-based carbon fibers. Int. J. Cast Met. Res. 2017, 30, 365–373. [Google Scholar] [CrossRef]
- Naito, K.; Tanaka, Y.; Yang, J.M. Transverse compressive properties of polyacrylonitrile (PAN)-based and pitch-based single carbon fibers. Carbon 2017, 118, 168–183. [Google Scholar] [CrossRef]
- Kim, B.; Kotegawa, T.; Eom, Y.; An, J.; Hong, I.P.; Kato, O.; Nakabayashi, K.; Miyawaki, J.; Kim, B.C.; Mochida, I.; et al. Enhancing the tensile strength of isotropic pitch-based carbon fibers by improving the stabilization and carbonization properties of precursor pitch. Carbon 2016, 99, 649–657. [Google Scholar] [CrossRef]
- Özsin, G.; Pütün, A.E.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.H. Environmental-friendly production of carbon fiber from isotropic hybrid pitches synthesized from waste biomass and polystyrene with ethylene bottom oil. J. Clean. Prod. 2019, 239, 118025. [Google Scholar] [CrossRef]
- Zabihi, O.; Shafei, S.; Fakhrhoseini, S.M.; Ahmadi, M.; Nazarloo, H.A.; Stanger, R.; Tran, Q.A.; Lucas, J.; Wall, T.; Naebe, M. Low-cost carbon fiber derived from sustainable coal tar pitch and polyacrylonitrile: Fabrication and characterisation. Materials 2019, 12, 1281. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.H. Preparation of pitch based carbon fibers using Hyper-coal as a raw material. Carbon 2016, 106, 28–36. [Google Scholar] [CrossRef]
- Kim, J.; Im, U.; Lee, B.; Peck, D.H.; Yoon, S.H.; Jung, D.H. Pitch-based carbon fibers from coal tar or petroleum residue under the same processing condition. Carbon Lett. 2016, 19, 72–78. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Shi, K.; Li, X.; Yoon, S.H. Preparation of isotropic spinnable pitch and carbon fiber from biomass tar through the co-carbonization with ethylene bottom oil. Carbon Lett. 2018, 25, 89–94. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.H. Preparation of isotropic pitch-based carbon fiber using hyper coal through co-carbonation with ethylene bottom oil. J. Ind. Eng. Chem. 2016, 34, 397–404. [Google Scholar] [CrossRef]
- Liu, J.; Shimanoe, H.; Nakabayashi, K.; Miyawaki, J.; Ko, S.; Jeon, Y.P.; Yoon, S.H. Preparation of isotropic pitch precursor for pitch-based carbon fiber through the co-carbonization of ethylene bottom oil and polyvinyl chloride. J. Ind. Eng. Chem. 2018, 67, 276–283. [Google Scholar] [CrossRef]
- Liu, J.; Shimanoe, H.; Nakabayashi, K.; Miyawaki, J.; Choi, J.E.; Jeon, Y.P.; Yoon, S.H. Enhancing the oxidative stabilization of isotropic pitch precursors prepared through the co-carbonization of ethylene bottom oil and polyvinyl chloride. J. Ind. Eng. Chem. 2018, 67, 358–364. [Google Scholar] [CrossRef]
- Yang, J.; Nakabayashi, K.; Miyawaki, J.; Yoon, S.H. Preparation of isotropic spinnable pitch and carbon fiber by the bromination–dehydrobromination of biotar and ethylene bottom oil mixture. J. Mater. Sci. 2017, 52, 1165–1171. [Google Scholar] [CrossRef]
- Ge, C.; Yang, H.; Miyawaki, J.; Mochida, I.; Yoon, S.H.; Qiao, W.; Long, D.; Ling, L. Synthesis and characterization of high-softening-point methylene-bridged pitches by visible light irradiation assisted free-radical bromination. Carbon 2015, 95, 780–788. [Google Scholar] [CrossRef]
- Kim, B.J.; Kil, H.; Watnabe, N.; Seo, M.H.; Kim, B.H.; Yang, K.S.; Kato, O.; Miyawaki, J.; Mochida, I.; Yoon, S.H. Preparation of novel isotropic pitch with high softening point and solvent solubility for pitch-based electrospun carbon nanofiber. Curr. Org. Chem. 2013, 17, 1463–1468. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Xie, Q.; Liang, D. Controllable synthesis of isotropic pitch precursor for general purpose carbon fiber using waste ethylene tar via bromination–dehydrobromination. J. Clean. Prod. 2020, 271, 122498. [Google Scholar] [CrossRef]
- Ge, C.Z.; Sun, Z.L.; Yang, H.X.; Long, D.; Qiao, W.M.; Ling, L.C. Preparation and characterization of high softening point and homogeneous isotropic pitches produced from distilled ethylene tar by a novel bromination method. New Carbon Mater. 2018, 33, 71–81. [Google Scholar] [CrossRef]
- Luo, F.; Ouyang, T.; Fei, Y. Thermal properties of isotropic pitch fibers characterized by thermomechanical analysis. J. Mater. Sci. 2016, 51, 3408–3414. [Google Scholar] [CrossRef]
- Kadla, J.F.; Kubo, S.; Venditti, R.A.; Gilbert, R.D.; Compere, A.L.; Griffith, W. Lignin-based carbon fibers for composite fiber applications. Carbon 2002, 40, 2913–2920. [Google Scholar] [CrossRef]
- Burgess, W.A.; Thies, M.C. Molecular structures for the oligomeric constituents of petroleum pitch. Carbon 2011, 49, 636–651. [Google Scholar] [CrossRef]
- Cristadoro, A.; Kulkarni, S.U.; Burgess, W.A.; Gervo, E.G.; Rader, H.J.; Mullen, K.; Bruce, D.A.; Thies, M.C. Structural characterization of the oligomeric constituents of petroleum pitches. Carbon 2009, 47, 2358–2370. [Google Scholar] [CrossRef]
- Diaz, C.; Blanco, C.G. NMR: A Powerful Tool in the Characterization of Coal Tar Pitch. Energy Fuels 2003, 17, 907–913. [Google Scholar] [CrossRef]


| Sample | Basic Pitch | Isotropic Pitch Precursor | Element Content | |||
|---|---|---|---|---|---|---|
| Yield (%) | Yield (%) | *SP (°C) | C (%) | H (%) | C/H | |
| EO-5 | 67.3 | 41.8 | 240 | 90.81 | 7.86 | 11.55 |
| EO-10 | 70.4 | 45.2 | 240 | 92.06 | 6.75 | 13.64 |
| EO-15 | 71.1 | 51.1 | 240 | 92.81 | 6.05 | 15.34 |
| EO-20 | 68.4 | 58.4 | 240 | 93.85 | 5.13 | 18.29 |
| Sample | *AMW | Molecular Composition (%) | ||||
|---|---|---|---|---|---|---|
| Small Molecules | Monomer | Dimer | Trimer | Tetramer | ||
| EO-5 | 638.04 | 0.12 | 6.70 | 54.37 | 25.58 | 12.94 |
| EO-10 | 667.92 | 0.13 | 6.95 | 49.09 | 28.41 | 15.42 |
| EO-15 | 707.87 | 0.05 | 5.25 | 43.28 | 32.38 | 19.04 |
| EO-20 | 737.24 | 0.05 | 5.20 | 39.87 | 33.45 | 21.43 |
| Sample | Aliphatic Carbon (%) | Aromatic Carbon (%) | fa | |||||
|---|---|---|---|---|---|---|---|---|
| CH3 a | CH2 b | CHa2 c | CHar d | Car3 e | Csar f | Car2 g | ||
| EO-5 | 6.88 | 10.04 | 14.23 | 1.94 | 30.02 | 7.95 | 28.94 | 0.69 |
| EO-10 | 5.47 | 10.23 | 14.22 | 1.85 | 30.95 | 8.73 | 28.55 | 0.70 |
| EO-15 | 5.58 | 9.21 | 11.69 | 1.76 | 33.52 | 7.42 | 30.82 | 0.74 |
| EO-20 | 5.01 | 9.43 | 10.23 | 1.59 | 35.42 | 6.58 | 31.74 | 0.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, D.; Liu, D.; Yang, S.; Lu, C.; Xie, Q.; Liu, J. Effects of Bromination-Dehydrobromination on the Microstructure of Isotropic Pitch Precursors for Carbon Fibers. Polymers 2020, 12, 3059. https://doi.org/10.3390/polym12123059
Liang D, Liu D, Yang S, Lu C, Xie Q, Liu J. Effects of Bromination-Dehydrobromination on the Microstructure of Isotropic Pitch Precursors for Carbon Fibers. Polymers. 2020; 12(12):3059. https://doi.org/10.3390/polym12123059
Chicago/Turabian StyleLiang, Dingcheng, Deqian Liu, Shuai Yang, Changyu Lu, Qiang Xie, and Jinchang Liu. 2020. "Effects of Bromination-Dehydrobromination on the Microstructure of Isotropic Pitch Precursors for Carbon Fibers" Polymers 12, no. 12: 3059. https://doi.org/10.3390/polym12123059





