The Effect of Halloysite Nanotubes on the Fire Retardancy Properties of Partially Biobased Polyamide 610
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Material Characterization
2.3.1. Cone Calorimeter Test (CCT)
2.3.2. Limiting Oxygen Index (LOI) and UL94
2.3.3. Toxicity and Opacity Test
2.3.4. Calorific Value
3. Results
3.1. Cone Calorimeter Test (CCT)
3.1.1. Heat Release Rate (HRR)
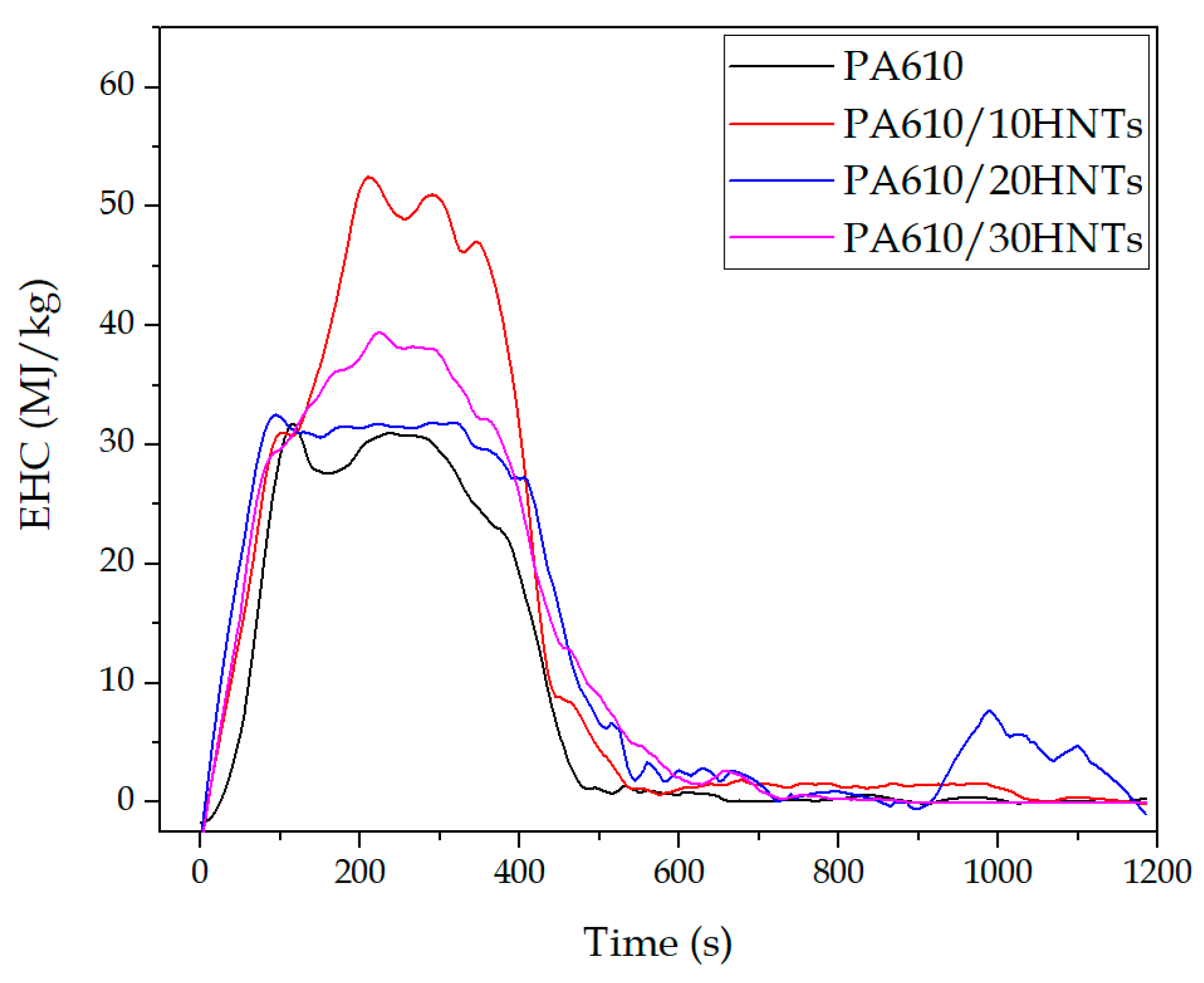
3.1.2. Effective Heat Combustion (EHC)
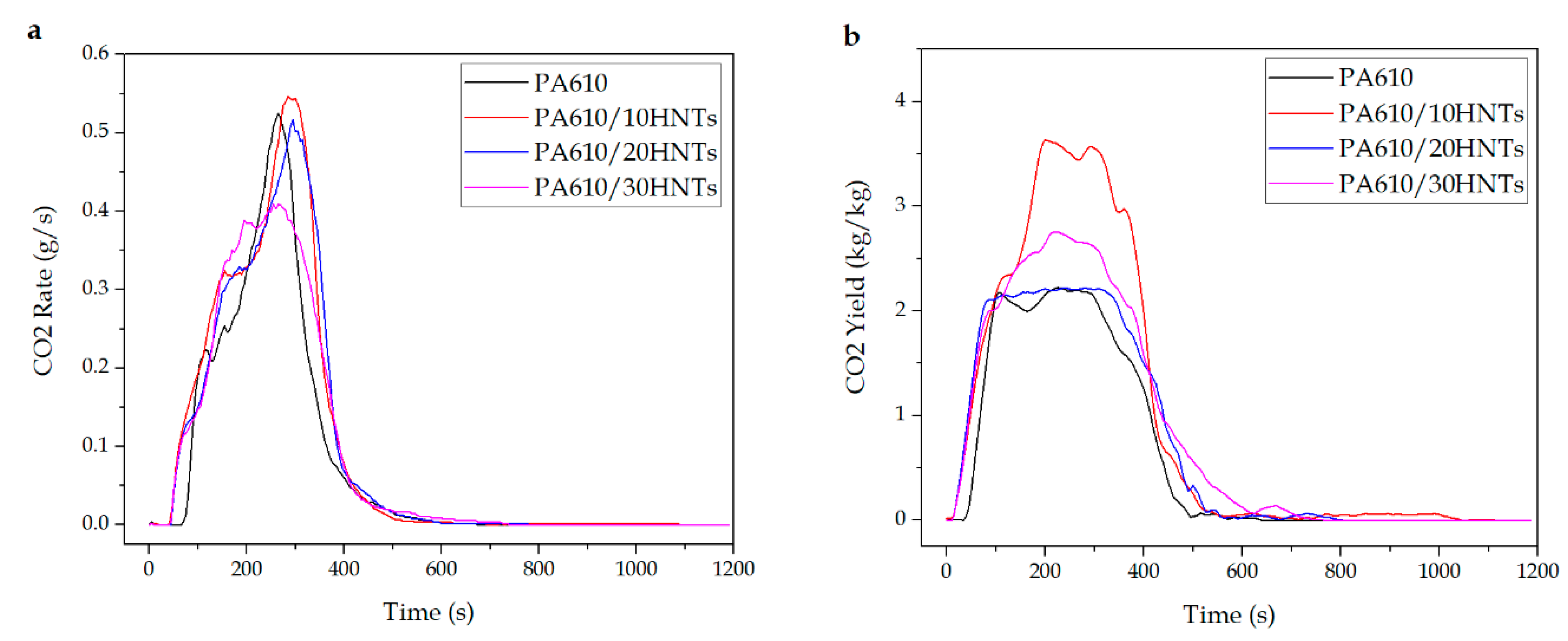
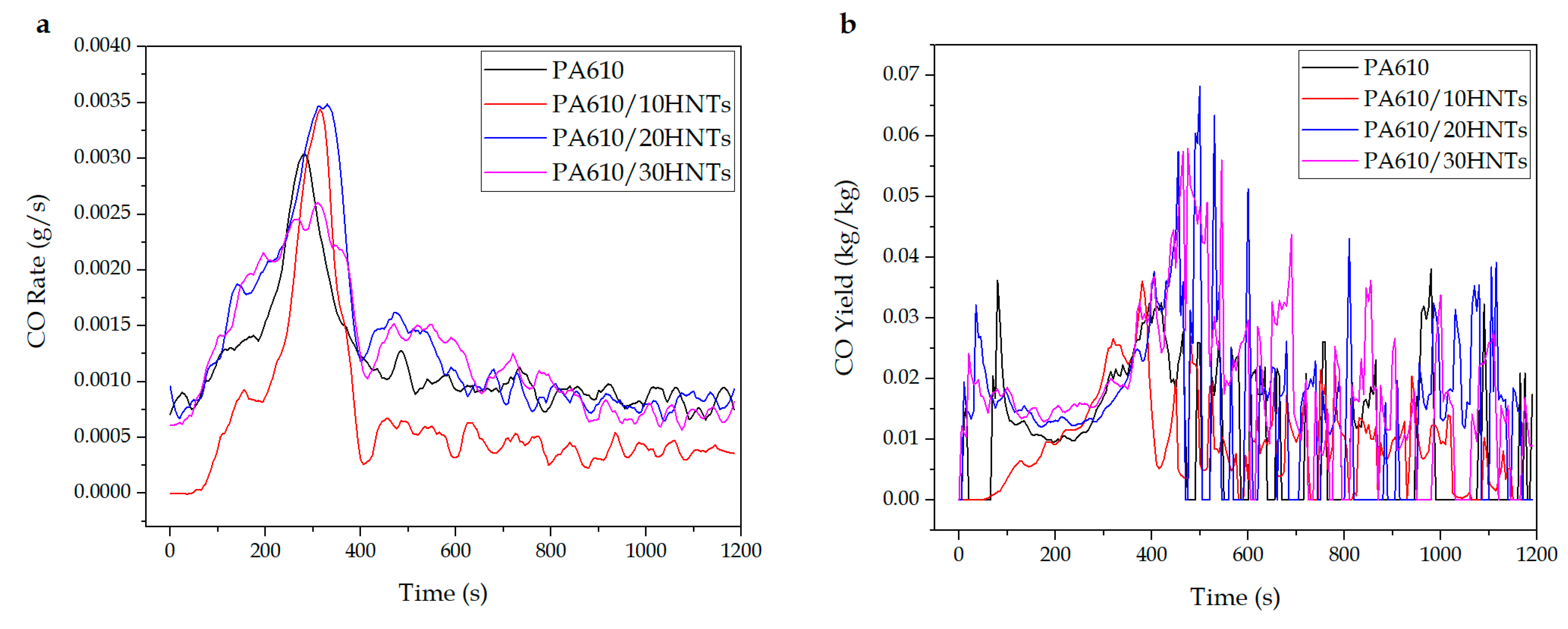
3.1.3. CO and CO2 Production
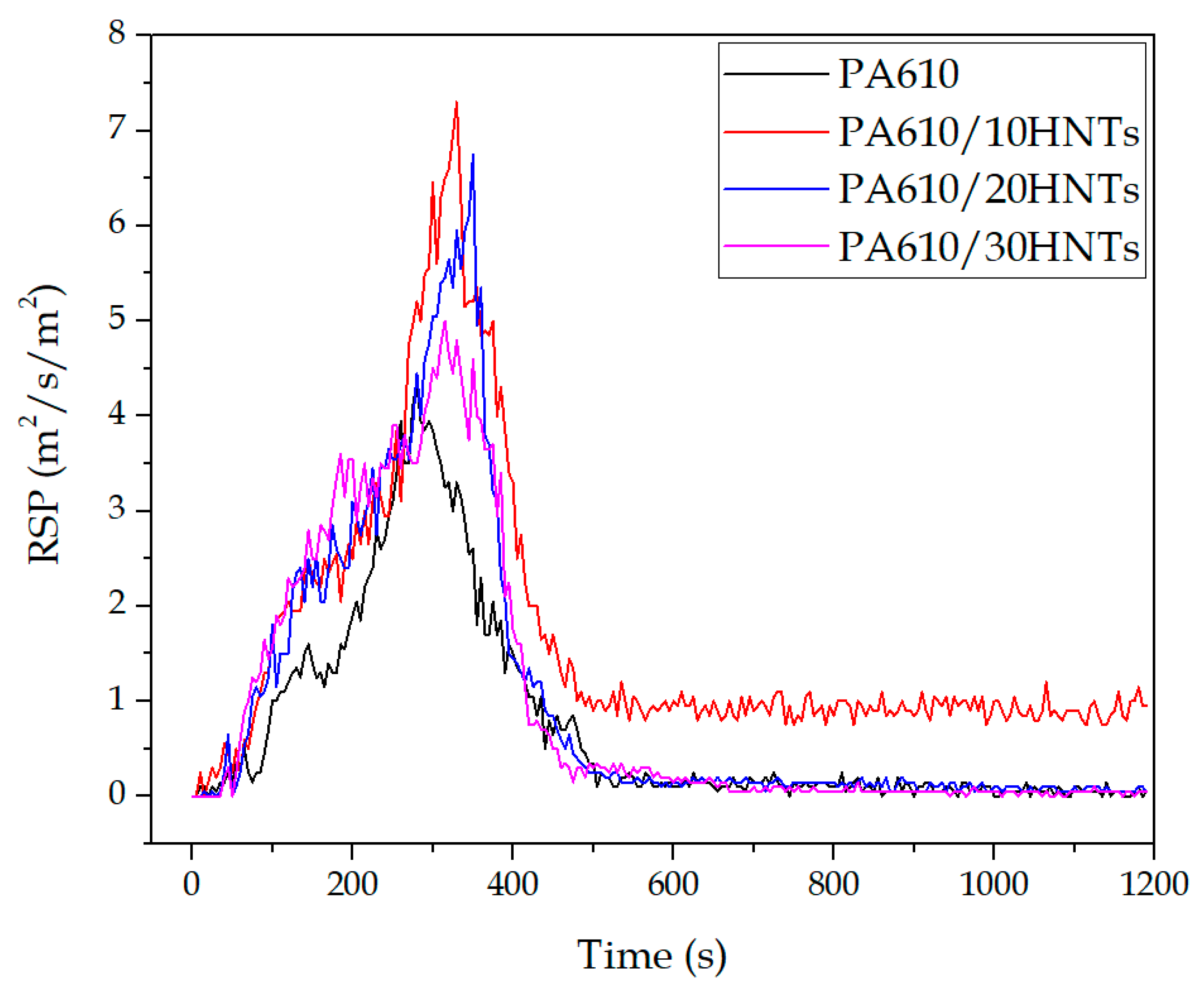
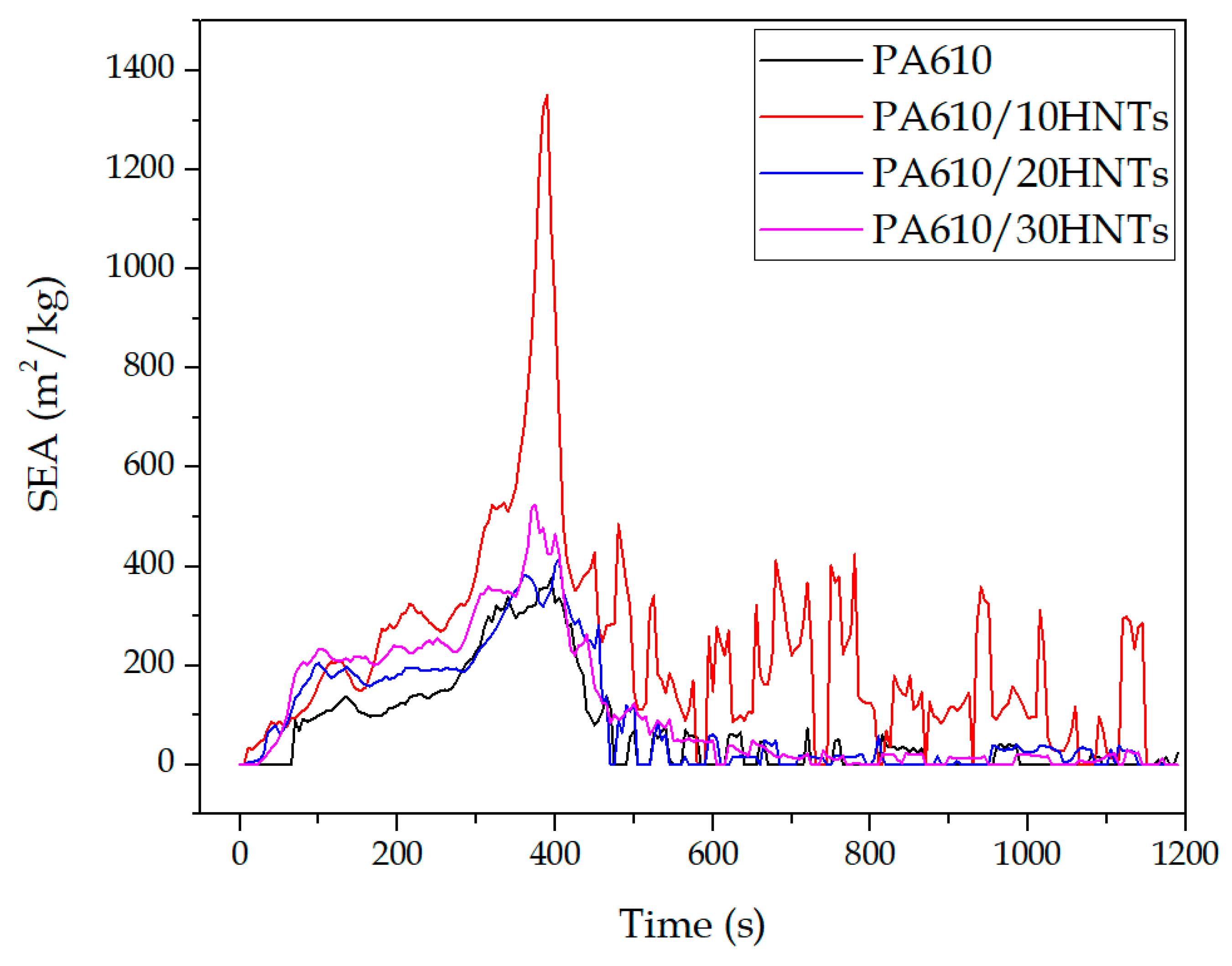
3.1.4. Rate of Smoke Production (RSP)
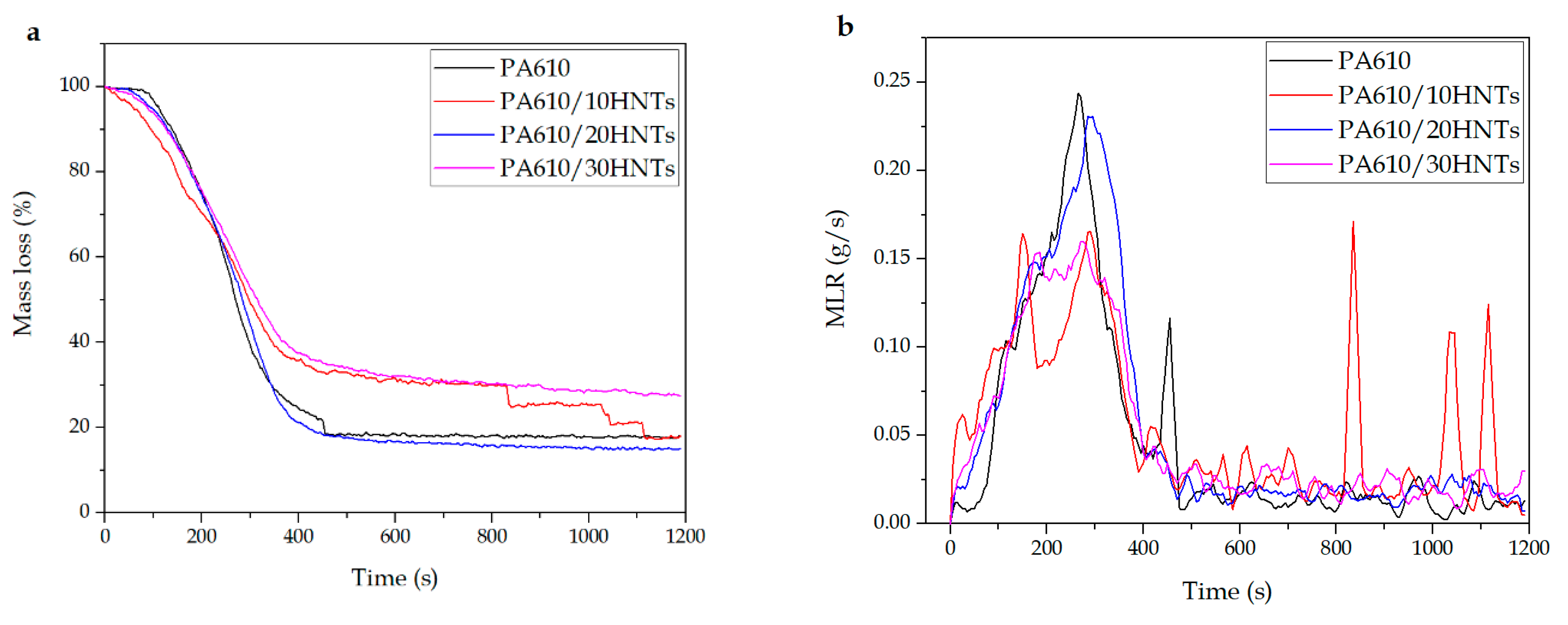
3.1.5. MASS
3.1.6. The Maximum Average Heat Rate of Emission (MARHE)
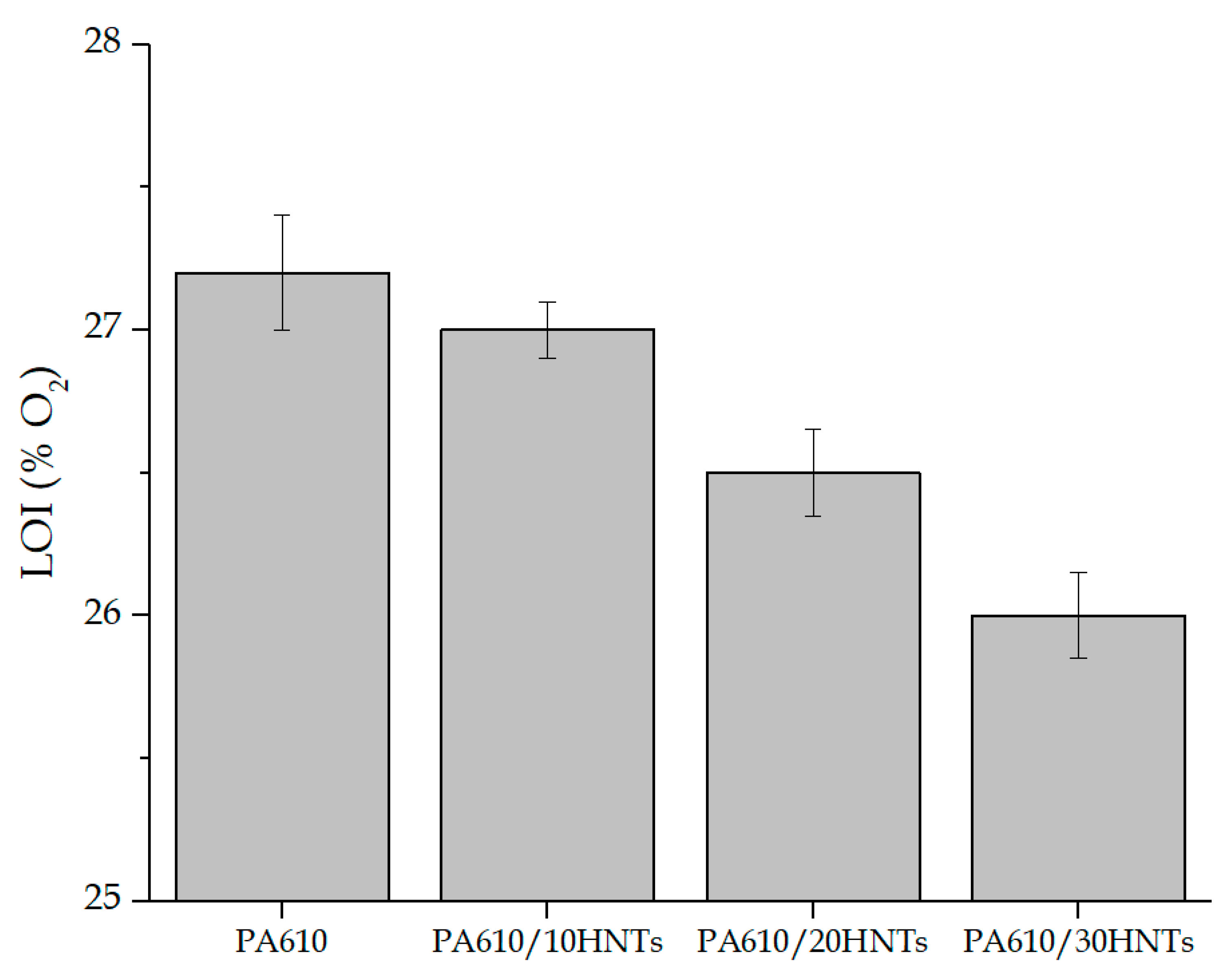
3.2. Limiting Oxygen Index (LOI) and UL94 Results
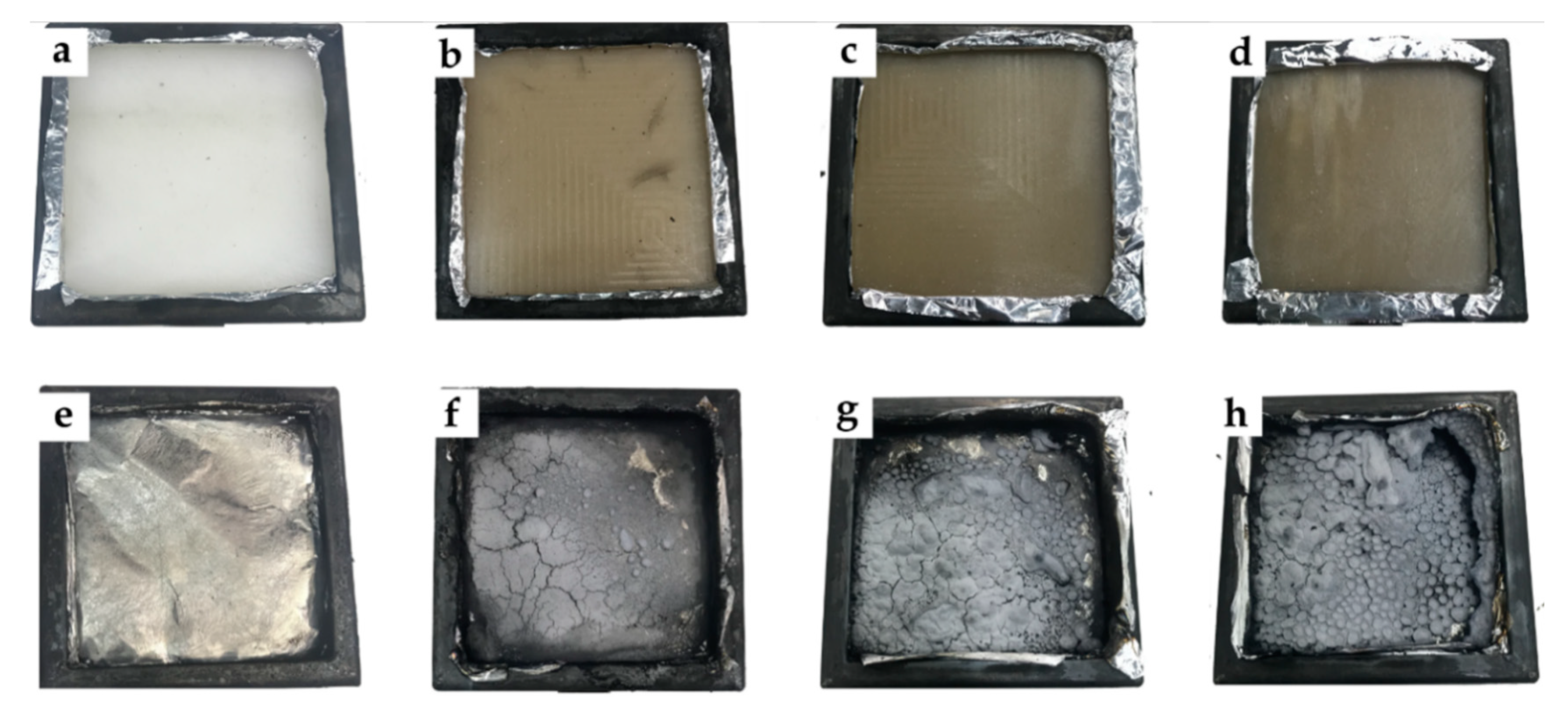
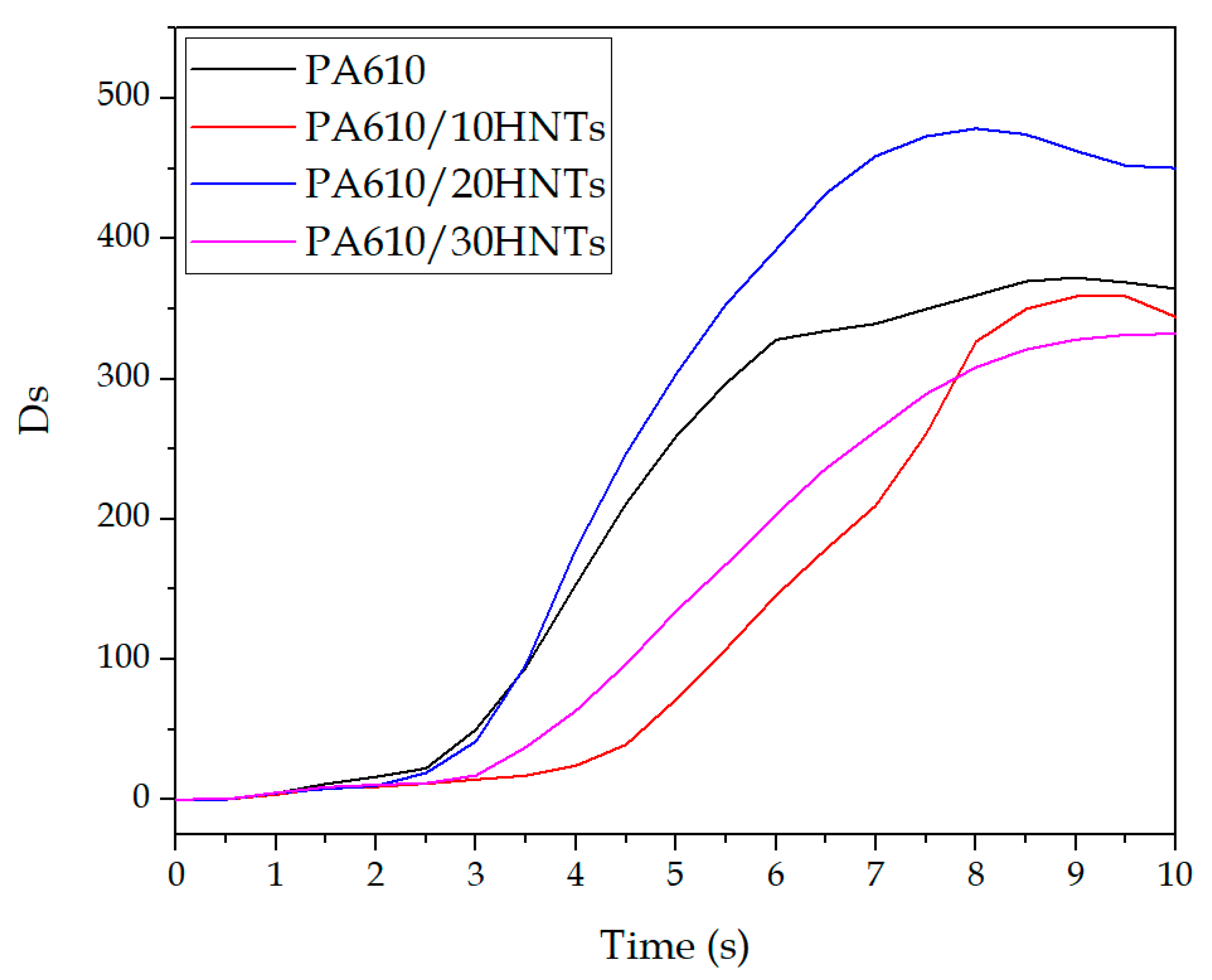
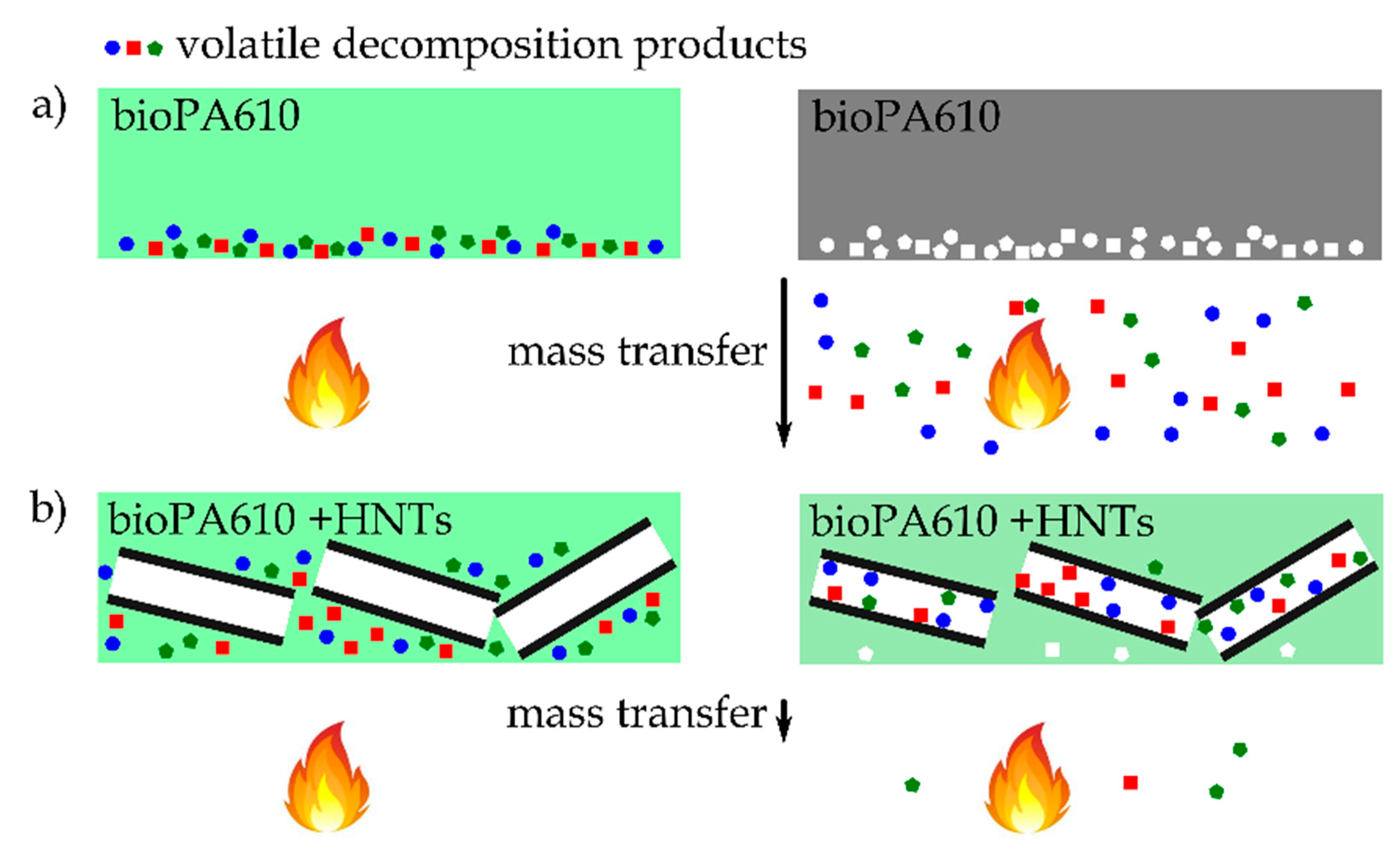
3.3. Smoke Density and Toxicity Analysis
3.4. Calorific Value
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ivorra-Martinez, J.; Manuel-Mañogil, J.; Boronat, T.; Sanchez-Nacher, L.; Balart, R.; Quiles-Carrillo, L. Development and Characterization of Sustainable Composites from Bacterial Polyester Poly (3-Hydroxybutyrate-co-3-hydroxyhexanoate) and Almond Shell Flour by Reactive Extrusion with Oligomers of Lactic Acid. Polymers 2020, 12, 1097. [Google Scholar] [CrossRef] [PubMed]
- Agüero, A.; Morcillo, M.d.C.; Quiles-Carrillo, L.; Balart, R.; Boronat, T.; Lascano, D.; Torres-Giner, S.; Fenollar, O. Study of the influence of the reprocessing cycles on the final properties of polylactide pieces obtained by injection molding. Polymers 2019, 11, 1908. [Google Scholar] [CrossRef] [Green Version]
- España, J.; Samper, M.; Fages, E.; Sánchez-Nácher, L.; Balart, R. Investigation of the effect of different silane coupling agents on mechanical performance of basalt fiber composite laminates with biobased epoxy matrices. Polym. Compos. 2013, 34, 376–381. [Google Scholar] [CrossRef]
- Garcia, D.; Balart, R.; Sanchez, L.; Lopez, J. Compatibility of recycled PVC/ABS blends. Effect of previous degradation. Polym. Eng. Sci. 2007, 47, 789–796. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Juárez, D.; Ferrand, S.; Fenollar, O.; Fombuena, V.; Balart, R. Improvement of thermal inertia of styrene–ethylene/butylene–styrene (SEBS) polymers by addition of microencapsulated phase change materials (PCMs). Eur. Polym. J. 2011, 47, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Carole, T.M.; Pellegrino, J.; Paster, M.D. Opportunities in the Industrial Biobased Products Industry. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Breckenridge, CO, USA, 4–7 May 2003; pp. 871–885. [Google Scholar]
- Quiles-Carrillo, L.; Boronat, T.; Montanes, N.; Balart, R.; Torres-Giner, S. Injection-molded parts of fully bio-based polyamide 1010 strengthened with waste derived slate fibers pretreated with glycidyl-and amino-silane coupling agents. Polym. Test. 2019, 77, 105875. [Google Scholar] [CrossRef]
- Jasinska, L.; Villani, M.; Wu, J.; van Es, D.; Klop, E.; Rastogi, S.; Koning, C.E. Novel, fully biobased semicrystalline polyamides. Macromolecules 2011, 44, 3458–3466. [Google Scholar] [CrossRef]
- Wu, J.; Jasinska-Walc, L.; Dudenko, D.; Rozanski, A.; Hansen, M.R.; Van Es, D.; Koning, C.E. An investigation of polyamides based on isoidide-2, 5-dimethyleneamine as a green rigid building block with enhanced reactivity. Macromolecules 2012, 45, 9333–9346. [Google Scholar]
- Kind, S.; Neubauer, S.; Becker, J.; Yamamoto, M.; Völkert, M.; von Abendroth, G.; Zelder, O.; Wittmann, C. From zero to hero–production of bio-based nylon from renewable resources using engineered Corynebacterium glutamicum. Metab. Eng. 2014, 25, 113–123. [Google Scholar] [CrossRef]
- Nguyen, A.Q.; Schneider, J.; Reddy, G.K.; Wendisch, V.F. Fermentative production of the diamine putrescine: System metabolic engineering of Corynebacterium glutamicum. Metabolites 2015, 5, 211–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winnacker, M.; Rieger, B. Biobased Polyamides: Recent Advances in Basic and Applied Research. Macromol. Rapid Commun. 2016, 37, 1391–1413. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Carrillo, L.; Montanes, N.; Boronat, T.; Balart, R.; Torres-Giner, S. Evaluation of the engineering performance of different bio-based aliphatic homopolyamide tubes prepared by profile extrusion. Polym. Test. 2017, 61, 421–429. [Google Scholar] [CrossRef]
- Fischer, M. Polyamides (PA). Kunstst. Plast Eur. 2004, 94, 90–95. [Google Scholar]
- Marchildon, K. Polyamides–Still Strong After Seventy Years. Macromol. React. Eng. 2011, 5, 22–54. [Google Scholar] [CrossRef]
- Nakayama, A. Development of biobased polyamides. Sen’i Gakkaishi 2010, 66, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Ogunsona, E.O.; Misra, M.; Mohanty, A.K. Sustainable biocomposites from biobased polyamide 6, 10 and biocarbon from pyrolyzed miscanthus fibers. J. Appl. Polym. Sci. 2017, 134, 44221. [Google Scholar] [CrossRef]
- Savas, L.A.; Dogan, M. Flame retardant effect of zinc borate in polyamide 6 containing aluminum hypophosphite. Polym. Degrad. Stab. 2019, 165, 101–109. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Lai, J.; Lu, C.; Wu, X.; Cai, Y.; Gu, L.; Yang, L.; Zhang, G.; Shi, G. Soy protein and halloysite nanotubes-assisted preparation of environmentally friendly intumescent flame retardant for poly (butylene succinate). Polym. Test. 2020, 81, 106174. [Google Scholar]
- Schartel, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef] [Green Version]
- Levchik, S.V.; Weil, E.D. A review of recent progress in phosphorus-based flame retardants. J. Fire Sci. 2006, 24, 345–364. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal degradation and flame retardance of biobased polylactide composites based on aluminum hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Guo, Y.; Song, L.; Liew, K.M.; Hu, Y. Preparation and characterization of flame-retardant aluminum hypophosphite/poly (vinyl alcohol) composite. Ind. Eng. Chem. Res. 2012, 51, 14065–14075. [Google Scholar] [CrossRef]
- Xu, M.J.; Liu, C.; Ma, K.; Leng, Y.; Li, B. Effect of surface chemical modification for aluminum hypophosphite with hexa-(4-aldehyde-phenoxy)-cyclotriphosphazene on the fire retardancy, water resistance, and thermal properties for polyamide 6. Polym. Adv. Technol. 2017, 28, 1382–1395. [Google Scholar] [CrossRef]
- Duquesne, S.; Futterer, T. Intumescent Systems; Wiley Online Library: New York, NY, USA, 2014; pp. 293–346. [Google Scholar]
- Tirri, T.; Aubert, M.; Aziz, H.; Brusentsev, Y.; Pawelec, W.; Wilén, C.-E. Sulfenamides in synergistic combination with halogen free flame retardants in polypropylene. Polym. Degrad. Stab. 2019, 164, 75–89. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Xu, L.; Qian, L. Phosphorus-containing silica gel-coated ammonium polyphosphate: Preparation, characterization, and its effect on the flame retardancy of rigid polyurethane foam. J. Appl. Polym. Sci. 2018, 135, 46334. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Fu, T.; Xu, Y.-J.; Li, D.-F.; Wang, X.-L.; Wang, Y.-Z. Novel phosphorus-containing halogen-free ionic liquid toward fire safety epoxy resin with well-balanced comprehensive performance. Chem. Eng. J. 2018, 354, 208–219. [Google Scholar] [CrossRef]
- Thirumal, M.; Singha, N.K.; Khastgir, D.; Manjunath, B.; Naik, Y. Halogen-free flame-retardant rigid polyurethane foams: Effect of alumina trihydrate and triphenylphosphate on the properties of polyurethane foams. J. Appl. Polym. Sci. 2010, 116, 2260–2268. [Google Scholar] [CrossRef]
- Zhu, Z.-M.; Rao, W.-H.; Kang, A.-H.; Liao, W.; Wang, Y.-Z. Highly effective flame retarded polystyrene by synergistic effects between expandable graphite and aluminum hypophosphite. Polym. Degrad. Stab. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Horrocks, R.; Sitpalan, A.; Zhou, C.; Kandola, B.K. Flame retardant polyamide fibres: The challenge of minimising flame retardant additive contents with added nanoclays. Polymers 2016, 8, 288. [Google Scholar] [CrossRef] [Green Version]
- Kausar, A. Flame retardant potential of clay nanoparticles. In Clay Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 169–184. [Google Scholar]
- Qi, Y.; Wu, W.; Liu, X.; Qu, H.; Xu, J. Preparation and characterization of aluminum hypophosphite/reduced graphene oxide hybrid material as a flame retardant additive for PBT. Fire Mater. 2017, 41, 195–208. [Google Scholar] [CrossRef]
- Jasinski, E.; Bounor-Legaré, V.; Taguet, A.; Beyou, E. Influence of halloysite nanotubes onto the fire properties of polymer based composites: A review. Polym. Degrad. Stab. 2020, 109407. [Google Scholar] [CrossRef]
- Rybiński, P.; Janowska, G. Influence synergetic effect of halloysite nanotubes and halogen-free flame-retardants on properties nitrile rubber composites. Thermochim. Acta 2013, 557, 24–30. [Google Scholar] [CrossRef]
- Chrissafis, K.; Bikiaris, D. Can nanoparticles really enhance thermal stability of polymers? Part I: An overview on thermal decomposition of addition polymers. Thermochim. Acta 2011, 523, 1–24. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.-M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Pascual, J.; Fages, E.; Fenollar, O.; García, D.; Balart, R. Influence of the compatibilizer/nanoclay ratio on final properties of polypropylene matrix modified with montmorillonite-based organoclay. Polym. Bull. 2009, 62, 367–380. [Google Scholar] [CrossRef]
- Prashantha, K.; Schmitt, H.; Lacrampe, M.-F.; Krawczak, P. Mechanical behaviour and essential work of fracture of halloysite nanotubes filled polyamide 6 nanocomposites. Compos. Sci. Technol. 2011, 71, 1859–1866. [Google Scholar] [CrossRef]
- Francisco, D.L.; de Paiva, L.B.; Aldeia, W.; Lugão, A.B.; Moura, E.A. Investigation on mechanical behaviors of polyamide 11 reinforced with halloysite nanotubes. In Characterization of Minerals, Metals, and Materials 2019; Springer: Berlin, Germany, 2019; pp. 693–701. [Google Scholar]
- Marney, D.; Russell, L.; Wu, D.; Nguyen, T.; Cramm, D.; Rigopoulos, N.; Wright, N.; Greaves, M. The suitability of halloysite nanotubes as a fire retardant for nylon 6. Polym. Degrad. Stab. 2008, 93, 1971–1978. [Google Scholar] [CrossRef]
- Karahan Toprakci, H.A.; Turgut, A.; Toprakci, O. Nailed-bat like halloysite nanotube filled polyamide 6, 6 nanofibers by electrospinning. Polym. Plast. Technol. Mater. 2020, 132, 1–14. [Google Scholar] [CrossRef]
- Sahnoune, M.; Taguet, A.; Otazaghine, B.; Kaci, M.; Lopez-Cuesta, J.M. Fire retardancy effect of phosphorus-modified halloysite on polyamide-11 nanocomposites. Polym. Eng. Sci. 2019, 59, 526–534. [Google Scholar] [CrossRef]
- Marset, D.; Dolza, C.; Boronat, T.; Montanes, N.; Balart, R.; Sanchez-Nacher, L.; Quiles-Carrillo, L. Injection-Molded Parts of Partially Biobased Polyamide 610 and Biobased Halloysite Nanotubes. Polymers 2020, 12, 1503. [Google Scholar] [CrossRef] [PubMed]
- Price, D.; Pyrah, K.; Hull, T.R.; Milnes, G.J.; Ebdon, J.R.; Hunt, B.J.; Joseph, P. Flame retardance of poly (methyl methacrylate) modified with phosphorus-containing compounds. Polym. Degrad. Stab. 2002, 77, 227–233. [Google Scholar] [CrossRef]
- Park, W.-H.; Yoon, K.-B. Optimization of pyrolysis properties using TGA and cone calorimeter test. J. Therm. Sci. 2013, 22, 168–173. [Google Scholar] [CrossRef]
- Zheng, T.; Ni, X. Loading an organophosphorous flame retardant into halloysite nanotubes for modifying UV-curable epoxy resin. RSC Adv. 2016, 6, 57122–57130. [Google Scholar]
- Marney, D.; Yang, W.; Russell, L.; Shen, S.; Nguyen, T.; Yuan, Q.; Varley, R.; Li, S. Phosphorus intercalation of halloysite nanotubes for enhanced fire properties of polyamide 6. Polym. Adv. Technol. 2012, 23, 1564–1571. [Google Scholar] [CrossRef]
- Vahabi, H.; Kandola, B.K.; Saeb, M.R. Flame retardancy index for thermoplastic composites. Polymers 2019, 11, 407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tewarson, A. Heat release rate in fires. Fire Mater. 1980, 4, 185–191. [Google Scholar]
- Porter, D.; Metcalfe, E.; Thomas, M. Nanocomposite fire retardants-a review. Fire Mater. 2000, 24, 45–52. [Google Scholar] [CrossRef]
- Doğan, M.; Bayramlı, E. The flame retardant effect of aluminum phosphinate in combination with zinc borate, borophosphate, and nanoclay in polyamide-6. Fire Mater. 2014, 38, 92–99. [Google Scholar] [CrossRef]
- Qin, H.; Su, Q.; Zhang, S.; Zhao, B.; Yang, M. Thermal stability and flammability of polyamide 66/montmorillonite nanocomposites. Polymer 2003, 44, 7533–7538. [Google Scholar] [CrossRef]
- Straka, P.; Nahunkova, J.; Brožová, Z. Kinetics of copyrolysis of coal with polyamide 6. J. Anal. Appl. Pyrolysis 2004, 71, 213–221. [Google Scholar] [CrossRef]
- Gilman, J.W.; Jackson, C.L.; Morgan, A.B.; Harris, R.; Manias, E.; Giannelis, E.P.; Wuthenow, M.; Hilton, D.; Phillips, S.H. Flammability properties of polymer−layered-silicate nanocomposites. Polypropylene and polystyrene nanocomposites. Chem. Mater. 2000, 12, 1866–1873. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Combustion and fire retardancy of aliphatic nylons. Polym. Int. 2000, 49, 1033–1073. [Google Scholar]
- Chen, X.; Wang, Y.; Jiao, C. Influence of TiO2 particles and APP on combustion behavior and mechanical properties of flame-retardant thermoplastic polyurethane. J. Therm. Anal. Calorim. 2018, 132, 251–261. [Google Scholar] [CrossRef]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. Int. J. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Lin, M.; Li, B.; Li, Q.; Li, S.; Zhang, S. Synergistic effect of metal oxides on the flame retardancy and thermal degradation of novel intumescent flame-retardant thermoplastic polyurethanes. J. Appl. Polym. Sci. 2011, 121, 1951–1960. [Google Scholar] [CrossRef]
- Nakamura, R.; Netravali, A.; Morgan, A.; Nyden, M.; Gilman, J. Effect of halloysite nanotubes on mechanical properties and flammability of soy protein based green composites. Fire Mater. 2013, 37, 75–90. [Google Scholar] [CrossRef]
- Li, L.; Wu, Z.; Jiang, S.; Zhang, S.; Lu, S.; Chen, W.; Sun, B.; Zhu, M. Effect of halloysite nanotubes on thermal and flame retardant properties of polyamide 6/melamine cyanurate composites. Polym. Compos. 2015, 36, 892–896. [Google Scholar] [CrossRef]
- Sun, J.; Gu, X.; Coquelle, M.; Bourbigot, S.; Duquesne, S.; Casetta, M.; Zhang, S. Effects of melamine polyphosphate and halloysite nanotubes on the flammability and thermal behavior of polyamide 6. Polym. Adv. Technol. 2014, 25, 1552–1559. [Google Scholar] [CrossRef]
- Vahabi, H.; Saeb, M.R.; Formela, K.; Cuesta, J.-M.L. Flame retardant epoxy/halloysite nanotubes nanocomposite coatings: Exploring low-concentration threshold for flammability compared to expandable graphite as superior fire retardant. Prog. Org. Coat. 2018, 119, 8–14. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, W.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Flame-retardant cyanate ester resin with suppressed toxic volatiles based on environmentally friendly halloysite nanotube/graphene oxide hybrid. J. Appl. Polym. Sci. 2018, 135, 46587. [Google Scholar] [CrossRef]
- Standard, I. 5659-2, Plastics--Smoke Generation-Part 2: Determination of Optical Density by a Single-Chamber Test; The International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Attia, N.F.; Hassan, M.A.; Nour, M.A.; Geckeler, K.E. Flame-retardant materials: Synergistic effect of halloysite nanotubes on the flammability properties of acrylonitrile–butadiene–styrene composites. Polym. Int. 2014, 63, 1168–1173. [Google Scholar] [CrossRef]
- Du, M.; Guo, B.; Jia, D. Thermal stability and flame retardant effects of halloysite nanotubes on poly (propylene). Eur. Polym. J. 2006, 42, 1362–1369. [Google Scholar] [CrossRef]
- Kashiwagi, T.; Grulke, E.; Hilding, J.; Harris, R.; Awad, W.; Douglas, J. Thermal degradation and flammability properties of poly (propylene)/carbon nanotube composites. Macromol. Rapid Commun. 2002, 23, 761–765. [Google Scholar]
- Smith, R.J.; Holder, K.M.; Ruiz, S.; Hahn, W.; Song, Y.; Lvov, Y.M.; Grunlan, J.C. Environmentally benign halloysite nanotube multilayer assembly significantly reduces polyurethane flammability. Adv. Funct. Mater. 2018, 28, 1703289. [Google Scholar] [CrossRef]
- Zhu, J.; Uhl, F.M.; Morgan, A.B.; Wilkie, C.A. Studies on the mechanism by which the formation of nanocomposites enhances thermal stability. Chem. Mater. 2001, 13, 4649–4654. [Google Scholar] [CrossRef]
- Goda, E.S.; Yoon, K.R.; El-sayed, S.H.; Hong, S.E. Halloysite nanotubes as smart flame retardant and economic reinforcing materials: A review. Thermochim. Acta 2018, 669, 173–184. [Google Scholar] [CrossRef]
- Hajibeygi, M.; Shabanian, M.; Omidi-Ghallemohamadi, M.; Khonakdar, H.A. Optical, thermal and combustion properties of self-colored polyamide nanocomposites reinforced with azo dye surface modified ZnO nanoparticles. Appl. Surf. Sci. 2017, 416, 628–638. [Google Scholar] [CrossRef]
- Majka, T.M.; Witek, M.; Radzik, P.; Komisarz, K.; Mitoraj, A.; Pielichowski, K. Layer-by-Layer Deposition of Copper and Phosphorus Compounds to Develop Flame-Retardant Polyamide 6/Montmorillonite Hybrid Composites. Appl. Sci. 2020, 10, 5007. [Google Scholar] [CrossRef]

| Code | PA610 (wt.%) | HNTs (wt.%) |
|---|---|---|
| PA610 | 100 | 0 |
| PA610/10HNTs | 90 | 10 |
| PA610/20HNTs | 80 | 20 |
| PA610/30HNTs | 70 | 30 |
| Code | TTI(s) | tsos. inflammability (s) | pHRR (kW/m2) | tpHRR (s) | EHC (MJ/kg) | THR (MJ/m2) | FRI |
|---|---|---|---|---|---|---|---|
| PA610 | 73.5 ± 0.5 | 621 ± 3 | 743 ± 4 | 272 ± 3 | 31.7 ± 1.9 | 128.1 ± 10.2 | 1 ± 0.1 |
| PA610/10HNTs | 45.5 ± 0.3 | 853 ± 4 | 800 ± 10 | 290 ± 4 | 52.2 ± 2.4 | 160.8 ± 8.3 | 0.46 ± 0.2 |
| PA610/20HNTs | 47.0 ± 0.4 | 694 ± 4 | 738 ± 10 | 300 ± 2 | 32.3 ± 1.6 | 164.3 ± 7.4 | 0.50 ± 0.3 |
| PA610/30HNTs | 45.0 ± 0.2 | 695 ± 3 | 581 ± 8 | 268 ± 2 | 39.3 ± 1.5 | 147.0 ± 9.9 | 0.68 ± 0.2 |
| Code | SEA (m2/kg) | CO2 Yieldmax (kg/kg) | CO Yieldmax (kg/kg) | Total Smoke (m2/m2) |
|---|---|---|---|---|
| PA610 | 360 ± 15 | 2.2 ± 0.5 | 0.037 ± 0.003 | 915.5 ± 16.5 |
| PA610/10HNTs | 1344 ± 32 | 3.6 ± 0.3 | 0.035 ± 0.002 | 1993.1 ± 25.1 |
| PA610/20HNTs | 403 ± 12 | 2.2 ± 0.4 | 0.067 ± 0.004 | 1245.4 ± 23.6 |
| PA610/30HNTs | 517 ± 26 | 2.7 ± 0.2 | 0.056 ± 0.002 | 1190.1 ± 19.8 |
| Code | MARHE (kW/m2) |
|---|---|
| PA610 | 337.8 ± 5.2 |
| PA610/10HNTs | 409.4 ± 8.1 |
| PA610/20HNTs | 396.9 ± 7.5 |
| PA610/30HNTs | 363.4 ± 9.8 |
| Code | Ds10 | Dsmax |
|---|---|---|
| PA610 | 364.3 ± 4.2 | 372.2 ± 5.9 |
| PA610/10HNTs | 344.2 ± 5.5 | 358.8 ± 4.8 |
| PA610/20HNTs | 450.5 ± 6.1 | 478.5 ± 5.4 |
| PA610/30HNTs | 332.1 ± 4.0 | 332.1 ± 4.5 |
| Volumetric Fraction (µL/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| PA | PA/10HNTs | PA/20HNTs | PA/30HNTs | |||||
| 4 min | 8 min | 4 min | 8 min | 4 min | 8 min | 4 min | 8 min | |
| CO2 | 169.51 | 437.59 | 253.99 | 479.68 | 114.16 | 102.94 | 112.31 | 151.19 |
| CO | 0.18 | 0.10 | 2.83 | 2.97 | 0.17 | 0.01 | 0.36 | 0.23 |
| HCl | 0.11 | 0.15 | 0.12 | 0.33 | 0.02 | 0.02 | 0.27 | 0.20 |
| HF | 0.05 | 0.04 | 0.16 | 0.17 | 0.05 | 0.07 | 0.03 | 0.06 |
| HCN | 0.16 | 0.03 | 0.81 | 0.95 | 0.38 | 0.46 | 0.12 | 0.06 |
| NO2 | 0.42 | 0.58 | 0.06 | 0.81 | 0.31 | 0.16 | 0.29 | 0.29 |
| SO2 | 33.15 | 29.38 | 58.73 | 57.71 | 22.85 | 22.51 | 25.63 | 26.03 |
| NO | 0.36 | 0.65 | 0.23 | 1.22 | 0.15 | 0.13 | 0.18 | 0.25 |
| Code | CITG | |
|---|---|---|
| 4 min | 8 min | |
| PA610 | 0.125 ± 0.005 | 0.111 ± 0.005 |
| PA610/10HNTs | 0.221 ± 0.005 | 0.220 ± 0.005 |
| PA610/20HNTs | 0.087 ± 0.005 | 0.085 ± 0.005 |
| PA610/30HNTs | 0.096 ± 0.005 | 0.098 ± 0.005 |
| Code | Heat Release (MJ/kg) | ∆T (°C) |
|---|---|---|
| PA610 | 33.2 ± 0.2 | 1.7 ± 0.1 |
| PA610/10HNTs | 30.2 ± 0.1 | 1.5 ± 0.1 |
| PA610/20HNTs | 27.3 ± 0.1 | 1.4 ± 0.1 |
| PA610/30HNTs | 23.9 ± 0.2 | 1.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marset, D.; Dolza, C.; Fages, E.; Gonga, E.; Gutiérrez, O.; Gomez-Caturla, J.; Ivorra-Martinez, J.; Sanchez-Nacher, L.; Quiles-Carrillo, L. The Effect of Halloysite Nanotubes on the Fire Retardancy Properties of Partially Biobased Polyamide 610. Polymers 2020, 12, 3050. https://doi.org/10.3390/polym12123050
Marset D, Dolza C, Fages E, Gonga E, Gutiérrez O, Gomez-Caturla J, Ivorra-Martinez J, Sanchez-Nacher L, Quiles-Carrillo L. The Effect of Halloysite Nanotubes on the Fire Retardancy Properties of Partially Biobased Polyamide 610. Polymers. 2020; 12(12):3050. https://doi.org/10.3390/polym12123050
Chicago/Turabian StyleMarset, David, Celia Dolza, Eduardo Fages, Eloi Gonga, Oscar Gutiérrez, Jaume Gomez-Caturla, Juan Ivorra-Martinez, Lourdes Sanchez-Nacher, and Luis Quiles-Carrillo. 2020. "The Effect of Halloysite Nanotubes on the Fire Retardancy Properties of Partially Biobased Polyamide 610" Polymers 12, no. 12: 3050. https://doi.org/10.3390/polym12123050





